Abstract
Transfer and receipt of seminal fluid proteins crucially affect reproductive processes in animals. Evolution in these male ejaculatory proteins is explained with post-mating sexual selection, but we lack a good understanding of the evolution of female post-mating responses (PMRs) to these proteins. Some of these proteins are expected to mediate sexually antagonistic coevolution generating the expectation that females evolve resistance. One candidate in Drosophila melanogaster is the sex peptide (SP) which confers cost of mating in females. In this paper, we compared female SP-induced PMRs across three D. melanogaster wild-type populations after mating with SP-lacking versus control males including fitness measures. Surprisingly, we did not find any evidence for SP-mediated fitness costs in any of the populations. However, female lifetime reproductive success and lifespan were differently affected by SP receipt indicating that female PMRs diverged among populations. Injection of synthetic SP into virgin females further supported these findings and suggests that females from different populations require different amounts of SP to effectively initiate PMRs. Molecular analyses of the SP receptor suggest that genetic differences might explain the observed phenotypical divergence. We discuss the evolutionary processes that might have caused this divergence in female PMRs.
Keywords: sexual conflict, cost of mating, sexually antagonistic coevolution, sex peptide, post-mating response
1. Introduction
Mating induces dramatic changes in female behaviour, physiology and gene expression that together constitute female post-mating responses (PMRs). In insects, two striking PMRs are increased egg production and a decreased receptivity towards courting males [1]. The act of mating, but particularly the receipt of sperm and proteins as part of the seminal fluid, induces female PMRs [2]. Male seminal fluid proteins (SFPs) are found in many taxa [2,3] and have been particularly well studied in Drosophila melanogaster where specific functions for several of the more than 130 SFPs have been identified and are responsible for invoking the majority of the female PMRs [4]. These SFPs are known to evolve rapidly [5,6] and coevolve with targets inside the female [7]. While male SFPs, their function and evolution have been intensively studied, the evolution of variance in female PMRs remains understudied.
In D. melanogaster, the transfer of SFPs benefits male reproductive success [1] and allows females to coordinate reproductive processes with the receipt of sperm as well as aid sperm management [4]. Despite these benefits, females suffer reduced longevity and lifetime reproductive success (LRS) from repeated receipt of SFPs [8] as part of the cost of mating. Therefore, Drosophila SFPs have been proposed as mediators of sexual conflict and targets of sexually antagonistic coevolution [8–10].
Sexual conflict can arise when the sexes follow different routes to maximize fitness, creating the potential for sexually antagonistic coevolution [11]. Theory predicts that the evolution of a trait that increases reproductive success in one sex while depressing the fitness of the other leads to coevolutionary cycles of adaptation and counteradaptation between the sexes [11,12]. This typically generates male persistence and female resistance traits. The latter evolve in response to male persistence traits to reduce the fitness costs imposed on females by reproductive interactions [11]. Hence, the evolution of female PMRs mediated by SFPs may be shaped by sexual conflict and sexually antagonistic coevolution.
Most empirical studies have focused on demonstrating and understanding the nature of male persistence traits but data on female resistance are scarce [11,13,14], probably because female traits are notoriously difficult to study. Notable exceptions include studies of morphological male antagonistic and corresponding female resistance traits which affect the mating rate, like the male grasping and female antigrasping traits in Gerris spp waterstriders [15,16] and diving beetles of the Dytiscidae family [17,18] (for a review, see [14]). Interestingly, the shape of the antigrasping traits in Gerris incognitus was moulded simultaneously by male–female coevolution and ecological factors [19]. Furthermore, the expression of female costs of mating in D. melanogaster is condition-dependent, e.g. on variation in available diet [20], demography [21,22] or the physical environment [23]. This can shift the economics of mating potentially altering coevolutionary dynamics [13,19,24]. Hence, the interplay between ecology and sexually antagonistic coevolution might shape the evolution of intraspecific variation in male and female reproductive traits.
In contrast to morphological traits, while we have started to identify the physiological and neuronal pathways and players mediating female PMRs in response to male chemical sexually antagonistic traits such as SFPs, we lack an understanding of how they coevolve with male traits [14]. Intriguingly, so far the female receptor for only one male SFP has been identified. The sex peptide receptor (SPR) is required for the PMRs mediated by receipt of male sex peptide (SP) [25]. Single nucleotide polymorphisms in the SP and SPR genes and their interaction affect sperm competition success and female remating [26]. This makes them promising candidates to study sexually antagonistic coevolution at the phenotypic and molecular level. The 36-amino acid long SP induces oviposition and decreases a female's willingness to remate [27], which directly benefits the male [28,29] and affects sperm competition outcomes [28,30]. Additionally, receipt of SP is one major contributor to the costs of mating in female D. melanogaster [10]. The opposing fitness consequences of SP transfer and receipt for, respectively, males and females, are expected to mediate sexual conflict over reproductive decisions and investment [10,28,31].
In this study, we investigated whether female PMRs to a specific sexually antagonistic signal, ejaculatory SP, diverged across three wild-type D. melanogaster populations, and whether we can detect signs of sexually antagonistic coevolution between SP and female PMRs. We combined tests for phenotypic variation in female PMRs with an investigation of expression levels of and genotypic variation in the SPR gene which is crucial for invoking the PMRs.
2. Methods
(a). Fly stocks and culturing methods
We used flies from three different D. melanogaster wild-type populations: Dahomey, Innisfail and Melbourne. All strains were kept in the laboratory at 25°C and 60% humidity on a 12 L : 12 D cycle and provided with standard sugar-yeast (SY) medium [28]. Dahomey was collected in Africa in the 1970s, while the Innisfail and Melbourne populations were collected in 2008 from the tropical and temperate ends, respectively, of a transect along the eastern coast of Australia [32]. We used SP knockout and appropriate control males [27] to test female responses to SP receipt. Males lacking SP (SP0) and genetically matched SP producing control males (SP+) were in the Dahomey genetic background and generated as described in [33].
Standardized females (see below) were from a randomly chosen isofemale (i.e. inbred) line of the Drosophila Genetic Reference Panel (line ID 776) [34]. For further details on fly stock maintenance, see electronic supplementary material.
To obtain experimental adults, the parental generation was allowed to oviposit on agar-grape juice plates supplemented with fresh yeast paste. Larvae were collected the following day at a density of 100 per vial containing 7 ml of SY medium supplemented with live yeast. We separated adults directly after eclosion to ensure virginity and held them in single sex groups of 20 individuals per vial until the start of the experiment.
All experiments were carried out at 25°C and 60% humidity in glass vials supplemented with 7 ml SY food and live yeast granules or paste.
(b). Single mating experiments
We carried out three separate experiments in which we measured female egg laying and remating behaviour 24 h after a single mating. In the first, we mated females of the three populations to their own males to measure the expression of female PMRs when mated to coevolved males. In the second, we mated males from the three populations to standardized females to compare the males' abilities to induce a PMR. While in the third, we mated females from the three populations to an SP0 or SP+ male to determine whether there were population differences in the strength of their SP-mediated PMRs. In all three experiments, we set up 40 pairs per treatment; for details on the experimental procedure, see electronic supplementary material.
(c). Costs of mating incurred by wild-type females continuously exposed to SP0 and SP+ males
Here, we tested the effects of continuous exposure to SP0 or SP+ males on LRS and lifespan in Dahomey, Innisfail and Melbourne females. We kept groups of three females continuously with three males of either genotype until their natural death. A total of 210 females (24–48 h post-eclosion) from each population were split evenly across the two male genotypes (i.e. 35 vials per population per male genotype). Males were 4 days post-eclosion to ensure sexual maturity [33]. Female survival was checked daily. We further recorded mating activity and the number of offspring produced twice a week. Mating activity was recorded for 3 h directly after lights on by scoring the number of mating pairs every 20 min. After the end of the spot check period, 21 randomly selected females per treatment were placed individually into fresh vials and allowed to oviposit for 18 h. The next morning, we moved females back into mating groups. We counted the number of eggs produced per female and incubated vials for 12 days and counted the number of eclosing adult offspring. For each treatment group (female population × male genotype), we stopped the assay when less than 21 females remained. In total, we measured six egg-laying time points for Melbourne females, five for Dahomey and Innisfail females mated to SP+ males, and four time points for Innisfail females mated to SP0 males. We totalled the number of eggs and adult offspring produced across all measured time points as a proxy of LRS.
Throughout the experiment, we maintained groups by transferring them to fresh food vials every 2 days using CO2 anaesthesia. At these transfers, we combined females across vials when dead females were found, to keep density constant. In addition, dead males were replaced at this opportunity when necessary. Once a week males were replaced with new 4-day-old males of the appropriate genotype to avoid male age as a confounding factor. At the same time, females were randomly mixed across vials within treatment to minimize the effects of mating group constellation.
(d). Injection of synthetic SP into virgin females
We injected different concentrations of synthetically derived SP (n = 21) into the abdomens of virgin 4-day-old females from the three populations to determine whether females differ in the amount of SP needed to display PMRs. Six hours after the injection, we measured the proportion of injected females (i) laying eggs (as not all females laid eggs) and (ii) mating with a 4-day-old Dahomey male. For details of the experimental procedure, see electronic supplementary material.
(e). SPR expression and SP and SPR haplotype networks
We used quantitative Real-Time PCR (qPCR) to measure relative expression levels of SPR in the female abdomen to quantify SPR expression in the reproductive tract [25]. RNA isolation from female abdomen, cDNA synthesis, qPCR and relative expression calculation were carried out as described in [35]. For detailed methods and primer sequences, see electronic supplementary material.
We sequenced the whole SP gene (FlyBase ID: FBgn0003034) and exon 6 of the SPR gene (FBgn0029768) in all three wild-type populations. A previous study identified 16 SNPs within exon 6 of the SPR of which nine had significant effects on egg laying, remating behaviour or egg hatchability [26]. We used the sequence information to build haplotype networks using statistical parsimony implemented in the TCS software [36]. To do this, we first isolated genomic DNA from single flies and amplified SP and exon 6 of SPR in a standard PCR with gene-specific primers (see electronic supplementary material). PCR products were then purified with alkaline phosphatase and exonuclease I (both from Thermo Scientific); correct product sizes were verified on 1%-agarose gels and then sent to GATC Biotech AG (Germany) for Sanger sequencing with the primers used for amplification. The resulting sequences were manually edited and aligned using PhyDE v. 0.997. In total, we used sequences from 14 Dahomey, 16 Innisfail and 10 Melbourne individuals for the SP network and six Dahomey, four Innisfail and five Melbourne individuals to build the SPR exon 6 network with the connection limit among haplotypes set to 97% and gaps treated as missing data.
(f). Statistical analysis
All statistical analyses were performed in RStudio v. 0.99.467 [37] using R v. 3.4.0 [38]. We specified generalized linear models (GLMs) [39] with the appropriate data distributions. Specifics on data distributions and use of the quasi-extension to account for overdispersion are given with the results. We tested for treatment effects using likelihood ratio tests by dropping terms. Post hoc tests for pair-wise comparisons were done using the glht function from the ‘multcomp’ package v. 1.4-8 (for main terms) [40] and the testInteractions function from the ‘phia’ package v. 0.2-1 (for interaction effects) [41]. Both tests use the Holm method to adjust p-values for multiple testing [42]. We performed the non-parametric Scheirer–Ray–Hare test [43] in cases where appropriate GLMs could not be fitted.
To analyse the mating data from the spot checks in the continuous exposure experiment, we calculated the total number of mating opportunities (sum of females alive for all days when checks were done) and summed the number of matings observed over all spot checks and used these sums in a χ2-test of association. We used the chisq.post.hoc function from the ‘fifer’ package v. 1.1 [44] to do pair-wise comparisons of the populations.
Graphs were made using the ‘gplots’ package v. 3.0.1. [45]. Unless stated otherwise, we present means ± s.e. calculated from raw data. For proportion data, we calculated the standard error as  where p is the proportion across all replicates and n is the sample size.
where p is the proportion across all replicates and n is the sample size.
3. Results
(a). Single mating experiments
(i). Population divergence in female PMRs
When mating once with their coevolved males, Innisfail females laid most eggs within the 24 h following mating, while Dahomey females produced the least eggs (GLM with quasi-poisson data distribution: F2,110 = 58.03, p < 0.001, electronic supplementary material, figure S1a). In addition, Innisfail females remated significantly more often than Dahomey females but not Melbourne females (GLM with binomial data distribution: 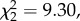 p = 0.01, electronic supplementary material, figure S1b). Hence, all three populations differed significantly in their coevolved PMRs for these two traits and, in the following, we picked apart male from female contributions to these responses.
p = 0.01, electronic supplementary material, figure S1b). Hence, all three populations differed significantly in their coevolved PMRs for these two traits and, in the following, we picked apart male from female contributions to these responses.
(ii). Wild-type male ability to elicit female PMRs
Male population origin had no significant effect on egg-laying rate after a single mating to the standard isoline females (GLM with quasi-poisson data distribution: F2,99 = 3.05, p = 0.052; electronic supplementary material, figure S2a). Similarly, there was no difference in the proportion of females remating (GLM with binomial data distribution: 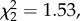 p = 0.466; electronic supplementary material, figure S2b). Hence, overall males from the three populations were similar in their ability to elicit SP-dependent female PMRs for this particular isoline.
p = 0.466; electronic supplementary material, figure S2b). Hence, overall males from the three populations were similar in their ability to elicit SP-dependent female PMRs for this particular isoline.
(iii). Response to receipt of sex peptide in wild-type females after a single mating
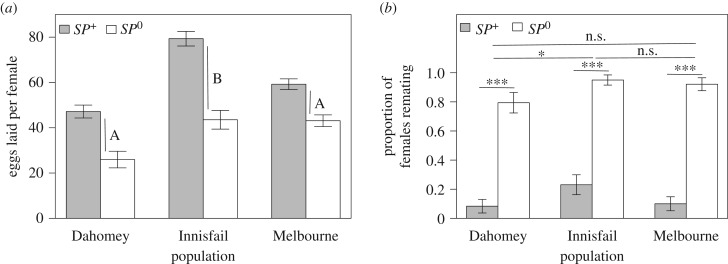
SP receipt had the expected effects on females in all three populations: females first mated to SP+ males laid more eggs than females mated to SP0 males. However, this egg boost was significantly stronger in Innisfail females than in Dahomey and Melbourne females as indicated by the significant male genotype × female population interaction (figure 1a and table 1). As expected, females first mated to SP+ males also had a reduced propensity to remate (SP+: 13.9 ± 3.2%, SP0: 89.3 ± 2.9%) with the extent of this reduction not significantly different among populations (figure 1b and table 1). However, across populations females overall differed significantly in their willingness to remate (table 1), with Innisfail females having a higher remating propensity (59.5 ± 5.5%) than Dahomey females (42.3 ± 5.9%). Melbourne females were intermediate, not differing significantly from either of the other two populations (50.0 ± 5.7%). The proportion of eggs that developed into adults (on average 86.1 ± 2.3%) was not affected by male genotype (Scheirer–Ray–Hare test, d.f. = 1, p = 0.27), female population (d.f. = 2, p = 0.97), nor their interaction (d.f. = 2, p = 0.70).
Figure 1.
Female PMRs to receipt of SP 24 h after a single mating to either an SP+ (grey bars) or an SP0 (white bars) male. (a) Mean number of eggs (±s.e.) produced per female. Different letters correspond to significant differences (p < 0.05) in contrasts of female population × male genotype interactions. (b) Proportion of females remating (± s.e.) with a Dahomey male within 1 h. Asterisks correspond to significant differences (***p < 0.001, **p < 0.01, *p < 0.05, n.s. = non-significant) in remating propensity depending on male genotype within populations and between populations.
Table 1.
Test statistics of GLMs testing for the effect of male genotype (SP0/SP+), female population and their interaction on the number of eggs laid by a once-mated female and on female remating behaviour 24 h after a first mating.
| factor/term | deviance | d.f. | test statistic | p-value |
|---|---|---|---|---|
| number of eggs (Gaussian data distribution) | F | |||
| female population | 115 791 | 2 | 29.10 | <0.001 |
| male genotype | 126 723 | 1 | 85.10 | <0.001 |
| female population × male genotype | 92 178 | 2 | 5.25 | 0.006 |
| remating behaviour (binomial data distribution) | χ2 | |||
| female population | 169.95 | 2 | 8.11 | 0.017 |
| male genotype | 310.39 | 1 | 149.45 | <0.001 |
| female population × male genotype | 160.94 | 2 | 0.70 | 0.71 |
(b). Cost of mating response of wild-type females continuously exposed to SP0 and SP+ males
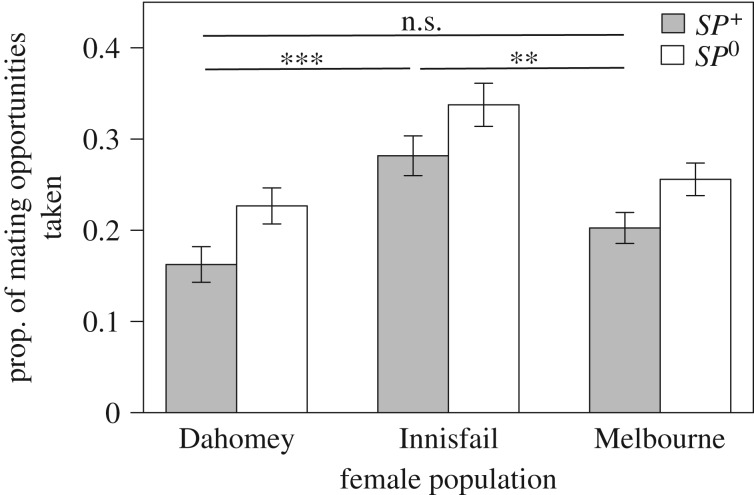
In general, females held with SP0 males mated significantly more often (Pearson's χ2-test: 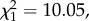 p = 0.002, figure 2). When comparing the three wild-types exposed to SP+ males separately, we found significant differences with Innisfail females showing the highest mating frequency (χ2-test:
p = 0.002, figure 2). When comparing the three wild-types exposed to SP+ males separately, we found significant differences with Innisfail females showing the highest mating frequency (χ2-test: 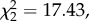 p < 0.001, figure 2).
p < 0.001, figure 2).
Figure 2.
Mating frequency of females continuously exposed to SP+ (grey bars) or SP0 (white bars) males throughout their lives. Mating frequency was recorded twice a week for 3 h directly after lights on by checking the number of mating pairs every 20 min until all females were dead. Asterisks correspond to significant contrasts (***p < 0.001, **p < 0.01, *p < 0.05, n.s. = non-significant) in mating frequency between females from different populations mated to SP+ males (the pattern was similar when comparing females from different populations mated to SP0 males).
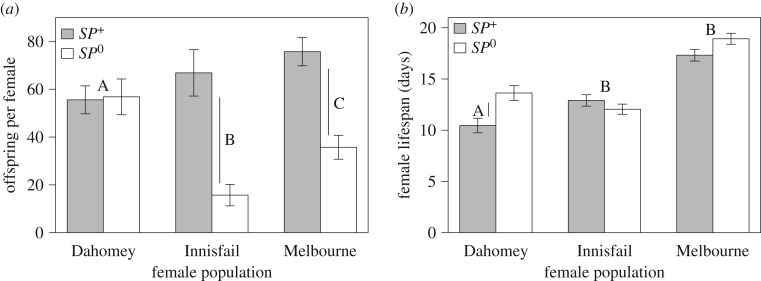
We estimated female LRS as the total number of adult offspring developed from vials in which 21 randomly chosen individualized females oviposited twice a week for 18 h. The LRS estimates were significantly affected by the male genotype × female population interaction (GLM with quasi-poisson distribution: F2,120 = 11.38, p < 0.001). Both Innisfail and Melbourne females had higher LRS estimates when exposed to SP+ males, but Innisfail females boosted offspring production more strongly when receiving SP, while Melbourne females still produced nearly half as many offspring even when not receiving SP. By contrast, Dahomey females produced similar numbers of offspring irrespective of male genotype (figure 3a).
Figure 3.
PMRs after continuous exposure to SP+ (grey bars) and SP0 (white bars) males in Dahomey, Innisfail and Melbourne females. (a) LRS was measured as mean number of adult offspring (±s.e.) per female. (b) Mean female lifespan (±s.e.) in days. Different letters indicate significant contrasts (p < 0.05) for female population × male genotype interactions on LRS and lifespan.
Female lifespan was not only affected by their population of origin, but also whether males transferred SP or not (GLM with Gamma data distribution: F2,615 = 9.27, p = 0.006). For both the Dahomey and Melbourne populations, females continuously exposed to SP+ males had a shorter lifespan than females exposed to SP0 males, while in the Innisfail population, this pattern reversed and females exposed to SP+ males lived on average longer than females exposed to SP0 males. However, only Dahomey and Innisfail females differed significantly from each other in the modulation of their lifespan in response to repeatedly receiving SP versus not at all (figure 3b).
We used the LRS estimates and lifespan data to calculate the intrinsic population growth rate r as an index of fitness with similar results (electronic supplementary material, figure S3).
(c). Injection of synthetic SP into virgin females
We first tried injecting synthetic SP at concentrations between 0 and 5 pmol as used in [46] in a few females (less than or equal to 10) but found that Innisfail and Melbourne females did not display PMRs at these concentrations (electronic supplementary material, figure S4). We therefore used higher concentrations ranging from 5 to 10 pmol.
The proportion of females that laid eggs within 6 h was not significantly affected by the concentration of SP injected into virgin females (GLM with binomial data distribution: 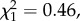 p = 0.50) nor by the interaction between concentration and population (
p = 0.50) nor by the interaction between concentration and population (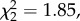 p = 0.40), but differed significantly between populations (
p = 0.40), but differed significantly between populations (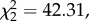 p < 0.001; electronic supplementary material, figure S5a). As we did not find an effect of concentration, we tested for an effect of SP receipt by comparing females injected with Ringer's solution with females injected with 10 pmol synthetic SP. Dahomey females reacted strongest to SP injection, with moderate and weak effects in Innisfail and Melbourne females, respectively (GLM with binomial data distribution: treatment × population:
p < 0.001; electronic supplementary material, figure S5a). As we did not find an effect of concentration, we tested for an effect of SP receipt by comparing females injected with Ringer's solution with females injected with 10 pmol synthetic SP. Dahomey females reacted strongest to SP injection, with moderate and weak effects in Innisfail and Melbourne females, respectively (GLM with binomial data distribution: treatment × population: 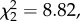 p = 0.012; electronic supplementary material, figure S5a).
p = 0.012; electronic supplementary material, figure S5a).
By contrast, we found significant effects of both concentration (GLM with binomial data distribution: 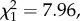 p = 0.005) and population (
p = 0.005) and population (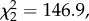 p < 0.001) but not their interaction (
p < 0.001) but not their interaction (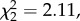 p = 0.348) on the proportion of females that mated 6 h after injection with synthetic SP (electronic supplementary material, figure S5b). Less females were willing to mate with increasing SP concentration injected in all three populations. Comparing 10 pmol SP versus Ringer's injected females showed that injection of SP overall decreased the proportion of females that mated (GLM with binomial data distribution:
p = 0.348) on the proportion of females that mated 6 h after injection with synthetic SP (electronic supplementary material, figure S5b). Less females were willing to mate with increasing SP concentration injected in all three populations. Comparing 10 pmol SP versus Ringer's injected females showed that injection of SP overall decreased the proportion of females that mated (GLM with binomial data distribution: 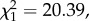 p < 0.001), with the extent significantly dependent on female population origin (treatment × population:
p < 0.001), with the extent significantly dependent on female population origin (treatment × population: 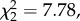 p = 0.02). Injection of 10 pmol SP strongly reduced female willingness to mate in Dahomey females but only weakly so in Innisfail and Melbourne females (electronic supplementary material, figure S5b).
p = 0.02). Injection of 10 pmol SP strongly reduced female willingness to mate in Dahomey females but only weakly so in Innisfail and Melbourne females (electronic supplementary material, figure S5b).
(d). SPR expression and SP/SPR haplotype networks
SPR was 1.04-fold higher expressed in the abdomen of Melbourne females compared to the other two populations (GLM with Gaussian data distribution: F2,12 = 7.65, p = 0.007; electronic supplementary material, figure S6).
The TCS haplotype network analysis of SP revealed two distinct haplotypes with only one mutational event separating them (figure 4a). Although all Dahomey samples shared the same haplotype, we detected a polymorphism for both haplotypes in the Australian populations. For exon 6 of SPR, we identified five distinct haplotypes with multiple events separating them with each population being characterized by its own distinct haplotype (figure 4b). Only the Melbourne population was made up entirely of one haplotype for this exon of SPR, while both Innisfail and Dahomey showed some low-level polymorphism composed of two different haplotypes separated by one mutational event.
Figure 4.
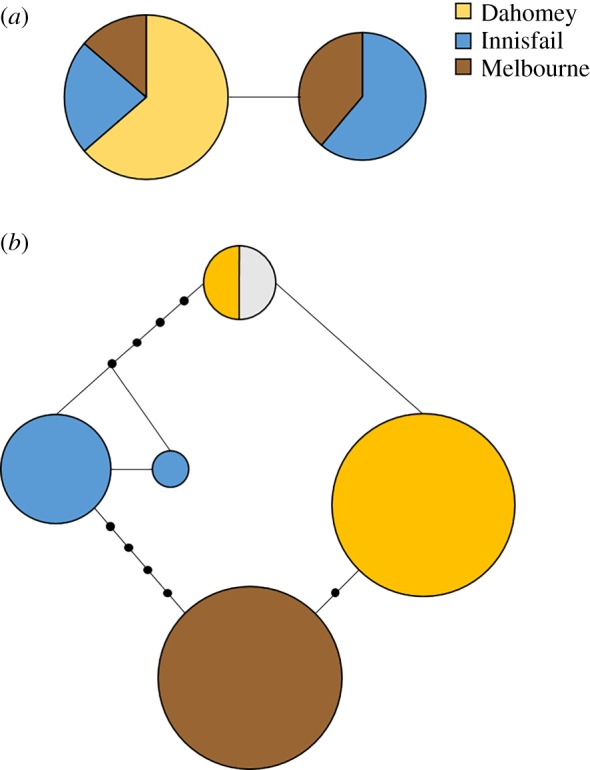
Haplotype networks of (a) the SP gene and (b) exon 6 of the SPR gene. Circle size represents the number of individuals carrying a specific haplotype and colour population affiliation. Each line segment represents a single mutational event with black dots indicating inferred haplotypes. Colours correspond to one of the three wild-type populations: yellow, Dahomey; blue, Innisfail; brown, Melbourne; grey, reference sequence from flybase (FBgn0029768).
4. Discussion
Our study shows for the first time significant variation in two female PMRs mediated by SP, egg-laying and remating behaviour [27], in three wild-type D. melanogaster populations and we hypothesized that SP and/or its receptor have diverged in these populations. While other SFPs might also be involved, SP and SPR have a major impact on these two PMRs [25,27]. In the next step, we disentangled male from female contributions by mating each sex separately to standardized mates. This revealed that males here did not differ in their ability to elicit female egg-laying and remating behaviour, suggesting that SP potency has not diverged. This was supported by the fact that genetic variability of the SP gene is low among these three populations. However, we found significant differences in female responses to SP indicating that females had diverged instead.
Our results reveal that Innisfail females are least responsive in terms of remating behaviour as they barely started to respond even to high doses of injected SP and, similarly, a single physiological dose from a single mating did not suppress remating as effectively as in Dahomey females. However, Innisfail females boosted their egg output by approximately 82% after receiving a physiological dose from a single mating (the two PMRs are regulated by independent pathways [47]). Interestingly, injecting synthetic SP at high doses did not induce an egg boost in Innisfail females. This could indicate that SP receipt alone is not enough to boost egg laying in these females and also the receipt of sperm and/or other SFPs are necessary. Alternatively, the concentration of synthetic SP used for injection might be simply too low to induce the egg boost (see below). While Melbourne females showed an intermediate phenotype, Dahomey females seemed most responsive. The latter react to both injection of synthetic SP and physiological receipt in single matings with increased egg-laying and suppressed remating behaviour and shortened lifespan when repeatedly receiving SP. Importantly, for females from all three populations, the effects of repeated SP receipt differently affected the estimate of LRS, female lifespan and the intrinsic rate of population increase r, which all serve as proxies of fitness, but not in the expected way.
Interestingly, we could not find any overall fitness costs of repeated SP receipt: SP was either beneficial (as in Innisfail and Melbourne) or neutral to fitness (Dahomey). This is in contrast to previous results also using Dahomey females, which showed a considerable cost [10]. One possible explanation is that females have overcome the cost of mating and evolved resistance to SP, i.e. females ‘won’ the sexual conflict at this particular time point and display no costs. Alternatively, the receipt of SP is only costly under certain conditions and otherwise beneficial to females. We will discuss both options in turn below.
Evolution of female resistance is a central part of sexually antagonistic coevolution theory [11] and is predicted to occur via two mechanisms [48–50]: either through shifts in (i) the female threshold value that elicits a PMR or (ii) female sensitivity to a male trait. According to theoretical models, the form of female resistance determines the dynamics of sexually antagonistic coevolution [48,50]. When sensitivity rather than the threshold evolves, then perpetual antagonistic changes are less likely [48] and females are predicted to suffer little fitness costs as we found here. By contrast, the evolution of the threshold leads to coevolutionary cycles [50]. However, both models highlight that the outcome depends on the strength of natural selection acting simultaneously and whether it constrains one form of resistance more than the other [48,50]. Empirical evidence for which of the two mechanisms is more likely to evolve is currently lacking. We tried to tackle this issue here by injecting females with different concentrations of synthetic SP to establish dose–response curves. A previous study [46] showed that SP elicited the PMR in a switch on/off manner indicating a threshold for SP, but here we needed much higher concentrations to effectively suppress remating or induce egg laying in the females. Importantly, for Innisfail and Melbourne females, we were unable to suppress remating or induce egg laying to the same extent as observed after a single mating (‘natural’ receipt of SP) even at the highest injected concentration (10 pmol). Remating suppression at least seemed to be concentration-dependent, in contrast to previous findings [46]. However, it is difficult here to judge conclusively whether resistance is modulated by a threshold or sensitivity as, particularly in Innisfail and Melbourne females, the SP concentrations we used did not induce a strong PMR, hence we might be well below their thresholds. Nonetheless, these females required a higher SP dose than e.g. Dahomey females, which supports the notion that females from these three populations differ in their responsiveness to SP, but sexually antagonistic coevolution is only one process that could explain the observed variation in female PMRs.
Alternatively, divergence in responsiveness to SP is not at all or only partially shaped by sexually antagonistic coevolution, and instead SP receipt is beneficial to females and intraspecific variation might be due to genetic drift or ecological variables. For example, the effect of SP is also dependent on female nutritional state [20] and shifts from antagonistic to beneficial. Hence, due to local adaptation, the underlying physiological trade-offs and/or metabolic pathways might be altered in these females potentially shifting life-history trade-offs as influenced by SP, and the effect on fitness across these populations. In addition, differences in population density and mate encounter rates might alter selection pressures as remating dynamics mediated through the SP receptor can shift reproductive interactions from expressing conflict to cooperation [31]. As the SPR also has another ligand (myoinhibiting peptides [51]) and is involved in other (unknown) functions, pleiotropic selection might have driven the observed sequence divergence in the SPR gene, which then altered the nature of male–female interactions and fitness effects of SP receipt. Hence, the expression of female mating costs might be condition-dependent and male–female coevolution as mediated by the SP–SPR molecular pair might be driven by neutral or positive selection under some conditions and shift towards an antagonistic interaction under others. Figuring out under which condition one or the other scenario occurs and how this affects male–female coevolutionary dynamics and population divergence needs further investigation.
Even though tentative, our genetic data for SPR support the phenotypic findings. We found that the three populations carry distinct haplotypes for exon 6 of the SPR suggesting that the observed phenotypic differences are rooted in the genetic variation found in SPR. Genetic variation in SPR and the interaction with SP have previously been found to affect male sperm defence success but not fertility or remating phenotypes [26].
We also found significant differences in SPR expression, but the difference was low (less than twofold change). Moreover, it is difficult to gauge to what extent this explains the phenotypic results, as intraspecific differences in SP and SPR expression are difficult to link to phenotypes [26]. However, one recent species comparison showed that overall high expression of SPR enhanced PMR responses [52]. Here, Melbourne females had a significantly higher SPR expression than Dahomey and Innisfail females, but they did not have the strongest response to SP receipt. This hints at SPR expression levels not correlating well with phenotypes, although testing more populations with known phenotypes like the DGRP isolines could provide further insights. Similarly, induction of egg laying and remating do not correlate linearly with SP expression in males [53,54]. Hence, expression levels might not explain phenotypic variation well and evolution at the SPR locus is more consistent with the divergence in female responsiveness to SP found here.
In conclusion, our study provides evidence for the evolution of female PMRs to receipt of SFPs such as SP. This opens up further research perspectives into male–female coevolution and whether SFPs are mediators of sexual conflict in general or only under specific conditions, taking into account the complexity of SFPs. In contrast to morphological traits that often have obvious fitness costs to females, SFPs can also be beneficial to females, e.g. through signalling the initiation of reproduction after receipt of sperm, and some SFPs need the cooperation of the female to be activated in the female reproductive tract after mating [4]. Previous ‘benefits to males and costs to females' schemes might simplify the complexity of these interactions too much. Another open question concerns the nature of female resistance traits: Do females modulate resistance via a threshold or sensitivity response? Hence, there is ample scope for future research to unravel the nature of female resistance that too often, at the level of physiological responses, is still a black box, and excitingly consider how these dynamics are influenced by ecology.
Acknowledgements
We thank Susann Wicke for invaluable help in constructing haplotype networks and Mareike Koppik for helpful discussions of statistical analyses. We are grateful to Toshiro Aigaki for providing the synthetic sex peptide and Tracey Chapman and Ary Hoffman for fly stocks. We thank Dolors Amorós-Moya, Hanna Ruhmann and Sonja Schindler for their help with mating assays and offspring counts. We thank the associate editor Alexei Maklakov and three anonymous reviewers for helpful comments.
Data accessibility
Phenotypic data and gene sequences are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.c394c12 [55].
Authors' contributions
K.U.W. and C.F. conceived of the study, designed experiments, carried them out and wrote the manuscript. K.U.W. analysed the data.
Competing interests
We have no competing interests.
Funding
This work was funded by the German Science Foundation (grant no. FR2973/1-1) to C.F.
References
- 1.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. 2011. Insect seminal fluid proteins: identification and function. Annu. Rev. Entomol. 56, 21–40. ( 10.1146/annurev-ento-120709-144823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillott C. 2003. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 48, 163–184. ( 10.1146/annurev.ento.48.091801.112657) [DOI] [PubMed] [Google Scholar]
- 3.Poiani A. 2006. Complexity of seminal fluid: a review. Behav. Ecol. Sociobiol. 60, 289–310. ( 10.1007/s00265-006-0178-0) [DOI] [Google Scholar]
- 4.Wolfner MF. 2009. Battle and ballet: molecular interactions between the sexes in Drosophila. J. Hered. 100, 399–410. ( 10.1093/jhered/esp013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begun DJ, Whitley P, Todd BL, Waldrip-Dail HM, Clark AG. 2000. Molecular population genetics of male accessory gland proteins in Drosophila. Genetics 156, 1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanson WJ, Clark AG, Waldrip-Dail HM, Wolfner MF, Aquadro CF. 2001. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc. Natl. Acad. Sci. USA 98, 7375–7379. ( 10.1073/pnas.131568198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Findlay GD, Sitnik JL, Wang W, Aquadro CF, Clark NL, Wolfner MF. 2014. Evolutionary rate covariation identifies new members of a protein network required for Drosophila melanogaster female post-mating responses. PLoS Genet. 10, e1004108 ( 10.1371/journal.pgen.1004108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244. ( 10.1038/373241a0) [DOI] [PubMed] [Google Scholar]
- 9.Rice WR. 1998. Intergenomic conflict, interlocus antagonistic coevolution, and the evolution of reproductive isolation. In Endless forms – species and speciation (eds Howard DJ, Berlocher SH), pp. 261–270. New York, NY: Oxford University Press. [Google Scholar]
- 10.Wigby S, Chapman T. 2005. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15, 316–321. ( 10.1016/j.cub.2005.01.051) [DOI] [PubMed] [Google Scholar]
- 11.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 12.Parker GA. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum MS, Blum NA), pp. 123–166. New York, NY: Academic Press. [Google Scholar]
- 13.Fricke C, Perry J, Chapman T, Rowe L. 2009. The conditional economics of sexual conflict. Biol. Lett. 5, 671–674. ( 10.1098/rsbl.2009.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry JC, Rowe L. 2015. The evolution of sexually antagonistic phenotypes. Cold Spring Harb. Perspect. Biol. 7, a017558 ( 10.1101/cshperspect.a017558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe L, Arnqvist G. 2002. Sexually antagonistic coevolution in a mating system: combining experimental and comparative approaches to address evolutionary processes. Evolution 56, 754–767. ( 10.1111/j.0014-3820.2002.tb01386.x) [DOI] [PubMed] [Google Scholar]
- 16.Arnqvist G, Rowe L. 1995. Sexual conflict and arms races between the sexes: a morphological adaptation for control of mating in a female insect. Proc. R. Soc. Lond. B 261, 123–127. ( 10.1098/rspb.1995.0126) [DOI] [Google Scholar]
- 17.Green K K, Kovalev A, Svensson EI, Gorb SN. 2013. Male clasping ability, female polymorphism and sexual conflict: fine-scale elytral morphology as a sexually antagonistic adaptation in female diving beetles. J. R. Soc. Interface 10, 20130409 ( 10.1098/rsif.2013.0409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergsten J, Töyrä A, Nilsson AN. 2001. Intraspecific variation and intersexual correlation in secondary sexual characters of three diving beetles (Coleoptera: Dytiscidae). Biol. J. Linn. Soc. 73, 221–232. ( 10.1006/bjls.2001.0540) [DOI] [Google Scholar]
- 19.Perry JC, Garroway CJ, Rowe L. 2017. The role of ecology, neutral processes and antagonistic coevolution in an apparent sexual arms race. Ecol. Lett. 20, 1107–1117. ( 10.1111/ele.12806) [DOI] [PubMed] [Google Scholar]
- 20.Fricke C, Bretman A, Chapman T. 2010. Female nutritional status determines the magnitude and sign of responses to a male ejaculate signal in Drosophila melanogaster. J. Evol. Biol. 23, 157–165. ( 10.1111/j.1420-9101.2009.01882.x) [DOI] [PubMed] [Google Scholar]
- 21.Edward DA, Fricke C, Gerrard DT, Chapman T. 2011. Quantifying the life-history response to increased male exposure in female Drosophila melanogaster. Evolution 65, 564–573. ( 10.1111/j.1558-5646.2010.01151.x) [DOI] [PubMed] [Google Scholar]
- 22.Fricke C, Green D, Mills WE, Chapman T. 2013. Age-dependent female responses to a male ejaculate signal alter demographic opportunities for selection. Proc. R. Soc. B 280, 20130428 ( 10.1098/rspb.2013.0428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yun L, Chen PJ, Singh A, Agrawal AF, Rundle HD. 2017. The physical environment mediates male harm and its effect on selection in females. Proc. R. Soc. B 284, 20170424 ( 10.1098/rspb.2017.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbuthnott D, Dutton EM, Agrawal AF, Rundle HD. 2014. The ecology of sexual conflict: ecologically dependent parallel evolution of male harm and female resistance in Drosophila melanogaster. Ecol. Lett. 17, 221–228. ( 10.1111/ele.12222) [DOI] [PubMed] [Google Scholar]
- 25.Yapici N, Kim Y-J, Ribeiro C, Dickson BJ. 2008. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451, 33–37. ( 10.1038/nature06483) [DOI] [PubMed] [Google Scholar]
- 26.Chow CY, Wolfner MF, Clark AG. 2010. The genetic basis for male×female interactions underlying variation in reproductive phenotypes of Drosophila. Genetics 186, 1355–1365. ( 10.1534/genetics.110.123174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Kubli E. 2003. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 100, 9929–9933. ( 10.1073/pnas.1631700100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fricke C, Wigby S, Hobbs R, Chapman T. 2009. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster. J. Evol. Biol. 22, 275–286. ( 10.1111/j.1420-9101.2008.01638.x) [DOI] [PubMed] [Google Scholar]
- 29.Fricke C, Chapman T. 2017. Variation in the post-mating fitness landscape in fruitflies. J. Evol. Biol. 30, 1250–1261. ( 10.1111/jeb.13090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avila FW, Ram KR, Bloch Qazi MC, Wolfner MF. 2010. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics 186, 595–600. ( 10.1534/genetics.110.119735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith DT, Clarke NVE, Boone JM, Fricke C, Chapman T. 2017. Sexual conflict over remating interval is modulated by the sex peptide pathway. Proc. R. Soc. B 284, 20162394 ( 10.1098/rspb.2016.2394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristensen TN, Overgaard J, Lassen J, Hoffmann AA, Sgrò C. 2015. Low evolutionary potential for egg-to-adult viability in Drosophila melanogaster at high temperatures. Evolution 69, 803–814. ( 10.1111/evo.12617) [DOI] [PubMed] [Google Scholar]
- 33.Ruhmann H, Wensing KU, Neuhalfen N, Specker J-H, Fricke C. 2016. Early reproductive success in Drosophila males is dependent on maturity of the accessory gland. Behav. Ecol. 27, 1859–1868. ( 10.1093/beheco/arw123) [DOI] [Google Scholar]
- 34.Mackay TFC, et al. 2012. The Drosophila melanogaster genetic reference panel. Nature 482, 173–178. ( 10.1038/nature10811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koppik M, Fricke C. 2017. Gene expression changes in male accessory glands during ageing are accompanied by reproductive decline in Drosophila melanogaster. Mol. Ecol. 26, 6704–6716. ( 10.1111/mec.14384) [DOI] [PubMed] [Google Scholar]
- 36.Clement M, Posada D, Crandall KA. 2000. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 9, 1657–1659. ( 10.1046/j.1365-294x.2000.01020.x) [DOI] [PubMed] [Google Scholar]
- 37.R Studio Team. 2015. R Studio: integrated development for R. Boston, MA: R Studio Inc. [Google Scholar]
- 38.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Developmental Core Team. [Google Scholar]
- 39.Nelder JA, Wedderburn RWM. 1972. Generalized linear models. J. R. Stat. Soc. A 135, 370–384. ( 10.2307/2344614) [DOI] [Google Scholar]
- 40.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 41.De Rosario-Martinez H.2015. phia: post-hoc interaction analysis. R package version 0.2–1. See https://cran.r-project.org/package=phia .
- 42.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70. [Google Scholar]
- 43.Dytham C. 2011. Choosing and using statistics: a biologist's guide, 3rd edn Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 44.Fife D.2017. fifer: a biostatisticians toolbox for various activities, including plotting, data cleanup, and data analysis. R package version 1.1. See https://cran.r-project.org/package=fifer .
- 45.Warnes GR, et al. 2016. gplots: Various R Programming Tools for Plotting Data. R package version 3.0.1. See https://cran.r-project.org/package=gplots .
- 46.Schmidt T, Choffat Y, Klauser S, Kubli E. 1993. The Drosophila melanogaster sex-peptide: a molecular analysis of structure-function relationships. J. Insect Physiol. 39, 361–368. ( 10.1016/0022-1910(93)90023-K) [DOI] [Google Scholar]
- 47.Haussmann IU, Hemani Y, Wijesekera T, Dauwalder B, Soller M. 2013. Multiple pathways mediate the sex-peptide-regulated switch in female Drosophila reproductive behaviours. Proc. R. Soc. B 280, 20131938 ( 10.1098/rspb.2013.1938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowe L, Cameron E, Day T. 2005. Escalation, retreat, and female indifference as alternative outcomes of sexually antagonistic coevolution. Am. Nat. 165, S5–S18. ( 10.1086/429395) [DOI] [PubMed] [Google Scholar]
- 49.Rosenthal GG, Servedio MR. 1999. Chase-away sexual selection: resistance to ‘resistance’. Evolution 53, 296–299. ( 10.2307/2640943) [DOI] [PubMed] [Google Scholar]
- 50.Gavrilets S, Arnqvist G, Friberg U. 2001. The evolution of female mate choice by sexual conflict. Proc. R. Soc. Lond. B 268, 531–539. ( 10.1098/rspb.2000.1382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim Y-J, et al. 2010. MIPs are ancestral ligands for the sex peptide receptor. Proc. Natl. Acad. Sci. USA 107 ( 10.1073/pnas.0914764107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuda M, Peyre JB, Asano T, Aigaki T. 2015. Visualizing molecular functions and cross-species activity of sex-peptide in Drosophila. Genetics 200, 1–10. ( 10.1534/genetics.115.177550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith DT, Hosken DJ, Ffrench-Constant RH, Wedell N. 2009. Variation in sex peptide expression in D. melanogaster. Genet. Res. Cambridge 91, 237–242. ( 10.1017/S0016672309000226) [DOI] [PubMed] [Google Scholar]
- 54.Smith DT, Sirot LK, Wolfner MF, Hosken DJ, Wedell N. 2012. The consequences of genetic variation in sex peptide expression levels for egg laying and retention in females. Heredity 109, 222–225. ( 10.1038/hdy.2012.32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wensing KU, Fricke C.. 2018. Data from: Divergence in sex peptide-mediated female post-mating responses in Drosophila melanogaster Dryad Digital Repository. ( 10.5061/dryad.c394c12) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Wensing KU, Fricke C.. 2018. Data from: Divergence in sex peptide-mediated female post-mating responses in Drosophila melanogaster Dryad Digital Repository. ( 10.5061/dryad.c394c12) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Phenotypic data and gene sequences are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.c394c12 [55].