Abstract
The standard procedure for remnant gastric cancer after esophago-proximal gastrectomy is total resection of the remnant stomach considering blood supply. However, sometimes surgery may be too invasive due to severe adhesion in the thoracic and mediastinal cavity. The blood supply to the remnant stomach depends on the right gastroepiploic artery and the right gastric artery. Therefore, preservation of the proximal region of the remnant stomach is thought to be anatomically impossible. We report a case of remnant gastric cancer that developed more than 12 years after lower thoracic esophagectomy plus proximal gastrectomy for Siewert Type I squamous cell carcinoma. We used intra-operative indocyanine green (ICG) venous-injection to evaluate blood flow and distal gastrectomy of the remnant stomach was performed by preserving the proximal stomach in the thoracic cavity through an abdominal approach. There were no complications of the remnant stomach or the anastomosis to the jejunum after surgery. In this case, we focused on the blood supply by collateral circulation through the anastomotic line from the remnant esophagus. After confirming blood supply with intra-operative evaluation using ICG fluorescence, less-invasive distal gastrectomy was successfully performed. As the intra-operative ICG-based evaluation for blood supply is a simple and safe method, it might be useful for determining the resection margin of various organs and be effective for the introduction of less invasive surgery. Here, we report a case and a review of the literature.
Keywords: blood-flow evaluation, esophageal carcinoma, indocyanine green, intra-operative, remnant gastric cancer
Lymphadenectomy is the standard procedure for gastro-intestinal (GI) cancer surgery. It is accompanied by vascular division, which requires the wide resection of the organ with the primary tumor. If intra-operative accurate evaluation for blood flow is available, less invasive and organ-preserving surgery can be achieved.
Recently, near-infrared fluorescence using indocyanine green (ICG) has been introduced into GI surgery to evaluate intra-operative blood supply in organs.1 Takahashi, et al. reported that preservation of the remnant stomach could be safely performed during distal pancreatectomy (DP) in patients who had previously undergone distal gastrectomy (DG) with intra-operative ICG evaluation of blood supply in the remnant stomach.2 After DG, the arterial blood supply to the remnant stomach primarily consists of arterial branches of the splenic artery (e.g., the short gastric artery). The standard DP procedure involves resection of the pancreatic body/tail combined with splenectomy and division of the splenic vessels, which, therefore, indicates that in patients with prior DG, the DP procedure is associated with the potential loss of blood supply to the remnant stomach. Takahashi demonstrated that intra-operative ICG fluorescence angiography revealed that blood circulated in a caudally direction from the esophagogastric junction through the intramural capillary in the remnant stomach. On reconstruction after esophagectomy, there have been some reports on the efficacy of ICG evaluation for blood supply in gastric or intestinal conduits to predict post-operative complications.3, 4
In this case report, we experienced a patient with gastric cancer in the remnant stomach who had undergone a lower esophagectomy plus proximal gastrectomy and subsequent radiation more than 12 years previously. We hypothesized that, in the long term, following esophagectomy revascularization through esophagogastric anastomosis might occur in the proximal region of the remnant stomach. Intra-operative ICG evaluation of blood supply enabled less-invasive surgery, and the preservation of the proximal part of the remnant stomach without a thoracic approach.
PATIENT REPORT
A 69-year-old woman underwent lower esophagectomy plus proximal gastrectomy and esophagogastric tube anastomosis through the retro-mediastinal route for abdominal esophageal squamous carcinoma at another hospital. She received subsequent radiation after surgery and suffered bilateral pleural effusion, which might be associated with radiation between 2 and 3 years after surgery. Twelve years after the initial surgery, local development of gastric cancer was identified in the middle portion of the remnant stomach by a follow-up esophagogastroduodenoscopy (EGD) and she was referred to our hospital. She had no other previous disease and no history of smoking, alcohol, or allergic episodes. Preoperative examinations including computed tomography (CT), upper gastrointestinal X-ray examination, and EGD diagnosed gastric cancer (cType0-IIc, por-sig) at the middle region of the remnant stomach with no apparent lymph node or distant organ metastasis (cStageIA, cT1bN0M0) (Fig. 1). In general, total resection of the remnant stomach through the thoracoabdominal approach is the standard surgery. However, severe adhesion in the thoracic and lower mediastinal cavities due to the initial surgery and subsequent radiation was expected and, what was more, she refused thoracotomy because of the experience of the initial surgery. Therefore, we planned to perform a less invasive surgery with intra-operative ICG-guided evaluation for blood supply. We have performed this surgical procedure as a clinical trial previously approved by institutional review board, and got informed consent from the patient. We performed a laparotomy with an upper abdominal incision from the xiphoid to the navel. As expected, there was severe adhesion in the upper abdominal cavity. There was no peritoneal dissemination or liver metastasis and intraoperative peritoneal lavage cytology tested negative for malignancy. The remnant stomach was carefully isolated from the adjacent organs including the liver, pancreas, spleen, and esophagus hiatus. The right gastroepiploic artery was identified and could be preserved, however, the right gastric artery was difficult to preserve because of severe adhesion to the liver. When the remnant stomach in the abdominal cavity was almost completely mobilized and separated from the surrounding tissues, we examined blood supply to the remnant stomach by means of intra-operative ICG fluorescence imaging with a near-infrared camera system. First, we clamped the distal part of gastric body, right gastroepiploic artery and vein to block the blood flow to the stomach wall from the anal side of the stomach (Fig. 2). Next, the ICG solution including 25 mg of ICG in 10 mL of saline was gently injected through a peripheral vein. The ICG fluorescence was illuminated with a near-infrared laser beam with a laparoscopic system (KARL STORZ GmbH & Co. KG, Tuttlingen, Germany). Imaging was generated using a high-end full high definition camera system (IMAGE 1 SPIES™, KARL STORZ) connected to a laparoscope with a 30° field of direction and 10 mm diameter equipped with a specific filter. Within a few minutes after injection of the ICG solution, the blood flow was confirmed by ICG fluorescence directed from the oral side of the remnant stomach up to approximately 2 cm over the cranial edge of tumor. Two cm past the oral side of the cranial edge of the tumor was decided as the resection line (Figs. 2 and 3). The pancreas head was attached to the pyloric region physiologically due to the anatomical anomaly. The pylorus ring was preserved; however, the supra- and infra-pyloric lymph nodes were completely dissected. Reconstruction with a Roux-en-Y gastro-jejunostomy was accomplished thorough the antecolic route. The operation time was 322 min. and total blood loss was 370 mL. Pathological examination revealed pStage IB gastric cancer (por2 > sig, pT2N0). There was no anastomotic leakage in an upper gastrointestinal X-ray examination and no stenosis or necrosis in EGD (Figs. 4A and B). Water and food intake were started on the 3rd and 7th post-operative day (POD), respectively. Her discharge was prolonged up to the 28th POD due to Grade 3 wound infection [Common Terminology Criteria for Adverse Events (CTCAE) version 4.0]. At 6 months after surgery the patient was doing well and no recurrence had been experienced.
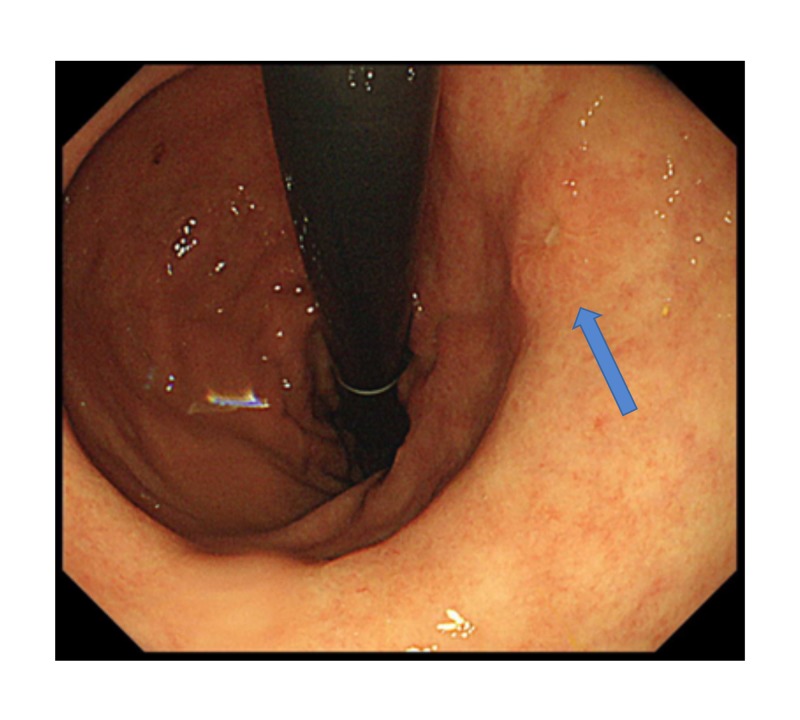
Fig. 1.
Preoperative EGD. Type 0-Ⅱc cancer was identified in the greater curvature posterior wall side of the middle portion of the remnant stomach (blue arrow). EGD, esophagogastroduodenoscopy.
Fig. 2.
The distal part of the gastric body, right gastroepiploic artery and vein was clamped to block the blood flow from the anal side of the stomach. The division lines were indicated as e and f. a; diaphragm line, b; proximal edge of the tumor (marking clips were placed), c; clamping line (blue rectangle), d; right gastric artery was cut before clamping, e; 2 cm oral side from the line placed with marking clips, f; 2 cm oral side from the pyloric ring, t; tumor (blue circle), the distance between a and b was approximately 5 cm.
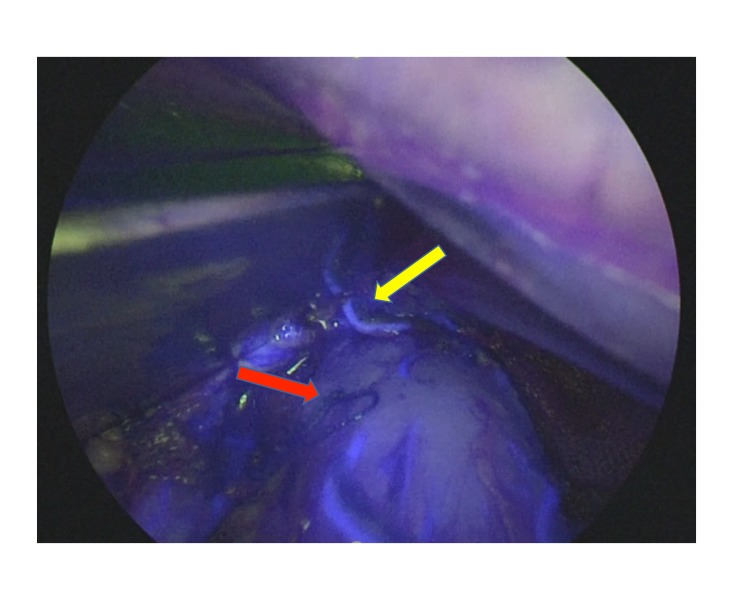
Fig. 3.
Intraoperative image and schema of division line. blood flow visualized with fluorescence was beyond the oral edge of the tumor (yellow arrow), which was marked with endoscopic clips (red arrow). The upper side is the proximal region of the gastric tube.
Fig. 4.

The residual stomach was observed with post-operative EGD. A: The mucosa of the residual stomach showed a bit of congestion, however with no ischemic change. B: The EGD view of the residual stomach-jejunostomy. Although fur was attached, there was no apparent anastomotic failure (blue arrow). EGD, esophagogastroduodenoscopy.
DISCUSSION
According to recent advances in early diagnosis and multidisciplinary treatment, patient prognosis after esophageal cancer surgery has improved and the incidence of remnant gastric cancer (RGC) has risen, especially in long-term survivors. It is reported that the RGC cumulative incidence rate for 10 years was 8.6%.5 The standard procedure for RGC after esophago-proximal gastrectomy is total resection of the remnant stomach considering appropriate lymph node dissection and blood supply. However, sometimes this surgery may be too invasive due to severe adhesion in the thoracic and mediastinal cavities. The blood supply to the remnant stomach is considered to depend on the right gastroepiploic artery and the right gastric artery, therefore it had been thought that preservation of the proximal part of the remnant stomach was impossible. Although it is unknown whether collateral circulation through the anastomotic line from the residual esophagus develops to maintain sufficient blood flow, Saito et al. reported two cases of gastric conduit cancer (GCC) after esophagectomy plus reconstruction with gastric tube, in which the proximal part was successfully preserved using intra-operative ICG evaluation for residual gastric tubes.6 In these two cases, distal gastrectomy was performed 4 and 5 years after esophagus surgery, respectively. In our case, distal gastrectomy of the residual stomach was performed 12 years after the initial surgery and we speculated that collateral circulation through the anastomotic line from the residual esophagus had sufficiently developed over the intervening years (Table 1).
Table 1.
Clinical characteristics of the three patients who underwent gastrectomy with ICG-guided blood flow evaluation
| Case No. | Age (years) | Sex | Location of esophageal cancer | Interval from esophagectomy (months) | Location of GTC | Operative time and blood loss | pStage | POD of oral intake (days) | Hospital stay (days) | Outcome after surgery |
| 1 | 52 | M | Ut | 67 | Lower | 538 min, 1,490 mL | T2N0 Adeno Ca | 14 | 25 | 5 Months alive |
| 2 | 50 | M | Mt | 47 | Middle | 783 min, 2,855 mL | T4N0 SCC (recurrence) | 31 | 62 | 4 Months alive |
| 3 | 69 | F | Ae | 154 | Middle | 322 min,370 mL | T2N0 Adeno Ca | 7 | 28 | 6 Months alive |
Cases 1 and 2 were reported by Saito et al. Case 3 is our case.
Adeno Ca, adenocarcinoma; Ae, abdominal esophagus; F, female; GTC, gastric tube cancer; min, minutes; ICG, Indocyanine green; M, male; Mt, middle thoracic; POD, postoperative days; pStage, pathological stage; SCC, squamous cell carcinoma; Ut, upper thoracic.
A previous study has reported that the median interval between esophagectomy and GCC detection was 86 months.5 Therefore, in most cases, blood flow by collateral circulation is supposed to be in effect at the time of GCC surgery. To evaluate the blood flow through the anastomotic line, it is necessary to use intra-operative evaluation for blood supply in the proximal stomach by clamping the right gastroepiploic artery, the right gastric artery, and gastric wall circulation.
ICG is commonly used for liver and circulatory function tests via intra-venous injection. Furthermore, it is widely used for the detection of sentinel lymph nodes6 and intra-operative determination of the detachment line in the digestive tract.7, 8
Moreover, there have been several reports on the ICG evaluation of blood flow in the gastric conduit to predict anastomosis leakage in esophagectomy reconstruction.9–11 In this case, blood flow was observed at up to 2 cm from the proximal edge of the tumor, so it was possible to resect the distal stomach in the abdominal cavity by preserving the proximal side of the remnant stomach located in the thoracic and mediastinal cavities. There was no necrosis or suture failure of the remnant stomach after surgery and feeding function was good. However, insufficient blood flow might have produced fatal complications such as stomach necrosis and empyema, therefore, careful observation including delayed oral intake was needed in this case.
In this report, intra-operative ICG fluorescent angiography enabled the preservation of the proximal remnant stomach after esophagogastric anastomosis, which was anatomically considered to be no blood supply. According to our experience, blood flow through anastomosis and intramural circulation might occur in the long-term after initial GI surgery. In conclusion, as intra-operative ICG-based evaluation for blood supply is simple and safe, it might be an effective method for determining the resection margin of various organs and be useful for the introduction of less invasive surgery.
Authors’ contributions: WM contributed to the drafting of the manuscript. YU, HS, YK, YM, HK, YF, TO, TS, SH, and KA contributed to the critical revision of the manuscript. YF contributed to the final approval of the manuscript. All authors read and approved the final manuscript.
Consent for publication: Consent for publication was obtained from the patients.
The authors declare no conflict of interest.
REFERENCES
- 1. Schaafsma B, Mieog J, Hutteman M, van der Vorst J, Kuppen P, Löwik C, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104:323-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takahashi H, Nara S, Ohigashi H, Sakamoto Y, Gotoh K, Esaki M, et al. Is Preservation of the Remnant Stomach Safe During Distal Pancreatectomy in Patients Who Have Undergone Distal Gastrectomy?. World J Surg. 2013;37:430-6. [DOI] [PubMed] [Google Scholar]
- 3. Noma K, Shirakawa Y, Kanaya N, Okada T, Maeda N, Ninomiya T, et al. Visualized Evaluation of Blood Flow to the Gastric Conduit and Complications in Esophageal Reconstruction. J Am Coll Surg. 2018;226:241-51. [DOI] [PubMed] [Google Scholar]
- 4. Shimada Y, Okumura T, Nagata T, Sawada S, Matsui K, Hori R, et al. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus. 2011;8:259-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bamba T, Kosugi S, Takeuchi M, Kobayashi M, Kanda T, Matsuki A, et al. Surveillance and treatment for second primary cancer in the gastric tube after radical esophagectomy. Surg Endosc. 2010;24:1310-7. [DOI] [PubMed] [Google Scholar]
- 6. Saito T, Yano M, Motoori M, Kishi K, Fujiwara Y, Shingai T, et al. Subtotal Gastrectomy for Gastric Tube Cancer After Esophagectomy: A Safe Procedure Preserving the Proximal Part of Gastric Tube Based on Intraoperative ICG Blood Flow Evaluation. J Surg Oncol. 2012;106:107-10. [DOI] [PubMed] [Google Scholar]
- 7. Miyashiro I, Hiratsuka M, Kishi K, Takachi K, Yano M, Takenaka A, et al. Intraoperative diagnosis using sentinel node biopsy with indocyanine green dye in gastric cancer surgery: an institutional trial by experienced surgeons. Ann Surg Oncol. 2013;20:542-6. [DOI] [PubMed] [Google Scholar]
- 8. Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa S, et al. ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc. 2017;31:4184-93. [DOI] [PubMed] [Google Scholar]
- 9. Kumagai Y, Ishiguro T, Haga N, Kuwabara K, Kawano T, Ishida H, et al. Hemodynamics of the reconstructed gastric tube during esophagectomy: assessment of outcomes with indocyanine green fluorescence. World J Surg. 2014;38:138-43. [DOI] [PubMed] [Google Scholar]
- 10. Karampinis I, Ronellenfitsch U, Mertens C, Gerken A, Hetjens S, Post S, et al. Indocyanine green tissue angiography affects anastomotic leakage after esophagectomy. A retrospective, case-control study. Int J Surg. 2017;48:210-4. [DOI] [PubMed] [Google Scholar]
- 11. Ohi M, Toiyama Y, Mohri Y, Saigusa S, Ichikawa T, Shimura T, et al. Prevalence of anastomotic leak and the impact of indocyanine green fluorescein imaging for evaluating blood flow in the gastric conduit following esophageal cancer surgery. Esophagus. 2017;14:351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]