Abstract
Background and Objectives
We aimed to investigate the relationship between the recurrence of atrial fibrillation (AF) and fibrosis marker soluble ST2 (sST2) in patients with nonvalvular paroxysmal AF (PAF).
Methods
We prospectively included 100 consecutive patients with PAF diagnosis and scheduled for cryoballoon catheter ablation for AF (47 males, 53 females; mean age 55.1±10.8 years). sST2 plasma levels were determined using the ASPECT-PLUS assay on ASPECT Reader device (Critical Diagnostics). The measurement range of these measurements was 12.5–250 ng/mL. Patients had regular follow-up visits with 12-lead electrocardiogram (ECG), medical history, and clinical evaluation. Twenty-four hours Holter ECG monitoring had been recorded 12 months after ablation.
Results
AF recurrence was detected in 22 patients after 1 year. Age, smoking history, diabetes mellitus,hypertension frequency, angiotensin converting enzyme inhibitor-angiotensin receptor blocker use, CHA2DS2VASc and HAS-BLED scores, serum sST2 level, left atrium (LA) end-diastolic diameter, LA volume and LA volume index were related to AF recurrence. In multivariable logistic regression analysis, sST2 was found to be only independent parameter for predicting AF recurrence (odds ratio, 1.085; p=0.001). Every 10-unit increase in sST2 was found to be associated with 2.103-fold increase in the risk of AF recurrence. The cut-off value of sST2 obtained by receiver operating characteristic curve analysis was 30.6 ng/mL for prediction of AF recurrence (sensitivity: 77.3%, specificity: 79.5%). The area under the curve was 0.831 (p<0.001).
Conclusions
sST2, which is associated with atrial fibrosis, can be thought to be a useful marker for detection of patients with high-grade fibrosis who will get less benefit from cryoablation.
Keywords: Soluble ST2, AF recurrence, Cryosurgery
INTRODUCTION
Atrial fibrillation (AF) is the most common continuous cardiac arrhythmia with a prevalence of 1.5–2% in developed countries.1) AF is associated with 5 times increase of stroke incidence and 3 times increase of congestive heart failure incidence. Electrical and structural remodeling that play important role in the beginning and continuing of AF, are also consequences of sustained AF. Structural remodeling results in electrical dissociation between muscle bundles and heterogeneity in local conductivity and provides reentry and continuity of AF.2) In many patients, structural remodeling begins before first AF episode.3) It seems that the early start of treatment is more beneficial because some components of structural remodeling may be irreversible.4) Fibrosis and inflammation are likely to correlate with atrial remodeling. Although several mechanisms of atrial remodeling are still unexplained, studies have helped to clarify the mechanisms associated with fibrosis and inflammation.5) Although there are a lot of studies about relationship of fibrosis markers with acute coronary syndrome and heart failure,6),7) there are not enough studies on AF patients. The association of fibrosis marker soluble ST2 (sST2) with incident AF had been investigated previously but no significant results were obtained.8) In this study, we planned to investigate the relationship between AF recurrence and sST2 after cryoablation in nonvalvular paroxysmal AF (PAF) patients.
METHODS
Study protocol and study population
We prospectively included 100 consecutive patients with PAF diagnosis and scheduled for cryoballoon catheter ablation for AF (47 males, 53 females; mean age, 55.1±10.8 years). AF was diagnosed on 12-lead electrocardiogram (ECG) during admission. Patients with spontaneous conversion to sinus rhythm or medical or electrical cardioversion within 7 days were considered as PAF. Patients with acute or end-stage liver or renal disease, severe chronic obstructive pulmonary disease, acute coronary syndrome, malignancy, active infection within the last 2 weeks, hemoglobin (Hb) value <8 g/dL, severe aortic and mitral valve disease, left atrium (LA) diameter >55 mm, left ventricular (LV) systolic dysfunction (LV ejection fraction [LVEF] <45) were excluded from the study.
After assessment of detailed medical history and a complete physical examination, the baseline characteristics of patients including age, sex, hypertension (HT), diabetes, hyperlipidemia, current smoking status, family history of cardiac disease and medications were recorded for all patients. ECG, telecardiography, complete blood count, fasting blood glucose, uric acid, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), serum electrolytes, serum lipids, prothrombin time, and renal and liver function tests were performed. Patients were classified according to the modified European Heart Rhythm Association (EHRA) score for AF related symptoms. CHA2DS2VASc and HAS-BLED staging systems for stroke and bleeding risk were also calculated. The local institutional ethics committee approved the study protocol (approval number: 2016-23), and each participant provided written informed consent.
Blood sampling
Venous blood samples were obtained following overnight fasting (8 hours), after 30-minute rest in the sitting position. Samples were taken from cubital vein into blood tubes and immediately stored on ice at 4°C. Blood counts were measured by a Sysmex K-1000 (Block Scientific, Bohemia, New York, NY, USA) autoanalyzer within 5 minutes of sampling. Plasma triglyceride, low-density lipoprotein (LDL), high-density lipoprotein (HDL), uric acid, NT-proBNP, creatinine, thyroid-stimulating hormone (TSH) concentrations were measured with an automated chemistry analyzer (Abbott Aeroset, Minneapolis, MN, USA) using commercial kits (Abbott Aeroset).
Measurement of sST2
Venous blood samples were obtained in the pre-ablation period. Patients were at sinus rhythm when blood samples were taken. Then, they were immediately centrifuged and stored at −80°C until analysis. Later, the frozen serum samples were rapidly dissolved for analysis. sST2 plasma levels were determined using the ASPECT-PLUS assay (Critical Diagnostics, San Diego, CA, USA) on ASPECT Reader device (Critical Diagnostics). The measurement range of these measurements was 12.5–250 ng/mL.9)
Echocardiographic assessment
Echocardiographic assessment was made by using a 2.5–3.5 MHz transducer (Philips HD11 ultrasound system; Philips, Bothell, WA, USA) with parasternal long- and short-axis, apical 2 and 4 chamber views. LV transverse axis dimensions at end-diastole, LV end-diastolic dimension (onset of the Q wave of the ECG) and LV end-systolic dimension were measured from M-mode recordings. This was obtained from the parasternal long-axis view with the M-mode cursor positioned adjacent to the tips of the mitral valve leaflets, using leading-edge methods. LV end-systolic volume, LV end-diastolic volume and LVEF were assessed using Simpson's equation using the apical 4-chamber view. Posterior wall (PW) Doppler, colour Doppler, PW and colour tissue Doppler evaluation were performed respectively. Echocardiographic wall motion anomalies, wall thickness, heart valves, left and right heart chambers were assessed. LA diameter was measured in anatomical M-mode in a parasternal long-axis view. The LA volume was calculated with biplane area-length method. The LA volume index was also calculated using the LA volume and the patient's body surface area.
Preprocedural evaluation
All patients underwent standard transthoracic echocardiography to rule out structural abnormality. A transesophageal echocardiogram was recorded to exclude left atrial thrombus. Oral anticoagulation was stopped before the procedure and the preprocedural interval was bridged with enoxaparin. Antiarrhythmic drugs were discontinued 5 half-lives before the procedure.
Cryoablation procedure
The method of cryoballoon ablation was described previously.10) All procedures were performed under sedation with midazolam. Invasive arterial blood pressure, oxygen saturation and ECG were continuously monitored throughout the entire procedure. Femoral venous access was achieved via the right and left femoral veins with the Seldinger technique. A 6 Fr steerable decapolar catheter (Dynamic Deca; Bard Electrophysiology, Lowell, MA, USA) was placed into the coronary sinus. Trans-septal access was obtained with modified Brockenbrough technique (BRK-1; St. Jude Medical, Minnetonka, MN, USA). A 8 Fr guiding trans-septal sheath (Biosense Webster, Irwindale, CA, USA) was placed into the LA. Boluses of heparin were used in order to ensure the activated clotting time between 300 and 350 seconds. After that, the sheath was changed with 12-F steerable (FlexCath; Medtronic CryoCath, Minneapolis, MN, US). Pulmonary vein (PV) recordings were done with an Achieve (Medtronic CryoCath) recording catheter. Pulmonary vein isolation (PVI) was achieved with a single big (28-mm) cryoablation balloon (Arctic Front; Medtronic CryoCath) in all patients. Occlusion was assessed with 50% diluted contrast injection. Two freezing cycles of 300 seconds were applied to each PV. At the end of the procedure, PV conduction was reevaluated with the achieve catheter. Successful PVI was defined as the elimination (or dissociation) of all the PV potentials recorded from the Achieve catheter. Direct palpation of the right hemi-diaphragmatic excursion was performed during phrenic nerve stimulation.
Postablation evaluation
Oral anticoagulation was initiated after 6 hours of the procedure continued for at least 3 months after the procedure. The need for oral anticoagulation was also evaluated after 3 months, based on the CHA2DS2VASc score. Antiarrhythmic drug treatment was also continued for 3 months. Patients had regular follow-up visits with 12-lead ECG, medical history, and clinical evaluation. The 24 hours Holter ECG monitoring had been recorded 12 months after ablation. Patients with symptoms consistent with AF earlier than 12 months had 24 hours Holter ECG monitoring earlier. Recurrence of AF is defined as detection of AF on 12-lead ECG or at least 30 seconds duration when detected with Holter ECG.
Statistical analyses
Statistical analyses were conducted using SPSS, version 14.0, (SPSS Inc., Chicago, IL, USA). Data are expressed as mean±standard deviation (SD) for continuous variables and percentage for categorical variables. The Shapiro-Wilk test was used to test normality and a p value >0.05 was defined as normally distributed data. Continuous variables that showed normal distribution were compared using the Student's t-test and analysis of variance (ANOVA), whereas the Mann-Whitney U test and Kruskal-Wallis test were used for nonnormally distributed samples. Categorical variables and frequencies were compared by means of the χ2 test. Statistical significance was defined as a p value <0.05 for all comparisons. Pearson's and Spearman's correlations were used to examine the relationship between continuous variables.
The factors associated with AF recurrence were tested by univariate and binary logistic regression analyses. Variables with a p value <0.05 in the univariate analysis were tested in the multivariate model. Results were expressed as the p value and odds ratio (OR) in confidence interval (CI) of 95%. Receiver operating characteristic (ROC) analysis was made to determine the cut-off value of sST2 to predict AF recurrence.
RESULTS
AF recurrence was detected in 22 patients after 1 year. Patients were divided into 2 groups according to the AF recurrence. All parameters were compared between the 2 groups.
Comparison of baseline characteristics in patients with and without AF recurrence
A comparison of the baseline characteristics is shown in Table 1. It was found that patients with AF recurrence were older and had higher rates of HT, diabetes mellitus (DM) and lower rates of smoking (p<0.05, for all). In the patients with AF recurrence, the CHA2DS2VASc and HAS-BLED scores were found to be significantly higher (p<0.05, for both). There was no significant difference between the groups in terms of modified EHRA score. The use of angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) was found to be significantly higher in patients with AF recurrence (p<0.05). Use of other cardiac drugs was found to be similar between the 2 groups.
Table 1. Baseline characteristics of patients with and without AF recurrence.
| Characteristics | Patients with recurrence (n=22) | Patients without recurrence (n=78) | p |
|---|---|---|---|
| Age (years) | 60.0±7.2 | 53.8±11.2 | 0.003 |
| Sex (female/male) | 14/8 | 39/39 | 0.258 |
| Weight (kg) | 79.1±12.3 | 76.6±10.2 | 0.333 |
| Length (cm) | 163.6±11.4 | 165.7±7.9 | 0.418 |
| BMI (kg/m2) | 29.3±4.9 | 28.0±4.5 | 0.263 |
| Smoking | 5 (22.7) | 36 (46.8) | 0.044 |
| Diabetes | 6 (27.3) | 7 (9.1) | 0.026 |
| Hypertension | 18 (81.8) | 29 (37.7) | <0.001 |
| CAD history | 3 (13.6) | 6 (7.8) | 0.400 |
| CHA2DS2VASc score | 2.22±1.41 | 1.29±1.20 | 0.018 |
| HAS-BLED score | 0.90±0.68 | 0.38±0.51 | 0.004 |
| Modified EHRA score | 1.86±0.35 | 1.84±0.39 | 0.803 |
| ACEI or ARB | 15 (68.2) | 25 (32.5) | 0.003 |
| Beta blocker | 22 (100) | 74 (96.1) | 0.347 |
| Amiadarone | 5 (22.7) | 7 (9.1) | 0.084 |
| Propafenon | 3 (13.6) | 7 (9.1) | 0.533 |
| Oral anticoagulants | 17 (77.3) | 42 (55.3) | 0.063 |
Values are presented as mean±SD or number of patients (%). Bold styled values are statistically significant.
ACEI = angiotensin converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin receptor blocker; BMI = body mass index; CAD = coronary artery disease; EHRA = European Heart Rhythm Association; SD = standard deviation.
Comparison of laboratory parameters in patients with and without AF recurrence
Laboratory parameters were similar in the 2 groups. Only sST2 serum level was significantly higher in the AF recurrence group (p<0.05, Table 2).
Table 2. Laboratory parameters of patients with and without AF recurrence.
| Parameters | Patients with recurrence (n=22) | Patients without recurrence (n=78) | p |
|---|---|---|---|
| Hematocrit (%) | 40.4±3.9 | 40.5±4.7 | 0.876 |
| Creatinine (mg/dL) | 0.69±0.20 | 0.84±0.61 | 0.274 |
| Total cholesterol (mg/dL) | 194.3±45 | 194.2±42 | 0.990 |
| LDL cholesterol (mg/dL) | 129.8±35 | 133.5±34 | 0.648 |
| HDL cholesterol (mg/dL) | 40.2±12.8 | 48.0±54.7 | 0.511 |
| Triglyceride (mg/dL) | 202±104 | 171±87 | 0.168 |
| Uric acid (mg/dL) | 4.77±1.36 | 5.12±1.43 | 0.308 |
| NT-proBNP (pg/mL) | 90.6±63.6 | 73.7±140.7 | 0.595 |
| TSH (uIU/dL) | 1.81±1.72 | 2.07±1.89 | 0.567 |
| HsCRP (mg/dL) | 0.73±0.67 | 0.52±0.88 | 0.340 |
| sST2 (ng/mL) | 54.6±35.1 | 22.8±11.5 | <0.001 |
Values are presented as mean±SD. Bold styled values are statistically significant.
AF = atrial fibrillation; HDL = high-density lipoprotein; HsCRP = high-sensitive C-reactive protein; LDL = low-density lipoprotein; NT-proBNP = N-terminal prohormone of brain natriuretic peptide; SD = standard deviation; sST2 = soluble ST2; TSH = thyroid-stimulating hormone.
Comparison of echocardiographic parameters in patients with and without AF recurrence
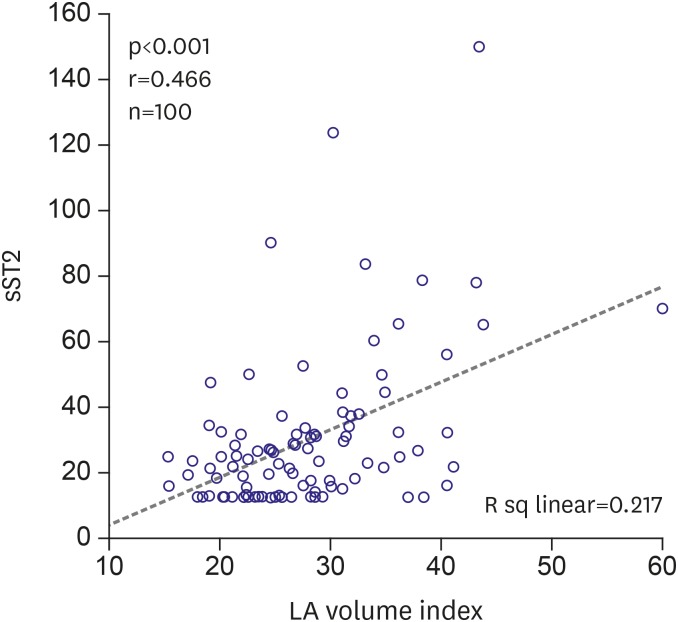
LA end-diastolic diameter, LA volume, and LA volume index were significantly higher in the patients with AF recurrence (p<0.05, for all). Other echocardiographic parameters were similar between the 2 groups (Table 3). LA volume index was also found to be significantly associated with sST2 (r=0.466; p<0.001) in bivariate analysis. Scatter plot diagram of the relationship of LA volume index with sST2 is shown in Figure 1.
Table 3. Echocardiographic parameters of patients with and without AF recurrence.
| Parameters | Patients with recurrence (n=22) | Patients without recurrence (n=78) | p |
|---|---|---|---|
| IVS diastolic thickness (mm) | 13.6±1.8 | 13.4±1.6 | 0.702 |
| PW diastolic thickness (mm) | 9.3±1.2 | 9.6±1.2 | 0.227 |
| LV ejection fraction (%) | 65.4±3.6 | 63.0±8.4 | 0.199 |
| LA end-diastolic diameter (mm) | 38.8±3.1 | 35.8±3.8 | 0.001 |
| LA volume (mL) | 61.1±10.6 | 47.4±12.5 | <0.001 |
| LA volume index (mL/m2) | 33.3±5.4 | 26.1±7.2 | <0.001 |
Values are presented as mean±SD. Bold styled values are statistically significant.
AF = atrial fibrillation; IVS = interventricular septum; LA = left atrium; LV = left ventricular; PW = posterior wall; SD = standard deviation.
Figure 1. Scatter plot diagram of the relationship of LA volume index with sST2.
LA = left atrium; sST2 = soluble ST2.
Univariate and multivariate relationships of AF recurrence
Age, smoking history, DM, HT frequency, ACEI-ARB use, CHA2DS2VASc and HAS-BLED score, serum sST2 level, LA end-diastolic diameter, LA volume, and LA volume index were related to AF recurrence. In multivariable logistic regression analysis, sST2 was found to be only independent parameter for predicting AF recurrence (2.103 [1.455–3.040]; p<0.001) (Table 4). Every 10 units increase in sST2 was found to be associated with 2.103-fold increase in the risk of AF recurrence.
Table 4. Multivariate relationships of AF recurrence.
| Variables | OR | p |
|---|---|---|
| Age (years) | 1.070 | 0.211 |
| Smoking history | 2.333 | 0.368 |
| DM | 0.250 | 0.244 |
| HT | 0.642 | 0.736 |
| ACEI-ARB use | 0.661 | 0.691 |
| CHA2DS2VASc score | 0.492 | 0.251 |
| HAS-BLED score | 2.198 | 0.308 |
| sST2 (every 10 units) | 2.103 | <0.001 |
| LA end-diastolic diameter | 2.738 | 0.418 |
| LA volume | 1.032 | 0.369 |
| LA volume index | 0.958 | 0.528 |
Bold styled values are statistically significant.
ACEI = angiotensin converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin receptor blocker; DM = diabetes mellitus; HT = hypertension; LA = left atrium; OR = odds ratio; sST2 = soluble ST2.
ROC curve analysis to determine predictive value of sST2 for AF recurrence
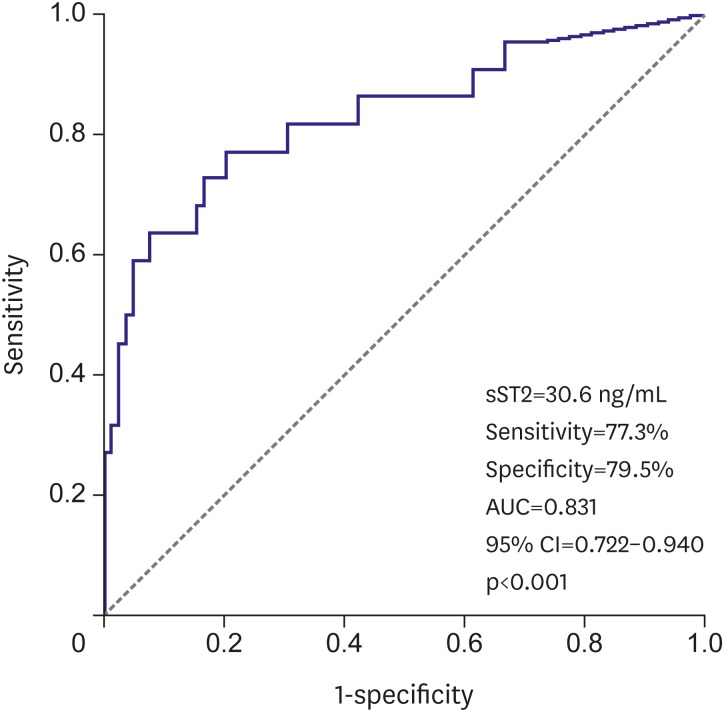
The cut-off value of sST2 obtained by ROC curve analysis was 30.6 ng/mL for prediction of AF recurrence (sensitivity: 77.3%, specificity: 79.5%). The area under the curve was 0.831 (0.722–0.940) (p<0.001, Figure 2).
Figure 2. ROC curve analysis to determine predictive value of sST2 for AF recurrence.
AF = atrial fibrillation; AUC = area under the curve; CI = confidence interval; ROC = receiver operating characteristic; sST2 = soluble ST2.
DISCUSSION
In our study, we aimed to demonstrate the relationship between cardiac fibrous tissue growth marker sST2 and AF recurrence in patients with cryoballoon ablation for PAF. To the best of our knowledge, this is the first study investigating the role of fibrosis marker sST2 to predict AF recurrence after catheter ablation of AF. The main findings of the present study are that: 1) After one year, the rate of patients without AF recurrence was 78% and 2) sST2 was found to be only independent parameter for predicting AF recurrence.
Catheter ablation of AF with the primary aim of PV isolation has become an effective and safe therapeutic option for patients with symptomatic and drug-refractory AF. In previous studies, the average annual success rate of cryoballoon ablation for AF was 60–70%.11),12) In the meta-analysis of 23 previously published studies, Andrade et al.11) found 1-year success after cryoballoon ablation to be 73% in PAF and 45.1% in persistan AF when the first 3-month period was accepted as blanking period. The most common reason for AF recurrence after cryoballoon operation is the reconnection of PV potentials with atrial tissue. This condition most commonly occurs in inferior PVs.13) No repeat cryoballoon procedure was performed in our study. Success rate was 78% after 1 year. The higher success rate in our study compared to other studies may be due to the fact that our study was conducted in one center and that the mean left atrial diameters were relatively low and only PAF patients were included in the study.
Detection of important predictors of AF recurrence after ablation will affect patient selection and increase success rate. A number of studies have been performed to predict AF recurrence after cryoballoon ablation. The predictors of AF recurrence were found to be non-PAF, increased LA diameter, sleep apnea, HT, advanced age, left atrial fibrosis detected by MR, and obesity.14) In a meta-analysis of 19 trials, D'Ascenzo et al.15) found the most important predictors of AF recurrence to be persisant AF and left atrial diameter >50 mm. Patients with sleep apnea, chronic obstructive pulmonary disease and heart failure were not included in our study. Patients with non-PAF were also excluded from the study. The results of our study are similar to the results of previous studies in regard of the effect of age on AF recurrence. In previous studies, older age was identified as a risk factor for AF recurrence. Similarly, the mean age of the patients with AF recurrence was higher than those without recurrence in our study. Similar to the results of previous studies, increased left atrial diameter was found to be associated with recurrence in our study. However, neither increased left atrial diameter nor advanced age and HT were found to be independent predictor for AF recurrence.
Although there are well-defined studies demonstrating the relationship between AF pathogenesis and inflammation and oxidative stress, whether initiation of AF promotes direct inflammatory and oxidative pathways or whether the presence of a pre-existing systemic inflammatory or pro-oxidant conditions result in AF development remains unclear.16) But, it is obvious that there will be a vicious circle after initiation of AF. The activity of inflammatory and oxidative pathways will cause an increase in calcium and a decrease in sodium channels. These changes at the ion channel level will then result in the pathological condition called electrical remodeling. Structural remodeling, which includes fibroblast proliferation, inflammation and apoptosis, follows electrical remodeling.17) sST2 is associated with inflammation, fibrosis and cardiac stress. sST2 has been included in the 2013 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guideline for additive risk stratification of patients with acute and chronic heart failure.18) sST2 blocks interleukin (IL)-33-ST2L interaction and inhibits inflammatory gene transcription and subsequent inflammatory cytokine release and immunologic response.19) So, the greater the level of IL-33 secretion and activity, the higher the serum sST2 levels are in response. sST2 is elevated in many diseases such as inflammatory and heart diseases and is used as prognostic marker in these diseases. sST2 has low specificity for heart failure. sST2 may be elevated in any disease where inflammation and fibrosis occur. Therefore, it is not used in the diagnose of heart failure. But it is a valuable predictor of severity and prognosis of heart failure.20) Although there are a lot of studies about relationship of fibrosis markers with acute coronary syndrome and heart failure,6),7) the effect of fibrosis markers on AF pathogenesis have not been widely investigated. In the study of Rienstra et al.,8) no significant association was found between fibrosis marker sST2 and incident AF. Our results are different from the results of Rienstra et al.8) Because, the vast majority of incident AF is associated with PV triggers. But the recurrence after AF ablation is mostly associated with reconnection of PVs and AF originating from larger areas of the atrium. Thus, while sST2 was not found to be helpful to predict incident AF, it was found to be useful to predict recurrence after AF ablation. In our study, sST2 was found to be independent predictor of AF recurrence after cryoablation treatment. sST2 may be useful for distinction of the patients with AF originating from only PV triggers who will get more benefit from ablation methods from the patients with AF originating from larger areas of the atrium who will get less benefit from ablation methods. The reason of the relation between sST2 and AF recurrence may be based on this distinctive effect of sST2.
There were some limitations in our study. As a single-center study, our patient cohort might be different from that in other centers. The sample size is relatively small and our results need to be confirmed in future large multi-center prospective trials. Because no repeat cryoballoon procedure was performed in our study, the recurrence rate after the second procedure could not be calculated. Furthermore, the electrophysiological mechanisms of AF recurrence and pulmonary venous connections to LA could not be assessed after recurrence. Patients with non-PAF were not included in our study. For this reason, no comparison could be made between patients with persistan and PAF in terms of recurrence. Patients with sleep apnea, chronic obstructive pulmonary disease or heart failure were not included in our study. Therefore, predictive value of these factors for AF recurrence could not be evaluated. We did not collect the data regarding AF duration. So, predictive value of AF duration could not be evaluated. Another limitation of our study is that long-term rhythm monitorization devices were not used during follow-up. In our study, Holter ECG monitorization was performed for only 24 hours. Longer duration of monitoring would have been beneficial such as 48–72-hour Holter monitoring at 6 and 12 months. Lack of long-term monitorization may have resulted in overestimation of the ablation success because of missed silent AF episodes. Furthermore, recurrence of AF is higher after the first year so 2–3-year follow-up may have been more appropriate.
Our study is the first study showing the relation between sST2 and recurrence of AF. sST2 was found to be only independent parameter for predicting AF recurrence. sST2, which is associated with atrial fibrosis, can be thought to be a useful marker for detection of patients with high-grade fibrosis who will get less benefit from cryoablation. Multiple ablation procedures or different ablation techniques may be used for such patients.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Okar S, Kaypakli O, Koç M.
- Data curation: Kaypakli O, Koç M.
- Formal analysis: Kaypakli O, Koç M.
- Investigation: Okar S, Koç M.
- Methodology: Koç M.
- Supervision: Şahin DY, Koç M.
- Validation: Koç M.
- Writing - review & editing: Kaypakli O, Şahin DY, Koç M.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–615. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 3.Anné W, Willems R, Roskams T, et al. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res. 2005;67:655–666. doi: 10.1016/j.cardiores.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation. 2002;105:2672–2678. doi: 10.1161/01.cir.0000016826.62813.f5. [DOI] [PubMed] [Google Scholar]
- 5.Sonmez O, Ertem FU, Vatankulu MA, et al. Novel fibro-inflammation markers in assessing left atrial remodeling in nonvalvular atrial fibrillation. Med Sci Monit. 2014;20:463–470. doi: 10.12659/MSM.890635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh RH, Seliger SL, Christenson R, Gottdiener JS, Psaty BM, deFilippi CR. Soluble ST2 for prediction of heart failure and cardiovascular death in an elderly, community-dwelling population. J Am Heart Assoc. 2016;5:e003188. doi: 10.1161/JAHA.115.003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rienstra M, Yin X, Larson MG, et al. Relation between soluble ST2, growth differentiation factor-15, and high-sensitivity troponin I and incident atrial fibrillation. Am Heart J. 2014;167:109–115.e2. doi: 10.1016/j.ahj.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieplinger B, Egger M, Gegenhuber A, Haltmayer M, Mueller T. Analytical and clinical evaluation of a rapid quantitative lateral flow immunoassay for measurement of soluble ST2 in human plasma. Clin Chim Acta. 2015;451:310–315. doi: 10.1016/j.cca.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Neumann T, Vogt J, Schumacher B, et al. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J Am Coll Cardiol. 2008;52:273–278. doi: 10.1016/j.jacc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Andrade JG, Khairy P, Guerra PG, et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011;8:1444–1451. doi: 10.1016/j.hrthm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 12.Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 13.Kühne M, Suter Y, Altmann D, et al. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: biomarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm. 2010;7:1770–1776. doi: 10.1016/j.hrthm.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Balk EM, Garlitski AC, Alsheikh-Ali AA, Terasawa T, Chung M, Ip S. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol. 2010;21:1208–1216. doi: 10.1111/j.1540-8167.2010.01798.x. [DOI] [PubMed] [Google Scholar]
- 15.D'Ascenzo F, Corleto A, Biondi-Zoccai G, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a meta-analysis. Int J Cardiol. 2013;167:1984–1989. doi: 10.1016/j.ijcard.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 17.Youn JY, Zhang J, Zhang Y, et al. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–79. doi: 10.1016/j.yjmcc.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg EO. ST2 protein in heart disease: from discovery to mechanisms and prognostic value. Biomarkers Med. 2009;3:495–511. doi: 10.2217/bmm.09.56. [DOI] [PubMed] [Google Scholar]
- 20.Miller AM, Liew FY. The IL-33/ST2 pathway--a new therapeutic target in cardiovascular disease. Pharmacol Ther. 2011;131:179–186. doi: 10.1016/j.pharmthera.2011.02.005. [DOI] [PubMed] [Google Scholar]