Abstract
The behavioural composition of a group and the dynamics of social interactions can both influence how social animals work collectively. For example, individuals exhibiting certain behavioural tendencies may have a disproportionately large impact on the group, and so are referred to as keystone individuals, while interactions between individuals can facilitate information transmission about resources. Despite the potential impact of both behavioural composition and interactions on collective behaviour, the relationship between consistent behaviours (also known as personalities) and social interactions remains poorly understood. Here, we use stochastic actor-oriented models to uncover the interdependencies between boldness and social interactions in the social spider Stegodyphus dumicola. We find that boldness has no effect on the likelihood of forming social interactions, but interactions do affect boldness, and lead to an increase in the boldness of the shyer individual. Furthermore, spiders tend to interact with the same individuals as their neighbours. In general, boldness decreases over time, but once an individual's boldness begins to increase, this increase accelerates, suggesting a positive feedback mechanism. These dynamics of interactions and boldness result in skewed boldness distributions of a few bold individuals and many shy individuals, as observed in nature. This group behavioural composition facilitates efficient collective behaviours, such as rapid collective prey attack. Thus, by examining the relationship between behaviour and interactions, we reveal the mechanisms that underlie the emergence of adaptive group composition and collective behaviour.
Keywords: collective behaviour, stochastic actor-oriented models, personality, keystone individual, social network analysis, Stegodyphus dumicola
1. Introduction
Collective phenomena, where interactions among individuals produce emergent behaviours, are ubiquitous in biology. Previous work on collective behaviour [1] typically assumes homogeneity of agents' intrinsic characteristics and interaction rules. However, heterogeneous interactions and consistent individual variation in behaviour over time, often described as ‘personality’, are both increasingly recognized as pervasive and important for emergent group function within animal societies [2–6]. Limited behavioural heterogeneity can be highly impactful. For example, just one ‘keystone’ individual, such as a leader or a tutor, can affect the whole group [7,8]. As such, the behavioural composition of a group can be crucial to its success [9], and thus it is a key research challenge to explain how such behavioural heterogeneity emerges.
The particular mechanism(s) by which keystone individuals' influence on other group members is imparted can be direct, e.g. by leading a collective behaviour, or indirect, e.g. by catalysing particular behaviours of others in the group [10]. Keystone individuals can induce long-term changes in others’ behaviour [11]; however, it is not known how these behavioural changes occur. The impact on the behaviour of group members may be mediated via social interactions, which can be studied using social networks [5,12–14]. Such network representations of social interactions often reveal highly heterogeneous interaction patterns that can influence collective outcomes [6,15].
Behavioural plasticity is often overlooked in consideration of animal personality [16], perhaps because of the seeming tension between behavioural consistency (the definition of personality) and development [17]. Nevertheless, social interactions probably have a strong influence on both short-term individual behaviour [18] and the development of group members' behavioural traits [19]. This influence can be manifest over an individual's lifetime: for example, in the long-tailed manakin, network position of juveniles predicts later social status [20]. Generally, however, the effect of social interactions on behavioural plasticity has been comparatively understudied, probably in part because it is methodologically challenging to estimate the relative influence of individual behavioural traits on dynamic social interactions, and vice versa. Perhaps as a result, personality has typically been assessed by observations across a short time period, often just a few days, because it may not be stable in the longer term [21,22].
Explicit empirical work to identify joint changes in both interaction networks and behaviour is therefore necessary to make further progress in attributing causal priority to either internal processes that affect personality, or external forces such as social interactions, in determining group behavioural compositions. Fortunately, a recently developed simulation-based method of statistical inference, known as stochastic actor-oriented models (SAOMs) [23], now enables such studies of dynamic animal social networks [24].
SAOMs represent network dynamics of longitudinal data, and can estimate the mutual effects of multiple micro-mechanisms that may be operating simultaneously, such as personality and social influence. Importantly, the SAOM framework allows one to study changing nodal variables alongside the network dynamics: behavioural characteristics can be dependent variables, whereby the social network influences the dynamics of behaviour, and the behaviour influences the dynamics of the network. Thus, one can establish the relative influence of networks and behaviour as they change over time (figure 1). The actor-oriented aspect of SAOM refers to the changes in network structure being modelled as stepwise choices by individuals, represented as nodes in a network. The framework describes the agency of individuals deciding with whom to form, maintain and dissolve social ties, as a function of their local social structure and neighbours' behavioural traits [23,25]. So far, there has been fairly limited use of SAOMs to study animal systems (but see examples in hyenas [26], vervet monkeys [27], rooks [28] and Drosophila flies [29]).
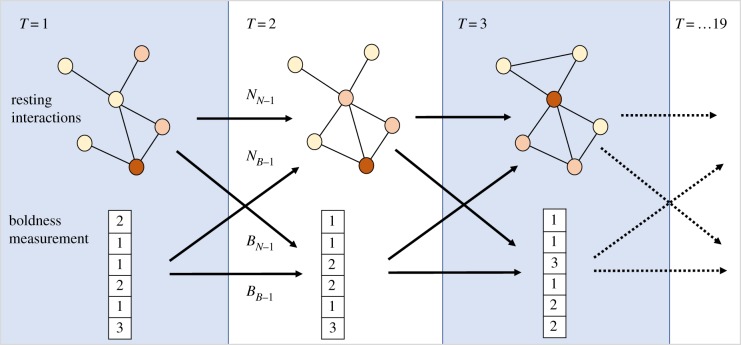
Figure 1.
Network–boldness co-dynamics in the SAOM framework. Each panel is one time point (observation), denoted as T = 1–19. Node (spider) colours indicate boldness which is also listed for each individual in the table below each network. Spider social interactions, which are physical contacts between resting spiders, are represented, for each observation, by undirected, unweighted edges. Arrows between observations indicate possible mechanisms of causal influence: current boldness measurement may depend on the social position of individuals in the previous observation (BN−1); network interactions may be shaped by the individuals' boldness in the previous observation (NB−1); network structure in one observation may result from the social interactions in the previous observation (NN−1), and boldness in one observation may result from the boldness in the previous observation (BB−1). SAOM allows us to estimate all four effects. Figure adapted from [24]. (Online version in colour.)
Stegodyphus dumicola are social spiders that live in colonies of up to several hundred individuals, and exhibit cooperative behaviours such as prey capture and allo-maternal care [30,31]. The presence of just one very bold individual (keystone) in a group of S. dumicola can substantially boost the prey capture success and mass gain of the whole colony [32], with that individual's presence having long-term effects on other spiders' boldness [11]. Boldness in this system is measured as the latency to resume movement after experiencing an aversive stimulus [33,34], and it is a repeatable behaviour, with a repeatability of 0.63 measured when spiders are kept in isolation [35]. Boldness has been shown to correlate with aggression [35], and thus provides insight into more general behavioural tendencies. However, behavioural consistency seems to be contingent on a stable social environment: boldness repeatability is much reduced following social disturbance [36], and such a disturbance reduces group performance [37]. This makes it challenging to assess the mechanism of influence and longer-term identity of keystone individuals. Furthermore, the identity of the boldest individual in the group does not influence its impact on prey capture dynamics [38]—in this system, keystone refers to a behavioural role rather than a specific individual [7].
Investigation into potential mechanisms of keystone influence on the group has been conducted using computer simulations [39]. A priori, one can expect behavioural variation among individuals in the same group to arise from either internal differences (genetics and development) or external conditions (social context and ecological conditions) [40]. Simulation investigation indicates that the effect of keystone individuals on social organization could be mediated through either internal (behavioural persistence) or external (social interaction) forces, as these models generate boldness distributions that match the empirical distribution of Stegodyphus colonies found in the field [39]. Here, we apply the SAOM framework to uncover the temporal dynamics of physical interaction patterns and boldness in the social spider S. dumicola, and to determine if social interactions affect boldness, and/or whether boldness affects who interacts with whom (figure 1).
2. Methods
(a). Animal collection and maintenance
Colonies of adult S. dumicola were collected from roadside Acacia trees in the Northern Cape of South Africa in March 2016. After transportation to the laboratory, they were fed crickets weekly. Laboratory colonies contained only females; males are short-lived and rare (12%) in natural colonies [41]. We created 24 groups of 10 adult female spiders each, from three source colonies. Groups were housed in large round containers (11 cm diameter, 10 cm depth) with a vertical wire mesh (a 5 × 5 cm sheet) to allow the spiders to build both a retreat and a capture web. Experimental observations were made during June–August 2016.
(b). Boldness
Each spider's boldness was measured once a week using an established assay that recorded the recovery of a spider from exposure to air puffs, which mimic the approach of an avian predator [33]. After placing spiders individually in a plastic container (15 × 15 cm), we waited for 30 s until the spiders were acclimated and stopped moving around the arena, as in recent studies [38,42–44]. We then administered two puffs of air to the anterior prosoma using an infant nose-cleaning bulb. Spiders react to the air puffs by huddling (i.e. pulling their legs under their body and remaining motionless). Boldness was measured as the latency to resume movement and move one body length. Because bolder individuals resume movement faster, the latency to resume movement was subtracted from the maximum duration of the procedure (600 s) to create a metric that increases with boldness. We designated as ‘shy’ those individuals with a latency to resume movement of 400–600 s (boldness of 0–200), while ‘bold’ individuals were those with a latency to resume movement of 0–200 s (boldness of 400–600). The abdomen of each spider was given a unique marking with acrylic paint to track their behaviour over time.
(c). Group boldness composition
We artificially created groups of 10 spiders with one of three boldness compositions: all bold spiders, all shy spiders and nine shy individuals with one bold individual. Overall, these groups contained more initially shy individuals than bold individuals because this represents the spiders' natural boldness distribution (fig. 4 in [39]). Group composition converged after the first week (electronic supplementary material, figure S1). To examine changes in groups’ boldness compositions, we compared the boldness distributions in week 1 to week 7.
(d). Social interactions
We manually recorded the physical contacts among spiders three times a week (see below), during the day, while spiders are inactive for long periods of time. Therefore, we refer to these interactions as ‘resting interactions' and define an interaction as a physical contact between any body parts of two spiders, when the colony is not active. Colony activity is minimal in the laboratory (initial web construction and collective predation when fed) and most of the time spiders are resting. Therefore, observing their interactions every 2–3 days samples most social interactions. We used the interactions to construct unweighted (binary), undirected (symmetrical), networks for each spider group during each observation.
We calculated the skewness of each resting network's degree distribution (N = 456), to assess whether the spiders in each observed network tended to have a similar number of interactions (skewness close to 0) or if degree was heterogeneous (skewness different from 0).
(e). Experimental procedure
Each group was observed for 6.5 weeks. Boldness was measured once a week and resting interactions, later translated into social networks, were observed three times a week with 2–3 days separating each observation. We recorded the first set of resting interactions each week immediately before measuring boldness (day 1). We recorded the second resting network on day 3, and the third resting network was recorded on day 5. After interactions were observed on day 5, we fed each colony a single four-week-old cricket; hence all colonies had an equal opportunity to consume prey. This spacing of measures of interactions allowed time for the spiders to recover from the disturbance caused by measuring boldness on day 1 (after observing the resting interaction). In week 7, we made a final observation of boldness and the resting network. In total, there were seven boldness measures for each individual spider and 19 resting networks for each group.
(f). Stochastic actor-oriented models
To determine the relationship between boldness and social interactions using the SAOM method, we first ensured that our data met the model assumptions. The SAOM method requires an appropriate level of tie turnover between successive network observations (i.e. edges being created, maintained or removed) measured using the Jaccard index of similarity between successive observation waves [45]. Because several spider groups did not have a Jaccard index greater than 0.2 when modelled individually, we aggregated groups by source colony and group composition treatment, such that 24 groups became 8 (electronic supplementary material, table S1). This aggregation allowed us to compare the different group composition treatments as detailed in the electronic supplementary material. We aggregated groups using structural zeros, whereby two or more networks are included in one adjacency matrix, but the two sets of nodes are not allowed to form edges between groups, only within them. This aggregation achieved the appropriate level of tie turnover and allowed us to proceed with the SAOM analysis. When nodes were removed because of spider death, we specified structural zeros for the relevant node in the time periods after its death, such that it can no longer participate in network dynamics, and is not included in statistical estimation from that time point.
Boldness was measured once per week, to minimize disruption to the spiders, and so we interpolated the boldness measure to obtain boldness measures for all three sets of network observations made each week. To calculate boldness at intermediate days (3 and 5), we used a linear interpolation between the two known points on day 1 of that week and the next week. The SAOM framework simulates network and behaviour changes through a series of microsteps (i.e. the addition, maintenance or dissolution of a single network tie, or a −1, 0 or + 1 change in a spider's boldness covariate). The boldness range of 0–600 is too wide for such microsteps, and therefore we translated it to a 1–3 scale, from 0 to 200, 200 to 400 and 400 to 600. These groupings match the criteria we used for creating group compositions of all shy and all-bold groups.
Although we created three group composition treatments, the behavioural composition of the groups converged after the first week, and thus after the first week, all treatments had similar boldness compositions.
We used the SIENA framework (Simulation Investigation for Empirical Network Analysis) to implement the SAOM analysis in the R package RSiena v. 1.2.3 [45,46], with R v. 3.3.3 [47]. To construct models, we followed an iterative approach guided by existing scientific insight and the hypotheses tested, as detailed in Fisher et al. [24]. We started with a simple set of core effects and then introduced further complexity to the model. We were primarily interested in the effect of boldness on tie formation and the effect of social ties on boldness, and used effects that are specific to undirected (symmetrical) ties.
(g). Stochastic actor-oriented model effects
We included the following structural and behavioural effects in our SAOMs:
(i) Network on boldness—to measure the influence of network ties on boldness, we included the average alter effect (avAlt). This is the influence of the (averaged) behaviour of alters (i.e. neighbouring spiders) upon interaction with a focal individual (i.e. the ‘actor’ in SAOM). Here, a positive effect indicates that the formation of a social interaction tends to increase boldness of the individual with lower boldness, while a negative effect indicates that interactions tend to reduce boldness.
(ii) Boldness temporal dynamics—to examine general tendencies in boldness over time across all the spiders, we included both a linear shape effect and a quadratic shape effect. A zero value for the linear shape effect indicates drift towards the midpoint of the range of the behavioural variable. A positive value indicates an increase, and a negative value a decrease, in boldness over time. The quadratic shape effect indicates the presence of feedback: positive values imply that an increase in boldness tends to be followed by another boldness increase, as a self-reinforcing, ‘addictive’ behaviour [46]. A negative value indicates a self-correcting negative feedback: boldness increases tend to be followed by reductions in boldness and when boldness decreases, the push towards further decreases is curtailed.
-
(iii) Boldness on interactions—to measure the effect of boldness on the tendency to form ties, we included the covariate effect (egoPlusAltX), the covariate being boldness in this case. A positive covariate effect would indicate that bolder spiders are more likely to form ties in general, while a negative effect would indicate that bolder spiders tend to be more isolated.
The following effects depend on the network itself, separately from individual behavioural covariates.
(iv) Structural equivalence—we examined two measures of structural equivalence. (i) Jaccard similarity effect (Jout)—the extent to which two actors (connected or not) are connected with the same third parties [48]. Thus, a positive Jout effect indicates that individuals share a similar social environment. (ii) Weighted structural equivalence effect (from.w.ind)—measures a preference to interact with individuals who have similar ties to other individuals, weighted by the degrees (number of neighbours) of those others. A positive from.w.ind suggests that structural equivalence is achieved by ties to third parties with high degree.
(v) Degree plus popularity (degPlus)—a feedback effect for undirected networks, representing (if positive) a tendency for nodes with high degree (many neighbours) to create and maintain relatively more ties than low-degree nodes. If negative, this indicates a constraint on node degrees becoming too dispersed.
Network density and period-specific network and boldness effects were also included. Network density (density), which is the ratio of observed ties to all possible ties, takes the role of an intercept in a regression model, by controlling for the overall density, given all the other effects included in the models. Thus, while it is a necessary effect, it is not biologically informative. Finally, because we model the change in network tie formation and boldness change over 19 observations, there are 18 period-specific rate constants for each of these (inter-)dependent variables. Similar to network density, these constants are not of focal interest [45].
To ensure that our data fit the SAOM, we ran post hoc statistical goodness-of-fit (GOF) tests. We ensured that the simulated networks and behaviour variables in the SAOM are sufficiently similar to empirical observations, across various relevant characteristics, and that model convergence has been obtained (maximum convergence ratio less than 0.25 [45]). We ran four such GOF tests, on the degree distribution, geodesic distribution (the number of nodes connected at a certain network distance), triad census (the number of node triplets with one, two or three edges) and behaviour distribution (the discrete behaviour-dependent variable ranged 1–3) (electronic supplementary material, table S2).
To assess the overall results of the SAOM analysis, we conducted meta-analysis of the eight SAOMs. When a common set of effects was identified that led to good model convergence and adequate post hoc GOF tests across all eight models, we performed a meta-analysis of the model effects, to see if they are significantly different from zero. We did this using the RSiena function siena08, which weights model effects according to their standard error, into a final mean effect value with associated estimated 95% confidence interval. The siena08 function provides means, standard errors and p-values under a normality assumption, and also under an alternative approach of modified iterated re-weighted least squares developed by Snijders & Baerveldt for meta-analysis [49]. We present the normality assumption results in the main text, but both sets of results are presented in electronic supplementary material, table S2, with the same overall results.
3. Results
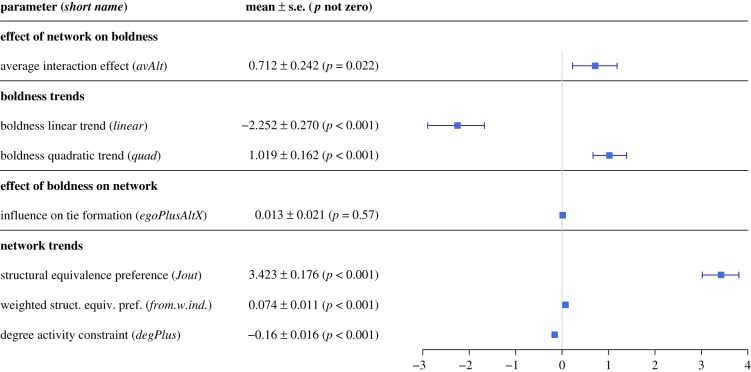
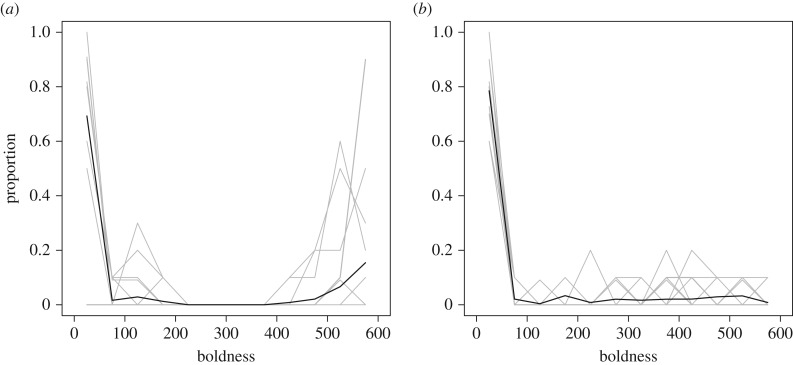
Interactions and time affected boldness, but boldness did not affect interactions. We found several significant effects in our meta-analysis of the eight SAOM models. When spiders interacted with others that had a different boldness than themselves, the spider with the lower boldness tended to increase its boldness in the next time step (significantly positive average alter effect; p = 0.024; figure 2). Boldness tended to decrease over time (significantly negative bold linear effect; p < 0.001; figure 2). However, once an individual's boldness increased, there was a positive feedback pushing towards higher boldness (positive bold quadratic effect; p < 0.001; figure 2). Boldness distributions changed over the course of seven weeks (figure 3) such that by the end of the seven weeks, boldness distributions resembled those observed in the field and generated by models in which boldness tends to decrease over time and increase when spiders interact [39].
Figure 2.
A forest plot showing meta-analysis results of the SAOMs’ parameters. Social interactions between spiders exhibiting different boldness tend to increase the boldness of the shyer spider (avAlt effect). There is a general decrease in boldness over time (linear) but boldness increases are self-reinforcing (quad). There is no effect of boldness on the likelihood of forming (or avoiding) social ties (egoPlusAltX). Positive Jout and from.w.ind indicate a tendency for spiders to form ties with nest-mates that interact with their neighbours: the positive from.w.ind effect suggests that individuals with high-degree centrality drive this trend. Negative degPlus implies a cap on the dispersion of spider degrees, likely because of physical restrictions on the maximum number of individuals a spider can touch.
Figure 3.
The empirical boldness distribution for each of the 24 groups (grey lines) and the average boldness distribution for all groups (black line), at (a) week 1 and (b) week 7.
Individuals’ social interactions were not impacted by boldness but they were influenced by the surrounding social environment. We did not detect a significant effect of boldness on the tendency to form (or avoid) ties (p = 0.445; figure 2). At the same time, spiders tended to interact with similar individuals as their nest-mates: both Jout and from.w.ind were positive effects (p < 0.001 in both cases; figure 2). Positive from.w.ind further indicates that individuals with high-degree make a larger contribution to achieving structural equivalence. Finally, we found a restriction on the dispersion of spider degrees (negative degPlus, p < 0.001; figure 2), likely because of physical limits on how many individuals a spider can touch at once (i.e. a cap on higher degrees).
These observed trends were seen in all three behavioural composition treatments (figure 2; electronic supplementary material, table S2). For example, all the SAOMs, including the all-shy and all-bold treatments, had a negative linear boldness trend. Furthermore, boldness compositions of the three treatments converged within the first week (electronic supplementary material, figure S1). Thus, our findings reveal that, in a social context, boldness is more plastic than in isolation, and artificially manipulated group boldness compositions are quickly rectified by endogenous group processes.
The average degree distribution across all networks was unimodal (electronic supplementary material, figure S2), and the skewness of the degree distributions of all networks was centred around zero (electronic supplementary material, figure S3). This indicates that all spiders in a network tend to physically interact with a similar number of nest-mates when resting.
4. Discussion
We found that social interactions promote changes in individual boldness in social spider groups. While boldness is a highly repeatable trait for spiders kept in isolation [35], in a social context, we find that individuals' boldness is plastic. Specifically, social interactions tend to increase boldness, such that a spider whose physical neighbours have overall higher average boldness at one point in time, tends towards higher boldness values itself. Here, where boldness has been analysed on a 1–3 scale, this finding is an aggregate trend that encompasses any interactions in which one individual is bolder and the other shyer (i.e. 3–1, 3–2 and 2–1). The boldness-promoting effect of social interactions is balanced against an overall decrease in boldness over time. Furthermore, spiders tend to interact with the same individuals as their neighbours, especially those that are well connected. This finding probably results from the spiders’ preference to huddle together in the nest retreat. This finding also suggests that an individual with high boldness (or even the highest boldness, i.e. the keystone individual) could promote increases in boldness across several individuals simultaneously, if more than one shyer individual is socially connected to that bold individual. Different spiders tend to interact with the same individuals, thus boldness increases to a few central spiders may have widespread effects. We did not find evidence that boldness influences the likelihood of forming social interactions. A question for future research is how social interactions influence boldness. Perhaps spiders cue on chemicals present on the body surface, like ants [50], or influence each other through small movements. For example, if bold individuals are more agitated than others, their proximity could affect their neighbours, directly or through web vibrations.
Our finding that bold spiders are no more or less likely to interact with other individuals than their shyer nest-mates could be seen as contrary to expectations. For example, social assortment according to behavioural type has been recognized in fish shoals [51]. On the other hand, bolder spiders might be thought to prefer social isolation: bolder three-spined stickleback fish have been observed to keep a greater distance from a partner, while showing more leadership behaviour [52]. However, it is possible that boldness does not correlate with sociability. Indeed, in a review of behavioural syndromes by Réale et al. [53], the shyness–boldness axis is distinguished from sociability. The natural distribution of boldness in Stegodyphus groups is a few bolder individuals among a majority of shyer individuals [39], and hence with no behavioural assortment bold individuals are more likely to interact with shy individuals by simple probability. Bolder individuals are more likely to interact with the environment outside the nest during foraging, given increased participation in prey capture in both the laboratory [32] and the field [54,55], and thus may be a source of disease vulnerability for other group members. Yet our findings here point towards bold–shy interactions being an indispensable element in determining the behavioural composition of the group, whereby boldness is ‘passed on’ by an as yet unidentified mechanism from bolder to shyer individuals. Bold–shy interactions thus maintain a suitable group-level boldness distribution that promotes effective prey capture [32,56].
A general trend towards decreases in boldness over time, occurring separately from the influence of the spiders' interaction network, is consistent with past findings. Recently disturbed colonies of S. dumicola become shyer over time before recovering in boldness [57]. However, the significantly positive quadratic shape effect on boldness that we identified indicates that an increase in spider boldness generally tends to be self-reinforcing, or ‘addictive’. In this way, a spider with a low boldness rating, that transitions to a medium rating, will be more likely to increase its boldness still further rather than reduce its boldness. Thus, individuals with small initial increases in their boldness are more likely to become a group's boldest group member in subsequent weeks. These boldest individuals are known to be major determinants of the behaviour and success of the colony as a whole [44]. Interestingly, despite different initial boldness compositions, by week 2 of the experiment, the average boldness of all groups was not different (see electronic supplementary material, figure S1). This change and the SAOM findings indicate that social interactions are apparently instrumental in changing artificially manipulated S. dumicola boldness distributions to resemble those found in nature [39], which facilitate rapid prey attack [32].
The results we present here corroborate the assumptions made in simulation work on how the dynamics of boldness and social interactions result in skewed behavioural distributions and can point to the model parameters that best fit the biological system [39]. The observed resting networks' degree distribution was unimodal (electronic supplementary material, figure S2), and the skewness of the degree distributions of all networks was centred around zero (electronic supplementary material, figure S3), similar to the uniform interaction rule in previous simulation work. These characteristics indicate that all individuals are equally likely to interact with one another, regardless of their boldness. Furthermore, our finding that boldness tends to decrease over time and that boldness is acquired from bolder neighbours points towards a scenario in the theoretical model in which there is low persistence of boldness and high acquisition of boldness from others. Indeed, the simulated boldness distributions for this parameter setting (low persistence, high acquisition) and a uniform interaction rule [39] qualitatively match well with the empirical observations presented here (figure 3).
One remaining open question, regarding influential keystone individuals in animal collectives, is their replaceability: whether the specific individual or the role performed by that individual is the most important [16]. Our results, indicating social plasticity of boldness in accordance with [38], point to the existence of a keystone role rather than a keystone individual [7]. With relatively low behavioural persistence, and high acquisition of behaviour from others via social interactions, the boldest spiders in the group—the keystone(s)—are highly influential, but likely to change in identity over time. Indeed, in the case of S. dumicola, while keystone individuals are important, they seem to be replaceable. For example, iteratively removing and replacing shy individuals has a greater impact on the colony's behaviour than replacing bold individuals [38].
One question arising from the boldness dynamics that we observed is what occurs when there are no bolder individuals to impart their positive catalytic influence on the boldness of other shyer individuals. In this case, one can see the importance of the significant quadratic shape effect (the ‘addictive’ boldness increase effect), to magnify even small boldness increases over time such that they become self-sustaining and do not require constant social contact to support them. In a real-world system, small boldness differences will always exist for such dynamics to work upon [39]. Such an inherent robustness of the group-level skewed boldness phenotype, dependent on social dynamics alone, seems to downplay the importance of internal, genetic or developmental differences for the ontogenesis of keystones. Instead, it indicates that external factors, such as social and ecological conditions, may be sufficient. In practice, boldness may be contingent on physiological factors such as satiation (i.e. the time since last feeding), though evidence for this idea are mixed [35,58]. For periods longer than a few weeks (i.e. beyond the observation range of the data examined here) life-history stages relating to reproduction are also likely to be important, given the relatively short lives of female Stegodyphus of 1–2 years [41].
5. Conclusion
Many animal groups are increasingly recognized to rely on heterogeneity in the behaviour and social interactions of the group members for effective group function. However, the relative importance of behaviour for shaping interactions, and interactions for shaping behaviour, is poorly understood. We show that social interactions promote the increase in boldness in social spiders, such that an optimal collective distribution in boldness is attained. Boldness, however, does not make individuals more or less likely to physically interact with others. Our findings are consistent with the uniform interactions, high acquisition, low persistence model of keystone influence on groups of Pinter-Wollman et al. [39], and thus suggest that it is the keystone role, rather than the identity of the individual acting it, that is important to such social groups [38]. Our findings have implications for the understanding of personality in social groups, indicating a priority of an animal's social environment for the development of personality. Future research should address in more detail the specific mechanisms of how social interactions promote boldness, and the dynamics of social networks and boldness in different ecological conditions in the field. This should further elucidate the relative importance of internal versus external factors for the emergence of adaptive collective phenotypes.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank T. A. B. Snijders for generous guidance on use of the RSiena software and SAOM models. We also thank the South Africa Department of Tourism, Environment, and Conservation for providing permits for animal collection (FAUNA 1072/2013 and 1691/2015) and Colin Wright and James Lichtenstein for collecting spiders in the field.
Data accessibility
The network and boldness data analysed in this paper are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.f78fc08 [59].
Authors' contributions
N.P.-W. and J.N.P. designed the study, E.R.H. analysed the data and wrote the first draft of the manuscript, and B.M., C.F., B.M.W. and N.P.-W. collected the data. All co-authors gave approval to the final version of the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Science Foundation IOS grants 1456010/1708455 to N.P.-W. and 1455895 to J.N.P., and National Institutes of Health grant GM115509 to N.P.-W. and J.N.P.
References
- 1.Sumpter DJ. 2010. Collective animal behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sih A, Bell AM, Johnson JC, Ziemba RE. 2004. Behavioral syndromes: an integrative overview. Q Rev. Biol. 79, 241–277. ( 10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 4.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198. ( 10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinter-Wollman N. 2015. Persistent variation in spatial behavior affects the structure and function of interaction networks. Curr. Zool. 61, 98–106. ( 10.1093/czoolo/61.1.98) [DOI] [Google Scholar]
- 6.Pinter-Wollman N, Wollman R, Guetz A, Holmes S, Gordon DM. 2011. The effect of individual variation on the structure and function of interaction networks in harvester ants. J. R. Soc. Interface 8, 1562–1573. ( 10.1098/rsif.2011.0059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modlmeier AP, Keiser CN, Watters JV, Sih A, Pruitt JN. 2014. The keystone individual concept: an ecological and evolutionary overview. Anim. Behav. 89, 53–62. ( 10.1016/j.anbehav.2013.12.020) [DOI] [Google Scholar]
- 8.Conradt L, Roper TJ. 2003. Group decision-making in animals. Nature 421, 155–158. ( 10.1038/nature01294) [DOI] [PubMed] [Google Scholar]
- 9.Pruitt JN, Goodnight CJ. 2014. Site-specific group selection drives locally adapted group compositions. Nature 514, 359–362. ( 10.1038/nature13811) [DOI] [PubMed] [Google Scholar]
- 10.Robson SK, Traniello JFA. 1999. Key individuals and the organisation of labor in ants. In Information processing in social insects (eds Detrain C, Deneubourg JL, Pasteels JM), pp. 239–259. Basel, Switzerland: Birkhäuser Basel. [Google Scholar]
- 11.Pruitt JN, Pinter-Wollman N. 2015. The legacy effects of keystone individuals on collective behaviour scale to how long they remain within a group. Proc. R. Soc. B 282, 89–96. ( 10.1098/rspb.2015.1766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sih A, Hanser SF, McHugh KA. 2009. Social network theory: new insights and issues for behavioral ecologists. Behav. Ecol. Sociobiol. 63, 975–988. ( 10.1007/s00265-009-0725-6) [DOI] [Google Scholar]
- 13.Wilson ADM, Krause S, Dingemanse NJ, Krause J. 2013. Network position: a key component in the characterization of social personality types. Behav. Ecol. Sociobiol. 67, 163–173. ( 10.1007/s00265-012-1428-y) [DOI] [Google Scholar]
- 14.Krause J, James R, Croft DP. 2010. Personality in the context of social networks. Phil. Trans. R. Soc. B 365, 4099–4106. ( 10.1098/rstb.2010.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royle NJ, Pike TW, Heeb P, Richner H, Kolliker M. 2012. Offspring social network structure predicts fitness in families. Proc. R. Soc. B 279, 4914–4922. ( 10.1098/rspb.2012.1701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause J, James R, Franks DW, Croft DP. 2015. Animal social networks. Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Groothuis TGG, Trillmich F. 2011. Unfolding personalities: the importance of studying ontogeny. Dev. Psychobiol. 53, 641–655. ( 10.1002/dev.20574) [DOI] [PubMed] [Google Scholar]
- 18.Firth JA, Voelkl B, Farine DR, Sheldon BC. 2015. Experimental evidence that social relationships determine individual foraging behavior. Curr. Biol. 25, 3138–3143. ( 10.1016/j.cub.2015.09.075) [DOI] [PubMed] [Google Scholar]
- 19.Bengston SE, Jandt JM. 2014. The development of collective personality: the ontogenetic drivers of behavioral variation across groups. Front. Ecol. Evol. 2, 81 ( 10.3389/fevo.2014.00081) [DOI] [Google Scholar]
- 20.McDonald DB. 2007. Predicting fate from early connectivity in a social network. Proc. Natl Acad. Sci. USA 104, 10 910–10 914. ( 10.1073/pnas.0701159104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamps JA, Groothuis TGG. 2010. Developmental perspectives on personality: implications for ecological and evolutionary studies of individual differences. Phil. Trans. R. Soc. B 365, 4029–4041. ( 10.1098/rstb.2010.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pronk R, Wilson DR, Harcourt R. 2010. Video playback demonstrates episodic personality in the gloomy octopus. J. Exp. Biol. 213, 1035–1041. ( 10.1242/jeb.040675) [DOI] [PubMed] [Google Scholar]
- 23.Snijders TAB, van de Bunt GG, Steglich CEG. 2010. Introduction to stochastic actor-based models for network dynamics. Social Netw. 32, 44–60. ( 10.1016/j.socnet.2009.02.004) [DOI] [Google Scholar]
- 24.Fisher DN, Ilany A, Silk MJ, Tregenza T. 2017. Analysing animal social network dynamics: the potential of stochastic actor-oriented models. J. Anim. Ecol. 86, 202–212. ( 10.1111/1365-2656.12630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snijders TAB. 2017. Stochastic actor-oriented models for network dynamics. Annu. Rev. Stat. Appl. 4, 343–363. ( 10.1146/annurev-statistics-060116-054035) [DOI] [Google Scholar]
- 26.Ilany A, Booms AS, Holekamp KE. 2015. Topological effects of network structure on long-term social network dynamics in a wild mammal. Ecol. Lett. 18, 687–695. ( 10.1111/ele.12447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borgeaud C, Sosa S, Bshary R, Sueur C, van de Waal E. 2016. Intergroup variation of social relationships in wild vervet monkeys: a dynamic network approach. Front. Psychol. 7, 915 ( 10.3389/fpsyg.2016.00915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boucherie PH, Sosa S, Pasquaretta C, Dufour V. 2017. A longitudinal network analysis of social dynamics in rooks Corvus frugilegus: repeated group modifications do not affect social network in captive rooks. Curr. Zool. 63, 379–388. ( 10.1093/cz/zox045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasquaretta C, Klenschi E, Pansanel J, Battesti M, Mery F, Sueur C. 2016. Understanding dynamics of information transmission in Drosophila melanogaster using a statistical modeling framework for longitudinal network data (the RSiena Package). Front. Psychol. 7, 539 ( 10.3389/fpsyg.2016.00539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilde T, Coates K, Birkhofer K, Bird T, Maklakov A, Lubin Y, Aviles L. 2007. Survival benefits select for group living in a social spider despite reproductive costs. J. Evol. Biol. 20, 2412–2426. ( 10.1111/j.1420-9101.2007.01407.x) [DOI] [PubMed] [Google Scholar]
- 31.Junghanns A, Holm C, Schou MF, Sørensen AB, Uhl G, Bilde T. 2017. Extreme allomaternal care and unequal task participation by unmated females in a cooperatively breeding spider. Anim. Behav. 132, 101–107. ( 10.1016/j.anbehav.2017.08.006) [DOI] [Google Scholar]
- 32.Pruitt JN, Keiser CN. 2014. The personality types of key catalytic individuals shape colonies’ collective behaviour and success. Anim. Behav. 93, 87–95. ( 10.1016/j.anbehav.2014.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riechert SE, Hedrick AV. 1993. A test for correlations among fitness-linked behavioural traits in the spider Agelenopsis aperta (Araneae, Agelenidae). Anim. Behav. 46, 669–675. ( 10.1006/anbe.1993.1243) [DOI] [Google Scholar]
- 34.Sloan Wilson D, Clark AB, Coleman K, Dearstyne T. 1994. Shyness and boldness in humans and other animals. Trends Ecol. Evol. 9, 442–446. ( 10.1016/0169-5347(94)90134-1) [DOI] [PubMed] [Google Scholar]
- 35.Keiser CN, Jones DK, Modlmeier AP, Pruitt JN. 2014. Exploring the effects of individual traits and within-colony variation on task differentiation and collective behavior in a desert social spider. Behav. Ecol. Sociobiol. 68, 839–850. ( 10.1007/s00265-014-1696-9) [DOI] [Google Scholar]
- 36.Laskowski KL, Pruitt JN. 2014. Evidence of social niche construction: persistent and repeated social interactions generate stronger personalities in a social spider. Proc. R. Soc. B 281, 20133166 ( 10.1098/rspb.2013.3166) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Laskowski KL, Montiglio P-O, Pruitt JN. 2016. Individual and group performance suffers from social niche disruption. Am. Nat. 187, 776–785. ( 10.1086/686220) [DOI] [PubMed] [Google Scholar]
- 38.Pinter-Wollman N, Mi B, Pruitt JN. 2017. Replacing bold individuals has a smaller impact on group performance than replacing shy individuals. Behav. Ecol. 28, 883–889. ( 10.1093/beheco/arx054) [DOI] [Google Scholar]
- 39.Pinter-Wollman N, Keiser CN, Wollman R, Pruitt JN. 2016. The effect of keystone individuals on collective outcomes can be mediated through interactions or behavioral persistence. Am. Nat. 188, 240–252. ( 10.1086/687235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schradin C. 2013. Intraspecific variation in social organization by genetic variation, developmental plasticity, social flexibility or entirely extrinsic factors. Phil. Trans. R. Soc. B 368, 20120346 ( 10.1098/rstb.2012.0346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henschel JR, Lubin YD, Schneider J. 1995. Sexual competition in an inbreeding social spider, Stegodyphus dumicola (Araneae: Eresidae). Insectes Soc. 42, 419–426. ( 10.1007/BF01242170) [DOI] [Google Scholar]
- 42.Keiser CN, Pinter-Wollman N, Ziemba MJ, Kothamasu KS, Pruitt JN. 2017. The index case is not enough: variation among individuals, groups, and social networks determine bacterial transmission dynamics. J. Anim. Ecol. 87, 369–378. ( 10.1111/1365-2656.12729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lichtenstein JL, Wright CM, Luscuskie LP, Montgomery GA, Pinter-Wollman N, Pruitt JN. 2017. Participation in cooperative prey capture and the benefits gained from it are associated with individual personality. Curr. Zool. 63, 561–567. ( 10.1093/cz/zow097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pruitt JN, Wright CM, Lichtenstein JLL, Chism GT, McEwen BL, Kamath A, Pinter-Wollman N. 2018. Selection for collective aggressiveness favors social susceptibility in social spiders. Curr. Biol. 28, 100–105.e4. ( 10.1016/j.cub.2017.11.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ripley RM, Snijders TAB, Boda ZO, Vörös AA, Preciado P. 2017. Manual for SIENA version 4.0 (version September 9, 2017). Oxford, UK: University of Oxford, Department of Statistics; Nuffield College. [Google Scholar]
- 46.Ripley R, Boitmanis K, Snijders TAB, Schoenenberger F. 2017. RSiena: Siena—simulation investigation for empirical network analysis. (R package version 1.2-3 ed).
- 47.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 48.Borgatti SP, Everett MG, Johnson JC. 2013. Analyzing social networks. Beverley Hills, CA: Sage Publications. [Google Scholar]
- 49.Snijders TAB, Baerveldt C. 2003. A multilevel network study of the effects of delinquent behavior on friendship evolution. J. Math. Soc. 27, 123–151. ( 10.1080/00222500305892) [DOI] [Google Scholar]
- 50.Greene MJ, Gordon DM. 2003. Cuticular hydrocarbons inform task decisions. Nature 423, 32 ( 10.1038/423032a) [DOI] [PubMed] [Google Scholar]
- 51.Croft DP, Krause J, Darden SK, Ramnarine IW, Faria JJ, James R. 2009. Behavioural trait assortment in a social network: patterns and implications. Behav. Ecol. Sociobiol. 63, 1495–1503. ( 10.1007/s00265-009-0802-x) [DOI] [Google Scholar]
- 52.Jolles JW, Fleetwood-Wilson A, Nakayama S, Stumpe MC, Johnstone RA, Manica A. 2015. The role of social attraction and its link with boldness in the collective movements of three-spined sticklebacks. Anim. Behav. 99, 147–153. ( 10.1016/j.anbehav.2014.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. Camb. Philos. Soc. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 54.Grinsted L, Pruitt JN, Settepani V, Bilde T. 2013. Individual personalities shape task differentiation in a social spider. Proc. R. Soc. B 280, 20131407 ( 10.1098/rspb.2013.1407) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Settepani V, Grinsted L, Granfeldt J, Jensen JL, Bilde T. 2013. Task specialization in two social spiders, Stegodyphus sarasinorum (Eresidae) and Anelosimus eximius (Theridiidae). J. Evol. Biol. 26, 51–62. ( 10.1111/jeb.12024) [DOI] [PubMed] [Google Scholar]
- 56.Pruitt JN, Grinsted L, Settepani V. 2013. Linking levels of personality: personalities of the ‘average’ and ‘most extreme’ group members predict colony-level personality. Anim. Behav. 86, 391–399. ( 10.1016/j.anbehav.2013.05.030) [DOI] [Google Scholar]
- 57.Modlmeier AP, Laskowski KL, DeMarco AE, Coleman A, Zhao K, Brittingham HA, McDermott DR, Pruitt JN. 2014. Persistent social interactions beget more pronounced personalities in a desert-dwelling social spider. Biol. Lett. 10, 20140419 ( 10.1098/rsbl.2014.0419) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Wright CM, Keiser CN, Pruitt JN. 2015. Personality and morphology shape task participation, collective foraging and escape behaviour in the social spider Stegodyphus dumicola. Anim. Behav. 105, 47–54. ( 10.1016/j.anbehav.2015.04.001) [DOI] [Google Scholar]
- 59.Hunt ER, Mi B, Fernandez C, Wong BM, Pruitt JN, Pinter-Wollman N. 2018. Data from: Social interactions shape individual and collective personality in social spiders Dryad Digital Repository. ( 10.5061/dryad.f78fc08) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hunt ER, Mi B, Fernandez C, Wong BM, Pruitt JN, Pinter-Wollman N. 2018. Data from: Social interactions shape individual and collective personality in social spiders Dryad Digital Repository. ( 10.5061/dryad.f78fc08) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The network and boldness data analysed in this paper are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.f78fc08 [59].