Abstract
Parental environments are regularly shown to alter the mean fitness of offspring, but their impacts on the genetic variation for fitness, which predicts adaptive capacity and is also measured on offspring, are unclear. Consequently, how parental environments mediate adaptation to environmental stressors, like those accompanying global change, is largely unknown. Here, using an ecologically important marine tubeworm in a quantitative-genetic breeding design, we tested how parental exposure to projected ocean warming alters the mean survival, and genetic variation for survival, of offspring during their most vulnerable life stage under current and projected temperatures. Offspring survival was higher when parent and offspring temperatures matched. Across offspring temperatures, parental exposure to warming altered the distribution of additive genetic variance for survival, making it covary across current and projected temperatures in a way that may aid adaptation to future warming. Parental exposure to warming also amplified nonadditive genetic variance for survival, suggesting that compatibilities between parental genomes may grow increasingly important under future warming. Our study shows that parental environments potentially have broader-ranging effects on adaptive capacity than currently appreciated, not only mitigating the negative impacts of global change but also reshaping the raw fuel for evolutionary responses to it.
Keywords: climate change, parental effects, transgenerational plasticity, genetic variance, marine invertebrates
1. Introduction
As environments become adversely affected by global change, populations can maintain or recover fitness in situ via plasticity in the short term (one or a few generations), and adaptation in the longer term [1]. Understanding how plasticity and adaptation potentially mitigate the negative impacts of global change, both separately and in tandem, is essential for predicting biodiversity loss and directing conservation strategies [2].
Individuals faced with environmental stress may respond through plastic changes in morphology, physiology and/or behaviour that help maintain fitness under that stress [2]. As environmental stressors such as temperature rise steadily owing to global change, individuals may increasingly need to respond plastically during their lifespans [3]. Plasticity can also be transgenerational if the effects of stressors on parents carry over to their offspring [4]. To the extent that offspring face similar environmental conditions, transgenerational plasticity can potentially buffer offspring fitness against environmental stress [5]. Such benefits of transgenerational plasticity are documented in several plant and animal taxa [3,4]. In other cases, however, parental exposure to environmental stress has only weak, or even detrimental, effects on offspring [6]. Thus, understanding population vulnerability to global change may hinge on understanding how exposure to environmental stress in one generation affects fitness in the next [7].
Nevertheless, plasticity alone is unlikely to buffer populations against sustained increases in environmental stress [1]. Population persistence in the longer term will often require adaptive evolution, which rests on the availability of adequate genetic variation in fitness and related traits [2,8]. Predictions of adaptive capacity focus on the additive component of this variation (i.e. the average effects of alleles summed across loci; [9]), which in principle constrains the rate that fitness increases from generation to generation. Nonadditive genetic variation can also arise owing to allele interactions within (dominance) and among (epistasis) loci, which may reflect how well the genes of parents function together in offspring [10]. However, speculation about its adaptive value [11,12] is often dismissed because such interactions are deemed unlikely to contribute much to trait variation in natural populations [13].
Crucially, levels of genetic variance for fitness and related traits are often environment-dependent [14], and should, therefore, be estimated under future levels of environmental stress to predict adaptive capacity under global change. However, past work on this front has shown mixed results [14] with additive genetic variance being abundant under some environmental stressors [15–17], but limited under others [18].
Adaptation to global change will not only depend on genetic variation within environments but also on genetic correlations across environments if similar alleles affect the phenotypes expressed in different environments [9]. In some cases, genetic correlations across environments can slow adaptation, or constrain it altogether, if alleles that are beneficial under current conditions become detrimental once those conditions change [19]. Alternatively, such correlations may accelerate adaptation if the alleles favoured in current environments are also favoured in future environments [8,16]. Overall, however, we still have little idea of how genetic correlations across traits or environments affect adaptation, and reviews of the existing literature have identified few general trends in their effects [20]. Nonetheless, genetic correlations are expected to influence the ability of populations to keep pace, in an evolutionary sense, with the rate of environmental change [8,21], meaning further research is needed to measure the strength and direction of genetic correlations across current and projected future environments.
It is clear that population persistence under global change will rely on plasticity and/or adaptation, yet it remains unclear how these two key processes interact [2]. In particular, the effects of parental environment on mean phenotypes of offspring are well-acknowledged [6], but genetic variation is typically assumed to be unaffected. Curiously, this assumption has rarely been tested (but see [17,22,23]), and was recently highlighted as a key knowledge gap [7]. Furthermore, studies have yet to consider how parental environment affects the multivariate structure of genetic variation, taking into account the potential for variances within environments and correlations across them to respond simultaneously. If parental environment does indeed alter genetic expression in offspring, failing to account for its effects could lead us to misjudge adaptive potential, and thereby misjudge the vulnerability of populations facing rising environmental stress under global change.
Here, we used the native marine tubeworm, Galeolaria caespitosa (henceforth described by genus name), to examine how parental exposure to projected ocean warming affects adaptive potential, in terms of genetic variation for survival during the most vulnerable life stage. Galeolaria is a habitat-forming ecosystem engineer on rocky shores of southeast Australia, where its dense colonies of calcareous tubes support endemic communities that cannot otherwise persist there [24]. Typical of marine invertebrates that are sessile as adults but have planktonic gametes and larvae, the viability of early life stages is the main bottleneck in the life cycle, dictating adult abundances. Our past work on Galeolaria showed that thermal environments of parents alter mean offspring survival [25], and that thermal environments of offspring alter genetic variation for offspring survival [16]. Here, we ask whether parental exposure to projected ocean warming alters levels of additive and nonadditive genetic variation for offspring survival at current and projected temperatures, plus genetic correlations for survival across temperatures. Our findings provide novel insights into the interplay of plasticity and adaptation in the probability of population persistence under global change.
2. Material and methods
(a). Study species and collection site
Galeolaria is an external fertilizer that spawns large numbers of gametes continuously year-round with no defined breeding season: at any time, populations contain spawned adults, ripe adults and adults with gametocytes at different stages of development [26]. Duration of gametogenesis is unknown, but spawned adults ripen new gametes in 7–10 days in closely related species [27]. Such reproductive biology gives ample scope for parental environments, and temperature especially, to influence offspring fitness through effects on developing gametes.
We collected adult Galeolaria (the parents in our study) from an intertidal population on pier pylons at Brighton Marina, Victoria, Australia. Here, sea-surface temperature has ranged from 9 to 25°C over the last decade, averaging approximately 16.5°C annually and approximately 20.5°C in summer [28]. Since our breeding design consisted of replicate superblocks (see figure 1 and below), we collected adults for one superblock at a time from January to April 2016, when seasonal variation in temperature is minimized [28]. Nonetheless, temperature ranged from 18 to 23°C across the period, which we accounted for by including superblock in statistical analyses (see below). Adults were transferred in insulated aquaria to Monash University, held for 2 h at temperatures matching those in nature on the day of collection, then ramped to the temperature treatments detailed below over another 24 h.
Figure 1.
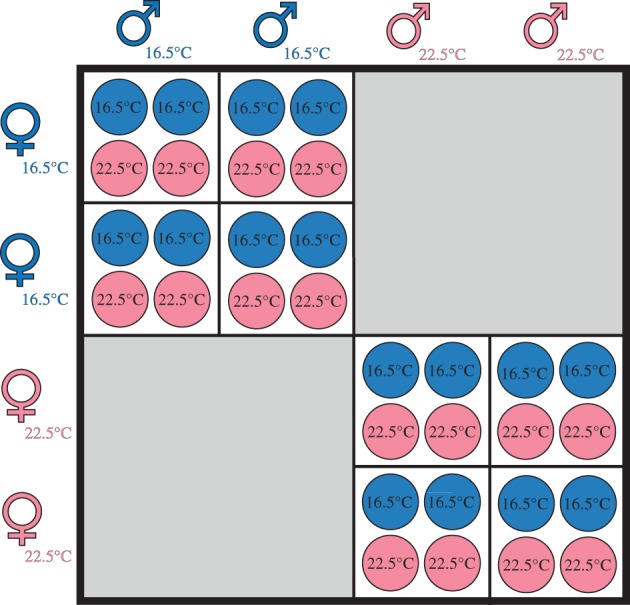
North Carolina II breeding design. Gametes collected from two sires and two dams per parental temperature (16.5 or 22.5°C) were crossed as shown, with each cross replicated four times. Embryos were then reared at either 16.5 or 22.5°C, such that each cross was replicated twice per rearing temperature. (Online version in colour.)
(b). Temperature treatment of parents
Parent Galeolaria were either non-warmed or warmed to an elevated temperature representing future conditions in coastal waters. To do so, non-warmed parents were held in seawater tanks at the annual mean sea-surface temperature (16.5°C), while warmed parents were held at a relatively stressful temperature (22.5°C) based on projected regional increases of approximately 2°C above the current summer mean by 2050, and approximately 3°C higher by 2070 [29,30]. This temperature is already exceeded during rare, extreme events, but is above the normal conditions where the vast majority of Galeolaria's breeding occurs [26] and is projected to persist long enough (weeks or months) in future to act as a reliable cue for transgenerational plasticity [5].
We held adults at approximately 1°C of their nominal temperatures (checked daily) for 14 days, reflecting thermal predictability at our collection site based on data loggers recently deployed there [25]. Water temperatures are uncorrelated beyond this period [25], limiting the capacity of parents to predict the thermal environments of offspring and influence offspring fitness through plasticity [5]. Nonetheless, a 14 day change in parental temperature can affect continuously developing gametes, and adults in our study were probably at similar stages of gametogenesis based on similar ripeness at the time of use. We did not simulate diurnal temperature variation because it is relatively small (approx. 0.7°C s.d. of the daily mean at our collection site) and too transient for parents to respond to [5]. To disentangle parental and tank effects, we split the parents used for each superblock across two tanks per temperature treatment and cross-fertilized individuals from different tanks.
(c). Gamete collection and fertilization protocol
After the treatment period, we collected parents' gametes by extracting each individual from its tube into a dish of seawater. Galeolaria release eggs or sperm soon after extraction, allowing gametes to be collected for use in fertilization crosses. All seawater for gamete collection was filtered and pasteurized.
To ensure a constant temperature for all crosses, we brought vials of gametes to an intermediate temperature of 19.5°C (±0.2°C) over approximately 30 min using mini dry-bath heaters [31]. This required ramping gametes from non-warmed parents up 3°C, and ramping those from warmed parents down 3°C, but we previously showed this did not affect Galeolaria's fertilization success under the same conditions [25]. We used this protocol because variation in fertilization environment can influence offspring traits in external fertilizers [32,33] and other small ectotherms [34,35]. Controlling this variation was, therefore, necessary to isolate the effects of parental environment on offspring survival here, but meant that other effects of fertilization environment could not be assessed. Once at 19.5°C, we diluted each male's sperm to approximately 5 × 105 cells ml−1, which optimized fertilization success in a pilot study. Since density of the limiting gamete has little influence on this success [36], we simply diluted each female's eggs to 1.0 ml, yielding densities of approximately 700–1500 eggs ml−1. Pilot data showed that fertilization success is unaffected by variation in egg density within this range (test of success across 100–4000 eggs ml−1: F1,16 = 0.51, p = 0.49).
We initiated each cross by adding 0.3 ml of sperm solution to 0.1 ml of egg solution, doing so three times at 5 min intervals to increase fertilization success and limit the risk of polyspermy [37]. Gamete solutions were left for 50 min, then rinsed twice through 0.25 µm Nitex mesh to remove sperm.
(d). Quantitative genetic breeding design
Using the protocol above, we subdivided and crossed the gametes of parents in a cross-classified North Carolina II (NCII) breeding design [9], with sperm from each male (sire) crossed with eggs from multiple females (dams) and vice versa. Such designs differ from the nested (NCI) ones often used to estimate genetic parameters by separating additive genetic variance (based on the covariance of paternal half-siblings) and nonadditive genetic variance (the covariance of full-siblings less those of paternal and maternal half-siblings) from maternal and residual variances (see analyses). In turn, the precision of genetic estimates always increases with more sires (which we therefore maximized), but NCII designs can double this precision relative to NCI designs, making the former more powerful [9,38].
We conducted crosses in replicate superblocks, each comprising a block of crosses using non-warmed parents and a block of crosses using warmed parents (figure 1). Parents were not crossed between temperature treatments owing to logistic constraints and because mates are likely to have similar thermal histories in nature, with previous studies suggesting most fertilizations occur between individuals within centimetres to metres of each other [37,39,40]. Within each block, sperm from two sires were crossed with eggs from two dams, yielding four families per block and eight families per superblock (figure 1). Each cross was replicated four times (using embryos from an independent fertilization), allowing two replicates per cross to be subsequently assigned to each of two offspring temperatures (see below). Each superblock, therefore, comprised 32 independent fertilizations (figure 1) and our experiment had 25 superblocks overall. Owing to a small number of inviable crosses, this yielded offspring from 87 families with non-warmed parents (44 sires and 44 dams), and 86 families with warmed parents (47 sires and 49 dams).
(e). Assays of offspring survival at parental treatment temperatures
Next, we reared offspring at the same temperatures as parents (16.5 or 22.5°C; figure 1). Embryos began cleaving approximately 90 min after fertilization, at which point we pipetted approximately 30 cleaved embryos per replicate cross (photographed beforehand on a glass slide at 40× magnification to recover their exact number) into a 1.5 ml vial of seawater. The tiny size of Galeolaria embryos (approx. 60 µm in diameter [41]) meant they were unlikely to become oxygen-limited at this volume. Pilot work also found embryos had negligible effect on oxygen concentrations under our experimental conditions (test of dissolved oxygen with and without embryos present; F1,8 = 0.07, p = 0.80).
Two vials per family were haphazardly assigned to each rearing temperature, and ramped to that temperature over 30 min using mini dry-bath heaters. Embryos were held within 0.2°C of nominal temperatures (per heater specifications; [31]) throughout a 48 h development period, before 0.1 ml of Lugol's solution was added to each vial to fix and stain the contents for counting. This period allowed sufficient time to assess whether embryos survived development and successfully hatched into trochophore larvae capable of swimming and feeding independently in the plankton, identified by previous studies as the most sensitive and reliable indicator of stress tolerance in Galeolaria [42]. More broadly, embryos and larvae are the life stages most sensitive to stress in marine invertebrates, and are key for assessing vulnerability to ocean warming [42,43]. Our measure of survival to this point is thus an ecologically-relevant measure of fitness in Galeolaria, and was ultimately scored for greater than 20 000 offspring (approx. 30 × 2 vials × 2 rearing temperatures for 173 families).
(f). Statistical analyses
We used restricted maximum likelihood (REML) and Bayesian approaches to estimate genetic matrices containing the variances of, and covariance between, offspring survival across rearing temperatures, and compare matrices across parental temperatures. Survival data were standardized to a variance of 1 by parental treatment, giving them a common scale for analysis.
(i). Genetic matrices for offspring from warmed and non-warmed parents
We started by analysing offspring survival in a multivariate animal model, fitted via REML in ASReml-R 3.0 [44]. An animal model is a linear mixed-effects model that estimates additive genetic effects on quantitative traits from pedigree information [45], constructed here from parental identities in NCII crosses. Our model also included family (sire × dam interaction) and dam identities to estimate nonadditive genetic and maternal effects on survival, respectively. Sire × dam interaction was assumed to estimate nonadditive genetic effects only, because replicate vials of siblings never shared a common environment [46]. Specifically, the model was:
where X was the design matrix for the fixed effects (B) of offspring and parental temperatures, while Za,
Zna, Zm and Zb were design matrices for the random effects estimating additive genetic variance 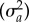 , nonadditive genetic variance
, nonadditive genetic variance 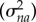 , maternal variance
, maternal variance 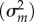 , and superblock variance
, and superblock variance 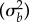 . The latter was modelled as a single variance (electronic supplementary material, table S1), while each of the other random effects (and the residual, ɛ) was initially modelled as a block-diagonal covariance matrix, containing a separate matrix per parental temperature. Each parental matrix contained the variances of, and covariance between, offspring survival across rearing temperatures. We tested the significance of all variance components using likelihood ratio tests, and removed maternal covariances from the final model without significant loss of fit (see the electronic supplementary material, table S1 for maternal variances).
. The latter was modelled as a single variance (electronic supplementary material, table S1), while each of the other random effects (and the residual, ɛ) was initially modelled as a block-diagonal covariance matrix, containing a separate matrix per parental temperature. Each parental matrix contained the variances of, and covariance between, offspring survival across rearing temperatures. We tested the significance of all variance components using likelihood ratio tests, and removed maternal covariances from the final model without significant loss of fit (see the electronic supplementary material, table S1 for maternal variances).
(ii). Comparisons of genetic matrices between parental temperatures
Next, we used two complementary approaches to explore the multivariate structures of genetic matrices and their responses to parental temperature (see figure 2 for a conceptual overview). First, we used a form of eigenanalysis, termed factor-analytic modelling, to identify how many independent dimensions (eigenvectors) occurred in each matrix, and how their variances (eigenvalues) were distributed (see details in [47]). By fitting these dimensions within our animal model [44], we could directly test how many were required to explain genetic effects on survival across offspring rearing temperatures, and do so separately by parental temperature.
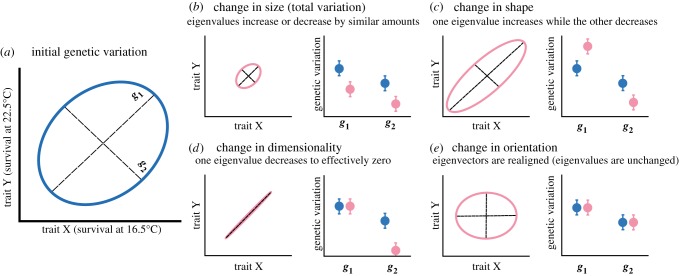
Figure 2.
(a–e) Changes in the multivariate structure of genetic variation. (a) The initial structure for two traits, X and Y (survival at 16.5°C and survival at 22.5°C). Genetic variation has two independent dimensions, g1 and g2, which are diagonal to plot axes because X and Y are genetically correlated. Each dimension has a direction (or eigenvector, representing a linear combination of X and Y) and a length (or eigenvalue, representing the amount of variation for that direction). If different parental temperature (with non-warmed parents in blue and warmed parents in pink) causes different alleles to affect X and Y, or the same alleles to affect them differently, this structure could change in several ways (b–e), with right-hand panels indicating what tensor analyses would show if initial and changed structures were compared. (b) The total variation changes owing to similar decreases in both eigenvalues (e.g. if alleles affecting both traits are lost or allele effects become more uniform, with parental warming). (c) Variation is re-distributed toward g1 and away from g2 (e.g. if the genetic correlation between traits grows stronger with parental warming). (d) Variation for g2 declines to the point where it is effectively zero. (e) The eigenvectors are reoriented owing to changes in the relationship between underlying traits (e.g. if parental warming changes allele effects more for one trait than the other), but eigenvalues are unchanged. (Online version in colour.)
Second, we used genetic covariance tensors to explore how parental temperature affected aspects of matrix structure other than dimensionality (figure 2b–e). Tensors compare any number of matrices (see [48] and [49]) but here, with only two parental temperatures, the comparison amounts to an eigenanalysis of E, the matrix of pairwise differences between genetic estimates. Eigenvectors (e1 and e2) of E are the dimensions that differentiate the original matrices, and corresponding eigenvalues are the amounts of genetic variation for those dimensions. For example, if matrices differ in size (total variation), both eigenvalues of e1 and e2 may be non-zero and similar in sign, meaning variation increased or decreased for both matrix dimensions (figure 2b). If matrices differ in shape (distribution of variation), both eigenvalues may be non-zero and different in sign, meaning variation increased for one matrix dimension and decreased for the other (figure 2c). If matrices differ in orientation, survival at each rearing temperature may contribute differently to e1 and/or e2, meaning a change in trait relationships across temperatures (figure 2e).
To facilitate tensor analyses, we re-fitted our animal model in a Bayesian framework, using the MCMCglmm package of R [50] to sample the marginal posterior distributions of genetic parameters. We used weakly informative inverse-Wishart priors (parameter-expanded priors were explored but gave similar results), with scale parameters defined as diagonal matrices containing values of one-fifth of the total variance in offspring survival per rearing temperature, and distribution parameters set to 0.001 for degrees of freedom [50]. Posterior distributions came from 220 000 Markov chain Monte Carlo (MCMC) iterations sampled every 200 iterations after an initial burn-in of 20 000 iterations. We checked convergence from plots of traces and posterior distributions, and autocorrelations between samples (all were below the recommended level of 0.1, yielding effective sample sizes close to 1000 for all parameters).
We applied the tensor analyses to our 1000 MCMC samples using the R routine in [49], modified to also compare nonadditive genetic matrices. Briefly, this routine compared the observed E to a null model constructed by randomizing additive (or nonadditive) genetic effects on offspring survival across parental temperatures. We then inferred whether parental temperature significantly altered these genetic effects from the posterior probability (the proportion of samples where differences in the observed E exceeded those under the null model; [51]) and 95% highest posterior density (HPD) intervals. Finally, to visualize how parental temperature altered these variances, we projected the eigenvectors (e1 and e2) of the observed E onto original matrices to find the relative amounts of variation in those directions.
3. Results
(a). Effects of parental temperature on mean offspring survival across rearing temperatures
We detected interactive effects of parental and offspring temperatures on mean offspring survival (F1,68 = 50.7, p < 0.05), which was approximately 6% higher when offspring were reared at the temperature of their parents than when they were not (figure 3).
Figure 3.
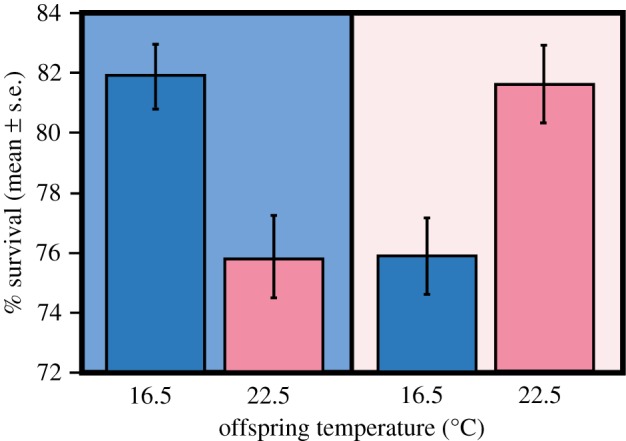
Mean (±s.e.) survival of offspring from non-warmed parents (blue background, left) and warmed parents (pink background, right) at each offspring rearing temperature. (Online version in colour.)
(b). Effects of parental temperature on genetic variation for offspring survival across rearing temperatures
Inspecting each genetic matrix implied that additive genetic variance was lower when parents were exposed to higher temperature (table 1a), whereas offspring rearing temperature had little effect on this variance (table 1a). Notably, additive genetic covariance across rearing temperatures was non-significant for offspring of non-warmed parents, but became significantly positive for offspring of warmed parents (table 1a).
Table 1.
Matrices of additive and nonadditive genetic variances (diagonal elements, in bold) and cross-environment covariances (below-diagonal elements) for survival of offspring reared at current (16.5°C) and projected (22.5°C) water temperatures. (Offspring of non-warmed parents are in blue (left) and offspring of warmed parents are in pink (right). The standard error of each estimate is in brackets; *p < 0.05. (Online version in colour.))
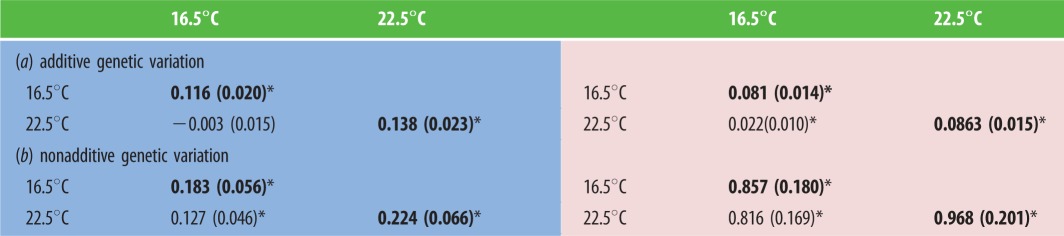 |
Parental exposure to warming substantially increased the nonadditive genetic variance for offspring survival. At both rearing temperatures, this variance was roughly four-fold greater for offspring of warmed parents relative to offspring of non-warmed parents (table 1b). It was also marginally greater when offspring were reared at a warmer temperature (table 1b). Nonadditive genetic covariance across rearing temperatures was positive regardless of parental treatment, but increased six-fold when parents were exposed to warming (table 1b).
(c). Comparisons of genetic matrices between parental temperatures
Our comparisons of these genetic matrices showed that parental temperature altered their multivariate structures. Since offspring were reared at two (current and projected) temperatures, each matrix could have up to two dimensions, depending on the amounts of genetic variance for survival at each temperature and covariance for survival across temperatures. For offspring of non-warmed parents, additive genetic variance was distributed quite evenly across two statistically supported dimensions that each associated strongly with survival at one rearing temperature (per figure 2e)—the first dimension with survival at 22.5°C, and the second dimension with survival at 16.5°C (table 2a). By contrast, offspring from warmed parents had effectively only one dimension of additive genetic variance (per figure 2d), with positive contributions from survival at both temperatures (table 2a). Dimensionality is reduced by lower variance or stronger covariance [52], and parental exposure to warming did both these things (table 1a), lowering additive genetic variance for survival at each rearing temperature, while strengthening covariance for survival across them. Hence, parental exposure to elevated temperatures may affect adaptive capacity by reducing the additive genetic variance on which it depends, but compensate by making adaptation to current temperatures facilitate adaptation to future ones.
Table 2.
Eigenanalyses of additive and nonadditive genetic matrices for survival of offspring at current (16.5°C) and projected (22.5°C) water temperatures. (Offspring from non-warmed parents are in blue (left) and offspring from warmed parents are in pink (right). Offspring from warmed parents had only one dimension (eigenvector) of additive genetic variance, while all other matrices had two dimensions of genetic variance (likelihood ratio tests identified significant loss of model fit if dimensionality was further reduced). Loadings on each eigenvector show the contribution from offspring survival at each rearing temperature, while eigenvalues show the amount of genetic variance for each eigenvector. (Online version in colour.))
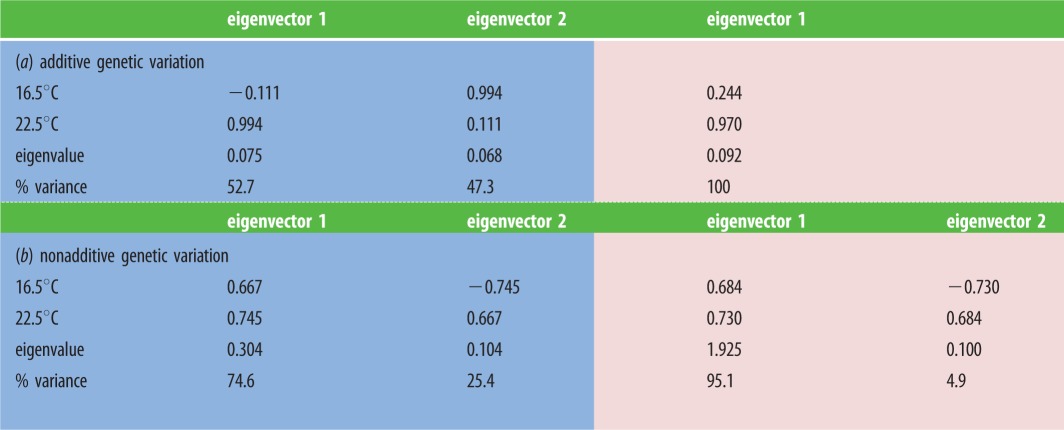 |
Parental exposure to warming also re-distributed the nonadditive genetic variance for offspring survival across rearing temperatures toward one dimension (per figure 2c), but without loss of dimensionality (because factor-analytic modelling also offered statistical support for the other, lesser dimension; table 2b). For offspring of non-warmed parents, 75% of nonadditive variance lay in a dimension with positive, evenly weighted contributions from survival at both rearing temperatures, while the other 25% lay in a dimension with opposing contributions. For offspring of warmed parents, nonadditive genetic variance remained associated with two dimensions that were very similar to those above (table 2b), but variance for the largest dimension rose to 95%. Hence, parental exposure to warming reshaped nonadditive genetic variance to enhance positive covariation between the compatibilities of parental genomes across current and future temperatures.
When genetic matrices were compared using tensors, parental temperature had no detectable effect on the additive genetic variance for offspring survival across rearing temperatures (observed differences between additive matrices often overlapped differences under the null model: p = 0.124, electronic supplementary material, figure S1a). This result in no way opposes our results from factor-analytic modelling, since Bayesian comparisons constrain matrices to be full rank [49] and cannot test changes in dimensionality. The tensor comparison did, however, detect a significant effect of parental temperature on the nonadditive genetic variance for survival (observed and null differences between nonadditive matrices did not overlap: p < 0.001; electronic supplementary material, figure S1b). The leading dimension of the tensor (e1, explaining 99.8% of differences between original matrices; table 3) had positive, even contributions from survival at both offspring temperatures. Projecting this dimension (and the lesser one, e2) onto each original matrix showed that parental exposure to warming significantly increased nonadditive genetic variance for e1 alone (figure 4). Overall, this supports the impression from factor-analytic modelling that warming re-distributed the nonadditive genetic variance for survival in line with figure 2c.
Table 3.
Dimensions (or eigenvectors, e1 and e2) of the tensor capturing the effects of parental temperature on the nonadditive genetic variation for offspring survival. (Loadings on each dimension show the contributions from survival when reared at current (16.5°C) and projected (22.5°C) water temperatures.)
| e1 | e2 | |
|---|---|---|
| 16.5°C | 0.686 | −0.727 |
| 22.5°C | 0.727 | 0.686 |
Figure 4.
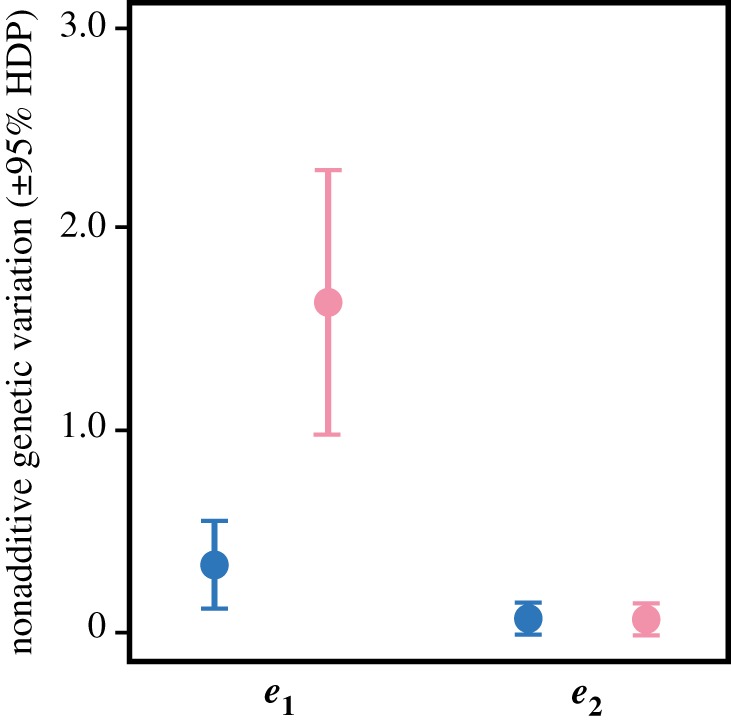
Effect of parental temperature on the nonadditive genetic variation for survival. Variation for e1 (the leading dimension of the tensor, capturing 99.8% of differences between original matrices) is greater for offspring from warmed parents (pink, right) than offspring from non-warmed parents (blue, left; note the lack of overlap between HPD intervals). Variation for e2 was unaffected by parental temperature. (Online version in colour.)
4. Discussion
Populations may respond to environmental change via plasticity and/or adaptation [1]. While these processes are predicted to interact [7], few experimental studies have tested how they may do so. Here, we examined how exposing parents to projected ocean warming altered the survival, and genetic variance for survival, of offspring during their most vulnerable life stage under current and projected temperatures. Offspring survival was higher when parent and offspring temperatures matched. Across offspring temperatures, moreover, parental exposure to warming altered the distribution of additive genetic variance, and increased levels of nonadditive genetic variance linked to the compatibilities of parental haplotypes. These results show that parental environments may potentially have broader-ranging effects on adaptive capacity under global change than currently appreciated.
Despite long-standing interest in the idea that parental environments may prime offspring to cope with similar conditions, evidence remains equivocal. Notable cases reporting adaptive parental effects of this kind [4,53], are countered by others where stressed parents simply yield poor-quality offspring with impaired tolerance of the same stressor [25,54]. When they occur, moreover, adaptive parental effects are often subtle [6]. Our results for Galeolaria support this observation, with parental exposure to projected warming buffering offspring against this stress (i.e. offspring from warmed parents survived just as well at 22.5°C as offspring from non-warmed parents at 16.5°C), but this stress only reduced absolute survival by 6%. Notably, our results differ from previous work on Galeolaria [25] where parental temperature had inconsistent effects on offspring survival. However, our sample sizes doubled those of the previous study [25], suggesting that evidence for adaptive parental effects may remain equivocal because previous tests lacked sufficient power to detect them [6].
While adaptive parental effects were subtle here, even this modest buffering may help natural populations maintain large enough sizes to persist under rising thermal stress, buying time for evolutionary rescue (which lowers extinction risk by restoring absolute fitness) to take effect [55]. Alternatively, such buffering could eliminate the need for rescue altogether as long as the loss of absolute fitness owing to thermal stress is fully restored [8]. This is a subtle, but important, distinction to the idea that plasticity in traits underlying relative fitness may limit the evolution of those traits by weakening selection on them [56,57], which ignores absolute fitness and takes persistence for granted [55]. The key issue now is whether transgenerational buffering will be equally effective under more extreme conditions, or even disadvantageous in the longer term—for instance, if conditions that induce it are not met, suddenly exposing buffered populations to severe stress [7].
Transgenerational effects on the genetic variation required for adaptation remain poorly understood [7]. Past work indicates that additive genetic variance for single traits is amplified by parental exposure to stress [17,23], or by mismatches between parental and offspring environments [22]. Here, if anything, parental exposure to thermal stress weakly reduced additive genetic variance for offspring survival at current and future temperatures, regardless of whether parental and offspring environments matched. We also build on past work by analysing parental effects on the multivariate structure of additive genetic variation [47]. These analyses revealed that exposing parents to warmer temperature not only reduced additive genetic variation for survival, but limited its distribution to a single dimension defined by positive genetic covariation for survival across current and future temperatures. Thus, loss of variance to fuel thermal adaptation may be offset by greater tendency for alleles that benefit survival at current temperatures to also do so at future ones. As such, our study population may progressively adapt to future warming using standing stocks of alleles with positive effects on survival under current-day conditions [8].
To our knowledge, ours is also the first study to show that parental environment not only alters, but increases, nonadditive genetic variance for any component of fitness. While nonadditive gene action is seemingly common in nature, its contribution to nonadditive genetic variance is assumed to be small and of limited evolutionary value [13]. Yet in breeding designs like ours, such variance manifests as sire × dam interaction (signalling variation in the compatibilities of parental haplotypes; [10]) and often increases with stress [58,59]. Here, exposing parents to thermal stress increased this variance more than four-fold, while stress at rearing increased it by another approximately 20%. However, the consequences of such increases are uncertain in the absence of clear links to theory ([11], but see [60]). At the very least, future warming may make evolutionary dynamics less predictable, if fitness increasingly depends on allele combinations shuffled by random segregation and recombination in parents [10]. Given growing evidence of nonadditive genetic effects on adaptation [12,60,61], and new or underused ways of linking them to the variances measured here [62], understanding their role in a warming world warrants further attention.
Adaptation requires genes to stably affect phenotypes from one generation to the next [9]. While individuals in our study population will probably experience persistent warming in future years [29], they may also experience periodic rises in water temperatures lasting weeks to months. In such cases, the population-wide impacts of parental effects will depend on how long they last within and across generations, which is unknown for Galeolaria. Past work has shown that plastic responses to stress affect some traits irreversibly, whereas others eventually revert to their original states once that stress eases [63]. Whether our manipulation of parents captured the full extent of their effects on offspring is also unclear, because environmental exposure at earlier life stages, or repeated across stages, has important phenotypic consequences in other species [34]. Hence, testing the duration and extent of environmental exposure in parents and offspring (including effects on other fitness components, like reproduction, later in the life cycle) is a key future step. Regardless, the transgenerational effects of parental environment may not need to be entirely stable to have evolutionary consequences. In theory, at least, even transient effects on offspring can produce major shifts in evolutionary dynamics and outcomes [64].
A key question unanswered by our study is how temperature at fertilization may affect adaptive capacity through genetic effects on offspring. Here, we had to control this temperature in order to isolate parental effects on offspring, but previous work indicates that phenotypically at least, offspring can be sensitive to environmental conditions at this life stage [32,33,43]. In the oyster Saccostrea glomerata, for instance, thermal stress was more detrimental to embryos when applied before fertilization than afterwards [33], and gamete exposure to stress could affect Galeolaria embryos in a similar way. To our knowledge, however, genetic effects of fertilization environment on offspring remain untested and are a key step for future studies.
In conclusion, we show that parental environment can potentially influence adaptation to projected future warming, not only by altering mean offspring fitness but also by altering the genetic variance for fitness. Yet phenomenological approaches like ours cannot identify the mechanistic basis of parental effects like those seen here. Recently, how various forms of non-genetic inheritance (e.g. epigenetic mechanisms including DNA methylation and microRNAs) contribute to plasticity within and across generations has been much-debated [65], and resolving this debate remains a priority. More work is also needed to dissect the thermal sensitivity of genetic effects on fitness, potentially involving interactions between nuclear and cytoplasmic genes [66] or, in external fertilizers like Galeolaria, effects on gametogenesis and/or fertilization dynamics [25,67]. Regardless of the underlying mechanisms, however, our study represents an important step towards understanding how plasticity and adaptation jointly shape population dynamics and extinction risk under global change.
Acknowledgements
We thank Henry Wootton and Annie Guillaume for assistance collecting data, Loeske Kruuk and Matt Hall for advice on analyses and Julian Beaman, Pieter Arnold, Sasha Mikheyev, Amanda Pettersen and Hayley Cameron for helpful discussions.
Ethics
All applicable international, national, and institutional guidelines were followed.
Data accessibility
The data used for this study have been deposited in Dryad as: http://dx.doi.org/10.5061/dryad.7fh10v7 [68].
Authors' contribution
All authors conceived and designed the study. E.C. collected the data. E.C. and K.M. performed analyses and drafted the manuscript. All authors contributed to revisions.
Competing interests
We have no competing interests.
Funding
The study was supported by grants and fellowships awarded under the Australian Research Council's Discovery Scheme.
References
- 1.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merila J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178. ( 10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 2.Chevin L-M, Collins S, Lefèvre F. 2013. Phenotypic plasticity and evolutionary demographic responses to climate change: taking theory out to the field. Funct. Ecol. 27, 967–979. ( 10.1111/j.1365-2435.2012.02043.x) [DOI] [Google Scholar]
- 3.Sgrò CM, Terblanche JS, Hoffmann AA. 2016. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 61, 433–451. ( 10.1146/annurev-ento-010715-023859) [DOI] [PubMed] [Google Scholar]
- 4.Agrawal AA, Laforsch C, Tollrian R. 1999. Transgenerational induction of defences in animals and plants. Nature 401, 60–63. ( 10.1038/43425) [DOI] [Google Scholar]
- 5.Burgess SC, Marshall DJ. 2014. Adaptive parental effects: the importance of estimating environmental predictability and offspring fitness appropriately. Oikos 123, 769–776. ( 10.1111/oik.01235) [DOI] [Google Scholar]
- 6.Uller T, Nakagawa S, English S. 2013. Weak evidence for anticipatory parental effects in plants and animals. J. Evol. Biol. 26, 2161–2170. ( 10.1111/jeb.12212) [DOI] [PubMed] [Google Scholar]
- 7.Donelson JM, Salinas S, Munday PL, Shama LNS. 2018. Transgenerational plasticity and climate change experiments: where do we go from here? Global Change Biol. 24, 13–34. ( 10.1111/gcb.13903) [DOI] [PubMed] [Google Scholar]
- 8.Bell G. 2013. Evolutionary rescue and the limits of adaptation. Proc. R. Soc. B 368, 20120080 ( 10.1098/rstb.2012.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch M, Walsh B. 1998. Genetics and the analysis of quantitative traits. Sunderland, MA: Sinauer. [Google Scholar]
- 10.Puurtinen M, Ketola T, Kotiaho JS. 2009. The good-genes and compatible-genes benefits of mate choice. Am. Nat. 174, 741–752. ( 10.1086/606024) [DOI] [PubMed] [Google Scholar]
- 11.Hansen TF. 2015. Measuring gene interactions. In Epistasis: methods and protocols (eds Moore JH, Williams SM), pp. 115–143. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 12.Carroll SP, Dingle H, Famula TR. 2003. Rapid appearance of epistasis during adaptive divergence following colonization. Proc. R. Soc. Lond. B 270, S80–S83. ( 10.1098/rsbl.2003.0019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill WG, Goddard ME, Visscher PM. 2008. Data and theory point to mainly additive genetic variance for complex traits. PLoS Genet. 4, e1000008 ( 10.1371/journal.pgen.1000008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowinski PK, Rogell B. 2017. Environmental stress correlates with increases in both genetic and residual variances: a meta-analysis of animal studies. Evolution 71, 1558–5646. ( 10.1111/evo.13201) [DOI] [PubMed] [Google Scholar]
- 15.Kelly MW, Padilla-Gamiño JL, Hofmann GE. 2013. Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Global Change Biol. 19, 2536–2546. ( 10.1111/gcb.12251) [DOI] [PubMed] [Google Scholar]
- 16.Chirgwin E, Monro K, Sgrò CM, Marshall DJ. 2015. Revealing hidden evolutionary capacity to cope with global change. Global Change Biol. 21, 3356–3366. ( 10.1111/gcb.12929) [DOI] [PubMed] [Google Scholar]
- 17.Munday PL, Donelson JM, Domingos JA. 2017. Potential for adaptation to climate change in a coral reef fish. Global Change Biol. 23, 307–317. ( 10.1111/gcb.13419) [DOI] [PubMed] [Google Scholar]
- 18.Kellermann V, van Heerwaarden B, Sgrò CM, Hoffmann AA. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325, 1244–1246. ( 10.1126/science.1175443) [DOI] [PubMed] [Google Scholar]
- 19.Teplitsky C, et al. 2014. Assessing multivariate constraints to evolution across ten long-term avian studies. PLoS ONE 9, e90444 ( 10.1371/journal.pone.0090444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agrawal AF, Stinchcombe JR. 2009. How much do genetic covariances alter the rate of adaptation? Proc. R. Soc. B 276, 1183–1191. ( 10.1098/rspb.2008.1671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Via S, Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522. ( 10.2307/2408649) [DOI] [PubMed] [Google Scholar]
- 22.Sgrò CM, Hoffmann AA. 1998. Effects of temperature extremes on genetic variances for life history traits in Drosophila melanogaster as determined from parent-offspring comparisons. J. Evol. Biol. 11, 1–20. ( 10.1046/j.1420-9101.1998.11010001.x) [DOI] [Google Scholar]
- 23.Sgrò CM, Hoffmann AA. 1998. Effects of stress combinations on the expression of additive genetic variation for fecundity in Drosophila melanogaster. Genet. Res. 72, 13–18. ( 10.1017/s0016672398003310) [DOI] [PubMed] [Google Scholar]
- 24.Cole VJ, Hutchings PA, Ross PM. 2017. Predicting biodiversity changes due to loss of bioengineers from an intertidal landscape, a case study from Sydney Harbour. Aust. Zool. 39, 194–206. ( 10.7882/az.2015.034) [DOI] [Google Scholar]
- 25.Guillaume AS, Monro K, Marshall DJ. 2016. Transgenerational plasticity and environmental stress: do paternal effects act as a conduit or a buffer? Funct. Ecol. 30, 1175–1184. ( 10.1111/1365-2435.12604) [DOI] [Google Scholar]
- 26.Kupriyanova EK. 2006. Fertilization success in Galeolaria caespitosa (Polychaeta: Serpulidae): gamete characteristics, role of sperm dilution, gamete age, and contact time. Sci. Mar. 70, 309–317. ( 10.3989/scimar.2006.70s3309) [DOI] [Google Scholar]
- 27.Kupriyanova EK, Nishi E, Ten Hove HA, Rzhavsky AV. 2001. Life-history patterns in serpulimorph polychaetes: ecological and evolutionary perspectives. In Oceanography and marine biology, vol. 39 (eds Gibson RB, Barnes M, Atkinson RJA), pp. 1–101. London, UK: Taylor & Francis Ltd. [Google Scholar]
- 28.CSIRO. 2017. CSIRO marine and atmospheric research. See http://www.cmar.csiro.au
- 29.Mills R, Womersley T, Hobday A. 2013. Implications of future climate for physicochemical conditions. In Implications of future climate for Victoria’s marine environment, ch. 1 (eds Klemke J, Arundel H), Victoria, Australia: Glenelg Hopkins Catchment Management Authority. [Google Scholar]
- 30.Hobday AJ, Lough JM. 2011. Projected climate change in Australian marine and freshwater environments. Mar. Freshw. Res. 62, 1000–1014. ( 10.1071/mf10302) [DOI] [Google Scholar]
- 31.MajorScience. 2015. Mini cooling dry bath incubator instruction manual. See https://www.n-genetics.com/file/IN_MC-0203.pdf, Major Science.
- 32.Ritchie H, Marshall DJ. 2013. Fertilisation is not a new beginning: sperm environment affects offspring developmental success. J. Exp. Biol. 216, 3104–3109. ( 10.1242/jeb.087221) [DOI] [PubMed] [Google Scholar]
- 33.Parker Laura M, Ross Pauline M, O'Connor Wayne A. 2009. The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Global Change Biol. 15, 2123–2136. ( 10.1111/j.1365-2486.2009.01895.x) [DOI] [Google Scholar]
- 34.Kellermann V, van Heerwaarden B, Sgrò CM. 2017. How important is thermal history? Evidence for lasting effects of developmental temperature on upper thermal limits in Drosophila melanogaster. Proc. R. Soc. B 284, 20170447 ( 10.1098/rspb.2017.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann AA, Sgrò CM. 2018. Comparative studies of vulnerability to environmental extremes in small ectotherms: how much should we control the environment? Integr Zool. 13, 355–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitan DR, Sewell MA, Chia FS. 1991. Kinetics of fertilization in the sea urchin Strongylocentrotus franciscanus: interaction of gamete dilution, age, and contact time. Biol. Bull. 181, 371–378. ( 10.2307/1542357) [DOI] [PubMed] [Google Scholar]
- 37.Hollows CF, Johnston EL, Marshall DJ. 2007. Copper reduces fertilisation success and exacerbates Allee effects in the field. Mar. Ecol. Prog. Ser. 333, 51–60. ( 10.3354/meps333051) [DOI] [Google Scholar]
- 38.Pederson DG. 1972. A comparison of four experimental designs for the estimation of heritability. Theoret. Appl. Genet. 42, 371–377. [DOI] [PubMed] [Google Scholar]
- 39.Pennington JT. 1985. The ecology of fertilization of echinoid eggs: the consequences of sperm dilution, adult aggregation, and synchronous spawning. Biol. Bull. 169, 417–430. ( 10.2307/1541492) [DOI] [PubMed] [Google Scholar]
- 40.Marshall DJ. 2002. In situ measures of spawning synchrony and fertilization success in an intertidal, free-spawning invertebrate. Mar. Ecol. Prog. Ser. 236, 113–119. [Google Scholar]
- 41.Monro K, Marshall DJ. 2016. Unravelling anisogamy: egg size and ejaculate size mediate selection on morphology in free-swimming sperm. Proc. R. Soc. B 283, 20160671 ( 10.1098/rspb.2016.0671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross KE, Bidwell JR. 2001. A 48-h larval development toxicity test using the marine polychaete Galeolaria caespitosa lamarck (fam. Serpulidae) . Arch. Environ. Contam. Toxicol. 40, 489–496. [DOI] [PubMed] [Google Scholar]
- 43.Byrne M. 2011. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. In Oceanography and marine biology: an annual review, vol. 49 (eds Gibson RN, Atkinson RJA, Gordon JDM), pp. 1–42. Boca Raton, FL: CRC Press. [Google Scholar]
- 44.Butler D, Cullis BR, Gilmour AR, Gogel BJ. 2007. ASReml-R reference manual. Brisbane, The State of Queensland, Australia: Department of Primary Industries and Fisheries. [Google Scholar]
- 45.Kruuk LEB. 2004. Estimating genetic parameters in natural populations using the ‘animal model’. Proc. R. Soc. Lond. B 359, 873–890. ( 10.1098/rstb.2003.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kruuk LEB, Hadfield JD. 2007. How to separate genetic and environmental causes of similarity between relatives. J. Evol. Biol. 20, 1890–1903. ( 10.1111/j.1420-9101.2007.01377.x) [DOI] [PubMed] [Google Scholar]
- 47.Hine E, Blows MW. 2006. Determining the effective dimensionality of the genetic variance–covariance matrix. Genetics 173, 1135–1144. ( 10.1534/genetics.105.054627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hine E, Chenoweth SF, Rundle HD, Blows MW. 2009. Characterizing the evolution of genetic variance using genetic covariance tensors. Proc. R. Soc. B 364, 1567–1578. ( 10.1098/rstb.2008.0313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aguirre JD, Hine E, McGuigan K, Blows MW. 2014. Comparing G: multivariate analysis of genetic variation in multiple populations. Heredity 112, 21–29. ( 10.1038/hdy.2013.12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. (doi:10.18637/jss.v033.i02)20808728 [Google Scholar]
- 51.Hadfield JD, Wilson AJ, Garant D, Sheldon BC, Kruuk LEB. 2010. The misuse of BLUP in ecology and evolution. Am. Nat. 175, 116–125. ( 10.1086/648604) [DOI] [PubMed] [Google Scholar]
- 52.Monro K, Marshall DJ. 2014. Faster is not always better: selection on growth rate fluctuates across life history and environments. Am. Nat. 183, 798–809. ( 10.1086/676006) [DOI] [PubMed] [Google Scholar]
- 53.Parker LM, Ross PM, O'Connor WA, Borysko L, Raftos DA, Portner HO. 2012. Adult exposure influences offspring response to ocean acidification in oysters. Global Change Biol. 18, 82–92. ( 10.1111/j.1365-2486.2011.02520.x) [DOI] [Google Scholar]
- 54.Shama LNS, Wegner KM. 2014. Grandparental effects in marine sticklebacks: transgenerational plasticity across multiple generations. J. Evol. Biol. 27, 2297–2307. ( 10.1111/jeb.12490) [DOI] [PubMed] [Google Scholar]
- 55.Bell G. 2017. Evolutionary rescue. Annu. Rev. Ecol. Evol. Syst. 48, 605–627. ( 10.1146/annurev-ecolsys-110316-023011) [DOI] [Google Scholar]
- 56.Ancel LW. 2000. Undermining the Baldwin expediting effect: does phenotypic plasticity accelerate evolution? Theor. Popul. Biol. 58, 307–319. ( 10.1006/tpbi.2000.1484) [DOI] [PubMed] [Google Scholar]
- 57.Price TD, Qvarnstrom A, Irwin DE. 2003. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B 270, 1433–1440. ( 10.1098/rspb.2003.2372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chirgwin E, Marshall DJ, Sgrò CM, Monro K. 2017. The other 96%: can neglected sources of fitness variation offer new insights into adaptation to global change? Evol. Appl. 10, 267–275. ( 10.1111/eva.12447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jinks JL, Jean MP, Pooni HS. 1973. The incidence of epistasis in normal and extreme environments. Heredity 31, 263–269. ( 10.1038/hdy.1973.81) [DOI] [Google Scholar]
- 60.Hill WG. 2017. ‘Conversion’ of epistatic into additive genetic variance in finite populations and possible impact on long-term selection response. J. Anim. Breed. Genet. 134, 196–201. ( 10.1111/jbg.12270) [DOI] [PubMed] [Google Scholar]
- 61.Forsberg SKG, Bloom JS, Sadhu MJ, Kruglyak L, Carlborg O. 2017. Accounting for genetic interactions improves modeling of individual quantitative trait phenotypes in yeast. Nat. Genet. 49, 497–503. ( 10.1038/ng.3800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Rouzic A. 2014. Estimating directional epistasis. Front. Genet. 5, 14 ( 10.3389/fgene.2014.00198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beaman JE, White CR, Seebacher F. 2016. Evolution of plasticity: mechanistic link between development and reversible acclimation. Trends Ecol. Evol. 31, 237–249. ( 10.1016/j.tree.2016.01.004) [DOI] [PubMed] [Google Scholar]
- 64.Day T, Bonduriansky R. 2011. A unified approach to the evolutionary consequences of genetic and nongenetic inheritance. Am. Nat. 178, E18–E36. ( 10.1086/660911) [DOI] [PubMed] [Google Scholar]
- 65.Futuyma DJ. 2017. Evolutionary biology today and the call for an extended synthesis. Interface Focus 7, 20160145 ( 10.1098/rsfs.2016.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dowling DK, Abiega KC, Arnqvist G. 2007. Temperature-specific outcomes of cytoplasmic-nuclear interactions on egg-to-adult development in seed beetles. Evolution 61, 194–201. ( 10.1111/j.1558-5646.2007.00016.x) [DOI] [PubMed] [Google Scholar]
- 67.Alavioon G, Hotzy C, Nakhro K, Rudolf S, Scofield DG, Zajitschek S, Maklakov AA, Immler S. 2017. Haploid selection within a single ejaculate increases offspring fitness. Proc. Natl Acad. Sci. USA 114, 8053–8058. ( 10.1073/pnas.1705601114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chirgwin E, Marshall DJ, Sgrò CM, Monro K. 2018. Data from: How does parental environment influence the potential for adaptation to global change? Dryad Digital Repository. ( 10.5061/dryad.7fh10v7) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Chirgwin E, Marshall DJ, Sgrò CM, Monro K. 2018. Data from: How does parental environment influence the potential for adaptation to global change? Dryad Digital Repository. ( 10.5061/dryad.7fh10v7) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data used for this study have been deposited in Dryad as: http://dx.doi.org/10.5061/dryad.7fh10v7 [68].