Abstract
As the most common genetic cause of Parkinson's disease (PD), the role of human leucine-rich repeat kinase 2 (hLRRK2) in the efficacy of PD treatment is a focus of study. Our previous study demonstrated that mushroom body (MB) expression of hLRRK2 in Drosophila could recapitulate the clinical feature of sleep disturbances observed in PD patients, and melatonin (MT) treatment could attenuate the hLRRK2-induced sleep disorders and synaptic dysfunction, suggesting the therapeutic potential of MT in PD patients carrying hLRRK2 mutations; however, no further study into the impacts on memory deficit was conducted. Therefore, in the current paper, the study of the effects of MT on hLRRK2 flies was continued, to determine its potential role in the improvement of memory deficit in PD. To achieve this, the Drosophila learning and memory phases, including short- and long-term memory, were recorded; furthermore, the effect of MT on calcium channel activity during neurotransmission was detected using electrophysiology patch clamp recordings. It was demonstrated that MT treatment reversed hLRRK2-induced long-term memory deficits in Drosophila; furthermore, MT reduced MB calcium channel activities. These findings suggest that MT may exerts therapeutic effects on the long-term memory of PD patients via calcium channel modulation, thus providing indication of its potential to maintain cognitive function in PD patients.
Keywords: melatonin, long-term memory, calcium channel, Drosophila melanogaster, Parkinson's disease
Introduction
Parkinson's disease (PD) is a long-term degenerative disorder of the central nervous system, which is reported to be the second most common neurodegenerative disorder after Alzheimer's disease (1). PD is an age-related disease, affecting 1% of the world population aged over 65 (2). PD is a complex syndrome, clinically characterized by motor symptoms including resting tremor, rigidity, bradykinesia and abnormal gait, as well as non-motor symptoms including autonomic insufficiency, sleep disorders and cognitive impairment (3).
Cognitive disturbances may occur in the early stages of PD in patients, in some cases occurring prior to diagnosis, and gradually increase in prevalence with disease duration(3). It has been reported that 84% of patients with PD exhibit cognitive decline and approximately 48% meet the diagnostic criteria for dementia within a 15-year follow-up period (4); furthermore, among patients with PD, the risk of developing dementia is almost 6 times higher than in the general population (5). A key issue is that there is no defined guidelines to clinically define cognitive deficit in old age, creating difficultly in determining whether cognitive deficit is prevalent in PD patients. Thus, our group aims to identify direct evidence for an association between clinical progression of PD and cognitive function deficit, to ultimately provide a basis for therapeutic approaches that may improve patient quality of life and functional status.
Memory, a complex term for cognitive processes and a set of mind faculties, involved in encoding, storage and retrieval of true and false information (6), may influence quality of life at all stages of life; in turn memory deficit is a source of burden for government and societies. Certain data indicate memory deficit and impaired memory processes in patients with PD (7), the extent of which often depends on age of onset, disease duration and the progression of clinical symptoms (8). More notably, although recognition memory deficits may be observed in PD with or without dementia; however, a greater recognition memory deficit has been observed in PD with dementia (9). These previous findings indicate a memory deficit associated with PD progression, though further studies are still required to specifically investigate the molecular mechanism and potential therapies for worsening memory deficits.
Melatonin (MT), produced by several tissues besides the pineal gland in animals (10), which is established to be an endogenous regulator of the sleep/wake cycle, may improve sleep quality and memory decline in both normal aging and dementia (11). The impacts of MT on intracellular Ca2+ concentration and calcium channel activities (12) have been reported as the potential mechanisms underlying the therapeutic effects of MT on degenerative disease; however, its specific mechanisms of action remain unclear. As a natural supplement, MT is non-toxic to the human body, which would supplement its efficacy as a potential treatment for patients. In this regard, larger scale and longer term studies are needed to evaluate the effect and mechanism of MT on intracellular Ca2+-related neurotransmission.
The fruit fly, Drosophila melanogaster, is typically used as a model to study neurological diseases due to the similarity of the nervous systems between fruit flies and humans (13,14). In a previous study by our group (15), the role of human leucine-rich repeat kinase 2 (hLRRK2), the most common genetic cause of PD (16), was investigated with regard to sleep problems, and the therapeutic potential of MT in hLRRK2-associated sleep problems was investigated in Drosophila. It was identified that 4 mM MT attenuated hLRRK2-induced sleep disorders and synaptic dysfunction, but the effects on memory deficits were undetermined. Therefore, in the current study, as a follow-on study of our previous work, the aim was to determine the potential molecular mechanism of MT with regard to Drosophila memory deficits by using a series of assays, including behavior tests and whole cell patch-clamp recordings in vitro, to thus elucidate the potential role of MT in hLRRK2-induced memory defects.
Materials and methods
Generation of hLRRK2 transgenic flies
The transgenic flies were constructed to bear different hLRRK2 constructs, Wild-type (WT) and G2019S [causing loss of dopamine cells to result in more severe symptoms of Parkinson's disease (17)], under the control of a yeast upstream activating sequence (UAS). A green fluorescent protein (GFP) XbaI-EcoRI fragment, gifted from Dr Pei Zhong (Department of Neurology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China), was first ligated into a pUAST vector to generate a UAS-GFP construct. Flag-tagged hLRRK2 (amino acids 1,333–1,516; gifted from Dr Pei Zhong) was then inserted between the KpnI and BglII sites of the UAS-GFP vector. The constructs were injected into w1118 embryos (Stock no. 3605, Bloomington Drosophila Stock Center) to obtain transformant lines. Two transgenic lines each of UAS-GFP-hLRRK2 and UAS-hLRRK2-GFP-G2019 were generated. The levels of hLRRK2 expression were examined by western blot analysis (15) using anti-Flag staining. OK107-GAL4 was used to selectively express UAS-GFP-hLRRK2 and UAS-GFP-hLRRK2-G2019S in the mushroom bodies (MBs) of Drosophila. The phenotypic characterization was conducted on hLRRK2-WT and hLRRK2-G2019S lines. w1118 served as a negative control. Drosophila were grown on standard cornmeal medium at 25°C under a 12-h light/dark cycle. For MT treatment, flies were transferred to regular fly food containing 4 mM MT [groups denoted as (M)]. All control groups (w1118, OK107, WT-LRRK2 and G1029S) and corresponding results are reported in our previous work (15).
Electrophysiological recordings from Kenyon cells (KCs) in isolated whole brains
All brains were obtained from adult flies 1–3 days in age. The entire brains including optic lobes were removed from the heads. The dissected brains were then mounted in an RC-26 perfusion chamber (Warner Instruments, LLC, Hamden, CT, USA) containing the recording solution bubbled with 95% O2 and 5% CO2 (2 ml/min) throughout the experiments with the ventral brain surface facing upward. The standard external solution contained (in mM): 101 NaCl, 1 CaCl2, 4 MgCl2, 3 KCl, 5 glucose, 1.25 NaH2PO4 and 20.7 NaHCO3, at pH 7.2, osmolarity 250 Osm. Whole-cell recordings were performed with pipettes (12–20 MΩ) filled with intracellular solution of the following composition (in mM): 102 D-gluconic acid, 102 CsOH, 0.085 CaCl2, 1.7 MgCl2, 17 NaCl, 0.94 EGTA and 8.5 HEPES, PH 7.2, 235 Osm. For calcium current, tetrodotoxin (TTX; 1 µM), tetraethylammonium (TEA; 10 mM) and 4 aminopyridine (4-AP; 1 mM) were added to the external solution and cesium (Cs+; 102 mM) to the internal solution. Giga-ohm seals were achieved prior to recording in an on-cell configuration, followed by whole-cell configuration while in voltage-clamp mode. Recordings were made at room temperature, and a single KC neuron was examined in each brain (n=6 per group). Voltage-clamp recordings were performed using patch-clamp electrodes. In initial experiments, KCs were held at a potential of −70 mV. The voltage pulse applied consisted of a 100 ms step to 30 mV from −70 mV (in 10 mV intervals). Calcium current recordings were performed using a BX51WI upright microscope (Olympus Corporation, Tokyo, Japan). Data were acquired by a MultiClamp 700B amplifier and an Axon Digidata1440A (Molecular Devices, LLC, Sunnyvale, CA, USA), and were filtered at 5 kHz using a built-in filter and digitized at 5 kHz. Amplitude and rise time of the calcium current were analyzed offline using Clampfit 10.2 (Molecular Devices, LLC).
Behavioral experiment
Classical olfactory conditioning in a T-maze for Drosophila is a well-documented paradigm that leads to conditioned odor-avoidance behavior and promotes memory formation (18). In the T-maze training tube, an electric flexible stimulus isolator (ISO-Flex; A.M.P.I., Jerusalem, Israel) was linked to an electrifiable copper grid. The training tube was controlled by a programmable pulse stimulator (Master-8 Channel Programmable Pulse Generator; A.M.P.I.). A vacuum pump (Tianjin Jinteng Experiment Equipment Co., Ltd., Tianjin, China) was used to maintain airflow at 800 ml/min in the training and collection tubes, and deliver odors to each tube.
3-octanol (3-OCT; purity 99%; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and benzaldehyde (BEN; purity 99%; Sigma-Aldrich; Merck KGaA) were used, since both wild-type and transgenic strains can learn 3-OCT/BEN combinations (19). The odors were dissolved in heavy mineral oil (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and delivered to the training tube or to the two collection tubes with a bubbler. The concentration of 3-OCT was 1.0×10−3 [in mineral oil (v/v)] and of BEN was 1.3×10−3 [in mineral oil (v/v)]. The exposure time to each odor and/or electric shock (ES) in the training sessions and the exposure time to odors in the testing sessions were 1 and 2 min respectively, as previously described (20). The ES mode was a series of 12×1.5-sec 70–V pulses in 5-sec intervals, yielding a total stimulus duration of 1 min during a single training cycle.
Behavioral analyses
A single conditioning performance was assayed using a standard protocol (18). Two groups were tested in one complete run to produce one score. The first group, with approximately 100 flies, was conditioned by exposure to 3-OCT (odor A) paired with an ES (conditioned stimulus, CS+) for 1 min, followed by exposure to BEN (odor B) without ES (CS−) for another minute. The flies were then forced into new feeding tubes and remained in the tube for 60 min. Then, flies were forced to choose between two odors during a 2 min testing period. Following the testing period, flies would be gathered from each T-maze collection tube, anesthetized by CO2, and counted. A second reciprocal group of flies was trained with odor B as the CS+ group and odor A as the CS− group. The memory performance index (PI) = NCS−-NCS+⁄NCS− +NCS+, where NCS− represents the number of flies approaching the CS− odor and NCS+ denotes the number of flies approaching the CS+ odor. The average of the two PIs from the reciprocal experiments was taken collectively as one complete PI. To avoid the possibility of odor bias, the two odors used for the tests were exchanged in the training sessions of the reciprocal experiments to generate one complete PI.
Data analysis
Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Data were analyzed by Student's t-tests, one-way analysis of variance (ANOVA) or χ2 tests. When appropriate, ANOVAs were followed by planned comparisons among the relevant groups with a Tukey's Honest Significant Difference test. All data were presented as mean ± standard error of the mean. All P-values were two-tailed and P<0.05 was considered to indicate statistical significance.
Results
MT rescues long-term memory deficits in hLRRK2 flies
hLRRK2 was selectively expressed in KCs using the OK107 promoter to target GAL4 expression in all MBs lobes, and structurally, MB lobes remained intact in flies expressing either hLRRK2-WT or hLRRK2-G2019S (15).
In the current study, the olfactory conditioning paradigm of Tully and Quinn (18) was used since it has been demonstrated to produce a robust memory for detailed analysis of a specific memory phase. In this study, to detect the cognitive impairment caused by hLRRK2 and investigate whether MT could improve the learning and memory deficits of flies expressing hLRRK2, the short- and long-term memory phases were measured (Table I). Our data indicated that hLRRK2 expressed in MBs could affect the long-term memory PI of Drosophila but not the short-term memory PI (Table I). The long-term memory PIs of hLRRK2-WT (0.554±0.036) and hLRRK2-G2019S (0.502±0.028) flies were significantly reduced compared with that of the w1118 control group (0.775±0.013; P<0.01 and P<0.001, respectively). Meanwhile, the long-term memory PI of the hLRRK2-G2019S(M) group (0.695±0.074; P<0.01) was significantly increased compared with that of hLRRK2-G2019S flies without MT, while being restored almost to that of the control group (P>0.05) following MT treatment; in contrast to the hLRRK2-G2019S group without MT treatment. The long-term memory PI of the hLRRK2-WT(M) group (0.593±0.065;) remained significantly reduced compared with that of the control group (P<0.05). Collectively, the data suggest that MB expression of hLRRK2 may induce the long-term but not short-term memory impairment, and the MT treatment could improve this memory deficit.
Table I.
Short- and long-term memory of flies expressing hLRRK2-WT and hLRRK2-G2019S (with and without melatonin treatment), driven by OK107-GAL4-mediated expression of upstream activating sequence transgenes.
| Group | Memory | w1118 | WT | G2019S | WT(M) | G2019S(M) |
|---|---|---|---|---|---|---|
| Performance | Short term | 0.675±0.019 | 0.655±0.021 | 0.064±0.055 | 0.666±0.015 | 0.670±0.019 |
| index | Long-term | 0.775±0.013 | 0.554±0.0356a | 0.502±0.028b | 0.593±0.065c | 0.695±0.074d |
Data are presented as the mean ± standard error of the mean (n=100; three independent experiments performed). Differences between means were determined by one-way analysis of variance followed by the Tukey's Honest Significant Difference test.
P<0.01
P<0.001
P<0.05 vs. w1118
P<0.01 vs. hLRRK2- G2019S mutant without MT. hLRRK2, human leucine-rich repeat kinase 2; WT, wild-type; G2019S, glycine 2019 serine; MT, melatonin; (M), melatonin-treated.
MT improved the calcium channel activity of KCs in the hLRRK2-G2019S mutants
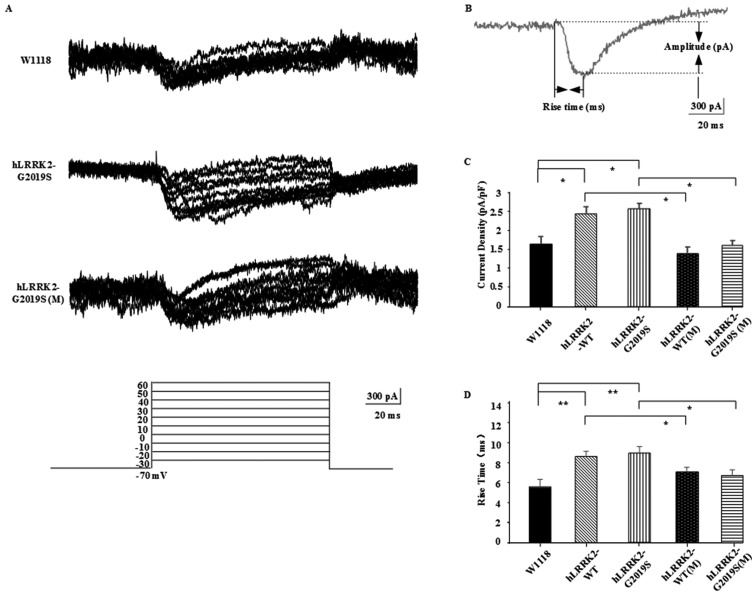
To test the hypothesis that MT may improve hLRRK2-induced learning and memory impairment by regulating calcium channel activity, Ca2+ currents were isolated by blocking NaC conductances with TTX in the external solution, and KC channels with a mixture of TEA and 4-AP in the external solution, and Cs+ in the internal solution. Fig. 1A depicts the current traces recorded in KCs of the w1118 group and hLRRK2-G2019 and hLRRK2-G2019 groups treated with MT, along with the excitation increments; figure 1B presents a diagram of calcium channel current, indicating the rise time (ms) and amplitude (pA) of calcium channel activity. For all groups, the current density (peak amplitude/capacitance, pA/pF) and rise time of the calcium current were detected (Fig. 1C and D). Current density in the hLRRK2-WT (2.399±0.208 pA/pF) and hLRRK2-G2019S (2.565±0.242 pA/pF) groups was significantly increased compared with in the w1118 group (1.653±0.319 pA/pF; P<0.05) due to hLRRK2-expression. Current density in the hLRRK2-WT(M) (1.403±0.138 pA/pF) and hLRRK2-G2019S(M) (1.620±0.286 pA/pF) groups was decreased compared with in the hLRRK2-WT and hLRRK2-G2019S groups (P<0.05), thus the excitation effect of hLRRK2 appeared inhibited by MT. Additionally, the mean open time of hLRRK2-WT (8.552±0.497 ms) and hLRRK2-G2019S (8.833±0.688 ms) channels was significantly increased compared with in the w1118 group (5.624±0.328 ms; P<0.01) due to hLRRK2-expression. The mean open time of hLRRK2-WT channels with MT treatment (6.966±0.451 ms) was significantly decreased compared with hLRRK2-WT channels without MT treatment (8.552±0.497 ms; P<0.05); and of hLRRK2-G2019S channels with MT treatment (6.666±0.557 ms) was significantly decreased compared with hLRRK2-G2019S channels without MT treatment (8.833±0.688 ms; P<0.05). These data indicated that opening of the calcium channel may reduce influx of calcium into the cell, resulting in inhibition of KCs.
Figure 1.
Calcium channel activity was improved in hLRRK2-G2019S mutants following treatment with MT. (A) Representative current traces recorded from KCs of w1118, hLRRK2-G2019, hLRRK2-G2019(M), hLRRK2-WT, hLRRK2-WT(M) (data not shown) in whole-cell current-clamp configuration. The graph (bottom) depicts the series of depolarizing voltage steps from a holding potential of −70 to 30 mV elicited by rapid activation of inward calcium currents in KCs. (B) Diagram of calcium channel current, showing the Rise time (ms), Delay time (ms) and Amplitude (pA). (C and D) MT decreased the hLRRK2-induced calcium channel current density and the mean rise time. Data are presented as the mean ± standard error of the mean (n=6; three independent experiments performed). Differences between means were evaluated by one-way analysis of variance followed by the Tukey's Honest Significant Difference test. hLRRK2, human leucine-rich repeat kinase 2; WT, wild-type; G2019S, glycine 2019 serine; KC, Kenyon cell; MT, melatonin; (M), melatonin-treated.
Discussion
Increasing clinical and animal studies have revealed dysregulation of the circadian rhythm in a variety of neurodegenerative diseases (21). MT, a hormone that regulates sleep and wakefulness (22), is proposed to have a physiological role in the aging process (23), since its secretion decreases with aging (24). It has been reported that there are greater reductions in MT secretion in populations with dementia than in those without dementia (25). More notably, it has been revealed that the level of MT may be significantly associated with degenerative changes and disease severity in patients with PD (26).
To detect the association between MT and PD, our previous research focused on the effect of MT on hLRRK2-induced sleep problems and hLRRK2-induced synaptic dysfunction (15). In addition to sleep problems, it was identified that the expression of hLRRK2 in MB did not result in any gross morphological damage; however, could significantly reduce the frequencies of cholinergic synaptic miniature excitatory postsynaptic current (mEPSC) and excitatory postsynaptic potential (EPSP), and increase the synaptic bouton density in transgenic flies. The frequency changes of cholinergic synaptic mEPSC and EPSP, and the increase of synoptic bouton, suggested a negative modulatory effect of hLRRK2 on presynaptic properties; meanwhile, a beneficial effect of MT on the promotion of presynaptic transmission in KCs was indicated. Therefore, our previous study suggested a potential clinical application of MT in patients with PD carrying LRRK2 mutations.
However, to the best of our knowledge, no previous study has detected the effect of MT on the learning and memory changes in PD, and its detailed mechanism particularly in relation to neurotransmitter release and synaptic transmission remains unclear. Our previous findings appeared associated with learning and memory abilities (15) and motivated further study into the effect of MT on learning and memory. Here, the present research primarily revealed that MT treatment could also attenuate hLRRK2-induced learning and memory impairments by regulating the presynaptic membrane calcium channel activity of KCs. Previous genetic study in Drosophila has identified distinct temporal memory phases, including short-term memory, middle-term memory, long-term memory and so-called anesthesia-resistant memory (27). In the Drosophila brain, the MBs are key components involved in olfactory learning and memory and are required during the different phases of memory processing (28). Here, the present study detected the memory traces of adult Drosophila, and the data indicated that the long-term but not the short-term memory of hLRRK2 flies was significantly decreased, suggesting that the expression of hLRRK2 could cause learning and memory deficit; furthermore. G2019S, which is reported to be the most common mutation in LRRK2-associated PD, leading to the loss of dopaminergic neurons, retinal degeneration, decreased climbing activity and early mortality (29,30), may cause a more severe phenotype than WT (17). As mentioned previously, the present data demonstrated that the long-term memory of hLRRK2-G2019S mutants appeared to be impacted to a greater extent than that of the hLRRK2-WT flies. With the treatment of 4 mM MT, the long-term memory of Drosophila expressing hLRRK2 was significantly increased compared with the groups without MT treatment. Notably, the hLRRK2-G2019S(M) flies exhibited a somewhat greater beneficial effect than the hLRRK2-WT(M) flies. In a word, our study was consistent with the hypothesis that MT should rescue the hLRRK2-induced learning and memory deficit.
Our previous study identified that MT could attenuate hLRRK2-induced dysfunction (15), and in the current study, the calcium channel activity, related to the neurotransmission function of the Drosophila brain, was detected by patch-clamp recordings. It was observed that MT inhibited calcium channel activity to reduce the intracellular Ca2+ levels of KCs in the hLRRK2 mutants, and decrease both calcium channel current density and mean rise time compared with in the groups lacking MT treatment. It is established that KCs are a key cell type responsible for learning and memory processes (31), and Ca2+ channels participate in dendritic integration and neurotransmission (32); therefore, the activation of presynaptic Ca2+ channels on KCs may influence postsynaptic output to restore the synaptic transmission and learning and memory dysfunctions caused by hLRRK2.
In conclusion, the present study demonstrated that MT could rescue long-term memory deficit by regulating the presynaptic membrane calcium activity of hLRRK2 mutants, suggesting that MT may be a potential novel therapy for the treatment of memory impairment in LRRK2-associated PD. However, the precise mechanisms underlying the beneficial effects of MT require further study.
Acknowledgements
The authors are thankful to Dr Pei Zhong at the Department of Neurology of the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) for providing constructs and fly stocks.
Funding
The current study was supported by the New Faculty Program of Pharmacy College of Chonqing Medical University (grant no. YXY2016×SZ03).
Authors' contributions
JY and HG conceived and designed the study. DR, ZG and XS performed the experiments. BX revised the manuscript critically. All authors contributed to the data analysis, interpretation and approved the final manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, Fratiglioni L, Lobo A, Martinez-Lage J, Trenkwalder C, et al. Neurologic Diseases in the Elderly Research Group: Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurology. 2000;54(Suppl 5):S21–S23. doi: 10.1212/WNL.54.5.21A. [DOI] [PubMed] [Google Scholar]
- 2.Farlow J, Pankratz ND, Wojcieszek J, Foroud T. Parkinson Disease Overview. In: Adam MP, Ardinger HH, Pagon RA, editors. NCBI Bookshelf. Gene, GeneReviews® (Internet) Seattle (WA): University of Washington, Seattle; 1993–2018. [Google Scholar]
- 3.Tolosa E, Gaig C, Santamaria J, Compta Y. Diagnosis and the premotor phase of Parkinson disease. Neurology. 2009;72:S12–S20. doi: 10.1212/WNL.0b013e318198db11. [DOI] [PubMed] [Google Scholar]
- 4.Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson's disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20:190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson's disease: A community-based, prospective study. Neurology. 2001;56:730–736. doi: 10.1212/WNL.56.6.730. [DOI] [PubMed] [Google Scholar]
- 6.Melton AW. Implications of short-term memory for a general theory of memory 1. J Verbal Learn Verbal Behav. 1963;2:1–21. doi: 10.1016/S0022-5371(63)80063-8. [DOI] [Google Scholar]
- 7.Sagar HJ, Sullivan EV, Gabrieli JDE, Corkin S, Growdon JH. Temporal ordering and short-term memory deficits in Parkinson's disease. Brain. 1988;111:525–539. doi: 10.1093/brain/111.3.525. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan EV, Sagar HJ. Double dissociation of short-term and long-term memory for nonverbal material in Parkinson's disease and global amnesia. A further analysis. Brain. 1991;114:893–906. doi: 10.1093/brain/114.2.893. [DOI] [PubMed] [Google Scholar]
- 9.Whittington CJ, Podd J, Kan MM. Recognition memory impairment in Parkinson's disease: Power and meta-analyses. Neuropsychology. 2000;14:233–246. doi: 10.1037/0894-4105.14.2.233. [DOI] [PubMed] [Google Scholar]
- 10.Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38:313–316. doi: 10.1016/j.biocel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Altun A, Ugur-Altun B. Melatonin: Therapeutic and clinical utilization. Int J Clin Pract. 2007;61:835–845. doi: 10.1111/j.1742-1241.2006.01191.x. [DOI] [PubMed] [Google Scholar]
- 12.Pappolla MA, Sos M, Omar RA, Bick RJ, Hickson-Bick DL, Reiter RJ, Efthimiopoulos S, Robakis NK. Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J Neurosci. 1997;17:1683–1690. doi: 10.1523/JNEUROSCI.17-05-01683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackay TF, Anholt RR. Of flies and man: Drosophila as a model for human complex traits. Annu Rev Genomics Hum Genet. 2006;7:339–367. doi: 10.1146/annurev.genom.7.080505.115758. [DOI] [PubMed] [Google Scholar]
- 14.Koh K, Evans JM, Hendricks JC, Sehgal A. A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci USA. 2006;103:13843–13847. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, Ran D, Zhao X, Huang Y, Long S, Liang F, Guo W, Nucifora FC, Jr, Gu H, Lu X, et al. Melatonin attenuates hLRRK2-induced sleep disturbances and synaptic dysfunction in a Drosophila model of Parkinson's disease. Mol Med Rep. 2016;13:3936–3944. doi: 10.3892/mmr.2016.4991. [DOI] [PubMed] [Google Scholar]
- 16.Greggio E, Cookson MR. Leucine-Rich Repeat Kinase 2 Mutations and Parkinson's Disease: Three Questions. Asn Neuro. 2009;1:e00002. doi: 10.1042/AN20090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Wang X, Yu Y, Li X, Wang T, Jiang H, Ren Q, Jiao Y, Sawa A, Moran T, et al. A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci USA. 2008;105:2693–2698. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 19.Akalal DB, Wilson CF, Zong L, Tanaka NK, Ito K, Davis RL. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem. 2006;13:659–668. doi: 10.1101/lm.221206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinton P, de Virgilio M, Fogarty KE, Rizzuto R. Behaviour, learning and memory. In: Connolly JB, Tully T, editors. Drosophila: A Practical Approach. IRL. Oxford University Press; Oxford: 1998. pp. 265–317. [Google Scholar]
- 21.Videnovic A, Lazar AS, Barker RA, Overeem S. ‘The clocks that time us’ - circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10:683–693. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: Nature's most versatile biological signal? FEBS J. 2006;273:2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 23.Pierpaoli W, Dall'Ara A, Pedrinis E, Regelson W. The pineal control of aging. The effects of melatonin and pineal grafting on the survival of older mice. Ann N Y Acad Sci. 1991;621:291–313. doi: 10.1111/j.1749-6632.1991.tb16987.x. (1 Physiological) [DOI] [PubMed] [Google Scholar]
- 24.Dori D, Casale G, Solerte SB, Fioravanti M, Migliorati G, Cuzzoni G, Ferrari E. Chrono-neuroendocrinological aspects of physiological aging and senile dementia. Chronobiologia. 1994;21:121–126. [PubMed] [Google Scholar]
- 25.Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatr Scand. 1994;89:1–7. doi: 10.1111/j.1600-0447.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 26.Breen DP, Nombela C, Vuono R, Jones PS, Fisher K, Burn DJ, Brooks DJ, Reddy AB, Rowe JB, Barker RA. Hypothalamic volume loss is associated with reduced melatonin output in Parkinson's disease. Mov Disord. 2016;31:1062–1066. doi: 10.1002/mds.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 28.McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- 29.Nichols WC, Pankratz N, Hernandez D, Paisán-Ruíz C, Jain S, Halter CA, Michaels VE, Reed T, Rudolph A, Shults CW, et al. Parkinson Study Group-PROGENI investigators: Genetic screening for a single common LRRK2 mutation in familial Parkinson's disease. Lancet. 2005;365:410–412. doi: 10.1016/S0140-6736(05)17828-3. [DOI] [PubMed] [Google Scholar]
- 30.Di Fonzo A, Rohé CF, Ferreira J, Chien HF, Vacca L, Stocchi F, Guedes L, Fabrizio E, Manfredi M, Vanacore N, et al. Italian Parkinson Genetics Network: A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson's disease. Lancet. 2005;365:412–415. doi: 10.1016/S0140-6736(05)70236-1. [DOI] [PubMed] [Google Scholar]
- 31.Akalal DB, Yu D, Davis RL. A late-phase, long-term memory trace forms in the γ neurons of Drosophila mushroom bodies after olfactory classical conditioning. J Neurosci. 2010;30:16699–16708. doi: 10.1523/JNEUROSCI.1882-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guadalupe A, Steffen H, Magdalena S, Juan B. TRP, TRPL and Cacophony Channels Mediate Ca2+ Influx and Exocytosis in Photoreceptors Axons in Drosophila. PLoS One. 2012;7:1036–1036. doi: 10.1371/journal.pone.0044182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.