Abstract
A key feature of human prosociality is direct transfers, the most active form of sharing in which donors voluntarily hand over resources in their possession. Direct transfers buffer hunter-gatherers against foraging shortfalls. The emergence and elaboration of this behaviour thus likely played a key role in human evolution by promoting cooperative interdependence and ensuring that humans' growing energetic needs (e.g. for increasing brain size) were more reliably met. According to the strong prosociality hypothesis, among great apes only humans exhibit sufficiently strong prosocial motivations to directly transfer food. The versatile prosociality hypothesis suggests instead that while other apes may make transfers in constrained settings, only humans share flexibly across food and non-food contexts. In controlled experiments, chimpanzees typically transfer objects but not food, supporting both hypotheses. In this paper, we show in two experiments that bonobos directly transfer food but not non-food items. These findings show that, in some contexts, bonobos exhibit a human-like motivation for direct food transfer. However, humans share across a far wider range of contexts, lending support to the versatile prosociality hypothesis. Our species' unusual prosocial flexibility is likely built on a prosocial foundation we share through common descent with the other apes.
Keywords: bonobo, chimpanzee, prosociality, cooperation, sharing, human evolution
1. Introduction
Prosocial behaviour is any positive social act—whether unselfish or selfish, costly or cost-free—that benefits another [1]. Of particular importance in considering the evolution of human prosociality is the phylogenetic origin of intentional direct transfer of food or objects, the most proactive form of sharing in which donors voluntarily hand over resources in their possession. Direct transfer of both objects and food emerges early in human ontogeny and likely played a key role in human evolution [2–4]. Direct transfers, including from non-kin, buffer modern hunter-gatherers against foraging shortfalls and, throughout our evolutionary history, likely helped ensure that humans more reliably met their increasing energetic needs [5,6].
Many have suggested that humans are derived or unique in exhibiting strong prosocial motivations—what we collectively refer to as the strong prosociality hypothesis—and, specifically, that among great apes only humans exhibit sufficiently strong prosocial motivations to directly transfer food in their physical possession [7–13]. The versatile prosociality hypothesis suggests instead that while other apes may make transfers in constrained contexts, only humans share flexibly across food and non-food contexts [14,15]. Based on both observations and experiments it appears that chimpanzees (Pan troglodytes) directly transfer objects but not food, supporting both hypotheses. Although chimpanzees sometimes share food and tools in the wild [16,17] and will help a conspecific access food or non-food that the actor cannot access herself ([18], but see [12]), experiments show that chimpanzees typically only transfer food in their possession when they cannot escape a begging recipient. When physically separated from the potential recipient and able to avoid harassment, they do not directly transfer easily monopolizable food [9,19,20]. However, in similar circumstances (i.e. when physically separated from the recipient), they reliably transfer tools and other objects in their possession [2,21–23].
Bonobos (Pan paniscus) exhibit a different prosociality profile than chimpanzees. In controlled dyadic contexts, they are more socially tolerant than chimpanzees, and often choose to co-feed in close proximity ([24–27], but see [28,29]). In the wild, females have even been observed sharing food from their mouths with other non-kin females even though more fruit of the same type is readily available to both—often within reaching distance of the recipient [30]. Their high levels of dyadic tolerance allow them to spontaneously outperform chimpanzees in instrumental cooperative tasks that require sharing monopolizable food [24]. In experiments, when given the choice of eating alone or releasing a conspecific to eat together, bonobos even share their food voluntarily [31,32]. Bonobos will also help groupmates or non-groupmates in obtaining out-of-reach food that they themselves cannot obtain, even without the potential for physical interaction or active solicitation by the recipient [14,32]. However, they do not share high-value food when they are unable to physically interact with the recipient or to control how much of their food the recipient receives [32].
Despite differences between chimpanzee and bonobo prosociality, the strong prosociality and versatile prosociality hypotheses were largely framed based on comparative data from chimpanzees and humans alone. A critical test of these hypotheses thus requires investigation of bonobos' tendency to directly transfer food and non-food items ([14], also see related work in more distant relatives of humans: e.g. [33,34]). According to the strong prosociality hypothesis, bonobos—like chimpanzees—will not exhibit any form of direct transfer of food. According to the versatile prosociality hypothesis, bonobos may show direct transfers but only in constrained contexts. For example, bonobos will not transfer both food and non-food items or they will only transfer low-value but not high-value food. We performed two experiments to test these competing predictions.
2. Experiment 1
(a). Methods
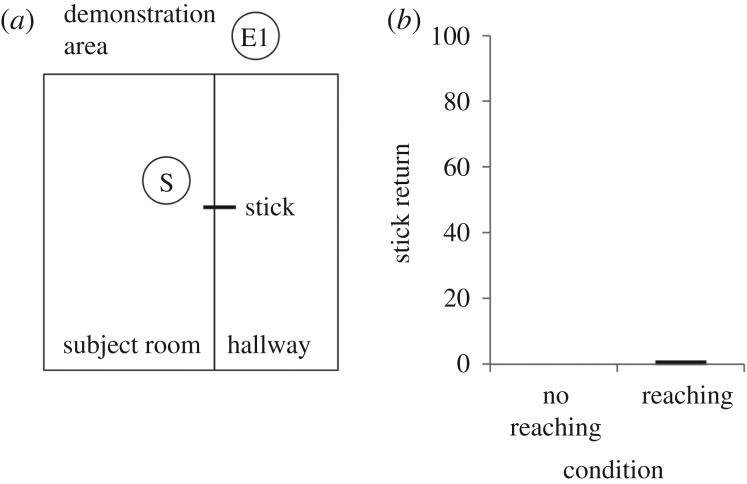
In Experiment 1, we tested whether bonobos (N = 18; 6M : 12F, ages 3–15; electronic supplementary material, table S1 and movie S1) would retrieve and transfer an out-of-reach object to help a human experimenter, using a method in which both human infants and chimpanzees readily do so [2]. Chimpanzees with extensive human socialization exhibit a similar motivation to help both conspecifics and humans retrieve out-of-reach objects. This frequently has been interpreted to mean that motivations towards humans can reveal how chimpanzees are motivated to interact with each other [2,3,21–23]. We pursued this experiment first because it facilitated a direct comparison between bonobos and chimpanzees under the maximally controlled settings that are only possible with human experimenters. Bonobos were situated in a mesh-walled room and witnessed E2, in a demonstration area, steal a stick from E1 (figure 1a). E2 then carried the stick into the hallway adjacent to the subject room and closed the door behind him. E1 grabbed the door, whimpering, and watched as E2 placed a small piece of banana under the mesh between the subject room and the hallway to position the subject at the starting location, and then placed the stick partially through the mesh about 1 m from the banana. E2 then walked further down the hallway out of the testing area.
Figure 1.
(a) Testing set-up and (b) results of Experiment 1.
Each trial began when the subject ate the piece of banana. In the first 30 s, E1 leaned against the door, looked, and vocalized towards the stick. If after 30 s the subject had not transferred the stick to E1, E1 became more communicative by calling the subject's name, banging the door and alternating his gaze between the subject and the stick. To determine whether an ostensive cue of desire can help elicit transfer, in the reaching condition (N = 9), E1 reached with effort towards the stick throughout the duration of the trial. In the no-reaching condition (N = 9), this additional cue was absent: E1 kept his arms at his side or on the door. Each trial ended when the subject transferred the stick, or after 1 min. Each subject participated in a 12-trial session with 10 test trials as just described and two baseline trials—one at the beginning and one at the end. Baseline trials were identical to test trials except that E1 was never present. Subjects were never rewarded for transfers to ensure that any transfer behaviour was spontaneous and did not occur in response to rewarding.
(b). Results and discussion
Bonobos did not transfer the stick. Although subjects often retrieved the stick (33.33% of reaching trials and 43.33% of no-reaching trials), they did not transfer it. Whereas chimpanzees and human infants in the same paradigm delivered the objects to the experimenter in approximately half of reaching trials—even when unrewarded at the time of testing [2], bonobos did so in only 1.1% of these trials and 0% of no-reaching trials (figure 1b) (see also [35]). Examining their behaviour qualitatively, subjects sometimes responded with what appeared to be teasing instead of helping (i.e. gesturing towards E1 with stick in hand, often moving the stick close and then pulling it back, and ultimately refusing to transfer the stick). Four subjects ‘teased’ the experimenter in a total of 11 trials in the reaching condition and two subjects ‘teased’ the experimenter in a total of two trials in the no-reaching condition. This behaviour, and previous work on bonobos' understanding of others' reaching goals [36–39], suggests that bonobos' lack of direct transfers is unlikely to be explained by a failure to understand E1's goal. The behaviour of bonobos here provides additional evidence against the idea that the direct transfer of objects by chimpanzees is simply the product of previous rewarding, unless there exists a species difference in susceptibility to reward history between chimpanzees and bonobos [2,3,12]. Sanctuary bonobos have highly similar rearing histories to sanctuary chimpanzees and caretakers are equally motivated to reward both species for returning objects, yet here bonobos have not developed a chimpanzee-like pattern of object transfer.
3. Experiment 2
While wild bonobos use a range of tools, they have not been observed using tools in extractive foraging. In contrast, captive bonobos exhibit tool-use in a range of food acquisition contexts that mirror chimpanzees [40–42]. Bonobos at Lola ya Bonobo sanctuary often use rocks to crack palm nuts. Although they can crack these nuts with their teeth, they prefer to crack them with rock hammers and can crack and consume nuts at a median rate of 2.8 nuts per minute [41,42]. In natural interactions at the sanctuary, bonobos have been observed to both passively and actively share nuts after cracking them (B Hare 2005, personal observation). Bonobos appear to assign nuts intermediate value between high-value fruit and low-value foliage [43]—likely, in part, because they require greater effort to open with teeth or to find a proper tool. Taking advantage of this natural context, as a second test of object and food transfer, we examined whether bonobos would directly transfer either a tool (i.e. a rock) or nuts to a conspecific when each only had access to one or the other resource (electronic supplementary material, movie S2).
(a). Methods
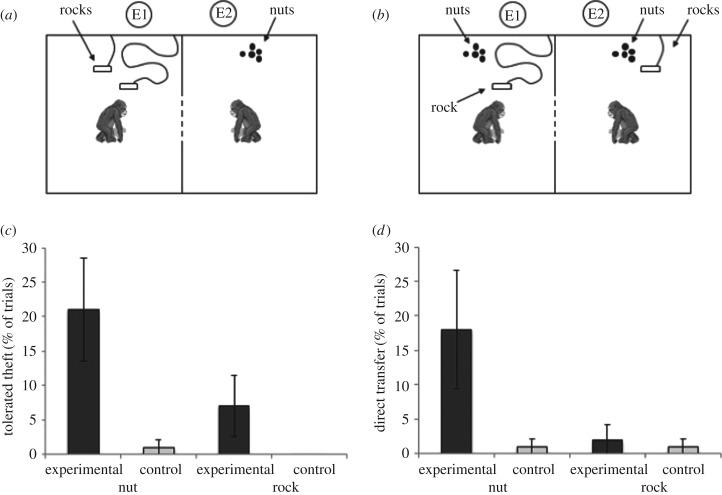
In Experiment 2, two bonobos (10 pairs comprised of 12 new subjects, 2M : 10F; aged 5–15 years; electronic supplementary material, table S2) were situated in adjacent rooms and could physically interact only through a 1 m2 mesh window with a 20 × 20 cm hole in the centre. Subjects could thus choose to transfer items or interact socially (e.g. grooming, ‘teasing’), or to avoid sharing or interacting. In each trial of the experimental condition, one individual (i.e. the rock-owner) was provisioned with two rocks that could be used to crack palm nuts and a second (i.e. the nut-owner) was provisioned with five nuts (figure 2a). In the rock-owner's room, each rock was approximately 20 × 15 × 5 cm in size (i.e. maximum length/width/height diameters) and approximately 2 kg in weight. Both rocks were tethered to the wall of the rock-owner's room, approximately 2 m away from the hole, so that they could reliably be returned to the rock-owner's room between trials. One rock was tethered with a short rope of approximately 1 m, and the other with a long rope of approximately 5 m. This set-up positioned both rocks out of the nut-owner's reach, but the rock with the long rope could be transferred through the hole into the nut-owner's room. In the nut-owner's room, the five nuts were provisioned approximately 4 m away from the transfer hole, well out of the rock-owner's reach. As a result, either subject had complete control over her items because the items were provisioned in a corner of the testing room far beyond the reach of her partner, but she could help her partner by transferring them [31,44]. In the control condition, transfer was not needed as each subject received one rock and five nuts, thus controlling for baseline rates of transfer and ensuring that transfer in the test could not be explained by a lack of motivation by the donor to crack and eat nuts (figure 2b). In both conditions, between trials, any transferred rocks were returned to the rock-owner's room via the rope but untransferred or uneaten nuts could not be recovered and remained in the nut-owner's room. Each pair participated in two five-trial sessions per condition (half of dyads received the conditions in ABBA order and half in BAAB order), for a total of 10 experimental and 10 control trials. A minimum of one day, but not more than six, elapsed between sessions. To control for currency-specific reciprocity across trials, within each dyad, roles were never reversed. Trials lasted 5 min. Note that for analyses the nut-owner and rock-owner maintained their designations across conditions, even though subjects received both resources in the control condition.
Figure 2.
Testing set-up and results of Experiment 2. (a) Experimental condition. (b) Control condition. Located in adjacent rooms, subjects could interact through a single window (dashed line). Round dots represent nuts provided to subjects in each trial while rectangles attached to rounded lines depict rocks and their tethers. Nuts and rocks were provisioned far beyond the reach of the bonobo in the adjacent room. E1 and E2 served as experimenters and camera-people. (c) Per cent of trials in which subjects exhibited tolerated theft of nuts and rocks in the experimental and control conditions. (d) Per cent of trials in which subjects exhibited direct transfer of nuts and rocks in the experimental and control conditions. Error bars denote standard error.
Before qualifying for the test phase, to demonstrate their knowledge of the task and motivation to consume nuts, each subject completed a self-regard pre-test in which in two 5-min trials they received three nuts and one rock [45]. To be included in the experiment, subjects had to crack at least two of six nuts (N = 12 passed, six others were excluded for not meeting this criterion); however, those that met this criterion tended to crack all or nearly all six (M = 4.83; electronic supplementary material, table S2), demonstrating both skill and high motivation to consume the nuts. To ensure their understanding that both the rock and nut were relevant for nut-cracking, in a subsequent tool-use mastery pre-test, subjects had to transport nuts to a rock on the other side of the room and crack at least one nut within 5 min. Subjects who did not meet this criterion after two trials were not included in the test phase (N = 0 excluded). To assess the role of dominance on transfer behaviour, each pair additionally participated in seven trials of a standard food dominance test (based on [26]) in which they were fed on opposite sides of a testing room and then allowed to compete over a monopolizable piece of food located directly between them. The individual who acquired the food in a majority of trials was scored as dominant (in all dyads the food-dominant individual acquired at least six of seven pieces of food, and reliability coding produced 100% agreement; electronic supplementary material, table S2).
During the test phase, we recorded whether or not in a trial the following behaviours occurred (i.e. as a binary measure) as well as the number of items transferred in each way (definitions largely followed [46]): (i) direct transfer, in which the possessor transferred an item through the test window into the adjacent room, (ii) tolerated theft, in which the recipient acquired an item from the possessor's side of the window (including on the floor and in the hands of the possessor) without resistance (or aggression) from the possessor, and (iii) forced claim, in which the recipient acquired an item from the possessor's side of the window while the possessor resisted by pulling back the recipient's hand, pulling back the item, or racing to grab the item off the floor. To assess whether sharing occurred proactively or in response to request, we also recorded gesturing (potential request behaviour) whenever an individual reached through the window empty-handed, as long as her hand remained empty when she retracted it (reliability on all measures was excellent, κ > 0.85; see electronic supplementary material for details). Much work suggests that chimpanzees tend to share and help reactively, whereas increasingly it appears that bonobos may be proactively prosocial [2,18,21,22,32].
(b). Results and discussion
Bonobos exhibited tolerated thefts and direct transfers but not forced claims (rates presented in figure 2c,d and electronic supplementary material, table S3). Bonobos shared nuts in significantly more trials in the experimental condition than in the control both by tolerated theft and by direct transfer (tolerated theft: M = 2.1 ± s.e. = 0.745 trials in experiment, M = 0.1 ± 0.105 trials in control, z = −2.207, N = 10, T+ = 6, ties = 4, p = 0.027; direct transfer: M = 1.8 ± 0.858 trials in experiment, M = 0.1 ± 0.105 trials in control, z = −1.980, N = 10, T+ = 6, ties = 3, p = 0.048, two-tailed related samples Wilcoxon signed-rank tests). However, frequency of rock-sharing by tolerated theft or by direct transfer did not differ between conditions (tolerated theft: M = 0.7 ± 0.446 trials in experiment, M = 0 ± 0 trials in control, z = −1.604, N = 10, T+ = 3, ties = 7, p = 0.109; direct transfer: M = 0.2 ± 0.211 trials in experiment, M = 0.1 ± 0.105 trials in control, z = −1, N = 10, T+ = 1, ties = 9, p = 0.317, two-tailed related samples Wilcoxon signed-rank tests).
There was no difference in the number of trials involving sharing of nuts versus rocks in the control condition (tolerated theft: z = −1.000, N = 10, T+ = 1, ties = 9, p = 0.317; direct transfer: z = 0, N = 10, T+ = 1, ties = 8, p = NS, two-tailed related samples Wilcoxon signed-rank tests). However, in the experimental condition subjects shared nuts in significantly more trials than rocks both by tolerated theft and by direct transfer (tolerated theft: z = −2.401, N = 10, T+ = 7, ties = 3, p = 0.016; direct transfer: z = −2.226, N = 10, T+ = 6, ties = 4, p = 0.026, two-tailed related samples Wilcoxon signed-rank tests). Of the 495 shareable nuts in the experimental condition (one dyad received only nine experimental trials), nut-owners directly transferred 40 nuts (8.08%) and shared an additional 41 nuts (8.28%) via tolerated theft, producing an overall sharing rate of 16.36% (electronic supplementary material, table S3). Three dyads never shared, meaning that the sharing rate for those that did was 23.14% (of 350 shareable nuts). We did not observe any form of aggression throughout and only witnessed instances of potential teasing with the rock in 2.22% of experimental trials and 4% of control trials (see electronic supplementary material for coding definitions). Together, these results reveal that bonobos both passively and actively shared nuts in their possession, in the absence of aggression or resistance. Most strikingly, in direct contrast to other primates, in which direct food transfers either never or almost never occur [47], bonobos' direct food transfers were not an occasional act; they occurred frequently (18.18% of experimental trials) and at comparable rates to tolerated thefts (21.21% of experimental trials). However, consistent with Experiment 1, bonobos almost never shared non-food items—tools in this case.
To further explore bonobos' food-sharing behaviour, we investigated the predictors of tolerated theft and direct transfer of nuts on a trial-by-trial basis in separate generalized linear mixed models (GLMMs) using the glmer function in lme4 in R. Both models included the same random effects and predictor variables, but differed in the dependent measure: tolerated theft or direct transfer of nuts. Both measures were binary (i.e. 0/1: whether or not, within the trial, the nut-owner transferred at least one nut by the given means). To account for multiple observations, we included the subject pair as a random intercept. Our models also included several fixed effects: condition (to account for differences in transfer between the experimental and control conditions), gesture by the rock-owner (to determine whether help was provided proactively, or in response to request; [18,21]), rock transfer in the same trial (combined tolerated theft and active transfer; to assess the influence of within-trial interchange), food-dominance (to determine whether transfers were directed up or down the hierarchy), and trial number (to account for change over time). It is possible that bonobos might have exchanged nuts for grooming or ‘teased’ their partner to reduce proximity; however, both behaviours occurred infrequently (grooming: 6.5% of trials; ‘teasing’: 3.0% of trials) and neither co-occurred with tolerated theft or direct transfer in more than a single trial. Therefore, we did not include either factor. We first compared our full models with null models that included only the random effects (and no fixed effects) using likelihood ratio tests. Both comparisons were significant (tolerated theft: χ2=31.428, d.f. = 5, p < 0.001; direct transfer: χ2 = 25.212, d.f. = 5, p < 0.001), permitting interpretation of the full models; p-values for fixed effects were generated using likelihood ratio tests comparing the full models with models in which individual fixed effects were removed.
Consistent with our previous analyses, both models showed that bonobos shared nuts significantly more in the experimental condition than in the control (p < 0.001 for both models; see electronic supplementary material, tables S4 and S5). However, there was no effect of trial number in either model, indicating that learning or changes in motivation did not influence nut-sharing of either type. We also found no effect of dominance, indicating that sharing did not simply occur up or down the hierarchy. Although gesturing by the rock-owner occurred in 26.5% of trials, there was no relationship between gesturing and nut-sharing of either type, consistent with sharing being unsolicited. This finding is in line with evidence that bonobos perform prosocial behaviours proactively [14,31,32,44], which contrasts with the reactive nature of chimpanzee helping ([2,18,21,22], but see [48]).
We found that tolerated theft of nuts (but not direct transfer of nuts) was predicted by sharing of rocks in the same trial (p = 0.040). This apparently reciprocal pattern might result from an intentional interchange of resources or, more parsimoniously, from the physical proximity shared by tolerant partners. The majority of rock transfers (7 of 10) were also tolerated thefts (i.e. symmetry-based reciprocity; [49,50]). Interestingly, we only documented two trials in which a nut-owner acquired any pieces of cracked nuts from the rock-owner after transferring uncracked nuts to her. Recovery of pieces of cracked nuts occurred in a single pair (nut-owner: Waka, rock-owner: Masisi) and only via tolerated theft. Finally, since some subjects participated in two dyads (once as the nut-owner and once as the rock-owner), in a separate model we confirmed that generalized reciprocity did not impact direct transfer of nuts (see electronic supplementary material and table S6).
Bonobos frequently shared food but not tools even though they had passed a self-regard pre-test and a tool mastery pre-test, demonstrating their motivation to crack and eat nuts and their understanding of the functionality of the tool. Although there was no cost to transferring the rock in the experimental condition, since subjects had a second rock and the rocks could be easily picked up with one hand (electronic supplementary material, figure S1), they did not exhibit transfers of this kind. Instead, subjects chose to transfer nuts in many trials. Because food was given to the subject approximately 4 m from the window between the subject and recipient, all food transfers required the subject to first bring food to within reach of the recipient and then actively or passively transfer it. It is possible that subjects did not transfer stones because they did not receive a pre-test in which they experienced transferring stones for their own use. The ability of subjects to spontaneously transfer nuts without a similar pre-test argues against this possibility. This account is also unlikely to explain differences in food versus non-food transfer for at least three additional reasons. First, bonobos were not motivated to transfer even much lighter non-food items in Experiment 1. Second, they are very familiar with large stones and often carry them around the sanctuary (see electronic supplementary material, figure S1 of an infant carrying a similarly sized rock). Finally, four pairs did transfer stones (via theft or direct transfer) through the window on at least one occasion, demonstrating that they were capable of doing so.
4. General discussion
These experiments support the versatile prosociality hypothesis by providing evidence that while bonobos will proactively transfer a type of food to non-relatives, they do not transfer toys or tools as chimpanzees do. Although neither bonobos nor chimpanzees demonstrate the range of prosocial behaviours observed in human infants and adults, each species exhibits forms of prosociality that have been hypothesized to be unique to our species. The current work suggests instead that it is the diversity and degree of prosociality that is derived in the human lineage [14].
In direct conflict with the predictions of the strong prosociality hypothesis, we provide the first experimental evidence that bonobos spontaneously hand conspecifics pieces of easily monopolizable food. Transfers required that a subject carry nuts several metres and within reach of the window separating the subject from the potential recipient. While bonobos did not transfer nuts or tools in the majority of trials, they did transfer nuts both passively and actively at relatively high rates. Importantly, both tolerated theft and direct transfer of nuts occurred more often in the experimental condition than in the control, and at higher rates than theft or direct transfer of rocks. We also never observed the bonobos discarding nuts by passing them out of the room except through the sharing window. This pattern is consistent with intentional sharing: subjects collected uncracked nuts, carried them within reach of the recipient, and either tolerated taking or actively handed them through the window for their partner to crack. The bonobos never attempted to prevent their partner from retrieving the food they had shared (i.e. no forced claims) and gesturing by the recipient was unrelated to nut transfers within each trial, suggesting that direct transfers were proactive. Subjects rarely transferred nuts in the control when both the subject and recipient possessed both nuts and stones. This pattern makes it difficult to characterize the observed sharing as an accidental by-product of stimulus enhancement or social facilitation (e.g. [12,45]). Even if some tolerated thefts occurred because tolerant nut-owners were attracted to the window by the rock in the adjacent room and brought the nuts with them, such behaviour cannot explain nuts that were actively shared via direct transfer. Subjects were also not sharing under pressure since neither subject could harass the other given their physical separation and size of the large testing rooms. Instead, the bonobos' food-sharing behaviour appears to be intentional [13,51].
There is little evidence that social or non-social rewards motivated the bonobos' direct food transfers. Since pairs of subjects never swapped roles in the experimental condition, rock-owners could not directly reciprocate by passing whole nuts to nut-owners, and we almost never observed subjects obtain cracked nuts after sharing them with recipients. There also was no interchange or generalized reciprocity associated with direct nut transfers. The direct transfer of uncracked nuts and stone tools between nut-owners and rock-owners did not correspond within trials, and the tendency for a subject to directly transfer nuts in a session was not related to whether or not she had recently received nuts when participating as a rock-owner. We found no effect of trial number on bonobos' direct transfer behaviour, suggesting that motivation to share was stable despite a lack of immediate rewards. The dominance relationship between the nut-owner and rock-owner was also unrelated to transfers, making it difficult to argue that sharing was motivated by status-striving [30].
Nut-owners incurred a moderate cost by directly transferring food. They had to carry the nuts across the room, within reach of the rock-owner, and transfer them. Although they prefer to use stone tools to open nuts, they are capable of cracking this type of nut with their teeth after some effort. They also could have brought the uncracked nuts into the outdoor enclosure, following the test session, and cracked them with naturally available rocks. By transferring nuts they were thus forfeiting edible food. Nonetheless, we rarely saw subjects use their teeth to crack nuts in this experimental setting. This may suggest that without a tool available, uncracked palm nuts are a relatively low-value food that only increases in value once cracked. It may therefore be that nut transfer was relatively low cost for the nut-owner but highly beneficial to the rock-owner. Despite the fact that nuts only have intermediate value as a food, the bonobos were highly motivated to eat them if they had a stone tool available to process them.
Although quantitative comparisons cannot be made between species due to differences in methodology, qualitative comparisons suggest that this instance of bonobo food-sharing is unlike that seen in chimpanzees and highly unusual among non-human primates. Although different empirical approaches have produced some differing results about food tolerance and sharing between species (see [14] for important discussion of this point) [24–26,28,29], controlled dyadic experiments can clarify rates of sharing when alternative motivations like harassment and group dynamics are controlled for. When chimpanzees are separated from a potential recipient, proactive and direct transfers are almost non-existent [9,19,52]. In contrast, bonobos exhibited direct transfers of nuts nearly as frequently as they did tolerated thefts (in 18.18% and 21.21% of experimental trials, respectively). In fact, 49.38% of nuts shared in the experimental condition were directly transferred and 50.62% were shared via tolerated theft. Even in capuchin monkeys (Sapajus apella), who have been described as tolerant food-sharers, direct transfers only account for 0.3% of sharing events [46]. Bonobos' rates of direct transfer are higher even than those reported for cooperative-breeding callitrichid adults sharing with other adults (M = 0% ± s.d. = 0% of sharing events) and with infants (16.44% ± 17.88% of sharing events) [8,47]. While we note that there are important differences between studies (e.g. in the specific types of food being shared and their potential values, the absolute amount of sharing, and the experimental set-ups), only bonobos have been observed to directly transfer food at such high rates without kinship, harassment, or mating opportunities as proximate motivators. Future work can use this paradigm to directly compare bonobos with chimpanzees and other species, and with bonobos from other groups and of other ages, that have experience with nut-cracking. Given that wild adult bonobos show the highest rates of sharing, it may be that bonobos' delayed development of social intolerance relative to chimpanzees contributed important pre-conditions for the emergence of proactive food-sharing [25,30].
It is equally interesting to consider what behaviours we did not observe from the bonobos. In Experiment 1, bonobos did not return an object they had seen forcefully taken from an experimenter [35]. While subjects often retrieved the experimenter's toy for themselves, they never responded to the experimenter's request to return it with anything but playful ‘teasing’ behaviours. These cases appeared to be an attempt to initiate a social interaction but it was not the helpful response displayed by chimpanzees in a nearly identical experimental context (i.e. [2]). The pattern seen in the current dyadic interaction is also consistent with the previous finding that bonobos even prefer individuals that hinder rather than help a third party trying to retrieve an object [53]. In Experiment 2, we also documented a striking absence of stone tool-sharing. Rock-owners had a surplus of rocks, yet rarely passed one of them through the sharing window. This is again unlike the response of chimpanzees who readily share tools that will help others obtain food [22].
Several explanations can be ruled out for the failure of bonobos to share objects in both experiments. Bonobos have as complex gestural repertoires and understand human gestures as well as or even better than chimpanzees [38,54,55]. Bonobos from this same sanctuary also successfully discriminate between helpful and unhelpful experimenters in a similar context [53]. It is unlikely that bonobos did not understand the experimenter's requests or the nut-owner's need for a stone tool [36,39]. A host of experimental and observational studies also show that bonobos are not more attracted to or possessive of novel objects or tools than chimpanzees [56–58]. However, in their everyday interactions, sanctuary bonobos have been observed to refuse to share nut-cracking stones and even carry them around for several consecutive days ([42]; C Krupenye 2012, personal observation) (electronic supplementary material, movie S2 and figure S1). Future research can test whether in some contexts bonobos perceive objects as having an unusually high value. For example, bonobos may especially value stone tools or toys that make them more attractive to other bonobos and increase opportunities for play, sex and food-sharing. Until then, the lack of object-sharing in bonobos remains enigmatic.
Any form of bi-directional direct transfer was also completely absent during Experiment 2. After cracking nuts that nut-owners had passed through the window, rock-owners rarely, if ever, passed any food back to the nut-owner. Rock-owners could have easily shared a small proportion of the nuts they cracked or at least provided a stone to help the nut-owner crack their remaining nuts. Communication was also limited and did not appear to influence sharing. Regardless of the role they were playing, bonobos could have persistently or more overtly gestured for help to initiate turn-taking and reciprocity. Future research can further explore if alternating the roles of the nut- and rock-owners can facilitate bi-directional sharing across trials, and continue to investigate any role of communication in mediating sharing levels. Work in the wild and in captivity suggests that sharing is goal-directed and has a social function [30,44]. However, it would also be interesting to specifically examine bonobos' sensitivity to others' needs of nuts and stones (or others' capacity to profit from sharing) by investigating whether nut-owners selectively transfer nuts to partners in possession of rocks.
Experiments have now demonstrated that both bonobos and chimpanzees are capable of the most active form of sharing—direct transfers—but the context in which each species does so is different. Here we show that bonobos exhibit this behaviour with at least one type of food. Given the xenophilic preferences previously observed in bonobos and their willingness to aid strangers attempting to obtain out-of-reach food, it's possible that bonobos would even transfer nuts to conspecifics with whom they have never had a social interaction [14,32,44]. The findings from the present studies (and other recent work with bonobos) suggest that the motivation driving human hunter-gatherers to proactively share may have evolved through a quantitative shift from their common ancestor with the other apes, rather than the radical qualitative shift that has previously been suggested [59,60]. This seems increasingly likely considering food-sharing in human hunter-gatherers, such as Hadza men, actually occurs after donors have already met their daily caloric needs [61], and across human populations highly costly altruism towards strangers is exceptionally rare [62,63]. While the quantity of food shared and its role in buffering group members against caloric shortfalls is unparalleled in humans [6], it is less difficult to explain provisioning with surplus food that is of high value to the recipient and of relatively low value to the possessor. This is analogous to the cost-benefit payoff seen in Experiment 2 for the bonobos sharing uncracked palm nuts. The challenge may not be in explaining how humans became extreme in our prosociality but instead understanding how our lineage evolved so much versatility in recognizing when low-cost helping is of greatest benefit to others [64,65].
Acknowledgements
We are thankful to Suzy Kwetuenda and Delphin Bilua for assistance collecting data, to Laura Lewis, Kyle Smith, Joe Sullivan, Danielle Su, and Malia Budd for coding our videos, and to Roger Mundry for statistical consultation. We thank Claudine Andre, Dominique Morel, Fanny Mehl, Pierrot Mbonzo and the animal caretakers at Lola ya Bonobo and the Ministry of Research and the Ministry of Environment in D.R. Congo (permit MIN.RS/SG/004/2009) for hosting our research.
Ethics
These non-invasive behavioural studies were approved by Duke University (IACUC #A078-08-03) and adhered to the legal requirements of the Ministry of Research and the Ministry of Environment in D.R. Congo (permit MIN.RS/SG/004/2009). Animal husbandry and care practices complied with the policies of Lola ya Bonobo, as well as the Pan-African Sanctuary Alliance Primate Veterinary Healthcare Manual.
Data accessibility
Data are tabulated in electronic supplementary material, tables S1–S3. Trial-by-trial data for the GLMMs in Experiment 2 are available in the ESM Data file.
Authors' contributions
C.K. and J.T. conducted the experiments; C.K., J.T. and B.H. designed the experiments, analysed the data and wrote the paper.
Competing interests
We have no competing interests.
Funding
This research was supported in part by National Science Foundation Graduate Research Fellowship DGE-1106401 and European Commission Marie-Sklodowska Curie European Fellowship MENTALIZINGORIGINS to C.K. and National Science Foundation grants NSF-BCS-08-27552-02 and NSF-BCS-10-25172 to B.H.
References
- 1.Eisenberg N, Fabes R, Spinrad T. 2006. Prosocial development. In Handbook of child psychology (ed. Eisenberg N.), pp. 646–718. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 2.Warneken F, Hare B, Melis AP, Hanus D, Tomasello M. 2007. Spontaneous altruism by chimpanzees and young children. PLoS Biol. 5, e184 ( 10.1371/journal.pbio.0050184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warneken F, Tomasello M. 2006. Altruistic helping in human infants and young chimpanzees. Science 311, 1301–1303. ( 10.1126/science.1121448) [DOI] [PubMed] [Google Scholar]
- 4.Birch LL, Billman J. 1986. Preschool Children's food-sharing with friends and acquaintances. Child Dev. 75, 387–395. ( 10.2307/1130594) [DOI] [Google Scholar]
- 5.Gurven M. 2004. To give and to give not: the behavioral ecology of human food transfers. Behav. Brain Sci. 27, 543–583. [Google Scholar]
- 6.Pontzer H, et al. 2016. Metabolic acceleration and the evolution of human brain size and life history. Nature 533, 390–392. ( 10.1038/nature17654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkart JM, Hrdy SB, Van Schaik CP. 2009. Cooperative breeding and human cognitive evolution. Evol. Anthropol. 18, 175–186. ( 10.1002/evan.20222) [DOI] [Google Scholar]
- 8.Jaeggi AV, Burkart JM, van Schaik CP. 2010. On the psychology of cooperation in humans and other primates: combining the natural history and experimental evidence of prosociality. Phil. Trans. R. Soc. B 365, 2723–2735. ( 10.1098/rstb.2010.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens JR. 2004. The selfish nature of generosity: harassment and food-sharing in primates. Proc. R. Soc. Lond. B 271, 451–456. ( 10.1098/rspb.2003.2625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silk JB, Brosnan SF, Vonk J, Henrich J, Povinelli DJ, Richardson AS, Lambeth SP, Mascaro J, Schapiro SJ. 2005. Chimpanzees are indifferent to the welfare of unrelated group members. Nature 437, 1357–1359. ( 10.1038/nature04243) [DOI] [PubMed] [Google Scholar]
- 11.Jensen K, Hare B, Call J, Tomasello M. 2006. What's in it for me? Self-regard precludes altruism and spite in chimpanzees. Proc. R. Soc. B 273, 1013–1021. ( 10.1098/rspb.2005.3417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tennie C, Jensen K, Call J. 2016. The nature of prosociality in chimpanzees. Nat. Commun. 7, 13915 ( 10.1038/ncomms13915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burkart JM, et al. 2014. The evolutionary origin of human hyper-cooperation. Nat. Commun. 5, 4747 ( 10.1038/ncomms5747) [DOI] [PubMed] [Google Scholar]
- 14.Tan J, Hare B. 2017. Prosociality among non-kin in bonobos and chimpanzees compared. In Bonobos: unique in mind, brain and behaviour (eds Hare B, Yamamoto S), pp. 140–154. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Warneken F. 2015. Precocious prosociality: why do young children help? Child Dev. Perspect. 9, 1–6. ( 10.1111/cdep.12101) [DOI] [Google Scholar]
- 16.Gilby IC. 2006. Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Anim. Behav. 71, 953–963. ( 10.1016/j.anbehav.2005.09.009) [DOI] [Google Scholar]
- 17.Pruetz JD, Lindshield S. 2012. Plant-food and tool transfer among savanna chimpanzees at Fongoli, Senegal. Primates 53, 133–145. ( 10.1007/s10329-011-0287-x) [DOI] [PubMed] [Google Scholar]
- 18.Melis AP, Warneken F, Jensen K, Schneider AC, Call J, Tomasello M. 2011. Chimpanzees help conspecifics obtain food and non-food items. Proc. R. Soc. B 278, 1405–1413. ( 10.1098/rspb.2010.1735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melis AP, Hare B, Tomasello M. 2006. Chimpanzees recruit the best collaborators. Science 311, 1297–1300. ( 10.1126/science.1123007) [DOI] [PubMed] [Google Scholar]
- 20.Hamann K, Warneken F, Greenberg JR, Tomasello M. 2011. Collaboration encourages equal sharing in children but not in chimpanzees. Nature 476, 328–331. ( 10.1038/nature10278) [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto S, Humle T, Tanaka M. 2009. Chimpanzees help each other upon request. PLoS ONE 4, e7416 ( 10.1371/journal.pone.0007416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto S, Humle T, Tanaka M. 2012. Chimpanzees’ flexible targeted helping based on an understanding of conspecifics’ goals. Proc. Natl Acad. Sci. USA 109, 3588–3592. ( 10.1073/pnas.1108517109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melis AP, Tomasello M. 2013. Chimpanzees’ (Pan troglodytes) strategic helping in a collaborative task. Biol. Lett. 9, 20130009 ( 10.1098/rsbl.2013.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hare B, Melis AP, Woods V, Hastings S, Wrangham R. 2007. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 17, 619–623. ( 10.1016/j.cub.2007.02.040) [DOI] [PubMed] [Google Scholar]
- 25.Wobber V, Wrangham R, Hare B. 2010. Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Curr. Biol. 20, 226–230. ( 10.1016/J.Cub.2009.11.070) [DOI] [PubMed] [Google Scholar]
- 26.Wobber V, Hare B, Maboto J, Lipson S, Wrangham R, Ellison PT. 2010. Differential changes in steroid hormones before competition in bonobos and chimpanzees. Proc. Natl Acad. Sci. USA 107, 12 457–12 462. ( 10.1073/pnas.1007411107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hare B, Wobber V, Wrangham R. 2012. The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Anim. Behav. 83, 573–585. ( 10.1016/j.anbehav.2011.12.007) [DOI] [Google Scholar]
- 28.Jaeggi AV, Stevens JM, Van Schaik CP. 2010. Tolerant food-sharing and reciprocity is precluded by despotism among bonobos but not chimpanzees. Am. J. Phys. Anthropol. 143, 41–51. ( 10.1002/ajpa.21288) [DOI] [PubMed] [Google Scholar]
- 29.Cronin KA, De Groot E, Stevens JM. 2015. Bonobos show limited social tolerance in a group setting: a comparison with chimpanzees and a test of the relational model. Folia Primatol. 86, 164–177. ( 10.1159/000373886) [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto S. 2015. Non-reciprocal but peaceful fruit sharing in wild bonobos in Wamba. Behaviour 152, 335–357. ( 10.1163/1568539x-00003257) [DOI] [Google Scholar]
- 31.Hare B, Kwetuenda S. 2010. Bonobos voluntarily share their own food with others. Curr. Biol. 20, R230–R231. ( 10.1016/j.cub.2009.12.038) [DOI] [PubMed] [Google Scholar]
- 32.Tan J, Hare B. 2013. Bonobos share with strangers. PLoS ONE 8, e51922 ( 10.1371/journal.pone.0051922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amici F, Mimo MC, von Borell C, Bueno-Guerra N. 2017. Meerkats (Suricata suricatta) fail to prosocially donate food in an experimental set-up. Anim. Cogn. 20, 1059–1066. ( 10.1007/s10071-017-1122-6) [DOI] [PubMed] [Google Scholar]
- 34.Horn L, Scheer C, Bugnyar T, Massen JJ. 2016. Proactive prosociality in a cooperatively breeding corvid, the azure-winged magpie (Cyanopica cyana). Biol. Lett. 12, 20160649 ( 10.1098/rsbl.2016.0649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liebal K, Vaish A, Haun D, Tomasello M. 2014. Does sympathy motivate prosocial behaviour in great apes? PLoS ONE 9, e84299 ( 10.1371/journal.pone.0084299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kano F, Call J. 2014. Great apes generate goal-based action predictions: an eye-tracking study. Psychol. Sci. 25, 1691–1698. ( 10.1177/0956797614536402) [DOI] [PubMed] [Google Scholar]
- 37.Wobber V, Herrmann E, Hare B, Wrangham R, Tomasello M. 2014. Differences in the early cognitive development of children and great apes. Dev. Psychobiol. 56, 547–573. ( 10.1002/dev.21125) [DOI] [PubMed] [Google Scholar]
- 38.MacLean EL, Hare B. 2015. Bonobos and chimpanzees exploit helpful but not prohibitive gestures. Behaviour 152, 493–520. ( 10.1163/1568539x-00003203) [DOI] [Google Scholar]
- 39.Krupenye C, Kano F, Hirata S, Call J, Tomasello M. 2016. Great apes anticipate that other individuals will act according to false beliefs. Science 354, 110–114. ( 10.1126/science.aaf8110) [DOI] [PubMed] [Google Scholar]
- 40.Furuichi T, Koops K, Ryu H, Sanz C, Sakamaki T, Morgan D, Tokuyama N. 2015. Why do wild bonobos not use tools like chimpanzees do? Behaviour 152, 425–460. ( 10.1163/1568539x-00003226) [DOI] [Google Scholar]
- 41.Gruber T, Clay Z, Zuberbühler K. 2010. A comparison of bonobo and chimpanzee tool use: evidence for a female bias in the Pan lineage. Anim. Behav. 80, 1023–1033. ( 10.1016/j.anbehav.2010.09.005) [DOI] [Google Scholar]
- 42.Neufuss J, Humle T, Cremaschi A, Kivell TL. 2017. Nut-cracking behaviour in wild-born, rehabilitated bonobos (Pan paniscus): a comprehensive study of hand-preference, hand grips and efficiency. Am. J. Primatol. 79, 1–16. ( 10.1002/ajp.22589) [DOI] [PubMed] [Google Scholar]
- 43.Rosati AG, Hare B. 2011. Chimpanzees and bonobos distinguish between risk and ambiguity. Biol. Lett. 7, 15–18. ( 10.1098/rsbl.2010.0927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan J, Ariely D, Hare B. 2017. Bonobos respond prosocially toward members of other groups. Sci. Rep. 7, 14733 ( 10.1038/s41598-017-15320-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan J, Kwetuenda S, Hare B. 2015. Preference or paradigm? Bonobos show no evidence of other-regard in the standard prosocial choice task. Behaviour 152, 521–544. ( 10.1163/1568539X-00003230) [DOI] [Google Scholar]
- 46.de Waal FB.M. 1997. Food transfers through mesh in brown capuchins. J. Comp. Psychol. 111, 370–378. ( 10.1037/0735-7036.111.4.370) [DOI] [PubMed] [Google Scholar]
- 47.Jaeggi AV, Gurven M. 2013. Natural cooperators: food-sharing in humans and other primates. Evol. Anthropol. 22, 186–195. ( 10.1002/evan.21364) [DOI] [PubMed] [Google Scholar]
- 48.Horner V, Carter JD, Suchak M, de Waal FB. 2011. Spontaneous prosocial choice by chimpanzees. Proc. Natl Acad. Sci. USA 108, 13 847–13 851. ( 10.1073/pnas.1111088108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brosnan SF, de Waal FBM. 2002. A proximate perspective on reciprocal altruism. Hum. Nat. 13, 129–152. ( 10.1007/s12110-002-1017-2) [DOI] [PubMed] [Google Scholar]
- 50.Schino G, Aureli F. 2010. Primate reciprocity and its cognitive requirements. Evol. Anthropol. 19, 130–135. ( 10.1002/evan.20270) [DOI] [Google Scholar]
- 51.Warneken F. 2013. Young children proactively remedy unnoticed accidents. Cognition 126, 101–108. ( 10.1016/j.cognition.2012.09.011) [DOI] [PubMed] [Google Scholar]
- 52.Bullinger AF, Burkart JM, Melis AP, Tomasello M. 2013. Bonobos, Pan paniscus, chimpanzees, Pan troglodytes, and marmosets, Callithrix jacchus, prefer to feed alone. Anim. Behav. 85, 51–60. ( 10.1016/j.anbehav.2012.10.006) [DOI] [Google Scholar]
- 53.Krupenye C, Hare B. 2018. Bonobos prefer individuals that hinder others over those that help. Curr. Biol. 28, 280–286. ( 10.1016/j.cub.2017.11.061) [DOI] [PubMed] [Google Scholar]
- 54.Pollick AS, de Waal FBM. 2007. Ape gestures and language evolution. Proc. Natl Acad. Sci. USA 104, 8184–8189. ( 10.1073/pnas.0702624104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herrmann E, Hare B, Call J, Tomasello M. 2010. Differences in the cognitive skills of bonobos and chimpanzees. PLoS ONE 5, e12438 ( 10.1371/journal.pone.0012438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koops K, Furuichi T, Hashimoto C. 2015. Chimpanzees and bonobos differ in intrinsic motivation for tool use. Sci. Rep. 5, 11356 ( 10.1038/srep11356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koops K, Furuichi T, Hashimoto C, van Schaik CP. 2015. Sex differences in object manipulation in wild immature chimpanzees (Pan troglodytes schweinfurthii) and bonobos (Pan paniscus): preparation for tool use? PLoS ONE 10, e0139909 ( 10.1371/journal.pone.0139909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herrmann E, Hare B, Cissewski J, Tomasello M. 2011. A comparison of temperament in nonhuman apes and human infants. Dev. Sci. 14, 1393–1405. ( 10.1111/j.1467-7687.2011.01082.x) [DOI] [PubMed] [Google Scholar]
- 59.Jensen K. 2016. The prosocial primate—a critical review. In Advances in the Study of Behavior (eds Naguib M, Brockmann J, Mitani JC), pp. 387–441. Waltham, MA: Academic Press, Ltd. [Google Scholar]
- 60.Silk JB, House BR. 2016. The evolution of altruistic social preferences in human groups. Phil. Trans. R. Soc. B 371, 20150097 ( 10.1098/rstb.2015.0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berbesque JC, Wood BM, Crittenden AN, Mabulla A, Marlowe FW. 2016. Eat first, share later: Hadza hunter–gatherer men consume more while foraging than in central places. Evol. Hum. Behav. 37, 281–286. ( 10.1016/j.evolhumbehav.2016.01.003) [DOI] [Google Scholar]
- 62.Marsh AA, Stoycos SA, Brethel-Haurwitz KM, Robinson P, VanMeter JW, Cardinale EM. 2014. Neural and cognitive characteristics of extraordinary altruists. Proc. Natl Acad. Sci. USA 111, 15 036–15 041. ( 10.1073/pnas.1408440111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vekaria KM, Brethel-Haurwitz KM, Cardinale EM, Stoycos SA, Marsh AA. 2017. Social discounting and distance perceptions in costly altruism. Nat. Hum. Behav. 1, 100 ( 10.1038/s41562-017-0100) [DOI] [Google Scholar]
- 64.Hare B. 2017. Survival of the friendliest: Homo sapiens evolved via selection for prosociality Annu. Rev. Psychol. 68, 155–186. ( 10.1146/annurev-psych-010416-044201) [DOI] [PubMed] [Google Scholar]
- 65.Hare B, Tan J. 2012. How much of our cooperative behavior is human? In The primate mind: built to connect with other minds (eds de Waal FBM, Ferrari PF), pp. 175–193. Cambridge, MA: Harvard University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are tabulated in electronic supplementary material, tables S1–S3. Trial-by-trial data for the GLMMs in Experiment 2 are available in the ESM Data file.