BoHV-1 is a ubiquitous pathogen affecting cattle populations worldwide. Infection can result in complicated, polymicrobial infections due to the immunosuppressive properties of the virus. While there are vaccines on the market, they only limit disease severity and spread but do not prevent infection. The financial and animal welfare ramifications of this virus are significant, and in order to develop more effective prevention and treatment regimens, a more complete understanding of the initial steps in viral infection is necessary. This research establishes the initial entry pathway of BoHV-1, which provides a foundation for future development of effective treatments and preventative vaccines. Additionally, it allows comparisons to the entry pathways of other alphaherpesviruses, such as HSV-1.
KEYWORDS: bovine herpesvirus 1, endocytosis, herpesviruses, low pH, viral entry
ABSTRACT
Bovine herpesvirus 1 (BoHV-1) is an alphaherpesvirus that poses a significant challenge to health and welfare in the cattle industry. We investigated the cellular entry route utilized by BoHV-1. We report that BoHV-1 enters Madin Darby bovine kidney (MDBK) cells, bovine turbinate cells, and African green monkey kidney (Vero) cells via a low-pH-mediated endocytosis pathway. Treatment of MDBK cells with hypertonic medium, which inhibits receptor-mediated endocytosis, prevented infection as measured by a beta-galactosidase reporter assay. Treatment of cells with noncytotoxic concentrations of the lysosomotropic agents ammonium chloride and monensin, which block the acidification of endosomes, inhibited BoHV-1 entry in a concentration-dependent fashion. The kinetics of endocytic uptake of BoHV-1 from the cell surface was rapid (50% uptake by ∼5 min). Time-of-addition experiments indicated that the lysosomotropic agents acted at early times postinfection, consistent with entry. Inactivation of virions by pretreatment with mildly acidic pH is a hallmark characteristic of viruses that utilize a low-pH-activated entry pathway. When BoHV-1 particles were exposed to pH 5.0 in the absence of target membrane, infectivity was markedly reduced. Lastly, treatment of cells with the proteasome inhibitor MG132 inhibited BoHV-1 entry in a concentration-dependent manner. Together, these results support a model of BoHV-1 infection in which low endosomal pH is a critical host trigger for fusion of the viral envelope with an endocytic membrane and necessary for successful infection of the target cell.
IMPORTANCE BoHV-1 is a ubiquitous pathogen affecting cattle populations worldwide. Infection can result in complicated, polymicrobial infections due to the immunosuppressive properties of the virus. While there are vaccines on the market, they only limit disease severity and spread but do not prevent infection. The financial and animal welfare ramifications of this virus are significant, and in order to develop more effective prevention and treatment regimens, a more complete understanding of the initial steps in viral infection is necessary. This research establishes the initial entry pathway of BoHV-1, which provides a foundation for future development of effective treatments and preventative vaccines. Additionally, it allows comparisons to the entry pathways of other alphaherpesviruses, such as HSV-1.
INTRODUCTION
Viruses exploit constitutive components of cellular infrastructure to invade the cell interior (1). Herpesviruses, like the majority of animal virus families, hijack endocytosis pathways to deliver their genetic material to the subcellular site of replication (2). Alphaherpesviruses, including the prototypical herpes simplex virus 1 (HSV-1), utilize cellular endosomal pathways in a cell-specific manner, and both low-pH and pH-neutral pathways have been described (3). Direct penetration at the plasma membrane is also a cell-specific means of entry (4).
Bovine herpesvirus 1 (BoHV-1) is a cattle-specific alphaherpesvirus with a worldwide distribution (5). BoHV-1 infection causes severe ulcerative rhinotracheitis, pustular vulvovaginitis and balanoposthitis, abortion, transient immunosuppression, and, critically, lifelong, latent infection (6–8). Host immune suppression by BHV-1 is considered a significant contributor to the polymicrobial disorder known as bovine respiratory disease complex, or shipping fever, which results in over $500 million a year in losses to the United States beef industry (9, 10). BoHV-1 entry and infection is initiated by virus interaction with cellular membranes mediated by viral glycoproteins gB, gC, and gD (7, 11, 12). BoHV-1 gC is nonessential for replication but plays a major role in attachment by binding to cell surface heparan sulfate proteoglycans (13, 14). BoHV-1 gB and gD are essential receptor-binding proteins that are involved in penetration and cell fusion (7, 15–19). BoHV-1 gD interacts with cellular receptors nectin-1 and poliovirus receptor (Pvr) (20–22). BoHV-1 gB or a gH/gL complex may also interact with cellular membrane proteins, including putative alphaherpesvirus gB-receptor PILRα, resulting in fusion of the viral envelope with a cellular membrane (23–28). The cellular entry pathways of BoHV-1 are poorly understood. Here, virus entry is defined as delivery of the uncoated viral genome into the host cell nucleus.
A better determination of effective infectious disease prevention and treatment strategies relies on a complete understanding of the replicative cycle of the pathogen. Since BoHV-1 establishes lifelong latency, understanding the entry pathway and developing ways to interfere with it are particularly important. We delineated the entry pathway of BoHV-1 into MDBK epithelial cells, a cell line commonly used to study BoHV-1 infection in culture. We implemented an array of assays to demonstrate that (i) BoHV-1 entry into MDBK cells requires low-pH-mediated endocytosis at an early, postinternalization step, (ii) BoHV-1 also utilizes low pH for entry into bovine turbinate (BT) and Vero cells, and (iii) BoHV-1 utilizes the host cell proteasome function for entry. In sum, BoHV-1 utilizes a low-pH-mediated endocytosis mechanism to enter certain target cells.
RESULTS AND DISCUSSION
Inhibition of endocytic uptake from the cell surface inhibits BoHV-1 entry into MDBK cells.
Treatment of cells with hypertonic medium inhibits receptor-mediated endocytosis and viral entry by endocytosis (29–32). Hypertonic medium does not inhibit virus entry via a nonendocytic mechanism, such as HSV-1 entry into Vero cells (3). MDBK cells were prebound with BoHV-1 v4a (lacZ+) and incubated with glucose-free medium containing 0.3 M sucrose for the first 30 min of infection. Beta-galactosidase activity at 6 h postinfection (p.i.) is an indicator of viral entry. Cells treated with hypertonic medium were resistant to BoHV-1 entry relative to untreated cells (Fig. 1). The level of inhibition produced by this treatment is consistent with reports of HSV-1 entry by endocytosis (3, 33). This treatment did not induce an evident inhibition of HSV-1 entry into Vero cells, as expected. This suggests that BoHV-1 employs an endocytosis mechanism for efficient entry into MDBK epithelial cells. This is the first report of an endocytic pathway for BoHV-1 entry. These results align with the endocytic pathway described for HSV-1 entry into human epithelial cells (34).
FIG 1.
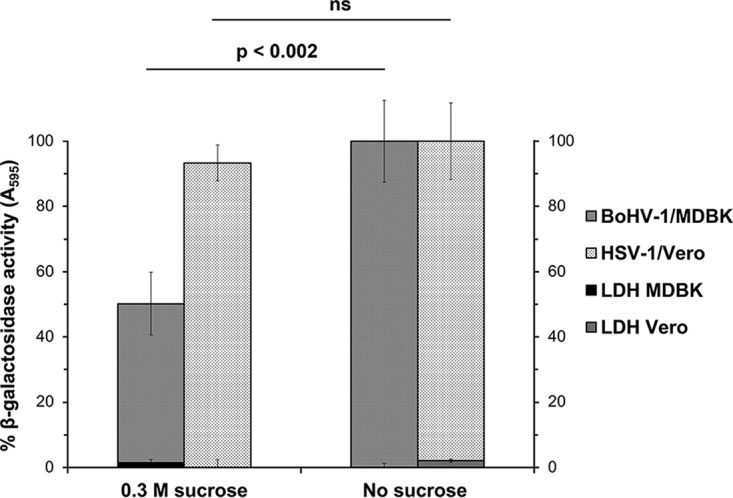
Effect of inhibition of endocytosis on BoHV-1 entry into cells. BoHV-1 was prebound to cells for 2 h at 4°C and then treated with medium containing either 0.3 M sucrose (hypertonic medium) or normal culture medium, and the cells were incubated at 37°C for 30 min. Treated and mock-treated cells were washed with PBS, and noninternalized virus was inactivated with medium buffered to pH 4.7. Infection proceeded for an additional 6 h in the presence of normal culture medium. The beta-galactosidase expression is calculated as a percentage of the activity in mock-treated cells. Percent LDH activity (cytotoxicity) is shown on the right axis. Values are the means of quadruplicate samples with standard deviations. The P values were determined using Student's t test. Results are representative of at least three independent experiments. ns, not significant (P > 0.05).
Lysosomotropic agents inhibit BoHV-1 infection of MDBK, BT, and Vero cells.
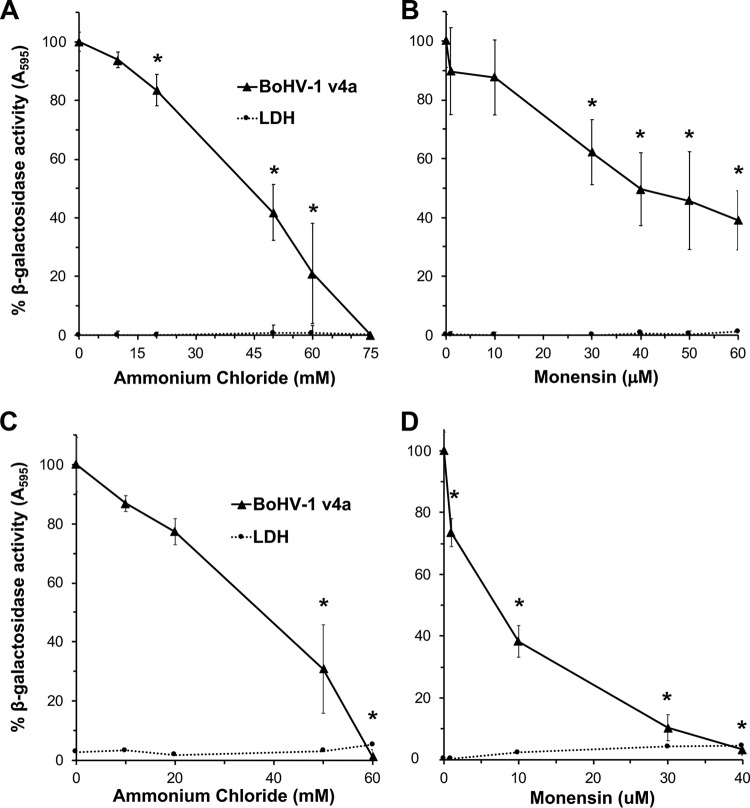
Viruses that enter by endocytosis may require delivery to an acidic endosome prior to successful penetration into the cytosol (35). Ammonium chloride is a weak base that elevates the low pH of acidic compartments and consequently inhibits entry of viruses that require low pH for entry. Monensin is a carboxylic ionophore that prevents endosomal acidification and also inhibits low-pH-dependent viral entry. To determine the role of low pH in BoHV-1 entry, MDBK or bovine turbinate (BT) cells were pretreated with ammonium chloride or monensin for 20 min, followed by infection with BoHV-1 v4a in the continued presence of agent. Beta-galactosidase expression at 6 h postinfection was an indicator of successful entry and infection. Both ammonium chloride (Fig. 2A) and monensin (Fig. 2B) inhibited BoHV-1-induced beta-galactosidase activity in a concentration-dependent manner in both cell types. The inhibitory concentrations of the agents were not cytotoxic to MDBK or BT cells under the tested conditions. This is consistent with BoHV-1 entry by endocytosis (Fig. 1). The results suggest that BoHV-1 requires endosomal acidification for successful entry and infection of MDBK and BT cells.
FIG 2.
Entry of BoHV-1 into cells treated with lysosomotropic agents. MDBK cells (A and B) and BT cells (C and D) were treated with ammonium chloride or monensin at the indicated concentrations for 20 min at 37°C. Cells were infected with BoHV-1 v4a (MOI of 2) for 6 h in the continued presence of the agents. Beta-galactosidase expression was calculated as a percentage of activity in untreated, infected cells. Cytotoxicity is shown as percent LDH activity. Values are the means from quadruplicate samples with standard deviations. The P values relative to no-drug samples were determined using Student's t test (*, P < 0.004). Results are representative of at least three independent experiments.
Vero cells are the prototype cells for pH-neutral entry of HSV-1, which utilizes cell-specific entry pathways. Vero cells also support productive infection by BoHV-1 (28, 36). The entry pathway taken by BoHV-1 in Vero cells was investigated to determine whether Vero cells could support low-pH entry by an alphaherpesvirus. Vero cells were pretreated with ammonium chloride for 20 min, followed by infection with either BoHV-1 v4a or HSV-1 tk12 in the continued presence of agent. Beta-galactosidase expression at 6 h p.i. was an indicator of successful entry and infection. When Vero cells were treated with increasing concentrations of ammonium chloride, there was a dose-dependent inhibition of entry of BoHV-1 v4a (Fig. 3). Ammonium chloride had no inhibitory effect on HSV entry into Vero cells, as reported previously (3). For successful entry, an enveloped virus must overcome a formidable energy barrier to mediate membrane fusion. This can be accomplished by host cell triggers acting on the virus, including receptor binding or low intracellular pH (37). Here, we suggest for the first time that BoHV-1 v4a relies on a low-pH trigger for entry into MDBK, BT, and Vero cell lines. The highest concentrations of ammonium chloride tested on Vero cells inhibited BoHV-1 by ∼43%, suggesting that BoHV-1 also utilizes pH-independent entry pathways into Vero cells. These results further support the role of endocytosis in BoHV-1 entry. HSV-1 is thought to enter Vero cells exclusively by pH-independent fusion at the plasma membrane (3, 4, 25). The current results suggest that Vero cells are not inherently defective in supporting herpesvirus entry by endocytosis or by a low-pH mechanism.
FIG 3.
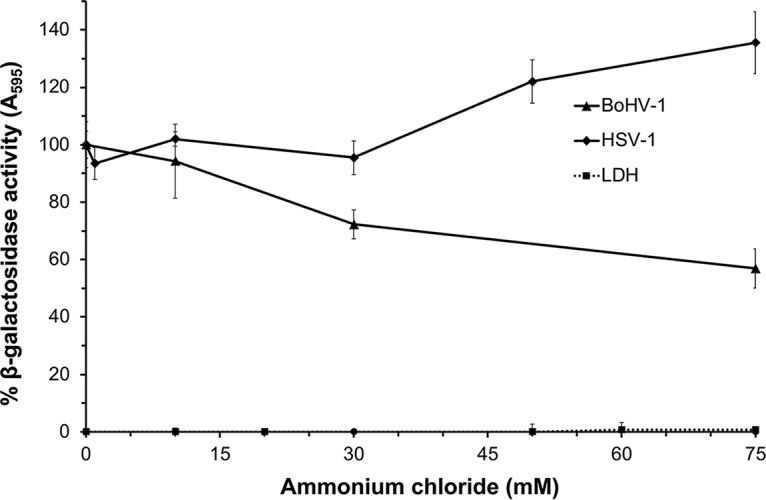
Effect of ammonium chloride on BoHV-1 and HSV-1 entry into Vero cells. Vero cells were treated with ammonium chloride at the indicated concentrations for 20 min at 37°C. Cells were infected with BoHV-1 v4a (MOI of 2) or HSV KOS tk12 (MOI of 7.5) for 6 h in the continued presence of agents. A higher MOI for HSV-1 tk12 was required to achieve beta-galactosidase expression levels comparable to those of BoHV-1 v4a. The beta-galactosidase expression was calculated as a percentage of activity in untreated, infected cells. Cytotoxicity is shown as percent LDH activity. Values are the means from quadruplicate samples with standard deviations. Results are representative of at least three independent experiments.
Ammonium chloride acts at an early time point in BoHV-1 infection.
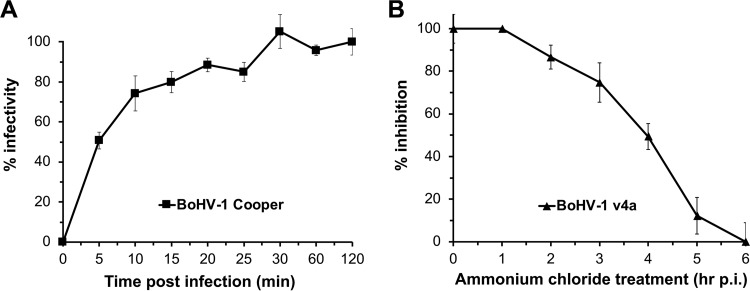
In order to specify the role of the lysosomotropic agents in BoHV-1 entry, we first established the rate of internalization of BoHV-1 into MDBK cells. To determine the kinetics of BoHV-1 uptake by endocytosis, we used a sodium citrate inactivation plaque assay of viral penetration. By 5 min p.i., 50% of BoHV-1 Colorado-1 strain was protected from citrate inactivation (Fig. 4A). By 30 min p.i., greater than 90% of BoHV-1 had been endocytosed. The entry kinetics are more rapid than those previously reported for a different strain of BoHV-1 but are consistent with the rapid penetration of several alphaherpesviruses, including pseudorabies virus and equine herpesvirus 1 (38–41).
FIG 4.
Kinetics of BoHV-1 entry by endocytosis. (A) BoHV-1 was prebound to MDBK cells in 6-well plates at 4°C for 120 min. Unbound virus was removed. At times from 0 to 120 min p.i., noninternalized virus was inactivated with sodium citrate buffer. At 44 h p.i., plaques were counted. Percent infectivity is calculated as a percentage of plaque formation in cells treated at 120 min p.i. Values are the means from quadruplicate samples with standard deviations. Results are representative of at least three independent experiments. To determine the effect of time of addition of ammonium chloride on BoHV-1 entry (B), BoHV-1 was prebound to MDBK cells at 4°C for 90 min. Unbound virus was removed, and 75 mM NH4Cl was added at time points from 1 to 6 h p.i. Infection proceeded for a total of 6 h. Beta-galactosidase expression was calculated as a percentage of activity in cells treated at 6 h p.i.
To determine when the lysosomotropic agents act on virus infection during the reporter assay, BoHV-1 was prebound to MDBK cells at 4°C. Following shift to 37°C at various times p.i., noninternalized virus was inactivated with sodium citrate buffer, followed by the addition of 75 mM ammonium chloride. The later the drug was added, the lower the inhibitory effect on BoHV-1 entry, indicating that lysosomotropic agents act on an early step in BoHV-1 infection (Fig. 4B). Taking into account the kinetics of BoHV-1 entry (Fig. 4A), these data also suggest that the inhibitory effect on entry was not due to an effect on virus interaction with the cell surface. Together, the results suggest that low pH is needed at an early step in BoHV-1 entry following viral binding to and internalization from the cell surface, such as penetration from an endosome. We propose that successful infection of specific cell types by BoHV-1 requires passage of the virus through an acidic intracellular compartment. The BoHV-1 entry pathways supported by other pathophysiologically relevant cell types, such as neurons, are of great interest. We speculate that BoHV-1 relies on low intraendosomal pH for conformational changes in viral envelope glycoproteins, particularly gB, that are necessary for penetration of the host cell cytosol, as for HSV-1 (42–44).
Role of the proteasome in BoHV-1 entry into MDBK cells.
To further characterize BoHV-1 entry into MDBK cells, we investigated the role of the host cell proteasome. The eukaryotic proteasome system is the primary pathway for degradation of intracellular proteins, regulating numerous cellular processes (45). The degradative activity of the 26S proteasome plays a critical role in HSV-1 entry at a postpenetration step (46). MG132 is a peptide aldehyde that competitively inhibits the degradative activity of the 20S proteasome (46, 47). While MG132 has been shown to reduce the release of BoHV-1 from infected cells, the effects of this drug on virus entry have not yet been determined (48). We used the beta-galactosidase reporter assay to evaluate the effect of MG132 on BoHV-1 entry. MG132 treatment of MDBK cells resulted in a concentration-dependent inhibition of BoHV-1-induced beta-galactosidase activity (Fig. 5). These results suggest that BoHV-1 requires proteasome-mediated proteolysis for successful entry and infection of MDBK cells. The precise step in the BoHV-1 infectious cycle that is facilitated by the proteasome is not known. HSV-1 specifically requires proteasome activity for transport of incoming capsids to the nuclear periphery, a process that is mediated by tegument ICP0 (49, 50). In contrast, BoHV-1 ICP0 has not been detected as a virion component, therefore the structural components that might contribute to the proteasome-dependent entry of BoHV-1 are of continued interest (51). The nature and identity of the substrate(s) targeted for degradation during alphaherpesvirus entry also remains to be determined.
FIG 5.
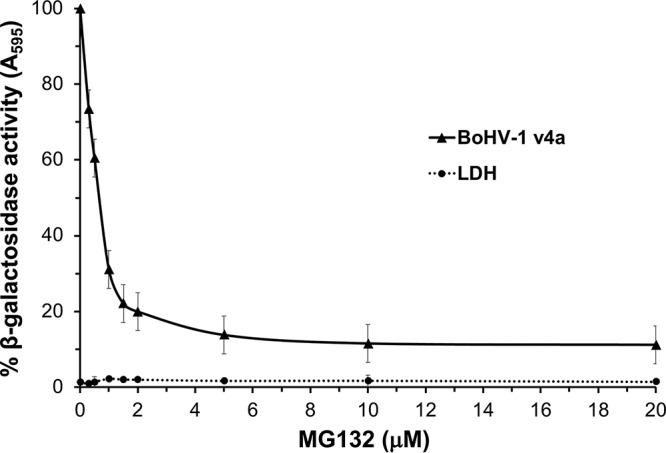
Effect of the proteasome inhibitor MG132 on BoHV-1 infectivity. MDBK cells were treated at the indicated concentrations for 20 min at 37°C. Cells were infected with BoHV-1 v4a (MOI of 2) for 6 h in the continued presence of the agent. The beta-galactosidase expression was calculated as a percentage of activity in untreated, infected cells. Cytotoxicity is shown as percent LDH activity. Values are the means from quadruplicate samples with standard deviations. Results are representative of at least three independent experiments.
Effect of mildly acidic pH pretreatment on infectivity of BoHV-1 virions.
A hallmark of viruses that utilize a low-pH entry pathway is that they are inactivated by exposure to mildly acidic pH in the absence of target membranes (44, 52, 53). Treatment of HSV-1 particles in vitro with buffers of pH 5.5 or below results in the inhibition of entry activity when the virions are added to cells. Experimental exposure of isolated virions to low pH is thought to prematurely activate fusion glycoproteins, rendering them incompetent for entry when virus is added to permissive cells. To support and extend the finding that BoHV-1 entry proceeds via a low-pH pathway, we characterized the effect of pH pretreatments of BoHV-1 particles. BoHV-1 Colorado-1 strain or v4a was incubated at pH 7.2, 6.0, 5.5, or 5.0 for 30 min, neutralized with NaOH, and then assayed for successful entry into MDBK cells. Virion infectivity decreased as the pH pretreatment decreased (Fig. 6). For both the wild-type (Colorado-1) and v4a strains of BoHV-1, approximately 50% of entry activity was abolished by pH 5.5 pretreatment, and greater than 90% of entry activity was abolished at pH 5.0. Under these conditions, the inactivation of BoHV-1 by low pH was irreversible, as neutralization of the treated virus did not restore infectivity to normal levels. These results support the notion that BoHV-1 requires low pH as a cellular cue for successful infection of MDBK cells and suggest that low-pH inactivation of BoHV-1 is irreversible. This property of inactivation is shared by HSV and a number of enveloped viruses. For HSV-1, a target of acid inactivation is gB (42). Whether this is the case for BoHV-1 remains to be determined. The primary portal of entry into the bovine host is the nasopharyngeal mucosal epithelia. The results presented here support a model of BoHV-1 infection of epithelial cells in which low endosomal pH serves as a critical host trigger for fusion of the viral envelope with an endocytic membrane.
FIG 6.
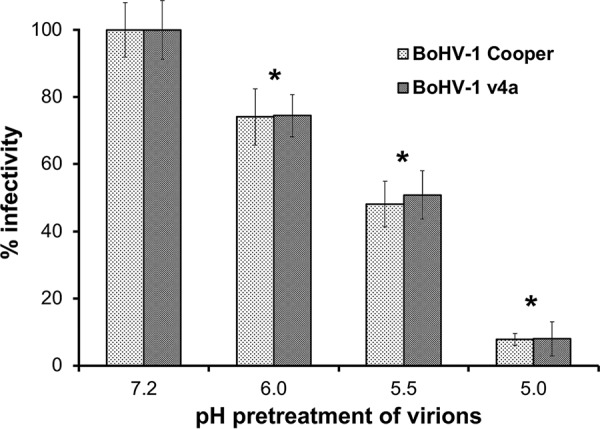
Inactivation of BoHV-1 by pretreatment with mildly acidic pH. BoHV-1 Colorado-1 (Cooper) or v4a was incubated at a range of pHs from 7.2 to 5.0, incubated at 37°C for 30 min, and then neutralized to pH 7.2. Treated virions were added to MDBK cells in 24-well plates. At 44 h p.i. plaques were enumerated as a measure of viral infectivity. The P values relative to the appropriate pH 7.2 sample were determined using Student's t test (*, P < 0.005).
MATERIALS AND METHODS
Cells and viruses.
MDBK, BT, and Vero cells (American Type Culture Collection, Manassas, VA) were propagated in Dulbecco's modified Eagle's medium (ThermoFisher Scientific, Grand Island, NY) supplemented with 5% (MDBK) or 10% (BT and Vero) fetal bovine serum (Atlanta Biologicals, Atlanta, GA). BoHV-1 strain Colorado-1 (Cooper) (American Type Culture Collection, Manassas, VA) was propagated on MDBK cells. A thymidine kinase-negative, beta-galactosidase-positive recombinant of BoHV-1 Colorado-1 containing the E. coli lacZ gene in place of the viral thymidine kinase gene (obtained from C. Whitbeck, G. Cohen, and R. Eisenberg, University of Pennsylvania) was propagated on MDBK cells (54). HSV-1 strain KOS tk12 (obtained from Patricia Spear, Northwestern University), which contains the lacZ gene under the control of an HSV-inducible promoter, was propagated on Vero cells (55).
Cell viability.
Cytotoxicity of agents was assessed by direct measurement of lactate dehydrogenase (LDH) leakage (56). Confluent cell monolayers grown in 96-well plates were treated with experimental concentrations of drugs or cell-culture-grade water for 5.5 h. Cells in triplicate wells were lysed, and plates were incubated for an additional 30 min. LDH leakage was determined using a Pierce LDH cytotoxicity assay kit (Thermo Scientific, Rockford, IL) by following the manufacturer's instructions.
Plaque assay.
Subconfluent cell monolayers grown in 6- or 24-well plates were infected with virus and rocked at room temperature for 2 h, followed by replacement of medium with 5% fetal bovine serum and 2% carboxymethyl cellulose (Sigma-Aldrich, St. Louis, MO). At 44 to 48 h p.i., 10% formalin (VWR International, Solon, OH) was added to dissolve the carboxymethyl cellulose and fix the cells. Monolayers were stained with crystal violet (Sigma-Aldrich, St. Louis, MO), and nonstaining regions (plaques) were counted.
Beta-galactosidase reporter assay of BoHV-1 entry.
Confluent cell monolayers grown in 96-well plates were infected with BoHV-1 (multiplicity of infection [MOI] of ∼2) for 6 h at 37°C. Cell lysates were prepared with 0.5% Igepal (Sigma-Aldrich, St. Louis, MO). Chlorophenol red-β-d-galactopyranoside (Roche Diagnostics, Indianapolis, IN) was added, and beta-galactosidase activity was read at 595 nm with a microtiter plate reader (BioTek Instruments, Winooski, VT). Mean results and standard deviations were calculated for four replicate samples.
Inhibition of endocytic uptake of BoHV-1 from the cell surface.
BoHV-1 v4a (MOI of 4) or HSV-1 KOS tk12 (MOI of 7.5) was prebound to cells on ice at 4°C for 1 h and then treated with glucose-free DMEM (ThermoFisher Scientific, Grand Island, NY) containing 0.3 M sucrose (J. T. Baker, Center Valley, PA) or normal culture medium for 30 min at 37°C. Cells were washed twice with warm phosphate-buffered saline (PBS). Bicarbonate-free DMEM buffered to pH 4.7 was added for 2 min at 37°C to inactivate any noninternalized virus. Cells were then incubated in normal culture medium for 6 h, and virus entry was assessed.
Effect of lysosomotropic agents on BoHV-1 entry.
Stock solutions of 1.5 M ammonium chloride (Sigma-Aldrich, St. Louis, MO) in water were prepared immediately prior to use. Stock solutions of 100 μM monensin (Sigma-Aldrich, St. Louis, MO) in ethanol were stored at −80°C. Confluent cell monolayers grown in 96-well plates were incubated with lysosomotropic agents at various concentrations at 37°C for 20 min. Virus was added and cells were incubated in the continued presence of agent for 6 h. For Vero cell experiments, drug treatments and infections were performed on confluent cell monolayers grown in 24-well plates. At 6 h p.i., cells were lysed and beta-galactosidase activity was determined.
Kinetics of entry assay.
Subconfluent MDBK cell monolayers grown in 6-well plates were preincubated with binding medium comprised of serum-free DMEM buffered with 20 mM HEPES (ThermoFisher Scientific, Grand Island, NY) and containing 0.2% bovine serum albumin (BSA; ThermoFisher Scientific, Fair Lawn, NJ). BoHV-1 Colorado-1 was prebound to cells on ice at 4°C for 90 min. Cells were washed with ice-cold PBS to remove any unbound virus, and then warm DMEM supplemented with fetal bovine serum (FBS) was added and cells were shifted to 37°C. Every 5 min from 0 min p.i. to 30 min p.i. and at 60 and 120 min, virions that were external to the cells were inactivated by incubation with sodium citrate buffer (pH 3.0) for 5 min. After inactivation, the cells were washed with warm PBS and entry was determined by plaque assay.
Proteasome inhibitor treatment of MDBK cells.
A 40 mM stock solution of MG132 (Sigma-Aldrich, St. Louis, MO) in dimethyl sulfoxide (DMSO) was diluted in DMEM. Confluent MDBK cell monolayers grown in 96-well plates were incubated with MG132 at various concentrations at 37°C for 20 min. BoHV-1 v4a was added, and cells were incubated in the continued presence of drug for 6 h. Entry was determined by beta-galactosidase reporter assay.
Low-pH treatment of BoHV-1 particles.
BoHV-1 virions were diluted in serum-free DMEM buffered with 5 mM HEPES, 5 mM 2-(N-morpholino)-ethanesulfonic acid, and 5 mM succinate containing 0.2% BSA. Pretitrated amounts of HCl were added directly to virus preparations to achieve final pHs ranging from 7.2 to 5.0. Samples were incubated at 37°C for 30 min and then neutralized to pH 7.2 by addition of pretitrated amounts of 0.05 N NaOH. Subconfluent cell monolayers grown in 24-well plates were infected with treated and control virus, and infectivity was determined by plaque assay.
ACKNOWLEDGMENTS
We thank Gary Cohen, Roselyn Eisenberg, Patricia Spear, Subramaniam Srikumaran, Naomi Taus, and J. Charles Whitbeck for generous gifts of reagents.
This work was supported by Public Health Service grants AI119159 (A.V.N.) and AI007025 (G.P.) from the National Institute of Allergy and Infectious Diseases and by a grant from the Stanley L. Adler Research Fund.
REFERENCES
- 1.Marsh M, Helenius A. 2006. Virus entry: open sesame. Cell 124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cossart P, Helenius A. 2014. Endocytosis of viruses and bacteria. Cold Spring Harb Perspect Biol 6:a016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicola AV, McEvoy AM, Straus SE. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol 77:5324–5332. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuller AO, Spear PG. 1987. Anti-glycoprotein-D antibodies that permit adsorption but block infection by herpes-simplex virus-1 prevent virion cell-fusion at the cell-surface. Proc Natl Acad Sci U S A 84:5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Office International des Epizooties. 2017. Manual of diagnostic tests and vaccines for terrestrial animals: mammals, birds and bees, p 1–15. Office International des Epizooties, Paris, France. [PubMed] [Google Scholar]
- 6.Jones C, Chowdhury S. 2007. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim Health Res Rev 8:187–205. doi: 10.1017/S146625230700134X. [DOI] [PubMed] [Google Scholar]
- 7.Babiuk LA, van Drunen Littel-van den Hurk S, Tikoo SK. 1996. Immunology of bovine herpesvirus 1 infection. Vet Microbiol 53:31–42. doi: 10.1016/S0378-1135(96)01232-1. [DOI] [PubMed] [Google Scholar]
- 8.Srikumaran S, Kelling CL, Ambagala A. 2007. Immune evasion by pathogens of bovine respiratory disease complex. Anim Health Res Rev 8:215–229. doi: 10.1017/S1466252307001326. [DOI] [PubMed] [Google Scholar]
- 9.Jones C, Chowdhury S. 2010. Bovine herpesvirus type 1 (BHV-1) is an important cofactor in the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract 26:303–321. doi: 10.1016/j.cvfa.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Gershwin LJ, Van Eenennaam AL, Anderson ML, McEligot HA, Shao MX, Toaff-Rosenstein R, Taylor JF, Neibergs HL, Womack J, Bovine Respiratory Disease Complex Coordinated Agricultural Project Research Team. 2015. Single pathogen challenge with agents of the bovine respiratory disease complex. PLoS One 10:e0142479. doi: 10.1371/journal.pone.0142479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang XP, Babiuk LA, Littelvandenhurk SV, Fitzpatrick DR, Zamb TJ. 1991. Bovine herpesvirus-1 attachment to permissive cells is mediated by its major glycoprotein-Gi, gycoprotein-Giii, and glycoprotein-Giv. J Virol 65:1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, van Drunen Littel-van den Hurk S, Babiuk LA, Liang X. 1995. Characterization of cell-binding properties of bovine herpesvirus 1 glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J Virol 69:4758–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang X, Babiuk LA, Zamb TJ. 1992. An in vivo study of a glycoprotein gIII-negative bovine herpesvirus 1 (BHV-1) mutant expressing beta-galactosidase: evaluation of the role of gIII in virus infectivity and its use as a vector for mucosal immunization. Virology 189:629–639. doi: 10.1016/0042-6822(92)90586-E. [DOI] [PubMed] [Google Scholar]
- 14.Okazaki K, Matsuzaki T, Sugahara Y, Okada J, Hasebe M, Iwamura Y, Ohnishi M, Kanno T, Shimizu M, Honda E, Kong Y. 1991. BHV-1 adsorption is mediated by the interaction of glycoprotein gIII with heparinlike moiety on the cell surface. Virology 181:666–670. doi: 10.1016/0042-6822(91)90900-V. [DOI] [PubMed] [Google Scholar]
- 15.Keil GM, Hohle C, Giesow K, Konig P. 2005. Engineering glycoprotein B of bovine herpesvirus 1 to function as transporter for secreted proteins: a new protein expression approach. J Virol 79:791–799. doi: 10.1128/JVI.79.2.791-799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ll YH, Van Drunen Littel-Van Den Hurk S, Liang XP, Babiuk LA. 1997. Functional analysis of the transmembrane anchor region of bovine herpesvirus 1 glycoprotein gB. Virology 228:39–54. doi: 10.1006/viro.1996.8372. [DOI] [PubMed] [Google Scholar]
- 17.Alves Dummer L, Pereira Leivas Leite F, van Drunen Littel-van den Hurk S. 2014. Bovine herpesvirus glycoprotein D: a review of its structural characteristics and applications in vaccinology. Vet Res 45:111. doi: 10.1186/s13567-014-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chase CC, Carter-Allen K, Lohff C, Letchworth GJ III. 1990. Bovine cells expressing bovine herpesvirus 1 (BHV-1) glycoprotein IV resist infection by BHV-1, herpes simplex virus, and pseudorabies virus. J Virol 64:4866–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miethke A, Keil GM, Weiland P, Mettenleiter TC. 1995. Unidirectional complementation between glycoprotein-B homologs of pseudorabies virus and bovine herpesvirus-1 is determined by the carboxyterminal part of the molecule. J Gen Virol 76:1623–1635. doi: 10.1099/0022-1317-76-7-1623. [DOI] [PubMed] [Google Scholar]
- 20.Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol 10:305–319. doi:. [DOI] [PubMed] [Google Scholar]
- 21.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 22.Spear PG, Eisenberg RJ, Cohen GH. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 23.Arii J, Uema M, Morimoto T, Sagara H, Akashi H, Ono E, Arase H, Kawaguchi Y. 2009. Entry of herpes simplex virus 1 and other alphaherpesviruses via the paired immunoglobulin-like type 2 receptor alpha. J Virol 83:4520–4527. doi: 10.1128/JVI.02601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Drunen Littel-van den Hurk S, Khattar S, Tikoo SK, Babiuk LA, Baranowski E, Plainchamp D, Thiry E. 1996. Glycoprotein H (gII/gp108) and glycoprotein L form a functional complex which plays a role in penetration, but not in attachment, of bovine herpesvirus 1. J Gen Virol 77(Part 7):1515–1520. [DOI] [PubMed] [Google Scholar]
- 25.Fuller AO, Santos RE, Spear PG. 1989. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol 63:3435–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer G, Hanon E, Georlette D, Pastoret PP, Thiry E. 1998. Bovine herpesvirus type 1 glycoprotein H is essential for penetration and propagation in cell culture. J Gen Virol 79:1983–1987. doi: 10.1099/0022-1317-79-8-1983. [DOI] [PubMed] [Google Scholar]
- 27.Khattar SK, van Drunen Littel-van den Harke S, Attah-Poku SK, Babiuk LA, Tikoo SK. 1996. Identification and characterization of a bovine herpesvirus-1 (BHV-1) glycoprotein gL which is required for proper antigenicity, processing, and transport of BHV-1 glycoprotein gH. Virology 219:66–76. doi: 10.1006/viro.1996.0223. [DOI] [PubMed] [Google Scholar]
- 28.Schroder C, Keil GM. 1999. Bovine herpesvirus 1 requires glycoprotein H for infectivity and direct spreading and glycoproteins gH(W450) and gB for glycoprotein D-independent cell-to-cell spread. J Gen Virol 80(Part 1):57–61. [DOI] [PubMed] [Google Scholar]
- 29.Mellman I. 1996. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol 12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 30.Daukas G, Zigmond SH. 1984. Selective-inhibition of receptor-mediated but not bulk-fluid-phase pinocytosis in polymorphonuclear leukocytes. J Cell Biol 99:A278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heuser JE, Anderson RG. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol 108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpentier JL, Sawano F, Geiger D, Gorden P, Perrelet A, Orci L. 1989. Potassium depletion and hypertonic medium reduce “non-coated” and clathrin-coated pit formation, as well as endocytosis through these two gates. J Cell Physiol 138:519–526. doi: 10.1002/jcp.1041380311. [DOI] [PubMed] [Google Scholar]
- 33.Delboy MG, Patterson JL, Hollander AM, Nicola AV. 2006. Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells. Virol J 3:105. doi: 10.1186/1743-422X-3-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicola AV. 2016. Herpesvirus entry into host cells mediated by endosomal low pH. Traffic 17:965–975. doi: 10.1111/tra.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh M, Helenius A. 1989. Virus entry into animal cells. Adv Virus Res 36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theodoridis A. 1985. Studies on bovine herpesviruses. Part 1. Isolation and characterization of viruses isolated from the genital tract of cattle. Onderstepoort J Vet Res 52:239–254. [PubMed] [Google Scholar]
- 37.Dimitrov DS. 2004. Virus entry: molecular mechanisms and biomedical applications. Nat Rev Microbiol 2:109–122. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicola AV, Straus SE. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol 78:7508–7517. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neubauer A, Osterrieder N. 2004. Equine herpesvirus type 1 (EHV-1) glycoprotein K is required for efficient cell-to-cell spread and virus egress. Virology 329:18–32. doi: 10.1016/j.virol.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 40.Kopp A, Mettenleiter TC. 1992. Stable rescue of a glycoprotein gII deletion mutant of pseudorabies virus by glycoprotein gI of bovine herpesvirus 1. J Virol 66:2754–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van de Walle GR, Peters ST, VanderVen BC, O'Callaghan DJ, Osterrieder N. 2008. Equine herpesvirus 1 entry via endocytosis is facilitated by alphaV integrins and an RSD motif in glycoprotein D. J Virol 82:11859–11868. doi: 10.1128/JVI.00868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dollery SJ, Delboy MG, Nicola AV. 2010. Low pH-induced conformational change in herpes simplex virus glycoprotein B. J Virol 84:3759–3766. doi: 10.1128/JVI.02573-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dollery SJ, Wright CC, Johnson DC, Nicola AV. 2011. Low-pH-dependent changes in the conformation and oligomeric state of the prefusion form of herpes simplex virus glycoprotein B are separable from fusion activity. J Virol 85:9964–9973. doi: 10.1128/JVI.05291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weed DJ, Pritchard SM, Gonzalez F, Aguilar HC, Nicola AV. 2017. Mildly acidic pH triggers an irreversible conformational change in the fusion domain of herpes simplex virus 1 glycoprotein B and inactivation of viral entry. J Virol 91:e02123-16. doi: 10.1128/JVI.02123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voges D, Zwickl P, Baumeister W. 1999. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochemistry 68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 46.Delboy MG, Roller DG, Nicola AV. 2008. Cellular proteasome activity facilitates herpes simplex virus entry at a postpenetration step. J Virol 82:3381–3390. doi: 10.1128/JVI.02296-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kisselev AF, Goldberg AL. 2001. Proteasome inhibitors: from research tools to drug candidates. Chem Biol 8:739–758. doi: 10.1016/S1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 48.Fiorito F, Iovane V, Cantiello A, Marullo A, De Martino L, Iovane G. 2017. MG-132 reduces virus release in bovine herpesvirus-1 infection. Sci Rep 7:13306. doi: 10.1038/s41598-017-13717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delboy MG, Nicola AV. 2011. A pre-immediate-early role for tegument ICP0 in the proteasome-dependent entry of herpes simplex virus. J Virol 85:5910–5918. doi: 10.1128/JVI.00267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delboy MG, Siekavizza-Robles CR, Nicola AV. 2010. Herpes simplex virus tegument ICP0 is capsid associated, and its E3 ubiquitin ligase domain is important for incorporation into virions. J Virol 84:1637–1640. doi: 10.1128/JVI.02041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barber KA, Daugherty HC, Ander SE, Jefferson VA, Shack LA, Pechan T, Nanduri B, Meyer F. 2017. Protein composition of the bovine herpesvirus 1.1 virion. Vet Sci 4:11. doi: 10.3390/vetsci4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bron R, Wahlberg JM, Garoff H, Wilschut J. 1993. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J 12:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards J, Mann E, Brown DT. 1983. Conformational changes in Sindbis virus envelope proteins accompanying exposure to low pH. J Virol 45:1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller JM, Whetstone CA, Bello LJ, Lawrence WC, Whitbeck JC. 1995. Abortion in heifers inoculated with a thymidine kinase-negative recombinant of bovine herpesvirus 1. Am J Vet Res 56:870–874. [PubMed] [Google Scholar]
- 55.Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 56.Korzeniewski C, Callewaert DM. 1983. An enzyme-release assay for natural cytotoxicity. J Immunol Methods 64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]