Abstract
Abundant expression of proinflammatory cytokines after a spinal cord injury (SCI) creates an inhibitory microenvironment for neuroregeneration. The mesenchymal stem cells help to mitigate the inflammation and improve neural growth and survival. For this purpose, we potentiated the function of adipose-derived mesenchymal stem cells (Ad-MSCs) by transfecting them with brain-derived neurotrophic factor (BDNF) and heme oxygenase-1 (HO-1), through a lentivirus, to produce BDNF overexpressed Ad-MSCs (BDNF-MSCs), and HO-1 overexpressed Ad-MSCs (HO-1-MSCs). Sixteen SCI beagle dogs were randomly assigned into four treatment groups. We injected both HO-1 and BDNF-overexpressed MSCs as a combination group, to selectively control inflammation and induce neuroregeneration in SCI dogs, and compared this with BDNF-MSCs, HO-1-MSCs, and GFP-MSCs injected dogs. The groups were compared in terms of improvement in canine Basso, Beattie, and Bresnahan (cBBB) score during 8 weeks of experimentation. After 8 weeks, spinal cords were harvested and subjected to western blot analysis, immunofluorescent staining, and hematoxylin and eosin (H&E) staining. The combination group showed a significant improvement in hindlimb functions, with a higher BBB score, and a robust increase in neuroregeneration, depicted by a higher expression of Tuj-1, NF-M, and GAP-43 due to a decreased expression of the inflammatory markers interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), and an increased expression of interleukin-10 (IL-10) (P ≤ 0.05). H&E staining showed more reduced intraparenchymal fibrosis in the combination group than in other groups (P ≤ 0.05). It was thus suggested that the cotransplantation of HO-1 and BDNF-MSCs is more effective in promoting the healing of SCI. HO-1-MSCs reduce inflammation, which favors BDNF-induced neuroregeneration in SCI of dogs.
Keywords: BDNF overexpressed MSCs, HO-1 overexpressed MSCs, neuroregeneration, spinal cord injury
Introduction
Primary spinal cord injury (SCI) inflicts neuronal damage and vascular disruption in spinal cord tissues. This proceeds toward a secondary SCI, which, with a series of inflammatory and other pathological events, initiates an expanding zone of destructed tissue with a concomitant inhibition of neuroregeneration1,2.
Following SCI, the expression of brain-derived neurotrophic factor is increased in astrocytes, microglia, and oligodendrocytes3, which is responsible for neuronal survival, growth, and maturation4. Simultaneously, HO-1 expression is induced after SCI, both in gray and white matter, primarily in microglial cells and other glial cells5–7 which have antioxidant and anti-inflammatory properties8,9, and attenuate neutrophil infiltration10 and CD-8 T-cell accumulation11.
Allogenic adipose-derived mesenchymal stem cells (Ad-MSCs) have been found to induce neuroregeneration and neuronal differentiation with improved functional outcomes in dogs with SCI12. The Ad-MSCs are further potentiated by lentivirus-mediated gene editing with neurotrophic factors, for more robust neural regeneration and functional outcomes13. BDNF-MSCs produce excessive amounts of BDNF, which promotes neural regeneration by upregulating neural markers such as Tuj-1, nestin, and NF-M in dogs with SCI14. Similarly, dogs with SCI showed improved functional recovery with intrathecal injections of HO-1-MSCs due to a decreased expression of inflammatory cytokines, such as TNF-α, IL-6, cyclooxygenase-2 (COX-2) as well as decreased astrogliosis and augmented neuroregeneration15.
Upon considering the essential role of BDNF and HO-1 in recovering from SCI, we hypothesize that BDNF and HO-1 selectively control inflammation and induce neuroregeneration with more favorable, functional clinical outcomes. Therefore, we produced and used, in combination, the two different types of Ad-MSCs highly expressing BDNF and HO-1.
Materials and Methods
Selection of Animal
The experiment was performed on 16 male dogs between the ages of 1 and 2 years, with an average weight of 10.5 ± 1.56 kg. The dogs were randomly divided into four groups, comprising four dogs each. The groups were assigned according to the type of cells used. Combination group – both BDNF and HO-1 overexpressed Ad-MSCs were used in combination; BDNF-MSCs group – only BDNF overexpressed Ad-MSCs were used; HO-1-MSCs group – only HO-1 overexpressed Ad-MSCs were used; GFP-MSCs group – only Ad-MSCs expressing green fluorescent protein (GFP) were injected at the injury site and were used as a control. The dogs were kept in accordance with the guidelines of the Institute of Laboratory Animal Resources, Seoul National University, Korea. The research was approved by the Animal Care and Use Committee of Seoul National University (SNU-160720-13). All the dogs were clinically sound and showed no neurological abnormality.
Isolation and Culture of ADSCs
The gluteal subcutaneous fat was aseptically collected from healthy beagle dog of age 1.5 years, under general anesthesia. The tissues were washed with phosphate buffer saline (PBS), minced in a six-well plate, and digested with collagenase type-1 (1 mg/ml, Sigma-Aldrich, St. Louis, MO, USA), for 2 h under shaking incubation. The suspension was strained through a nylon mesh of pore size 100 µm and centrifuged to obtain the stromal vascular fraction (SVF). The SVF was resuspended and cultured in commercially available low-glucose Dulbecco’s modified Eagle’s medium (DMEM; GenDEPOT, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS; Gibco BRL, Grand Island, NY, USA), and 1% penicillin and streptomycin (PS), for 24 h in a humidified incubator at 37°C and 5% CO2. After 24 h, the Ad-MSCs were washed with PBS to remove the cells debris, and the medium was changed. Next time the medium was changed after an interval of 48 h, until the cells reached 80–90% confluence. The cells were either stored or subcultured, and further processed for the experiment16.
Preparation of Transgenic Lentivirus and Ad-MSCs Transduction
Ad-MSCs were transduced with lentivirus vectors encoding GFP, canine BDNF with GFP, or canine HO-1 with GFP, depending on the group to which the cells belonged. For the preparation of the edited plasmid with the desired gene, the canine HO-1 and BDNF genes were cloned by referring to the Pub-Med database. The genes encoding Flag-tagged BDNF, HO-1, and GFP were amplified by Phusion DNA Polymerases with the cDNA of canine peripheral blood (Thermo Scientific, Pittsburgh, PA, USA). The canine-specific primer set for BDNF (forward, 5′-ATGACCATCCTTTTCCTTAC-3′; reverse, 3′-GATAGAAGGGGAGAATTACC-5′), and HO-1 (forward, 5′-GACAGCATGCCCCAGGAT-3′; reverse, 3′-TCACAGCCTAAGGAGCCAGT-5′), with restriction enzymes EcoRI and BamHI, were inserted into a pCDH-EF1-MCS-pA-PGK-copGFP-T2A-Puro vector (System Biosciences, Mountain View, CA, USA). Dharmacon Trans-Lentivirus packaging system was used to prepare the transgenic viruses (GE Healthcare, Lafayette, CO, USA). First, the HEK293 T cells were cultured in a 100 mm culture plate using a high-glucose medium (DMEM) containing 10% non-heat-inactivated FBS and 1% PS, until the cell density of 5.5 × 106 cells in 14 ml was reached. The suspension of DNA transfer vectors encoding GFP, BDNF, and HMOX-1, the lentiviral packaging mix (Fisher Scientific Cat #14-432-23) encoding viral proteins Gag-Pol, Rev, and VSV-G, and CaCl2, was made and added, drop-wise, to the cultured cells for transfection. The cells were incubated for 10–16 h at 37°C and 5% CO2. The medium was changed and the viral particles were collected from the culture medium after 48 h of incubation. Successful transfection was confirmed by observing GFP expression during incubation under a fluorescent microscope. Finally, three types of lentiviruses, Lenti-BDNF-GFP, Lenti-HO-1-GFP, and Lenti-GFP, were obtained for gene delivery into the Ad-MSCs. The concentration of viral particles was determined according to the manufacturer’s guidelines. The MSCs were transduced at passage 1, during 50–60% confluence, by the lentivirus at MOI = 10017, and were subcultured. The transduced cells were cultured with 3 μg/ml puromycine to get a higher percentage of transduced stem cells. We obtained three types of cells: Ad-MSCs overexpressing BDNF (BDNF-MSCs), Ad-MSCs overexpressing HO-1 (HO-1-MSCs), and Ad-MSCs expressing only GFP (GFP-MSCs). The modified stem cells were subcultured up to three passages and were subsequently used for in vivo study.
Induction of SCI
The SCI was induced by the balloon compression method under general anesthesia18. A mini hemi-laminectomy was performed by creating 3–5 mm hole at the fourth lumbar vertebral arch (L4) using a high-speed pneumatic burr. A 6 Fr embolectomy catheter (Sorin, Biomedica, Salugia, VC, Italy) was inserted and advanced to the cranial margin of the first lumbar vertebra (L1), using fluoroscopic guidance. The balloon was distended with contrast agent (Omnipaque, Amersham Health, Carrington Hill, Ireland) diluted in normal saline (50:50) at a dose rate of 50 µl/kg body weight. The catheter was removed after 12 h. This technique was found to be reliable and repeated with satisfactory outcomes in our previous studies12,14,18–20. As found by computed tomography, this SCI model occludes 80% of the spinal canal which inflicts compression injury to the spinal cord12,18. All the dogs were kept under close supervision during the experimental period. Each dog was placed in a big cage with soft bedding comprising 12 mm thick soft mats to prevent bed sores. Food and water were provided ad libitum. Manual bladder compression was performed three times a day to assist with urination.
Transplantation of Overexpressed Ad-MSCs
After 1 week of SCI, BDNF-MSCs, HO-1-MSCs, and GFP-MSCs were injected. The injured segment of the spinal cord at L1 was exposed through dorsal laminectomy, and about 1 × 107 cells diluted in 150 µl PBS were injected 2–3 mm deep into the spinal cord parenchyma. Three injections of 50 µl each were made: two at the epicenter and one in the center of the injured spinal cord segment. The cells were injected slowly, within an interval of 5 min. In the combination group, we mixed 5 × 106 BDNF-MSCs with 5 × 106 HO-1-MSCs to make a full dose of 1 ×107 cells. After Ad-MSCs transplantation, the dogs were kept for 8 weeks and observed for functional improvement.
Behavioral Assessment
All the dogs were evaluated for gait analysis before SCI and after Ad-MSCs injection. The motor activity was assessed by 19-point scoring system of canine Basso, Beattie, and Bresnahan (cBBB)21. All the dogs were observed for improvement in functional recovery on a daily basis, when they were resting in the cage or set free during cleaning and washing of the cage. On a weekly basis, the improvement in the cBBB score was recorded. Each dog was allowed to move freely in a contained area for 4-5 min to observe improvement in locomotion, weight-bearing ability, and hindlimb coordination. The movement of the joints and muscle contraction was recorded when the dog’s hindquarters were lifted with the base of the tail while standing, and flexion of the joints was recorded while the dog was lifted by an attendant. For deep nociception, the phalanges were pinched with an artery forceps and the degree of pain perception was evaluated by the degree of limb protraction, lip licking, vocalization, and facial expressions. The gait evaluation was performed by three persons blind to the study and the average values were presented for each dog and group.
Western Blot Analysis
At the end of the experimental period, each dog was euthanized and the spinal cords were extracted through dorsal laminectomy, from the twelfth thoracic vertebra (T12) to the third lumbar vertebra (L3). The spinal cord segments were immersed for 12 h in 10% sucrose at 4°C, followed by 20% sucrose at 4°C for the next 24 h. The duramater was carefully removed and the spinal cord segments were embedded and frozen in the optimal cutting temperature compound (Rica Biosystems, Richmond, VA, USA). The spinal cord segments were longitudinally incised into two equal halves; one half was kept for western blot analysis and the other half was employed for cryosectioning, for performing immunofluorescent staining, and histopathological assessment.
For western blotting, the entire injured segment of the spinal cord tissue was properly minced and incubated with 300 μl Ripa Lysis Buffer (Gen Depot,Grand Island, NY, USA), and 3 μl proteinase inhibitor solution (Gen depot) for 30 min on ice, followed by cold centrifugation (4°C, 10 min, 13,000 rpm). In the case of transgenic cells, for the confirmation of successful expression of the transfected gene, the cells of each treatment group were harvested at 90% confluence from three 150 mm petri dishes, pelleted, incubated with Ripa Lysis Buffer, and centrifuged (4°C, 10 min, 13,000 rpm). The supernatant was collected and the protein concentration was determined by the Bradford assay. About 20 μg of protein of each sample was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and electrically transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% skimmed milk for 1 h, followed by overnight incubation with primary antibodies. The following primary antibodies were used: for neural markers, Tuj-1 (sc69966, β-III-tubulin, immature neurons), neurofilament (NF-M, mature neurons, sc-398532), GAP-43 (growth associated proteins for neuro-regeneration, ab-12274), GFAP (astrocytes, sc65343), nestin (neural differentiation, neural stem cells, ab7695); for inflammatory markers, COX2 (cyclooxygenase-2, sc19999), TNF-α (sc1350), IL-6 (interleukin-6, ab-6672), phosphorylated STAT3 (sc1008 R); for anti-inflammatory markers, IL-10 (interleukin-10, R&D, MAB7352), HO-1 (ab-13243); and for neurotrophic factor, BDNF (ab-101747). The membrane was incubated for 1 h at 4°C with anti-mouse secondary antibody (ab6728), and anti-rabbit secondary antibody (ab6721). The β-actin antibody (sc-47778) was used as an internal reference. Protein bands were visualized using Cyanagen WESTAR chemiluminescent substrates (Cyanagen, Bologna BO, Italy), through the image quant LAS 4000 mini system (GE Health Care Biosciences, Uppsala, Sweden).
Immunohistological and Histopathological Assessments
For immunofluorescent staining, the spinal cord segment was sliced at 10 μm by the cryomicrotome, at –20°C. The sections were mounted on silane-coated slides, fixed, and permeated for 10 min with 0.1% v/v Triton X-100, followed by an incubation with 1% bovine serum albumin in PBS for 30 min (BSA; Sigma-Aldrich). The slides were incubated with primary antibodies of neural marker Tuj-1, NF-M, GFAP, and nestin for 24 h at 4°C. This was followed by 2 h incubation of fluorescein iso-thio-cyanate conjugated anti-mouse (Alexa flour, ab-150111), and anti-rabbit secondary antibodies (Flamma 648). DAPI was used to stain the nuclei, and a coverslip was placed over the sections. The slides were examined under the microscope (EVOS FL Imaging System, Stanwood, Washington USA). Cells positive for specific markers were counted from the five randomly selected areas at the injury site, on a slide, through the integrated cell counting system in the microscope. The values were expressed as a percentage representing the average of each slide, and the relative group.
In order to measure the degree of fibrosis, hemorrhages, and vacuoles, slides were stained with H&E. The images were obtained to include the entire injured area of the spinal cord – that is, rostral, caudal, and middle segments of the injured spinal cords22. The lesions were measured for degree of fibrosis by computer-associated image analysis system (Image-J version 1.37; National Institute of Health, Bethesda, MS, USA). Average values were presented for each slide and group.
Statistical Analysis
The data was presented as a mean ± standard error (SE). All experiments were analyzed for the presence of general significance among the groups by a nonparametric two-way analysis of variance (ANOVA), and a post hoc Tukey’s HSD test was used to analyze the significant difference between two groups. A Student t test was used to examine the significantly increased expression of HO-1 and BDNF by HO-1-MSCs and BDNF-MSCs. OriginPro 8.5 software (Northampton, MA, USA) was used to analyze the data and P ≤ 0.05 was taken as a significant value. P value was expressed together with an F or Q value.
Results
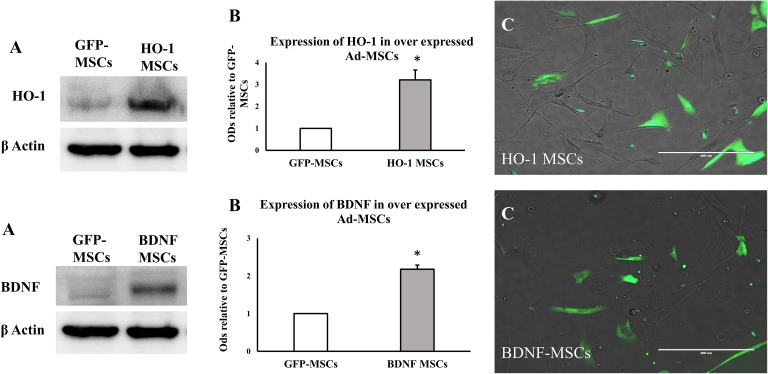
Ad-MSCs transfected with BDNF and HO-1 genes were successfully expressed, as shown by the expression of GFP and western blot analysis (Figure 1). Western blot showed significant increase in the expression of BDNF and HO-1 in transfected Ad-MSCs, relative to GFP-MSCs (Figure 1(b), t test *P ≤ 0.05).
Figure 1.
Expression of transfected HO-1 and BDNF genes in Ad-MSCs. (a) The densities of HO-1 and BDNF proteins in HO-1-, BDNF-, and GFP-MSCs. (b) Quantitative data obtained by densitometry showing optical densities (ODs) relative to GFP-MSCs, bar represent average of three samples and standard error, * indicates significance (P ≤ 0.05). (c) The fluorescence of GFP depicting the successful expression of a transfected gene.
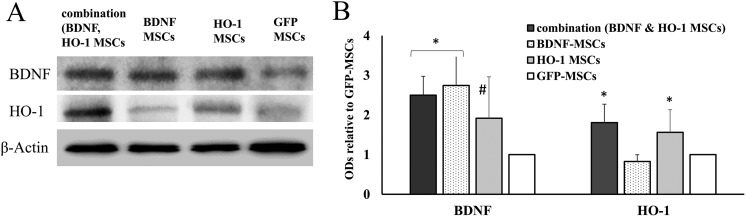
Corresponding to the type of cells used in vivo, BDNF was highly upregulated in the combination and the BDNF-MSCs groups (two-way ANOVA F = 10.35, *P ≤ 0.05). The expression of BDNF was not significantly different between the combination and BDNF-MSCs group; however, its expression was significantly higher in the BDNF-MSCs group than in the HO-1-MSCs (q = 7.99, *P ≤ 0.05) and GFP-MSCs groups (q = 10.26, *P ≤ 0.05) (Figure 2). It also showed upregulation in the HO-1 group compared to the GFP-MSCs group (q = 8.5, #P ≤ 0.05) (Figure 2). Regarding in vivo expression of HO-1, it was upregulated in combination and HO-1 groups (two-way ANOVA F = 9.55, *P ≤ 0.05). It was significantly higher in the HO-1-MSCs group compared to the BDNF (q = 5.73, *P ≤ 0.05) and GFP-MSCs groups (q = 4.69, *P ≤ 0.05).
Figure 2.
In vivo expression of HO-1 and BDNF, 8 weeks after transplantation of HO-1- and BDNF-MSCs. BDNF was highly upregulated in combination and BDNF groups (*P ≤ 0.05) compared to HO-1 and GFP-MSCs groups, while it was also upregulated in the HO-1 group compared to the GFP-MSCs group (#P ≤ 0.05). HO-1 was upregulated in combination and HO-1 groups (*P ≤ 0.05).
Behavioral Assessment
The functional outcomes of the use of modified Ad-MSCs was assessed using the cBBB score. All the dogs were physically normal with a cBBB score of 19 before the SCI. After SCI, the score for all the dogs was zero. The hindquarters of all the dogs were completely paralyzed, showing no deep pain. After 1 week of SCI, Ad-MSCs were injected (intrathecal), and at the time most of the dogs exhibited few reflexes. The cBBB score recorded at the time of Ad-MSCs transplantation was 0.25 ± 0.5 for combination, BDNF, and GFP-MSCs groups, and 0 for the HO-1-MSCs group. During the course of the experimental period the combination, BDNF, and HO-1-MSCs groups showed improvement in their BBB score up to an intermediate stage of improvement – that is, 8–13 – however, the GFP-MSCs group remained in the early stage of BBB score improvement at 0–7.
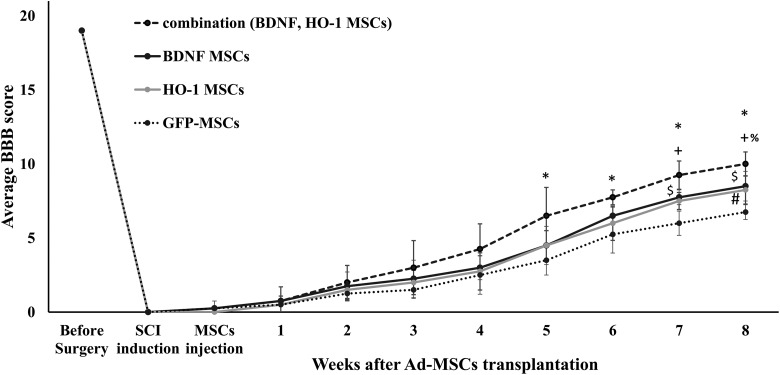
After 5 weeks the cBBB score become significantly different among the groups (two-way ANOVA F = 2.34, P ≤ 0.05) and remained significant after 6 weeks (two-way ANOVA F = 5.04, P ≤ 0.05), 7 weeks (two- way ANOVA F = 13.07, P ≤ 0.05), and 8 weeks (two-way ANOVA F = 2.34, P ≤ 0.05) (Figure 3). A Tukey’s post hoc test showed that the cBBB score was significantly improved by the combination group after 5 weeks (q = 4.43, *P ≤ 0.05), 6 weeks (q = 5.34, *P ≤ 0.05), 7 weeks (q = 8.83, *P ≤ 0.05), and 8 weeks (q = 7.72, *P ≤ 0.05) compared to the GFP-MSCs group. Compared to the HO-1-MSCs group, the combination group attained significant improvement in cBBB score after 7 weeks (q = 4.75, +P ≤ 0.05) and 8 weeks (q = 5.40, +P ≤ 0.05); compared to the BDNF-MSCs group it attained a significant level after 8 weeks of transplantation (q = 4.7, %P ≤ 0.05) (Figure 3). Comparing the BDNF group with the HO-1 group, both showed similar patterns of improvement in the cBBB score (q = 0.67, ns) after 8 weeks (Figure 3); comparing the BDNF-MSCs group with the GFP-MSCs group, a significantly higher BBB score was found after 7 (q = 4.75, $P ≤ 0.05) and 8 weeks (q = 5.33, $P ≤ 0.05). A significant improvement in the score of the HO-1-MSCs group was seen after 8 weeks (q = 4.66, #P ≤ 0.05) compared to the GFP-MSCs group (Figure 3). In the combination group, three out of four dogs were able to stand and support their body weight; they were able to support their own body weight when forced to walk as well, but coordination between the legs was poor. In the BDNF and HO-1 groups, only one out of four dogs was able to bear its weight. In the GFP-MSCs group, the dogs were only able to bear their weight when support was provided at the tuber coxae. Collectively, the average BBB score obtained after 8 weeks was 10 ± 0.8, 8.5 ±1, 8.25 ± 1.2, and 6.75 ± 0.5 for combination, BDNF, HO-1, and GFP-MSCs groups respectively. Dogs that were able to support their body weight showed stronger limb protraction upon phalangeal pinching; however, no dog vocalized during the pinching process, which may indicate spinal reflex action with weak recovery of pain perception. After SCI, all the dogs failed to urinate voluntarily. At the end of the experimental period, voluntary urination recovered in two dogs in the combination group, one dog each in the BDNF and HO-1 groups, and none in the GFP-MSCs group after 8 weeks of Ad-MSCs injection. Defecation was normal in all dogs.
Figure 3.
Changes in BBB score until 8 weeks after transplantation of MSCs. The BBB score of the combination group, BDNF-MSCs group, and the HO-1-MSCs group was significantly higher. *P ≤ 0.05, combination vs. GFP-MSCs; %P ≤ 0.05, combination vs. BDNF-MSCs; +P ≤ 0.05, combination vs. HO-1-MSCs; $P ≤ 0.05, BDNF-MSCs vs. GFP-MSCs; #P ≤ 0.05, HO-1-MSCs vs. GFP-MSCs. Each group represents the average score of four dogs (n = 4).
Neuroregeneration and Differentiation
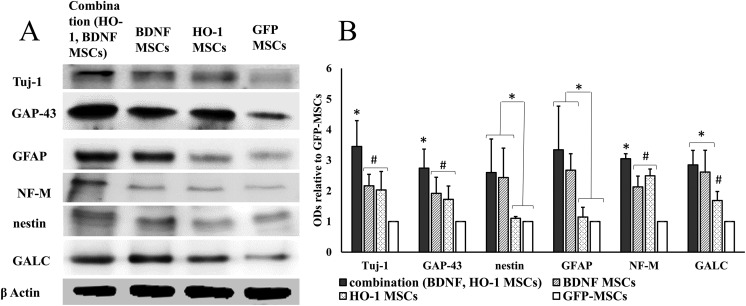
The expression of Tuj-1, GAP-43, and NF-M was the highest in the combination group. A Tukey’s post hoc analysis showed a significant upregulated expression of Tuj-1 (q = 5.14, *P ≤ 0.05), GAP43 (q = 4.40, *P ≤ 0.05), and NF-M (q = 4.95, *P ≤ 0.05) in the combination group compared to other groups (Figure 4). It was the same in the BDNF and HO-1-MSCs groups; however, compared to GFP-MSCs, the BDNF and HO-1-MSCs groups showed significantly higher expression for Tuj-1 (q = 4.23, #P ≤ 0.05), GAP-43 (q = 5.39, #P ≤ 0.05), and NF-M (q = 6.52, #P ≤ 0.05). The expression of nestin, GFAP, and GALC was the same in the combination group and the BDNF-MSCs group; however, both groups showed a significantly higher expression of nestin (q = 6.71, *P ≤ 0.05), GFAP (q = 5.64, *P ≤ 0.05), and GALC (q = 5.13, *P ≤ 0.05) compared to the HO-1-MSCs and GFP-MSCs groups (Figure 4). The expression of GALC was also higher in the HO-1-MSCs group than in the GFP-MSCs group (q = 4.38, #P ≤ 0.05). Collectively, the process of neuroregeneration might be upregulated in the combination group, evidenced by the highest expression of Tuj-1, GAP-43, and NF-M, since an equal level of upregulated expression of nestin, GALC, and GFAP, was found in the combination and BDNF groups (Figure 4).
Figure 4.
Expression of neural markers. (a) The densities of neural markers. (b) Quantitative data obtained by densitometry showing optical densities (ODs) relative to GFP-MSCs. The expression of Tuj-1, GAP-43, and NF-M was significantly increased in the combination group compared to the other groups (*P ≤ 0.05), while it was the same between BDNF and HO-1-MSCs groups, but significantly higher than the GFP-MSCs group (#P ≤ 0.05). The expression of nestin, GFAP, and GALC was the same in the combination and BDNF groups, but significantly higher than the HO-1- and GFP-MSCs groups (*P ≤ 0.05). GALC was also significantly higher in the HO-1 group compared to the GFP-MSCs group (#P ≤ 0.05). The graph represents mean ± standard error of four dogs per groups, as determined by densitometry relative to β-actin.
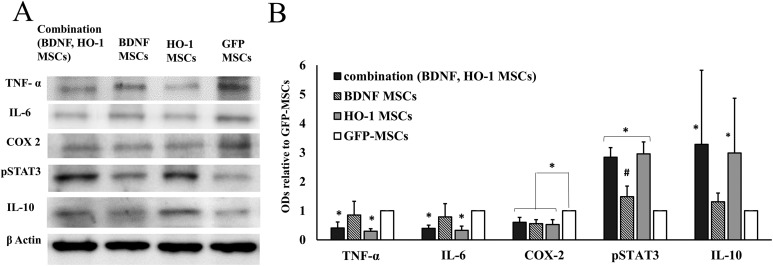
Expression of Inflammatory and Anti-Inflammatory Markers
The expressions of inflammatory markers were significantly reduced in the combination group. A Tukey’s post hoc analysis showed that the expression of TNF-α and IL-6 was significantly reduced in both combination and HO-1-MSCs groups compared to BDNF-MSCs (q = 4.12, *P ≤ 0.05) and GFP-MSCs (q = 4.27, *P ≤ 0.05) (Figure 5). Additionally, the expression was not different between the BDNF-MSCs group and the GFP-MSCs group (q = 1.13, ns). COX-2 represented similar expression within the combination, BDNF-MSCs, and HO-1-MSCs groups; however, this was lower than the expression in the GFP-MSCs group (q = 6.88, *P ≤ 0.05).
Figure 5.
Expression of inflammatory and anti-inflammatory markers. (a) The densities of inflammatory and anti-inflammatory markers. (b) Quantitative data obtained by densitometry showing optical densities (OD) relative to GFP-MSCs. The expression of TNF-α and IL-6 was significantly lower in combination and HO-1-MSCs groups compared to BDNF and GFP-MSCs groups (*P ≤ 0.05). The expression of COX2 was significantly decreased in all combination, BDNF-, and HO-1-MSCs groups compared to the GFP-MSCs group (*P ≤ 0.05). pSTAT3 was upregulated in both combination and HO-1-MSCs group compared to BDNF- and GFP-MSCs groups (*P ≤ 0.05); its expression was also higher in the BDNF group compared to the GFP-MSCs group (#P ≤ 0.05). IL-10 was significantly higher in the combination and HO-1 groups (*P ≤ 0.05) compared to the BDNF and GFP-MSCs groups. The graph represents mean ± standard error of four dogs per groups, as determined by densitometry relative to β-actin.
pSTAT3 was upregulated in the combination and HO-1 groups compared to the BDNF-MSCs and GFP-MSCs groups (two-way ANOVA F = 10.35, *P ≤ 0.05) (Figure 5). The combination group showed significantly higher expression of pSTAT3 compared to BDNF-MSCs (q = 7.56, *P ≤ 0.05) and GFP-MSCs (q = 7.46, *P ≤ 0.05). Within the BDNF-MSCs and GFP-MSCs groups, a higher expression in the BDNF-MSCs group was evidenced than in the GFP-MSCs group (q = 4.29, #P ≤ 0.05) (Figure 5). IL-10, as an anti- inflammatory marker, showed upregulation in both the combination and HO-1 groups, but remained downregulated in the BDNF and GFP-MSCs groups (two-way ANOVA, F = 9.56, *P ≤ 0.05) (Figure 5). A significantly higher expression of IL-10 was observed in the combination group compared to the BDNF-MSCs group (q = 6.26, *P ≤ 0.05) and GFP-MSCs group (q = 7.45, *P ≤ 0.05) (Figure 5).
Immunohistochemistry
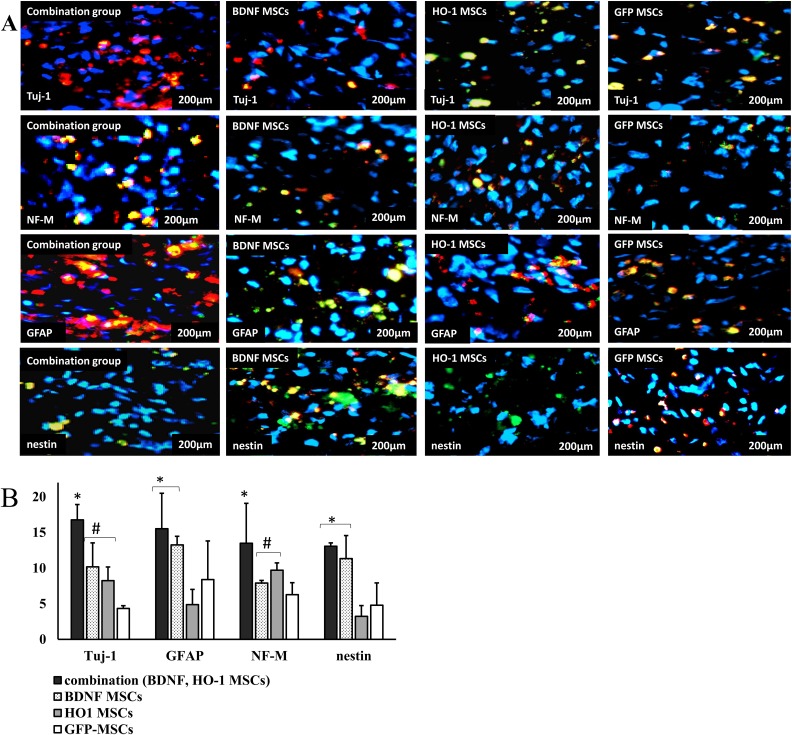
Immunohistochemistry was performed to evaluate the expression of neural markers Tuj-1, GFAP, NF-M, and nestin in transplanted MSCs and host cells at the injured segment of the spinal cord. The transplanted cells survived until 8 weeks. They were evenly distributed in the injured spinal cord segment. Based upon the presence of GFP expression, the percentage of live transplanted cells observed after 8 weeks was 14.9 ± 5.8%, 11 ± 4.36%, 14.1 ± 4.65%, 10.89 ± 5.28% for combination, BDNF-MSCs, HO-1-MSCs, and GFP-MSCs groups respectively. No clustering of the transplanted cells was observed. The cells were found together with endogenous neurons and glial cells, because GFP-positive cells shown by green and blue color were found among the cells that were negative for GFP and positive for blue and red color of the neural marker.
Regarding the expression of neural markers, we found a similar pattern of expression, compared to that found in western blot results. We obtained the highest percentage of Tuj-1 positive cells, at 16.78 ± 2.14%, in the combination group compared to BDNF-MSCs (q = 5.93, *P ≤ 0.05), HO-1-MSCs (q = 7.65, *P ≤ 0.05) and GFP-MSCs (q = 8.64, *P ≤ 0.05). The percentage value was not significantly different between the BDNF- and HO-1-MSCs groups, at 10.17 ± 3.378% and 7.13 ± 2.86%, respectively, although it was higher than that of the GFP-MSCs group, at 4.34 ± 0.30% (q = 5.2 #P ≤ 0.05). The percentage expression of GFAP was similarly high in the combination group, at 16.87 ± 3.30% compared to the BDNF group, at 14.25 ± 1.21% (Figure 6). The percentage expression of GFAP in the combination group was higher than that of the HO-1 (q = 5.49, *P ≤ 0.05) and GFP-MSCs groups (q = 4.22, *P ≤ 0.05), at 4.9 ± 2.1% and 8.40 ± 5.40%, respectively. The BDNF-MSCs group showed a similar pattern of expression for GFAP compared to HO-1-MSCs and GFP-MSCs (Figure 6(a,b)). In case of NF-M-positive cells, the percentage was the highest in the combination group, at 13.50 ± 5.50% compared to BDNF-MSCs (q = 7.07, *P ≤ 0.05), HO-1-MSCs (q = 7.23, *P ≤ 0.05), and GFP-MSCs (q = 7.56, *P ≤ 0.05) (Figure 6(a,b)), followed by the BDNF- and HO-1-MSCs groups, at 9.70 ± 1.03% and 7.91 ± 0.30%, respectively compared to the GFP-MSCs group, at 6.27 ± 1.68% (Figure 6(a,b)).
Figure 6.
Immunofluorescent staining for the expression of neural markers. (a) images of stained slides. The slides were stained with Tuj-1, NF-M, GFAP, and nestin as red, and the nucleus was stained with DAPI as blue. Each image represents the four samples per group with a scale bar of 200 μm. (b) Percentage expression of neural marker positive cells. The percentage of cells positive for Tuj-1 and NF-M was highest in the combination group, followed by higher percentages in BDNF- and HO-1-MSCs groups compared to the GFP-MSCs group (*#P ≤ 0.05). While the cells positive for GFAP and nestin were similar between combination and BDNF-MSCs groups, they were higher than the HO-1- and GFP-MSCs groups (*P ≤ 0.05).
Nestin, as a differentiation and stem cells marker, was high only in the combination and BDNF-MSCs groups, at 13.07 ± 0.47% and 11.33 ± 3.23%, respectively. These values were significantly higher than those of the HO-1 (q = 6.45, *P ≤ 0.05) and GFP-MSCs groups (q = 6.45, *P ≤ 0.05), at 3.23 ± 1.50%, and 4.80 ± 3.10%, respectively (Figure 6(a,b)).
Histopathological Assessment
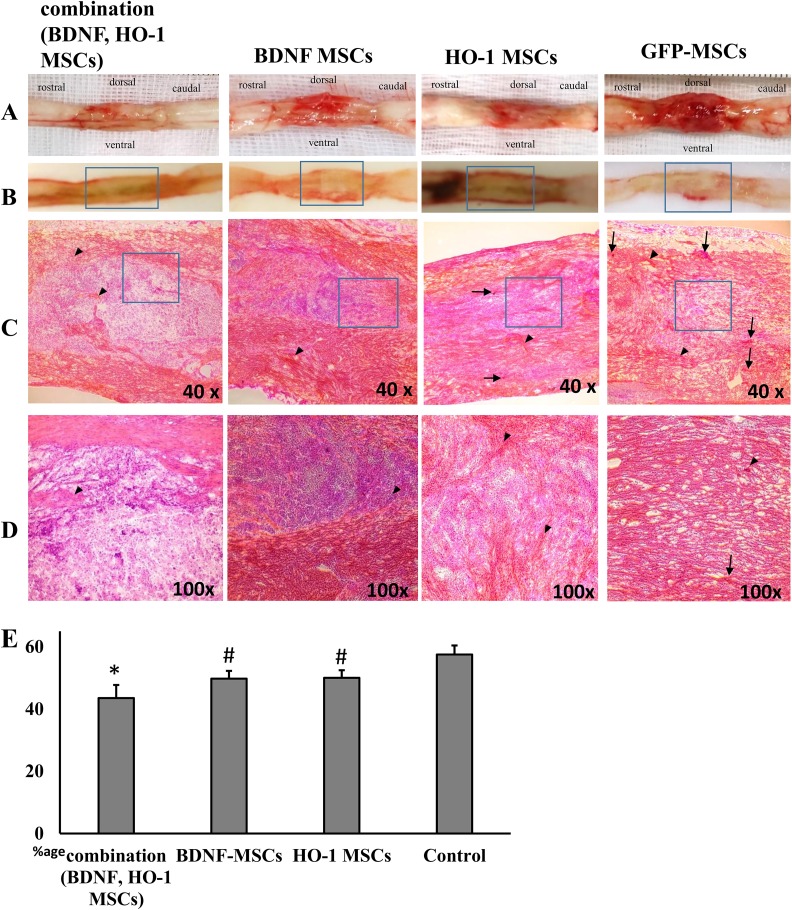
The injured spinal cord showed atrophy, fibrosis, and hemorrhages (Figure 7(a)). The degree of fibrosis was significantly the lowest in the combination group, compared to other groups (Figure 7(e)). This was followed by higher fibrosis in the BDNF and HO-1 groups, although lower compared to the GFP-MSCs (two-way ANOVA F = 10.23, *#P ≤ 0.05) (Figure 7(e)). Based on the visual examination, the combination group showed a well-organized parenchymal matrix with very few intervening fibrotic areas in the healthy area (Figure 7(c,d), arrowheads). The parenchymal matrix was comparatively less organized in the BDNF and HO-1-MSCs groups, with more intervening fibrotic areas in these groups than in the combination group. More robust fibrosis was observed in the GFP-MSCs group, with a more distorted parenchymal matrix (Figure 7(c,d)). More hemorrhagic areas were observed in the GFP-MSCs group than in the BDNF, HO-1, and combination groups (Figure 7(c,d), arrows).
Figure 7.
Histopathology of spinal cord lesions stained with H&E stain. (a) An injured spinal cord showed fibrosis, atrophy, and hemorrhages. (b) Spinal cords sliced at the injured segment. (c) The fibrotic (red and pink) and healthy areas (purple) at the injured spinal cord section, at low magnification (40×), and (d) at high magnification (100×); scale bar 200 μm. (e) Percentage expression of fibrotic area. There is fibrosis at the epicenter that covers most of the healthy areas at the injured segment (c). The percentage fibrosis in the combination group was lower than in the other groups ((e), *P ≤ 0.05), followed by lower fibrosis in BDNF- and HO-1-MSCs groups compared to GFP-MSCs ((e), #P ≤ 0.05). Arrowheads denote fibrotic areas, while arrows indicate hemorrhages. The degree of hemorrhage was lower in combination, BDNF, and HO-1-MSCs groups compared with the GFP-MSCs group. Each picture (c) and bar (e) represents four samples per group (n = 4).
Discussion
The present study indicates that using the transgenic Ad-MSCs of different strengths – that is BDNF-MSCs and HO-1-MSCs in combination – has a more favorable effect on healing than using the BDNF-MSCs and HO-1-MSCs alone. The results indicated that anti-inflammatory functions of HO-1 positively manipulate the microenvironment of the SCI, which allows the BDNF to promote nerve cell growth more than the BDNF cells transplanted alone.
Ad-MSCs have been used in SCI dogs with improved functional recovery due to enhanced neuroregeneration, differentiation, and reduced intraparenchymal fibrosis. The treatment groups had shown higher Olby, modified Tarlov, and BBB scores with an improvement in conduction velocities observed through somatosensory evoked potentials12,18,23,24. Similarly, clinical cases of dogs with a chronic SCI showed improved Olby score after an intramedullary injection of human dental pulp stem cells and physical therapy25. We potentiated the usefulness of Ad-MSCs by transfecting them with BDNF and HO-1 genes, through a lentivirus that showed successful expression in HO-1-MSCs and BDNF-MSCs, both in vitro and in vivo. As previously described, the transfection did not negatively affect the viability and proliferation of both HO-1-MSCs and BDNF-MSCs26,27. In our previous studies, we found that both BDNF-MSCs and HO-1-MSCs were more potent than MSCs in terms of healing of the SCI and improvement in functional recovery14,15. In the present study we injected both HO-1 and BDNF-MSCs, called the “combination group” and compared it with HO-1-MSC, BDNF-MSC, and GFP-MSC groups. We found improved functional ability in terms of a higher cBBB score in the combination group compared to other groups. This improvement might be due to a favorable microenvironment created by the types of cells that were transplanted.
BDNF is a neurotrophic factor that is responsible for neuronal survival, growth, and maturation4. HO-1 is a kind of anti-stress protein that is normally expressed in spinal cord neurons and induced after an SCI, both in gray and white matter, primarily in microglial and glial cells5,28 that reduce inflammation, apoptosis, and oxidative injury29. Because the pathophysiological events with an expression of proinflammatory cytokines during SCI create an unfavorable microenvironment for the regeneration of injured neurons30, it is desirable to control inflammation to get an optimum healing effect of BDNF. BDNF-MSCs have been found to induce regeneration in a contused spinal cord of mice by upregulating GAP-43 and neuron-specific enolase positive cells (NSE)31. Similarly, in SCI dogs, BDNF-MSCs and HO-1-MSCs induce neuroregeneration by upregulating the expression of Tuj-1, NF-M, and nestin14,15. In the present study, we found an increase in neuroregeneration in both HO-1- and BDNF-MSC groups, evidenced by a higher expression of Tuj-1, GAP-43, and NF-M. In the BDNF group it was due to the enhanced expression of BDNF by BDNF-MSCs, which stimulated the neuroregeneration. Similar increase in neuroregeneration in the HO-1-MSCs group is attributed to an upregulation of BDNF in the group. This happen because HO-1 upregulates the expression of BDNF by activating ERK and PI3K-AKT pathways32, which subsequently activate the TrkB, PI3K/Akt signaling pathways for neuroregeneration and survival33. However, we found more robust neuroregeneration in the combination group, which might be due to the different contribution of two different types of cells – that is, the control of inflammation by HO-1-MSCs and neuroregeneration by BDNF-MSCs.
As previously described, the BDNF-MSCs did not reduce the expression of inflammatory cytokines in chronic SCI of dogs, but it was reduced with higher expression of neural and glial markers when the dogs were intravenously (IV) injected with Ad-MSCs14. The MSCs injected IV cause systemic immunomodulation, which affects the inflammatory status of SCI and promotes healing34. We also found compatible results, regarding the expression of TNFα and IL-6 in the combination group. It seems that BDNF continues its role of nerve growth regardless of the presence of proinflammatory cytokines, but it is more significant when the inflammation is controlled. We got the highest expression of neural markers in the combination group with GAP43 than both BDNF and HO-1-MSCs groups, which represent more favorable neuroregeneration due to the local anti-inflammatory effect of HO-1-MSCs.
HO-1 mediates the anti-inflammatory effect of IL-10 in mice19, and confers protection to the hepatic ischemia reperfusion injury by inhibiting TNF-α mediated activation of apoptotic pathways35. IL-10 inhibits the expression of proinflammatory cytokines and pro-apoptotic factors such as IL-6, TNF-α, etc., which increases nerve tissue sparing and improves functional recovery36. In the present study, the expression of inflammatory markers TNF-α and IL6 was significantly reduced in both combination and HO-1 groups, which is compatible with the previously reported data of canine SCI treated with HO-1-MSCs15. We further confirmed that the anti-inflammatory effect was due to the upregulation of IL-10. As an anti-inflammatory marker, IL-10 was significantly upregulated in both the combination and HO-1-MSCs groups, compared to the BDNF and GFP-MSCs groups, which suppressed the expression of TNF-α and IL-6, increasing nerve tissue sparing by impeding further progress into the secondary SCI, and this might be the reason for more potent action of BDNF in the combination group.
COX2 expression is induced by TNF-α, which further induces inflammation by regulating prostaglandin E2 synthesis37. HO-1 overexpression attenuates the lipopolysaccharide-induced COX2 expression in the mouse brain38. Additionally, BDNF overexpression suppresses COX2 expression in a transgenic mice model of Alzheimer’s Disease39. In the present study, COX2 was downregulated in the combination, BDNF, and HO-1-MSCs groups, which confirms the inhibitory effect upon COX2 in dogs with SCI; however, this effect was not observed in a chronic SCI dog model treated with BDNF-MSCs14. This may indicate that BDNF-MSCs act differently in controlling the expression of COX2 when transplanted in different stages of SCI.
Previously, both HO-1-MSCs and BDNF-MSCs were found to reduce the degree of fibrosis and hemorrhages at the injury site14,19; however, we report a reduction in the degree of fibrosis, more significantly in the combination group than in the HO-1- and BDNF-MSCs groups.
As a result of SCI, a blood–brain barrier is disrupted, which allows the infiltration of leukocytes at the injured segment of the spinal cord, which together with glial cells build a glial scar. The glial scar inhibits further injury to the injured spinal cord segment but it also interferes with the healing process40. Resident cells in the glial scar produce copious amounts of inhibitory factors, such as chondroitin sulfate proteoglycans (CSPG) and matrix metalloproteinases, that inhibit axonal regeneration41. Various experimental data suggest that inhibiting the glial scar ameliorates the microenvironment of the injured spinal cord for the growth of neurofilaments. Various agents such as decorin, hepatocyte growth factors, curcumin macrophage colony stimulating factors, etc. have been experimentally found effective in reducing the glial scar, with improved functional recovery and increased gray matter40. There are also conflicting data available in the support of astrogliosis. Astrocyte scar formation has been found to support the regeneration of corticospinal and serotonergic axons in SCI of rats42. The role of astrocytes in neuroregeneration is significant because they inhibit microglial activation and control inflammation43. We also found a favorable effect of astrocytes on neuroregeneration, as both GFAP and nestin were equally upregulated in both the BDNF-MSCs and combination groups, and neuroregeneration was not affected by astrocytes, as evidenced by a higher expression of Tuj-1, GAP-43, and NF-M.
A challenge during the repair of SCI is the re-myelination of newly formed neurons, and the neurons that get demyelinated during secondary SCI. Fibroblasts overexpressing BDNF were found to promote myelination of newly formed neurons by inducing oligodendrocyte and Schwan cell proliferation44, and the expression of myelin basic protein45. The marker specific for oligodendrocytes and re-myelination, GALC, was upregulated equally in both the combination and BDNF-MSCs groups, compared to the HO-1- and GFP-MSCs groups, which may represent a stimulatory effect of BDNF upon re-myelination. GALC was also upregulated in the HO-1 group compared to GFP-MSCs group, and this may be due to a higher expression of BDNF in the HO-1-MSCs group. These results do not comply with our previous findings, which showed the downregulation of GALC in both BDNF-MSCs and HO-1-MSCs used in chronic and subacute SCI models of dogs respectively14,15. GALC expression was reduced with systemic immunosuppression due to MSCs injected IV14; however, the local immune suppression in the combination group of the present study did not reduce the expression of GALC, which may indicate the different behavior of BDNF-MSCs in the chronic stage of SCI.
Signal transducer and activator of transcription 3 (pSTAT3) is a transcription factor that mediates multiple pathways related to neuroregeneration and differentiation. STAT3 activation enhances axonal regeneration and neuroprotection after an SCI by activating the JAK/STAT3 and PI3K/AKT/mTOR pathways33,46. STAT3 activation mediates the activation of tyrosine kinase-A receptors by BDNF, which are required for nerve growth and survival47. Pertaining to HO-1 expression, IL-10 induces HO-1 expression by activating STAT348. In the present study, STAT3 was highly upregulated in both the combination and HO-1 groups, which suggests the activation of IL-10/pSTAT3/HO-1, as well as the pathways associated with neuronal regeneration, as previously described. STAT3 was also upregulated in the BDNF group compared to the GFP-MSCs group, which may indicate the BDNF activation of STAT3 for TrkB signaling, required for neural regeneration49.
Collectively, we get enhanced neuroregeneration in the combination group, that is, a higher expression of Tuj-1, GAP-43, and NF-M, with BDNF-associated benefits of nestin and GFAP expression favored because of the noninhibitory environment created by HO-1-MSCs, by controlling the expression of TNF-α, IL-6, and COX2. Thus, this could be a novel strategy to selectively control inflammation and induce neuroregeneration by using two different types of MSCs, with good clinical outcomes. We used dogs as an experimental SCI model because large animals’ experimental SCI models are considered more appropriate than those of rodents in order to translate these studies into human beings. Because of most of the anatomical and histopathological similarity with human beings, the studies conducted on dogs and primates with experimental SCI can be applied in human beings as well50. The injury pattern of the spinal cord in dogs, such as naturally occurring intervertebral disk herniation (IVDH), has a close resemblance with IVDH in humans. Magnetic resonance imaging showed similar post-traumatic myelopathies, such as cystic cavities, spinal cord atrophy, and syringomyelia in dogs and humans due to IVDH51. We used balloon compression SCI model in dogs, which closely mimic the contusion or compression caused by IVDH in dogs and humans52. Therefore, the methodology and techniques used in the present study could have favorable outcomes for SCI in humans as well. Moreover, it will also help to investigate more combinations of suitable cell types in the future, to selectively manipulate various factors for better clinical outcomes.
Conclusion
From the present study, it can be concluded that HO-1-MSCs inhibit the expression of inflammatory cytokines, which establishes a favorable environment for BDNF-induced neuroregeneration in dogs with subacute SCI.
Footnotes
Ethical Approval: This study was approved by Institute of Animal Care and Use Committee of Seoul National University (see Materials and Methods section).
Statement of Human and Animal Rights: This article does not contain any studies with human subjects. The study was performed on animals using the dog as an experimental subject.
Statement of Informed Consent: In the present study, human subjects are not used for experimental purpose, therefore informed consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Research Foundation of Korea (NRF-10 2015R1D1A1A01057415).
References
- 1. Mortazavi MM, Verma K, Harmon OA, Griessenauer CJ, Adeeb N, Theodore N, Tubbs RS. The microanatomy of spinal cord injury: A review. Clin Anat. 2015;28(1):27–36. [DOI] [PubMed] [Google Scholar]
- 2. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg Focus. 2008;25(5):E2. [DOI] [PubMed] [Google Scholar]
- 3. Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7(6):574–585. [DOI] [PubMed] [Google Scholar]
- 4. Wollen KA. Alzheimer’s disease: The pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern Med Rev. 2010;15(3):223–244. [PubMed] [Google Scholar]
- 5. Liu Y, Tachibana T, Dai Y, Kondo E, Fukuoka T, Yamanaka H, Noguchi K. Heme oxygenase-1 expression after spinal cord injury: The induction in activated neutrophils. J Neurotrauma. 2002;19(4):479–490. [DOI] [PubMed] [Google Scholar]
- 6. Mautes AE, Bergeron M, Sharp FR, Panter SS, Weinzierl M, Guenther K, Noble LJ. Sustained induction of heme oxygenase-1 in the traumatized spinal cord. Exp Neurol. 2000;166(2):254–265. [DOI] [PubMed] [Google Scholar]
- 7. Vargas MR, Pehar M, Cassina P, Martínez-Palma L, Thompson JA, Beckman JS, Barbeito L. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes consequences for motor neuron survival. J Biol Chem. 2005;280(27):25571–25579. [DOI] [PubMed] [Google Scholar]
- 8. Abraham NG, Asija A, Drummond G, Peterson S. Heme oxygenase-1 gene therapy: Recent advances and therapeutic applications. Curr Gene Ther. 2007;7(2):89–108. [DOI] [PubMed] [Google Scholar]
- 9. Choi A, Alam J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15(1):9–19. [DOI] [PubMed] [Google Scholar]
- 10. Yamauchi T, Lin Y, Sharp FR, Noble-Haeusslein LJ. Hemin induces heme oxygenase-1 in spinal cord vasculature and attenuates barrier disruption and neutrophil infiltration in the injured murine spinal cord. J Neurotrauma. 2004;21(8):1017–1030. [DOI] [PubMed] [Google Scholar]
- 11. Chora ÂA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, Soares MP. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007;117(2):438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, Kim WH, Kang KS, Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10(4):273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ji XC, Dang YY, Gao HY, Wang ZT, Gao M, Yang Y, Zhang HT, Xu RX. Local injection of lenti-BDNF at the lesion site promotes M2 macrophage polarization and inhibits inflammatory response after spinal cord injury in mice. Cell Mol Neurobiol. 2015;35(6):881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SH, Kim Y, Rhew D, Kim A, Jo KR, Yoon Y, Choi KU, Jung T, Kim WH, Kweon OK. Combinational transplantation of chondroitinase ABC and BDNF-expressing mesenchymal stromal cells (MSCs) with intravenous injection of MSCs in chronic spinal cord injury model dogs. Cytotherapy. 2016;33(1):14–15. [Google Scholar]
- 15. Lee SH, Kim Y, Rhew D, Kim A, Jo KR, Yoon Y, Choi KU, Jung T, Kim WH, Kweon OK. Effect of canine mesenchymal stromal cells overexpressing heme oxygenase-1 in spinal cord injury. J Vet Sci. 2017;18(3):377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neupane M, Chang CC, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Engineering Part A. 2008;14(6):1007–1015. [DOI] [PubMed] [Google Scholar]
- 17. McGinley L, McMahon J, Strappe P, Barry F, Murphy M, O’Toole D, O’Brien T. Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia. Stem Cell Res Ther. 2011;2(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007;8(3):275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. [DOI] [PubMed] [Google Scholar]
- 20. Park SS, Lee YJ, Lee SH, Lee D, Choi K, Kim WH, Kweon OK, Han HJ. Functional recovery after spinal cord injury in dogs treated with a combination of matrigel and neural-induced adipose-derived mesenchymal stem cells. Cytotherapy. 2012;14(5):584–597. [DOI] [PubMed] [Google Scholar]
- 21. Song RB, Basso DM, da Costa RC, Fisher LC, Mo X, Moore SA. Adaptation of the Basso–Beattie–Bresnahan locomotor rating scale for use in a clinical model of spinal cord injury in dogs. J Neurosci Methods. 2016;268:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ninomiya K, Iwatsuki K, Ohnishi YI, Ohkawa T, Yoshimine T. Intranasal delivery of bone marrow stromal cells to spinal cord lesions. J Neurosurg Spine. 2015;23(1):111–119. [DOI] [PubMed] [Google Scholar]
- 23. Park SS, Byeon YE, Ryu HH, Kang BJ, Kim Y, Kim WH, Kang KS, Han HJ, Kweon OK. Comparison of canine umbilical cord blood-derived mesenchymal stem cell transplantation times: Involvement of astrogliosis, inflammation, intracellular actin cytoskeleton pathways, and neurotrophin-3. Cell Transplant. 2011;20(11–12):1867–1880. [DOI] [PubMed] [Google Scholar]
- 24. McMahill BG, Borjesson DL, Sieber-Blum M, Nolta JA, Sturges BK. Stem cells in canine spinal cord injury: promise for regenerative therapy in a large animal model of human disease. Stem Cell Rev Rep. 2015;11(1):180–193. [DOI] [PubMed] [Google Scholar]
- 25. Feitosa MLT, Sarmento CAP, Bocabello RZ, Beltrão-Braga PCB, Pignatari GC, Giglio RF, Miglino MA, Orlandin JR, Ambrósio CE. Transplantation of human immature dental pulp stem cell in dogs with chronic spinal cord injury. Acta Cir Bras. 2017;32(7):540–549. [DOI] [PubMed] [Google Scholar]
- 26. Mijung K, Yongsun K, Seunghoon L, Minyoung K, Kim AY, Wanhee K, Kweon OK. Comparison of viability and antioxidant capacity between canine adipose-derived mesenchymal stem cells and heme oxygenase-1-overexpressed cells after freeze-thawing. J Vet Med Sci. 2016;78(4):619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ritfeld GJ, Patel A, Chou A, Novosat TL, Castillo DG, Roos RA, Oudega M. The role of brain-derived neurotrophic factor in bone marrow stromal cell-mediated spinal cord repair. Cell Transplant. 2015;24(11):2209–2220. [DOI] [PubMed] [Google Scholar]
- 28. Stahnke T, Richter-Landsberg C, Stadelmann C, Netzler A, Brück W. Differential upregulation of heme oxygenase-1 (hsp32) in glial cells after oxidative stress and in demyelinating disorders. J Mol Neurosci. 2007;32(1):25–37. [DOI] [PubMed] [Google Scholar]
- 29. Negi G, Nakkina V, Kamble P, Sharma SS. Heme oxygenase-1, a novel target for the treatment of diabetic complications: Focus on diabetic peripheral neuropathy. Pharmacol Res. 2015;102:158–167. [DOI] [PubMed] [Google Scholar]
- 30. Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J Stem Cells. 2014;6(2):120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ji W, Qiu Y. An experimental study on the treatment of spinal cord injury in rats by tissue engineering method that uses AsSCs modified by BDNF and NT-3 double genes combing the use of SFCs. Global Spine J. 2016;6(S 01):GO171. [Google Scholar]
- 32. Hung SY, Liou HC, Fu WM. The mechanism of heme oxygenase-1 action involved in the enhancement of neurotrophic factor expression. Neuropharmacology. 2010;58(2):321–329. [DOI] [PubMed] [Google Scholar]
- 33. Qi D, Ouyang C, Wang Y, Zhang S, Ma X, Song Y, Yu H, Tang J, Fu W, Sheng L. Ho-1 attenuates hippocampal neurons injury via the activation of BDNF–TRKB–PI3K/AKT signaling pathway in stroke. Brain Res. 2014;1577:69–76. [DOI] [PubMed] [Google Scholar]
- 34. Badner A, Vawda R, Laliberte A, Hong J, Mikhail M, Jose A, Dragas R, Fehlings M. Early intravenous delivery of human brain stromal cells modulates systemic inflammation and leads to vasoprotection in traumatic spinal cord injury. Stem cells Transl Med. 2016;5(8):991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim SJ, Eum HA, Billiar TR, Lee SM. Role of heme oxygenase 1 in TNF/TNF receptor-mediated apoptosis after hepatic ischemia/reperfusion in rats. Shock. 2013;39(4):380–388. [DOI] [PubMed] [Google Scholar]
- 36. Thompson CD, Zurko JC, Hanna BF, Hellenbrand DJ, Hanna A. The therapeutic role of interleukin-10 after spinal cord injury. J Neurotrauma. 2013;30(15):1311–1324. [DOI] [PubMed] [Google Scholar]
- 37. Miyamoto H, Saura R, Harada T, Doita M, Mizuno K. 2000. The role of cyclooxygenase-2 and inflammatory cytokines in pain induction of herniated lumbar intervertebral disc. Kobe J Med Sci. 46(1–2):13–28. [PubMed] [Google Scholar]
- 38. Shih RH, Yang CM. Induction of heme oxygenase-1 attenuates lipopolysaccharide-induced cyclooxygenase-2 expression in mouse brain endothelial cells. J Neuroinflammation. 2010;7(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koo JH, Kwon IS, Kang EB, Lee CK, Lee NH, Kwon MG, Cho IH, Cho JY. Neuroprotective effects of treadmill exercise on BDNF and PI3-K/AKT signaling pathway in the cortex of transgenic mice model of Alzheimer’s disease. J Exerc Nutrition Biochem. 2013;17(4):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orlandin JR, Ambrósio CE, Lara VM. Glial scar-modulation as therapeutic tool in spinal cord injury in animal models. Acta Cir Bras. 2017;32(2):168–174. [DOI] [PubMed] [Google Scholar]
- 41. Yuan YM, He C. The glial scar in spinal cord injury and repair. Neurosci Bull. 2013;29(4):421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim Jh, Min KJ, Seol W, Jou I, Joe Eh. Astrocytes in injury states rapidly produce anti-inflammatory factors and attenuate microglial inflammatory responses. J Neurochem. 2010;115(5):1161–1171. [DOI] [PubMed] [Google Scholar]
- 44. McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18(14):5354–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koda M, Murakami M, Ino H, Yoshinaga K, Ikeda O, Hashimoto M, Yamazaki M, Nakayama C, Moriya H. Brain-derived neurotrophic factor suppresses delayed apoptosis of oligodendrocytes after spinal cord injury in rats. J Neurotrauma. 2002;19(6):777–785. [DOI] [PubMed] [Google Scholar]
- 46. Mehta ST, Luo X, Park KK, Bixby JL, Lemmon VP. Hyperactivated STAT3 boosts axon regeneration in the CNS. Exp Neurol. 2016;280:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ng YP, Cheung ZH, Ip NY. STAT3 as a downstream mediator of TRK signaling and functions. J Biol Chem. 2006;281(23):15636–15644. [DOI] [PubMed] [Google Scholar]
- 48. Ricchetti GA, Williams LM, Foxwell BM. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol. 2004;76(3):719–726. [DOI] [PubMed] [Google Scholar]
- 49. Chen MR, Dai P, Wang SF, Song SH, Wang HP, Zhao Y, Wang TH, Liu J. BDNF overexpression exhibited bilateral effect on neural behavior in SCT mice associated with AKT signal pathway. Neurochem Res. 2016;41(10):2585–2597. [DOI] [PubMed] [Google Scholar]
- 50. Gabel BC, Curtis EI, Marsala M, Ciacci JD. A review of stem cell therapy for spinal cord injury: Large animal models and the frontier in humans. World Neurosurg. 2017;98:438–443. [DOI] [PubMed] [Google Scholar]
- 51. Alisauskaite N, Spitzbarth I, Baumgärtner W, Dziallas P, Kramer S, Dening R, Stein VM, Tipold A. Chronic post-traumatic intramedullary lesions in dogs, a translational model. PLoS One. 2017;12(11):e0187746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheriyan T, Ryan D, Weinreb J, Cheriyan J, Paul J, Lafage V, Kirsch T, Errico T. Spinal cord injury models: A review. Spinal Cord. 2014;52(8):588–595. [DOI] [PubMed] [Google Scholar]