Abstract
AIM
To reveal the protective mechanism of the combined use of vitamin D and puerarin in the progression of hepatic fibrosis induced by carbon tetrachloride (CCl4).
METHODS
Eight-week-old male Wistar rats were randomly divided into a normal control group (C group), a CCl4 group (CCl4 group), a vitamin D group (V group), a puerarin group (P group), and a combined group of vitamin D and puerarin (V + P group), each of which contained ten rats. In this way, we built a rat model of CCl4-induced hepatic fibrosis with intervention by vitamin D, puerarin, or a combination of the two. After eight weeks, the mice were sacrificed to collect serum and liver specimens. Blood was collected to detect the hyaluronic acid (HA). We also measured hydroxyproline (Hyp) and prepared paraffin sections of liver. After Sirius red staining, the liver specimens were observed under a microscope. RT-PCR and western blot analysis were adopted to detect the mRNA and the protein levels of Collagen I, Collagen III, Wnt1, and β-catenin in the liver tissues, respectively.
RESULTS
Hepatic fibrosis was observed in the CCl4 group. In comparison, hepatic fibrosis was attenuated in the V, P, and V + P groups: the HA level in blood and the Hyp level in liver were reduced, and the mRNA levels of Collagen I, Collagen III, Wnt, and β-catenin in liver were also decreased, as well as the protein levels of Wnt1 and β-catenin. Among these groups, the V + P group demonstrated the greatest amelioration of hepatic fibrosis.
CONCLUSION
The combined application of vitamin D and puerarin is capable of alleviating CCl4-induced hepatic fibrosis of rats. As to the mechanism, it is probably because the combined use is able to silence the Wnt1/β-catenin pathway, suppress the activation of hepatic stellate cells, and reduce the secretion of collagen fibers, therefore improving the anti-hepatic fibrosis effect.
Keywords: Carbon tetrachloride, Hepatic fibrosis, Vitamin D, Puerarin, Wnt/β-catenin
Core tip: The proliferation of hepatic stellate cells (HSCs) is associated with hepatic fibrosis. The activated HSCs, as well as Wnt1 and β-catenin, have become important targets in anti-hepatic fibrosis therapy. This research investigated the protective effect of the combined use of vitamin D and puerarin against CCl4-induced hepatic fibrosis in rats. The protective effect of the combined use of vitamin D and puerarin in the progression of hepatic fibrosis is closely associated with the function of silencing the Wnt1/β-catenin pathway, suppressing the activation of HSCs, and decreasing the secretion of collagen fibers, which provided a useful reference for those in clinical practice.
INTRODUCTION
Hepatic fibrosis is the change of pathological structures due to all kinds of chronic liver diseases. Essentially, it occurs because extracellular matrix (ECM) synthesized by hepatic stellate cells (HSCs) under the actions of various pathogenic factors substantially increases, exceeding the degradation ability of the liver itself. The long-term accumulation of ECM will lead to hepatic fibrosis and thus cause liver cirrhosis[1,2]. In recent years, it has been proven that the β-catenin protein plays a critical role in the occurrence and development of hepatic fibrosis[3,4]. Previous studies revealed that the more serious the hepatic fibrosis, the higher the expression of β-catenin in liver tissues compared with normal liver tissues, and silencing β-catenin is able to suppress the secretion of collagen and the proliferation of HSCs and mediate cell apoptosis. β-catenin participates in many signalling pathways, among which the Wnt/β-catenin pathway is the most common along which β-catenin plays its role. Apart from this, β-catenin also participates in alternative pathways including E-cadherin, NF-κB, and TGF-β[5-7]. Therefore, β-catenin is key to the intersection of multiple signalling pathways. Existing experiments revealed that HSC-T6 cell membranes and cytoplasm with activated phenotypes show β-catenin expression, and β-catenin expression is also observed in nuclei. This indicates that the Wnt/β-catenin signalling pathway is activated in activated HSCs. The expression of α-SMA in HSC-T6 cells was down-regulated by blocking the transduction of the Wnt/β-catenin signalling pathway, and the expression of types I and III collagen (Collagen I and Collagen III) was also significantly down-regulated. The analysis based on the String database finds that DVL1, DVL2, and DVL3 play their functions in signal transduction pathways mediated by multiple Wnt genes. They are regulatory factors for the Wnt signalling pathway and ER-to-Golgi transport and participate in the ER-to-Golgi transport, thus KLHL12 plays a critical role in the export of collagen, as shown in Figure 1. This implies that the Wnt/β-catenin signalling pathway is closely associated with the activation of HSCs.
Figure 1.
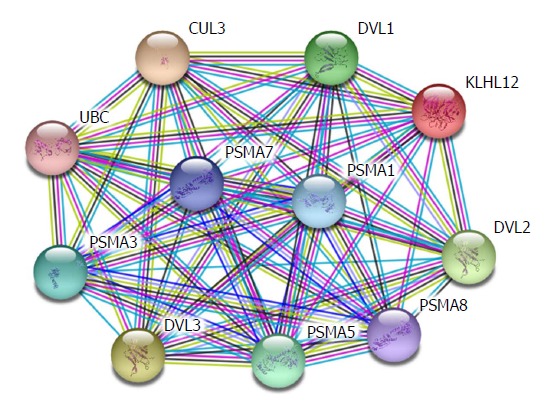
Interaction between WNT-related genes and collagen-related genes.
Carbon tetrachloride (CCl4) is one of the classical poisons used in establishing hepatic fibrosis models and is therefore widely used in fundamental research[8]. Previous research has proven in vivo that vitamin D is able to alleviate hepatic fibrosis, and in vitro experiments confirmed that vitamin D can reduce the secretion of collagen fibers of HSCs[9,10]. There are also evidences from existing research that puerarin is capable of attenuating hepatic fibrosis, which is probably associated with the inhibition of the activation of HSCs by blocking the TNF-α signalling pathway[11,12]. A preliminary study by the present research team has confirmed that the combined use of vitamin D and puerarin is able to enhance the anti-hepatic fibrosis effect; however, the related mechanism has not been revealed. Therefore, the current research investigated the effects of vitamin D combined with puerarin on the expression of key factors, including Wnt1, β-catenin, Collagen I, and Collagen III, in the Wnt/β-catenin signalling pathway based on the aforementioned preliminary study. By doing so, we attempted to further clarify the mechanism of the combined use of vitamin D and puerarin in anti-hepatic fibrosis.
MATERIALS AND METHODS
Experimental materials
Fifty clean-grade healthy male Wistar rats (with a body mass of about 200 g) of similar age were provided by the Animal Center of Youjiang Medical College for Nationalities. Analytically pure CCl4 was purchased from Sinopharm Chemical Reagent Co., Ltd, and vitamin D and puerarin were purchased from Sigma. In addition, corn oil, Sirius red staining solution, and real-time fluorescence quantitative PCR kits were purchased from Wako Pure Chemical Industries, Ltd, Beijing Leagene Biotech Co., Ltd, and Roche (Switzerland), respectively. Wnt1 and β-catenin antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, United States). Table 1 shows the designations and sequences of primers.
Table 1.
Designations and sequences of primers
| Designation (mice) | Sequence (5’-3’) |
| GAPDH | F: GGCATCCTGACCCTCAAGTA |
| R: GGGGTGTGAACCTCTCAAA | |
| Collagen I | F: GGACACTACTGGATCGACCTAAC |
| R: CTCACCTGTCTCCATGTTGCA | |
| Collagen III | F: CTACCTTGCTCAGTCCTATGAGTCTAGA |
| R: TCCCGAGTCGCAGACACATAT | |
| Wnt1 | F: GAAACCGCCGCTGGAACT |
| R: CCCTGCCTCGTTATTGTGAAG | |
| β-catenin | F: ACC TCC CAAGTC CTG TAT |
| R: CCT GGT CCT CGT CAT TTA |
Experimental methods
Fifty Wistar rats were randomly divided into five groups. Rats in the CCl4 group were administered 3 mL/kg of corn oil solution containing 45% CCl4 through intraperitoneal injection twice a week. The normal control group (C group) was administered the same dose of normal saline through intraperitoneal injection twice a week. The V, P, and V + P groups were the drug intervention groups. On the basis of the models established using corn oil solution containing CCl4, mice in the three groups received 2 μg/kg of vitamin D, 0.4 g/kg of puerarin, and a combination of D vitamin (2 μg/kg) and puerarin (0.4 g/kg) through intragastric administration twice a week. The mice were subjected to fasting for one hour before each administration of treatment. The C group received the same quantity of normal saline through intragastric administration. After eight weeks, the mice were fasted overnight the day before the end of the experiments and sacrificed the next day. Whole blood was collected and stood for 20 min at room temperature. Then, the blood was centrifuged at 5000 rpm for 15 min. Thereafter, the supernatant and then the serum were obtained, followed by the detection of hyaluronic acid (HA) in blood using the aforementioned kit. After collecting the liver of the rats, the kit was used to detect hydroxyproline (Hyp) in the liver. Part of the liver was fixed in a paraformaldehyde solution, and paraffin sections were prepared. After Sirius red staining, liver tissue slices were placed under the microscope to observe their pathological changes and degree of fibrosis. Moreover, real-time fluorescence quantitative PCR was applied to detect the mRNA levels of Collagen I, Collagen III, Wnt1, and β-catenin, and a western blot assay was used to measure the protein levels of Wnt1 and β-catenin.
Statistical analysis
The experiments were repeated three times for each group. SPSS 17.0 statistical software was used for subsequent analysis. The analytical results were expressed using mean ± SD, and a t-test was used when comparing paired groups. The difference was deemed statistically significant when P < 0.05.
RESULTS
Determination of biochemical indices of blood and liver
HA is a type of proteoglycan distributed around hepatic cells, and its content significantly increases in blood when hepatic fibrosis occurs[13]. Hyp in liver is an important part of the collagen fibers in liver, and the amount thereof also increases when hepatic fibrosis occurs[14]. Therefore, these two factors are important diagnostic indices of hepatic fibrosis. As shown in Table 2, the HA and Hyp of the CCl4 group were significantly higher than those of the C group, which indicated, to some extent, that the hepatic fibrosis model had been successfully established. The HA and Hyp levels of the V, P, and V + P groups were much lower than those of the CCl4 group. Among these groups, the V + P group showed the most significant reduction in HA and Hyp levels.
Table 2.
Biochemical indices of blood and liver
| Groups | HA (μg/L) | Hyp (μg/g) |
| C | 61 ± 20.6bcde | 195.6 ± 10.5bcde |
| CCl4 | 157.3 ± 44.3acde | 503 ± 31.7acde |
| V | 70.2 ± 12.9b | 375.2 ± 26.9ab |
| P | 79.5 ± 11.6b | 361.3 ± 24.1ab |
| V + P | 65 ± 12.1b | 353.7 ± 21.6ab |
In the comparison of the mean ± standard deviation (SD) of various groups, differences are statistically significant when P < 0.05, with n = 10. In addition, a, b, c, d, and e represent statistical differences vs C, CCl4, V, P, and V + P groups, respectively. C: Control; V: Vitamin; P: Puerarin.
Observation of the pathological sections of liver tissues
The Sirius-red-stained sections of each group were observed under the microscope. As illustrated in Figure 2A-F, mice in the CCl4 group and the medication groups showed larger areas of fibrosis in their liver tissues than in the C group. In addition, the areas of fibrosis of liver tissues in rats in each of the medicated groups were smaller than that of the CCl4 group (P < 0.05). Among the three groups, the V + P group presented the smallest area of fibrosis.
Figure 2.
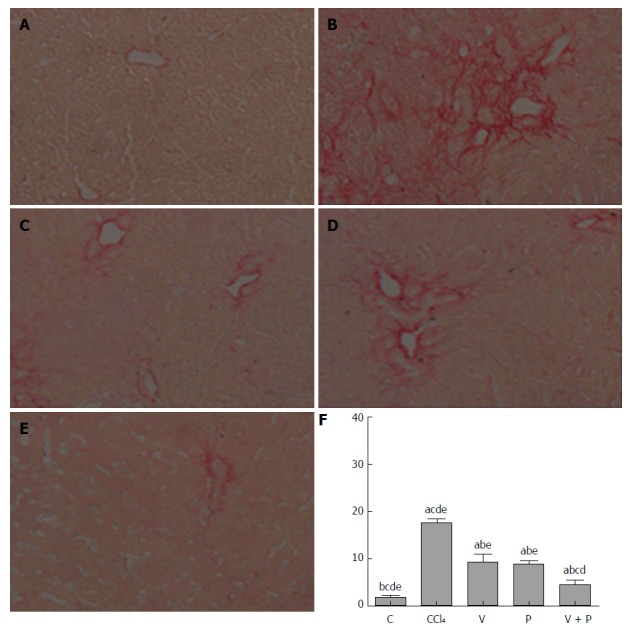
Sirius red staining. A: Control (C) group; B: CCl4 group; C: Vitamin D (V) group; D: Puerarin (P) group; E: V + P group (200 × magnification); F: Statistical graph of positive Sirius-red-stained areas (in the comparison of the mean ± SD of various groups, differences are statistically significant when P < 0.05, n = 10. a, b, c, d, and e represent statistical differences vs C, CCl4, V, P, and V + P groups, respectively).
mRNA levels of collagen I, collagen III, Wnt1, and β-catenin
RT-PCR detection was conducted to test the mRNA levels of Collagen I, Collagen III, Wnt1, and β-catenin of the rats in each group. It can be seen from Table 3 that the mRNA levels of Collagen I, Collagen III, Wnt1, and β-catenin of liver tissues of rats in the CCl4 group and the medicated groups are all higher than those of the C group. The mRNA levels of these indices of rats in the three medicated groups were all lower than those of the CCl4 group (P < 0.05). Among the medicated groups, the V + P group showed the lowest mRNA levels.
Table 3.
The mRNA levels of collagen I, collagen III, Wnt1, and β-catenin of liver
| Groups | Collagen I | Collagen III | Wnt1 | β-catenin |
| C | 1.17 ± 0.16bcde | 0.62 ± 0.15bcde | 0.56 ± 0.13bcde | 0.48 ± 0.21bcde |
| CCl4 | 4.73 ± 0.76acde | 2.47 ± 1.12acde | 1.77 ± 0.32acde | 2.16 ± 0.42acde |
| V | 1.97 ± 0.31b | 1.29 ± 0.19ab | 1.06 ± 0.35ab | 1.25 ± 0.33ab |
| P | 1.75 ± 0.46b | 1.35 ± 0.17ab | 0.83 ± 0.19ab | 0.71 ± 0.46ab |
| V + P | 1.53 ± 0.12abcd | 1.03 ± 0.08abcd | 0.61 ± 0.24abcd | 0.59 ± 0.14abcd |
Differences are considered to exhibit statistical significance when P < 0.05 (n = 10) in the comparison of the mean ± standard deviation (SD) between various groups. a, b, c, d, and e indicate that there are statistical differences vs C, CCl4, V, P, and V + P groups, respectively. C: Control; V: Vitamin; P: Puerarin.
Protein levels of Wnt1 and β-catenin of liver
As the Wnt/β-catenin signalling pathway plays a significant role in the progression of hepatic fibrosis, a western blot assay was applied to detect the protein levels of Wnt1 and β-catenin in the liver. Figure 3 shows that the protein levels of Wnt1 and β-catenin in the liver tissues of rats in the CCl4 group and the medicated groups were higher than the C group. The medicated groups showed lower protein levels than the CCl4 group (P < 0.05), among which the V + P group exhibited the lowest protein levels (P < 0.01).
Figure 3.
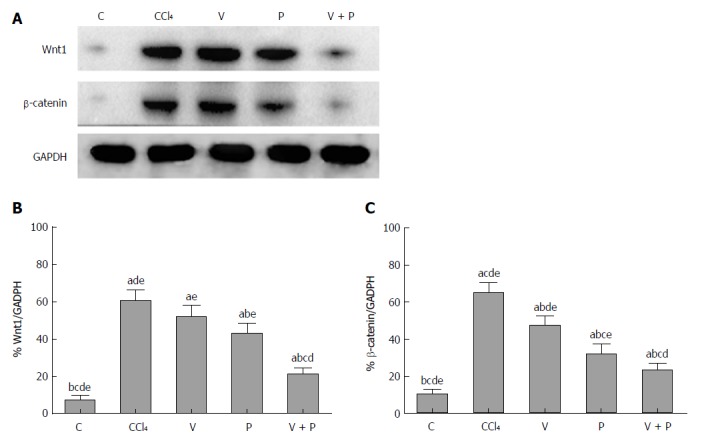
The protein levels of Wnt1 and β-catenin of liver tissues in rats in the CCl4 group and the medicated groups were higher than the Control (C) group. A: Detection of protein levels of Wnt1, and β-catenin using western blot assay; B: Grey analysis of Wnt1; C: Grey analysis of β-catenin [in the comparison of mean ± standard deviation (SD) of various groups, differences are statistically significant when P < 0.05, n = 10. In addition, a, b, c, d, and e represent statistical differences vs C, CCl4, V, P, and V + P groups, respectively]. C: Control; V: Vitamin; P: Puerarin.
DISCUSSION
Hepatic fibrosis is the inevitable pathological change during the development of chronic liver diseases. If it is not blocked, its further development would cause liver cirrhosis and even liver cancer. Hepatic fibrosis can be induced by many pathogenic factors, including viruses, parasites, alcohol, and some poisons such as CCl4. It is essentially a disturbance of the balance between the production and degradation of ECM outside hepatocytes. The ECM accumulates in the liver and its main component is collagen fibers[15,16]. HSCs, as the principal participants in the production and degradation of ECM, when activated, are the key link necessary for the occurrence of hepatic fibrosis. Existing in the Disse space, HSCs mainly play their roles in metabolizing and storing vitamin A in normal conditions. They can synthesize and secrete small amounts of ECM and produce collagenase. When hepatic fibrosis occurs, HSCs in their resting state are activated. Active oxygen, lipid peroxide, and molecules secreted by nearby cells, such as activated Kupffer cells, and liver sinusoidal endothelial cells, as well as damaged hepatic cells, all can facilitate the activation of resting HSCs. After activation, HSCs have different morphologies, and the changes include the secretion of alpha smooth muscle actin (α-SMA), loss of vitamin A stored in cells, and increase in the rough endoplasmic reticulum. The activation of HSCs is also accompanied by a series of changes in genetic expression, including the appearance of receptors that can respond to paracrine stimulation on the cytomembrane of activated HSCs and a series of signalling cascade reactions in cells. The presence of the signalling cascade reactions in cells is beneficial to maintaining the phenotype of activated cells and controlling the occurrence of fibrosis, the proliferation of HSCs, and the increases in the transcription and translation of Collagen I and Collagen III[17-19].
The canonical Wnt signal transduction pathway is also known as the Wnt/β-catenin signal transduction pathway. It is the pathway that has been the subject of most of the research to date. As a highly conserved signalling pathway activated during the evolution of some species, it is a basic pathway that is able to regulate cell proliferation and cell polarity, control cell fate, and maintain homeostasis in the embryo, and influence tissue development. Therefore, the pathway plays significant roles in various physiological processes including early development of animal embryos, organogenesis, and tissue regeneration, as shown in Figure 4[20]. When Wnt is deficient, β-catenin in the cytoplasm is generally degraded via Axin complexes; in addition, glycogen and casein kinase 1 (CK1) synthesize glycogen synthase kinase 3 (GSK3), which sequentially phosphorylates the amino terminus of β-catenin. As a result, β-catenin is recognized by β-Trcp and the sub-unit of E3 ubiquitin ligases and is then degraded by the ubiquitination pathway. The continuous removal of β-catenin inhibits the nuclear import of β-catenin and the combination of the protein family of T cell factor/lymphoid enhancer factor (TCF/LEF) with DNA, thus suppressing the transcription of Wnt target genes[21,22]. When Wnt ligands are bound with transmembrane Frizzled (Fz) receptors and their co-receptor–low-density lipoprotein receptor related protein 6 (LRP6) or similar LRP5, the Wnt/β-catenin signalling pathway and dishevelled Dsh/Dvl proteins in cells are activated. Consequently, the GSK-3β activity is suppressed, Axin falls off, and the formation of biodegradable composite film (mainly composed of Axin, APC, and GSK-3β) of β-catenin is inhibited. Therefore, β-catenin will aggregate, not be recognized, and degraded via the ubiquitination pathway[23,24]. As β-catenin accumulates to a certain level, it dissociates. Under these conditions, β-catenin is likely to undergo nuclear translocation, thus combining with the transcription factor TCF/LEF, forming transcriptional activation complex, and finally upregulating or downregulating the expressions of some downstream genes. At present, the known target downstream genes include D1 (cyclin D1), c-myc, MMP7, survivin, CD44, and growth factor, and new target genes are constantly being discovered[25,26]. In the Wnt/β-catenin pathway, Wnt1 is the key gene participating in the aggregation and disappearance of β-catenin and is closely related to the occurrence and development of hepatic fibrosis and tumors.
Figure 4.
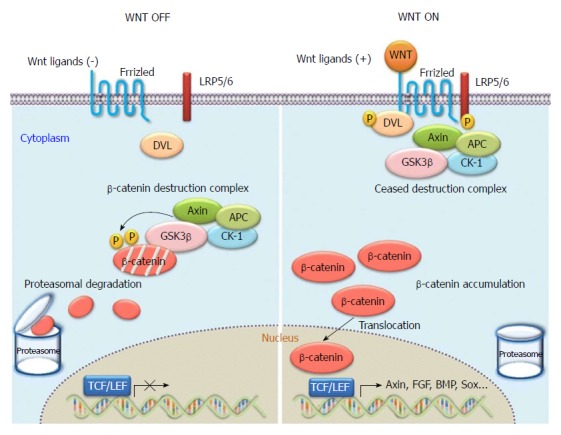
Overview of WNT/β-catenin signalling. Without WNT signalling (“WNT OFF”), the destruction complex phosphorylates cytosolic β-catenin, and phosphorylated β-catenin is recognized and degraded by the proteasomes. With active WNT signalling (“WNT ON”), the function of the “destruction complex” is inhibited to phosphorylate cytosolic β-catenin. Then unphosphorylated β-catenin accumulates in the cytosol, translocates into the nucleus, and activates WNT target gene expression, such as the targets of the T-cell factor and lymphoid enhancer factor-1 (TCF/LEF1) family of transcription factors.
The Wnt/β-catenin pathway and the activation and proliferation of HSCs are therefore associated with hepatic fibrosis. The Wnt/β-catenin pathway, dominated as it is by Wnt and β-catenin, plays a significant role in regulating the activation of HSCs and the secretion of Collagen I and III. Although the mechanism via which the Wnt/β-catenin signalling pathway takes part in hepatic fibrosis by activating HSCs is not completely understood, activated HSCs, as well as Wnt1 and β-catenin, have become important targets in anti-hepatic fibrosis therapy.
Most of the vitamin D in the body is produced by the liver, which is also one of the target organs of vitamin D. Previous studies have revealed that vitamin D is capable of ameliorating CCl4-induced hepatic fibrosis in mice. In vitro experiments have shown that this is probably because it reduces the activation of HSCs and the secretion of collagen fibers. Puerarin, as a type of flavonoid drug that is widely applied in clinical practice, is also able to alleviate CCl4-induced hepatic fibrosis (according to the evidence of existing research): however, there is no report on the issue as to whether the Wnt/β-catenin signalling pathway can be significantly suppressed after the combined use of vitamin D and puerarin. Whether using vitamin D or puerarin alone, their protective effects on the liver during the progression of hepatic fibrosis have been found to be closely associated with their functions of suppressing HSCs. Our preliminary research revealed that the combined use of the two drugs is able to impart greater protective function, so we speculatd that the mechanism of the combined use is probably related to HSCs, Wnt, and β-catenin.
The research demonstrated that CCl4 greatly increased the HA level in blood and Hyp level in rat liver. By observing the Sirius-red-stained specimens under a microscope, it was found that CCl4 caused the deposition of collagen fibers in the liver of the rats. All these results indicated that the rat model of hepatic fibrosis was successfully established. Vitamin D and puerarin can both reduce the HA level in blood and Hyp level in liver of the rats, thus greatly decreasing the amount of collagen fibers in the liver. The combined use of vitamin D and puerarin most significantly reduced various damage indices and alleviated the deposition of collagen fibers, which proved that the two drugs have synergistic effects. Meanwhile, we detected the mRNA and protein levels of Collagen I, Collagen III, Wnt1, and β-catenin molecules in the liver of the rats. The results indicated that both vitamin D and puerarin are capable of decreasing the mRNA and protein levels of these molecules. Likewise, the most significant reduction effect was also observed in the V + P group. This suggested that the protective effect of the combined use of vitamin D and puerarin in the progression of hepatic fibrosis is closely associated with the functions of silencing the Wnt1/β-catenin pathway, suppressing the activation of HSCs, and decreasing the secretion of collagen fibers.
The research investigated the protective effect of the combined use of vitamin D and puerarin against CCl4-induced hepatic fibrosis in rats. The results revealed that the combined use of the two drugs exhibited a superior anti-hepatic fibrosis effect compared to that of each of the agents used as a single medication. It was preliminarily found that the protective mechanism of the combined use was related to the Wnt/β-catenin pathway, which provided a useful reference for those in clinical practice. However, the regulatory mechanism is complex and involves multiple factors and pathways. Other mechanisms of action warrant further investigation.
ARTICLE HIGHLIGHTS
Research background
Hepatic fibrosis is seriously endangering the safety of life. At present, there is no ideal way to treat hepatic fibrosis. The combination of vitamin D and puerarin can improve the effects of anti-hepatic fibrosis, but the mechanism is not clear. Therefore, the aim of this study is to explore the mechanism of the combined use of vitamin D and puerarin in the treatment of hepatic fibrosis, so as to improve the theoretical basis for the treatment of hepatic fibrosis.
Research motivation
The combination of vitamin D and puerarin can improve the effect of anti-hepatic fibrosis, but its mechanism is not clear. The Wnt/β-catenin pathway is closely related to liver fibrosis. In this study, we found that vitamin D and puerarin are closely related to the regulation of Wnt/β-catenin pathway in hepatic fibrosis, which provided a useful reference for those in clinical practice.
Research objectives
The research aimed to reveal the protective mechanism of the combined use of vitamin D and puerarin in the progression of hepatic fibrosis induced by carbon tetrachloride (CCl4).
Research methods
In this study, a Wistar rat model of hepatic fibrosis was constructed by CCl4. Vitamin D combined with puerarin was used in the treatment of hepatic fibrosis rats, and the liver pathology and the genes related to the Wnt/β-catenin pathway were detected. The protective mechanism of the combined use of vitamin D and puerarin in the progression of hepatic fibrosis was explored at the molecular level.
Research results
The research demonstrated that CCl4 greatly increased the HA level in blood and Hyp level in the rat liver. CCl4 caused the deposition of collagen fibers in the liver of the rats. Vitamin D and puerarin can both reduce the HA level in blood and Hyp level in liver of the rats, thus greatly decreasing the amount of collagen fibers in the liver. The combined use of vitamin D and puerarin most significantly reduced the various damage indices, and alleviated the deposition of collagen fibers, which proved that the two drugs had synergistic effects.
Research conclusions
The combined application of vitamin D and puerarin is capable to alleviating the CCl4-induced hepatic fibrosis of rats.
Research perspectives
We have learned to induce an animal model of hepatic fibrosis and study the drug effects by the animal model, but also because we were not skilled enough in the experimental technique and spent more time doing repetitive work. The future research direction is to explore the prevention and treatment measures of hepatic fibrosis. The drug targets will be studied by transcriptome, proteomics and other methods.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: This study was reviewed and approved by the Youjiang Medical University for Nationalities.
Conflict-of-interest statement: All authors have no conflict of interest.
Data sharing statement: No additional data are available.
ARRIVE guidelines statement: The manuscript has been revised according to the ARRIVE guidelines.
Peer-review started: June 5, 2018
First decision: June 21, 2018
Article in press: August 1, 2018
P- Reviewer: Facciorusso A, Musquer N, Shin T S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
Contributor Information
Gan-Rong Huang, Youjiang Medical University for Nationalities, School of Basic Medical Sciences, Baise 533000, Guangxi Zhuang Autonomous Region, China.
Si-Jun Wei, Affiliated Hospital of Youjiang Medical University for Nationalities, Baise 533000, Guangxi Zhuang Autonomous Region, China.
Yan-Qiang Huang, Youjiang Medical University for Nationalities, School of Basic Medical Sciences, Baise 533000, Guangxi Zhuang Autonomous Region, China.
Wei Xing, Youjiang Medical University for Nationalities, School of Basic Medical Sciences, Baise 533000, Guangxi Zhuang Autonomous Region, China. 116895009@qq.com.
Lu-Yao Wang, Youjiang Medical University for Nationalities, School of Basic Medical Sciences, Baise 533000, Guangxi Zhuang Autonomous Region, China.
Ling-Ling Liang, Youjiang Medical University for Nationalities, School of Basic Medical Sciences, Baise 533000, Guangxi Zhuang Autonomous Region, China.
References
- 1.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 3.Ge WS, Wang YJ, Wu JX, Fan JG, Chen YW, Zhu L. β-catenin is overexpressed in hepatic fibrosis and blockage of Wnt/β-catenin signaling inhibits hepatic stellate cell activation. Mol Med Rep. 2014;9:2145–2151. doi: 10.3892/mmr.2014.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Zhu C, Li Y, Wu Q, Gao R. Mest attenuates CCl4-induced liver fibrosis in rats by inhibiting the Wnt/β-catenin signaling pathway. Gut Liver. 2014;8:282–291. doi: 10.5009/gnl.2014.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci USA. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian J, Niu M, Zhai X, Zhou Q, Zhou Y. β-catenin pathway is required for TGF-β1 inhibition of PPARγ expression in cultured hepatic stellate cells. Pharmacol Res. 2012;66:219–225. doi: 10.1016/j.phrs.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Nejak-Bowen K, Kikuchi A, Monga SP. Beta-catenin-NF-κB interactions in murine hepatocytes: a complex to die for. Hepatology. 2013;57:763–774. doi: 10.1002/hep.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma ZG, Lv XD, Zhan LL, Chen L, Zou QY, Xiang JQ, Qin JL, Zhang WW, Zeng ZJ, Jin H, et al. Human urokinase-type plasminogen activator gene-modified bone marrow-derived mesenchymal stem cells attenuate liver fibrosis in rats by down-regulating the Wnt signaling pathway. World J Gastroenterol. 2016;22:2092–2103. doi: 10.3748/wjg.v22.i6.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramovitch S, Dahan-Bachar L, Sharvit E, Weisman Y, Ben Tov A, Brazowski E, Reif S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728–1737. doi: 10.1136/gut.2010.234666. [DOI] [PubMed] [Google Scholar]
- 10.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R, Xu L, Liang T, Li Y, Zhang S, Duan X. Puerarin mediates hepatoprotection against CCl4-induced hepatic fibrosis rats through attenuation of inflammation response and amelioration of metabolic function. Food Chem Toxicol. 2013;52:69–75. doi: 10.1016/j.fct.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 12.Kaji K, Yoshiji H, Ikenaka Y, Noguchi R, Aihara Y, Douhara A, Moriya K, Kawaratani H, Shirai Y, Yoshii J, et al. Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats. J Gastroenterol. 2014;49:481–491. doi: 10.1007/s00535-013-0783-4. [DOI] [PubMed] [Google Scholar]
- 13.Lei X, Wang YL, Tu-er-xun AL, Zhang H, Zhang RL, Yang Y, Wu G, Cheng W. Correlation between radiation-induced hepatic fibrosis and serum levels of hyaluronic acid, type IV collagen and laminin in rats. Zhonghua Quanke Yixue. 2015;13:18–20. [Google Scholar]
- 14.Wang ZL, Liu ZJ, Song T, Sun XH, Luo WM, Liu S. Effects of FuzhengHuoxue recipes on liver fibrosis associated serum indicators and hepatic hydroxyproline in liver fibrosis rats. Zhongyao Xinyao Yu Linchuang Yaoli. 2010;21:611–613. [Google Scholar]
- 15.Lu J, Chen B, Li S, Sun Q. Tryptase inhibitor APC 366 prevents hepatic fibrosis by inhibiting collagen synthesis induced by tryptase/protease-activated receptor 2 interactions in hepatic stellate cells. Int Immunopharmacol. 2014;20:352–357. doi: 10.1016/j.intimp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Zhao Z, Yan Y, Qiang X, Zhou C, Li R, Chen H, Zhang Y. Demethyleneberberine Protects against Hepatic Fibrosis in Mice by Modulating NF-κB Signaling. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17071036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman SL. Hepatic fibrosis -- overview. Toxicology. 2008;254:120–129. doi: 10.1016/j.tox.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Atzori L, Poli G, Perra A. Hepatic stellate cell: a star cell in the liver. Int J Biochem Cell Biol. 2009;41:1639–1642. doi: 10.1016/j.biocel.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Ota C, Baarsma HA, Wagner DE, Hilgendorff A, Königshoff M. Linking bronchopulmonary dysplasia to adult chronic lung diseases: role of WNT signaling. Mol Cell Pediatr. 2016;3:34. doi: 10.1186/s40348-016-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi B, Wang Y, Chen ZJ, Li XN, Qi Y, Yang Y, Cui GH, Guo HZ, Li WH, Zhao S. Down-regulation of miR-30a-3p/5p promotes esophageal squamous cell carcinoma cell proliferation by activating the Wnt signaling pathway. World J Gastroenterol. 2017;23:7965–7977. doi: 10.3748/wjg.v23.i45.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Lu G, Shao Y, Xu D. MiR-182 promotes prostate cancer progression through activating Wnt/β-catenin signal pathway. Biomed Pharmacother. 2018;99:334–339. doi: 10.1016/j.biopha.2018.01.082. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Zhu H, Sun Z, Xiang Z, Ge Y, Ni C, Luo Z, Qian W, Han X. Inhibition of Wnt/β-catenin signaling promotes epithelial differentiation of mesenchymal stem cells and repairs bleomycin-induced lung injury. Am J Physiol Cell Physiol. 2014;307:C234–C244. doi: 10.1152/ajpcell.00366.2013. [DOI] [PubMed] [Google Scholar]
- 24.Dijksterhuis JP, Petersen J, Schulte G. WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br J Pharmacol. 2014;171:1195–1209. doi: 10.1111/bph.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JT, Liu C, Zaytseva YY, Weiss HL, Townsend CM Jr, Evers BM. Neurotensin, a novel target of Wnt/β-catenin pathway, promotes growth of neuroendocrine tumor cells. Int J Cancer. 2015;136:1475–1481. doi: 10.1002/ijc.29123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbons GS, Owens SR, Fearon ER, Nikolovska-Coleska Z. Regulation of Wnt signaling target gene expression by the histone methyltransferase DOT1L. ACS Chem Biol. 2015;10:109–114. doi: 10.1021/cb500668u. [DOI] [PubMed] [Google Scholar]


