Abstract
Ischemic heart disease, also known as coronary artery disease (CAD), poses a challenge for regenerative medicine. iPSC technology might lead to a breakthrough due to the possibility of directed cell differentiation delivering a new powerful source of human autologous cardiomyocytes. One of the factors supporting proper cell maturation is in vitro culture duration. In this study, primary human skeletal muscle myoblasts were selected as a myogenic cell type reservoir for genetic iPSC reprogramming. Skeletal muscle myoblasts have similar ontogeny embryogenetic pathways (myoblasts vs. cardiomyocytes), and thus, a greater chance of myocardial development might be expected, with maintenance of acquired myogenic cardiac cell characteristics, from the differentiation process when iPSCs of myoblastoid origin are obtained. Analyses of cell morphological and structural changes, gene expression (cardiac markers), and functional tests (intracellular calcium transients) performed at two in vitro culture time points spanning the early stages of cardiac development (day 20 versus 40 of cell in vitro culture) confirmed the ability of the obtained myogenic cells to acquire adult features of differentiated cardiomyocytes. Prolonged 40-day iPSC-derived cardiomyocytes (iPSC-CMs) revealed progressive cellular hypertrophy; a better-developed contractile apparatus; expression of marker genes similar to human myocardial ventricular cells, including a statistically significant CX43 increase, an MHC isoform switch, and a troponin I isoform transition; more efficient intercellular calcium handling; and a stronger response to β-adrenergic stimulation.
Keywords: Cardiac differentiation, cardiomyocyte maturation, iPSCs, skeletal myoblasts, cardiomyogenesis
Introduction
Coronary heart disease, also known as coronary artery disease, is one of the main causes of death worldwide1. Post myocardial infarction (MI), the heart is unable to regenerate itself. Currently, one of the guidelines for treatment of the post-infarction heart is autologous stem cell intervention. Nevertheless, obtaining an appropriate number of cardiac progenitor cells (CPCs) through biopsy from the patient has proven to be highly impractical. This is the reason for identification of other adult stem cell sources available in human tissues, such as the bone marrow, skeletal muscle, fetal or mature heart muscle, adipose tissue, or umbilical cord blood2. However, thus far, only cardiac progenitor cells seemed to possess sufficient plasticity to form highly functional cardiomyocytes3.
The development of induced pluripotent stem cell (iPSC) technology holds great promise for significant progress in understanding the basis mechanisms underlying cardiomyogenesis, and may aid in further biomedical analysis, including screening of new drugs and possible important clinical applications4. Importantly, the development of efficient protocols for cell differentiation towards desired somatic cell types may also contribute to providing a new source for personalized cardiac myocytes (CMs).
The first attempts to differentiate pluripotent stem cells into myogenic cardiac cells followed observations of embryonic stem cell (ESC) lines derived from inner cell mass (ICM) at the blastocyst stage5. Certain potent protein families were found to play an essential role in triggering cardiac signaling pathways, namely, WNTs, transforming growth factor beta (TGF-β), fibroblast growth factor (FGF), and activin A6. More recently, cardiac induction has been supported by replacing existing protocols for pluripotent stem cell induction with small molecule chemical compounds or organic compounds, including histone deacetylase (HDAC) inhibitors such as trichostatin A7 and/or ascorbic acid8.
Acquiring a mature human cardiomyocyte phenotype in vivo takes from 6 to 10 years9. Obviously, in laboratory conditions, speeding up this process is desired; however, in vitro differentiated cardiomyocytes often demonstrate phenotypic similarities to native cardiac cells at a fetal stage. For example, after a couple of weeks of in vitro cell culture, human embryonic stem cell (hESC)- and human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes (ESC-CMs and iPSC-CMs, respectively) featured underdeveloped contractile performance, weak subcellular structural organization, relatively low release of intracellular calcium, and defective Ca2+ uptake kinetics10.
Likewise, mouse neonatal cardiomyocytes and human ESC-CMs were reputed to exhibit hypertrophy in serum-free medium11, which was manifested at the ultrastructural level12. It was only after a year of human iPSC-CM maintenance under laboratory conditions that the M band was confirmed to exhibit sarcomere structural maturity. Electrophysiological assays revealed that within 3 months of human ESC-CM culture, a gradual maturation occurs that can be observed in kinetics of the ion currents13. However, in this period, contraction was not increased14. There are some controversies over the tendency of the beating rate during cell in vitro maturation – in some studies the beating rate rose gradually11, and in others it slowed14. Nevertheless, conduction velocity increased after 2 months of in vitro cell culture15.
Thus, some protocols involved additional stimuli applied at the time of cell differentiation to improve this process. Many factors promote in vitro cardiac differentiation, including substrate stiffness16, cell patterning17–19, electrical pacing20,21, β-adrenergic stimulation11, and supplementation with other compounds affecting hormonal mechanisms associated with cytokine, chemokine, and protein growth factor secretion22. Other driving elements include microRNA, that is, let-7 and miR-123, plating cardiomyocytes onto 3D structures, and mechanical stretching24. Furthermore, iPSC-CM incubation with triiodothyronine or insulin-like growth factor 1 (IGF-1) was shown to obtain the desired effects of a mature phenotype25–27.
The incubation time of iPSC-CM in vitro cultures positively influences cell morphology, structural organization and functional properties, including electrophysiology. Long-term cardiac cell maintenance, 3 months or longer, results in cell hypertrophy and anisotropy, increased myofibril density, and a more organized sarcomeric arrangement. A significant increase in multinucleated cardiomyocytes has also been observed10.
Here, we report an in vitro maturation process for the development of derived SMiPSC-CMs (skeletal muscle-induced iPSC-derived cardiomyocytes) within 40 days of in vitro culture and examine their resulting morphology, structural organization, gene expression, contractile apparatus, calcium handling, and contractile performance. To the best of our knowledge, this is the first attempt at cardiac myogenic differentiation of human induced pluripotent cells obtained by genetic reprogramming from skeletal myoblasts (SkMCs). Due to the close embryogenetic paths of myoblasts and cardiac myocytes and some evidence of common ‘epigenetic memory’ in iPSC lines derived from ontogenetically similar cell types, this approach may be advantageous and better mimic myogenic cardiac cell properties. The set of assays performed here can contribute to further comparative experiments with the other origin-derived iPSC-cardiomyocytes when studying a variety of biochemical or biophysical factors promoting cardiac maturation. Moreover, 40-day in vitro culture of CM-like cells may find application for modeling heart disease, new in vitro drug screening, cardiotoxicity analysis, and basic knowledge of cardiomyogenesis.
Materials and Methods
The Local Ethical Committee, University of Medical Sciences (Permission No. 818/13), Poznan approved the protocol for human tissue collection, and all donors provided written informed consent.
Myoblast Cell in Vitro Culture
Skeletal muscle stem cells were obtained from a 19-year-old patient undergoing a surgical procedure for cruciate ligament reconstruction in Poznan Voivodship Hospital. Cells were cultured in standard Modified Eagle’s Medium with 4.5 g/l glucose, supplemented with 20% fetal bovine serum (Lonza, Bazylea, Switzerland), 1% antibiotics, 1% ultra-glutamine and bFGF (Sigma-Aldrich, St. Louis, MO, USA) as previously described28. The cells were maintained in vitro under standard cell culture conditions (95% humidity, 5% CO2 at 37°C).
Human iPSC Derivation and Maintenance
The 194 cell line of SMiPSCs was derived from isolated SkMCs by using Sendai virus provided by a CytoTune®-iPS 2.0 Sendai Reprogramming Kit (Thermo Fisher, Waltham, MA, USA). The procedure was performed according to the manufacturer’s instructions for feeder-free reprogramming of fibroblasts. Briefly, 5 × 104 human myoblasts were plated onto six-well dishes, and after 2 days, when they reached 60–70% confluency (approx. 2.5 × 105 per well), they were subjected to genetic reprogramming (Supplementary Figure 1). The most effective multiplicity of infection (MOI) set was 10–10–6, respectively, for KOS (the Yamanaka factors: human KLF4, OCT, SOX2) – hc-MYC – hKLF4 gene components. After 24 hours, the transduction medium was replaced with regular myoblast medium and changed every other day. On day 7, the transduced cells were seeded onto Geltrex-coated culture dishes. The next day, the medium for myoblasts was exchanged with complete Essential 8TM medium (Life Technologies, Carlsbad, CA, USA). The medium was then replaced every day, and culture wells were monitored for the appearance of iPSC colonies. Starting from the third week of the procedure, all reprogrammed individual cell colonies typical for ESC morphology were picked and clonally expanded. iPSC colonies were checked for pluripotency by performing live staining with SSEA-4 (1:100, Abcam, Cambridge, UK). Clones of the 194 iPSC line were routinely maintained on Geltrex-coated wells in complete Essential 8TM medium. Cells were passaged every 4–5 days using 0.5 mM EDTA (Thermo Fisher, Waltham, MA, USA) in Dulbecco’s phosphate-buffered saline (D-PBS) without CaCl2 or MgCl2. For the first day of culture after passaging, 10 μM Rho kinase inhibitor Y-27632 (Sigma-Aldrich, St. Louis, MO, USA) was added. The in vitro cell culture was maintained in standard conditions at 95% humidity, 5% CO2, and 37°C.
Guided Cardiac Differentiation
Two different cardiac myogenic differentiation protocols were used, as follows.
BMP4 and Other Small Molecule Induction29
At 90% cell confluency, on day 3 or 4 after SMiPSC generation, cardiac differentiation was induced by adding 25 ng/mL BMP4 (Life Technologies, Carlsbad, USA) and 5 μM CHIR99021 (http://Selleckchem.com, Houston, TX, USA) in RPMI1640 medium (Life Technologies, Carlsbad, USA), which activated the WNT pathway, and 3 days later, 10 μM IWR1 (Sigma-Aldrich, St. Louis, USA) was added to inhibit this signaling. After 7 days of cardiac differentiation, insulin-depleted medium was exchanged with insulin-supplemented medium to promote further cell proliferation. On day 12, the differentiated cell population was metabolically selected via a 4-day incubation with 4 mM lactate-supplemented DMEM w/o glucose (Thermo Fisher, Waltham, USA). After day 16, enrichment medium was exchanged with basal medium (RPMI+B27+glutamine). The differentiation scheme is presented in Supplementary Figure 2.
PSC Cardiomyocyte Differentiation Kit
When iPSCs reached 70% confluency on day 4, cardiac differentiation was induced by applying a 2-day incubation in Medium A provided in a PSC Cardiomyocyte Differentiation Kit (Life Technologies, Carlsbad, USA). Next, medium B was added for another 2 days and exchanged with Cardiomyocyte Maintenance Medium (M) every other day. Additionally, from day 12 to day 16, cells were subjected to metabolic selection and maintained for 4 days in enrichment medium – DMEM w/o glucose supplemented with 4 mM lactate. A scheme of the protocol is presented in Supplementary Figure 3.
Karyotype Analysis
SMiPSCs were incubated with colcemid (10 μg/mL) (Life Technologies, Carlsbad, USA) for 30 minutes. The supernatant was aspirated, and cells were trypsinized, split into single cells, and collected for a 5-minute centrifugation at 1600 rpm. Afterwards, 2 mL of warm 0.075 M KCl (0.56%) solution was added dropwise while vortexing, and the cells were incubated at 37°C for 30 minutes. After this time, six to eight drops of fresh chilled 3:1 methanol: acetic acid fixative was added, and the cells were incubated for 20 minutes.
Samples were centrifuged at 2000 rpm at 4°C for 10 minutes. The supernatant was removed, another solution was added dropwise with 5 mL of cold fixative under vortexing, and the cells were finally spun down at 4°C, 2000 rpm for 10 minutes. This step was repeated twice, and cells were observed on cover glasses to detect iPSC chromosomes arrested in metaphase. Samples were frozen at –20°C and subjected to G-band staining and cytogenetic analysis.
Spontaneous Differentiation by Embryoid Bodies
Embryoid bodies (EBs) were generated after passaging of iPSC and ESC colonies using type IV collagenase (1 mg/mL) (Life Technologies, Carlsbad, USA) and cultured in suspension culture on Petri dishes. Differentiation medium consisted of Essential 8 supplemented with
4 mg/mL polyvinyl alcohol (PVA) (Sigma-Aldrich, St. Louis, USA) to prevent colony adhesion. After 5 days of incubation, EBs were transferred onto cover glasses and placed in adhesive culture dishes. They were fixed after 14 days according to the abovementioned protocol for immunofluorescence staining.
EBs were immunostained against derivatives of the three germ layers, AFP, SMA, and TUJ1, using a 3-Germ Layer Immunocytochemistry Kit (Thermo Fisher Scientific, Carlsbad, USA) according to the manufacturer’s instructions.
Teratoma Formation
The Local Bioethical Committee for Animal Research in Poznan approved the protocol for experiments performed in the mice post-infarction heart model and for teratoma formation (Permission No. 13/2017). Approximately 3 × 106 iPSCs and ESCs were administered subcutaneously into athymic mice (NUDE strain). Earlier, cell cultures were passaged and resuspended in 100 µL PSC medium and Matrigel (BD Biosciences, San Jose, CA, USA). Mice were previously anesthetized by applying a mixture of ketamine and xylazine. Mouse termination and teratoma derivation occurred after 3 months. Paraffin sections were histologically prepared for detection of the three germ layer derivatives in the Department of Clinical Pathology, Heliodor Swiecicki Clinical Hospital No. 2 of the Poznan University of Medical Sciences and the Department of Clinical Pathology, Poznan University of Medical Sciences (Supplementary Figure 4).
Immunofluorescence Assay
Staining was performed with the antibodies specified in Supplementary Table 1. The cells were fixed in 4% paraformaldehyde in PBS. After three washes with PBS, the cells were incubated for 15 min with 0.1% Triton X-100 in PBS to permeabilize cell membranes. After being washed with PBS, the cells were incubated in 10% goat serum diluted in PBS with 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, USA) to block unspecific epitopes for another 60 minutes at room temperature. After removal of the blocking serum, the cells were incubated overnight at 4°C with primary antibody diluted in 10% goat serum. The next day, cells were incubated for 1 hour with secondary antibody conjugated with fluorochromes. After three washes with PBS, DAPI (Sigma-Aldrich, St. Louis, USA) was added to visualize cell nuclei (Sigma-Aldrich, St. Louis, USA). The stained preparations were observed under Leica DMi8 and Olympus BX40 fluorescence microscopes.
Mitochondrial Staining
Mitochondrial assays were performed on days 20 and 40 of cardiomyocyte differentiation in in vitro culture. Previously, SMiPSC-CMs were seeded onto cover glasses covered with Geltrex. The cells were loaded with 200 nM MitoTracker Green FM (Thermo Fisher, Waltham, USA) for 30 minutes at 37°C. Next, cells were washed twice with D-PBS and monitored using a fluorescence microscope. Additionally, nuclei were stained with 1 μM Hoechst 34580 (live cell dye) (Thermo Fisher, Waltham, USA) to detect their localization within the cells.
Immunofluorescent staining of mitochondria was performed with JC-1 dye to detect mitochondrial membrane potential (Δψm). In functional mitochondria (with a highly developed membrane potential), this cationic lipophilic calcium probe generates complexes called J-aggregates, which emit a red fluorescence signal (585 nm). In inactive organelles or apoptotic cells with a low Δψm, JC-1 occurs in monomeric form, which can be observed as green fluorescence (530 nm). JC-1 (1.5 μM) was added to the CM medium for 30 minutes. After two washes with D-PBS, the cells were immediately observed. For signal detection, a Zeiss Imager.D1 microscope was applied.
Flow Cytometry Evaluation
The iPSC-CMs were evaluated by flow cytometry using an anti-cardiac troponin T (TNNT2) primary antibody (1:200, Abcam, Cambridge, UK) and a fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:1000, Abcam, Cambridge, UK). Briefly, cardiac cells were cultured in 12-well dishes and harvested on days 20 and 40 of culture with collagenase II (300U) and 0.25% trypsin, dissociated by pipette, centrifuged and resuspended in 1 mL of 1% fetal bovine serum (FBS). Further cell preparation was conducted according to a previously described protocol30. The proportion of TNNT2-positive cells was analyzed with a Cell Lab Quanta Flow cytometer (Beckman Coulter, Brea, CA, USA).
RNA Isolation
Cultured in vitro cells were harvested, centrifuged, and resuspended in 1 mL of TRI Reagent (Sigma-Aldrich, St. Louis, USA). Afterwards, a standard protocol for RNA isolation was followed according to the manufacturer’s instructions. Isolated total RNA was further purified from DNases with a Turbo DNA free™ Kit from Thermo Fisher Scientific (Waltham, USA). Moreover, purified RNA was used as a template for quantitative polymerase chain reaction (qPCR) to check for any remaining DNA in the analyzed samples. SuperScript IV (Invitrogen, Carlsbad, CA, USA) was then used in a reverse transcriptase reaction to obtain cDNA. For samples used for pluripotency validation, isolated RNA was purified with Oligo(dT)25 (Invitrogen, Carlsbad, USA) to obtain a pure mRNA fraction. cDNA was synthesized with SuperScriptIII reverse transcriptase (Invitrogen, Carlsbad, USA). cDNA quality was evaluated with a regular PCR reaction for β-actin gene expression.
Quantitative PCR Analysis
The expression of pluripotent and cardiac myocyte genes was evaluated by qRT-PCR using SYBRGreen (iQ SYBR Green Supermix, Bio-Rad, Hercules, CA, USA). Relative expression was calculated in respect to two housekeeping genes – β-actin and GAPDH. All the applied primers and product sizes are listed in Supplementary Table 2. qRT-PCR was performed using a CFXConnect Real-Time System by Bio-Rad (USA). All the PCR and qRT-PCR reaction conditions are shown in Supplementary Table 3. Reaction efficiency and the correlation coefficient for every selected gene are listed in the supplementary data (Supplementary Table 4).
Calcium Imaging
Intracellular calcium kinetics was measured using the ratiometric indicator dye FURA2-AM (Thermo Fisher, Waltham, USA). In brief, cells were incubated with 5 μM FURA2-AM for 20 min at 37°C, washed with D-PBS, and transferred to fresh cardiac maintenance medium. Spontaneous calcium transients were monitored under a 40× objective embedded in a fluorescence microscope (Leica DMi8) using a Calcium Imaging module (hardware synchronized mode enabled signal registering even below 100 ms per channel). The substantial advantage of the system was fluorescence detection directly in the culture dishes. Thus, the same cardiomyocyte culture sample could be examined under specified conditions (at 37°C and 5% CO2), both at day 20 and 40 of differentiation. Cytoplasmic Fura-2 was excited at 340 nm (excitation wavelength of the Ca2+-bound form; exposure time was set to 50 ms) and 387 nm (excitation wavelength of the Ca2+-unbound form; exposure time 8 ms). The fluorescent signal was detected with a 510 nm filter. The parameters of calcium handling were inferred from the emitted fluorescence intensity ratio F (F340 nm/F 380 nm). Calcium parameters were determined with LASX software by Leica Microsystems (Wetzlar, Germany).
Additionally, the beating stimulation effect of beta-adrenergic receptors was documented via a 3-minute cell incubation with 10 µM isoproterenol (Sigma-Aldrich, St. Louis, USA). Intracellular Ca2+ content in sarcoplasmic reticulum (SR) was estimated by applying 20 mM caffeine (Sigma-Aldrich, St. Louis, USA) to induce SR Ca2+ release. For the 20-day differentiation time point, the medium was refreshed after the signal measurement, and iPSC-CMs were maintained for the next 20 days in in vitro culture to monitor the calcium performance at the 40-day time point. During all fluorescence measurements, the cells were kept in standard culture conditions at 37°C and 5% CO2.
Statistical Analysis
Relative gene expression levels were quantified using the geNorm tool31. iPSC, ESC, myoblast, and cardiomyocyte samples were compared by using one-way ANOVA with a Bonferroni multiple comparison post hoc test at an α=0.05 significance level.
Samples for calcium handling parameters were compared using Student’s t-test at a 95% confidence interval. The statistical analysis of the data was achieved using GraphPad Prism software, version 5.03 for Windows.
Results
Characterization of a Skeletal Myoblast-Derived iPSC Line
A human iPSC 194 line (SMiPSCs) was generated through genetic reprogramming of skeletal myoblasts (2 × 105 cells per well) with a Sendai virus vector after the sixth passage. The suggested MOI values were elevated compared with the producer’s recommendation to yield high efficiency, although the initial cell cytotoxicity was slightly increased. In the third week of genetic reprogramming, the first colonies appeared, and within the next month of iPSC in vitro culture, three clones (iPSC 194 cl. 10, 11, and 13) were propagated and validated for their pluripotency (Supplementary Figure 5). Following the exclusion of any remaining Sendai virus or transgene (Supplementary Figure 1), gene and protein expression were evaluated. Human embryonic stem cells (ESC P27) after the 27th passage were used as positive control samples, and primary skeletal myoblasts served as the negative reference.
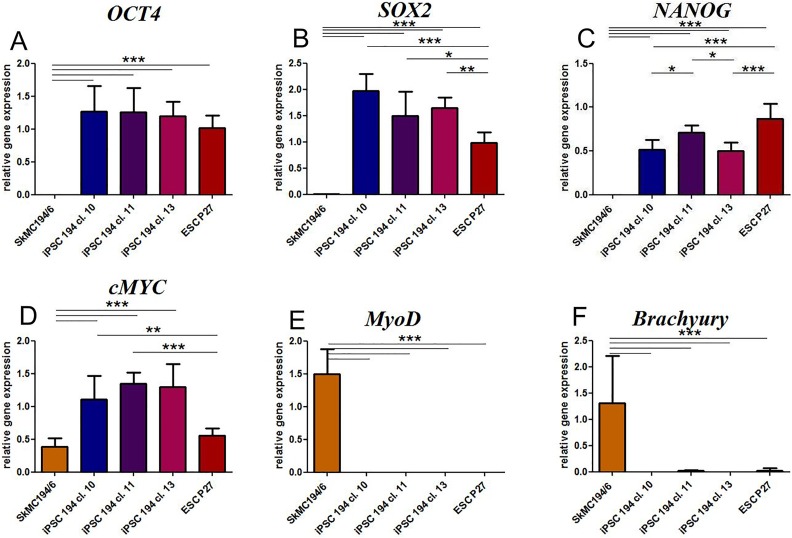
In all the three clones obtained from the SMiPSC line, the transcription level for the endogenous pluripotential genes OCT4, SOX2, NANOG, and c-MYC was similar and even higher than in ESC P27 cells (Figure 1 (a) to (d)). Moreover, there were significant differences between iPSCs and myoblasts with respect to the expression of these genes (p<0.001). In fact, in human myoblasts, OCT4, SOX2, and NANOG were not detected, while a low number of c-MYC transcripts were visible. The c-MYC levels expressed in myoblasts and in the ESC P27 cell line were similar. As a matter of fact, as a regulatory factor, this gene should not be strictly associated with pluripotent genes, while myoblasts per se are not completely considered differentiated cells. It is also worth mentioning that the low c-MYC level in ESCs in comparison to SMiPSC clones might be the result of their spontaneous differentiation in in vitro culture. The validity of the myoblast population was assessed by elevated expression of the MyoD gene (Figure 1(e)). With respect to the pluripotent cell line, MyoD as well as the mesodermal Brachyury (Figure 1(f)) gene levels were negligible.
Figure 1.
Endogenous gene expression markers in established cell clones of the iPSC 194 cell line: (a) OCT4, (b) SOX2, (c) NANOG, (d) c-MYC (typical of pluripotent cells), (e) MyoD (skeletal myoblast marker), and (f) BRACHYURY (mesoderm marker). Human embryonal cells (ESC P27) served as a positive control. The expression of the studied genes was normalized to the expression of two housekeeping genes (ACTB and GAPDH). Samples: SkMC 194/6: skeletal myoblast cells from patient no. 194 after the sixth passage; iPSC 194 cl. 10/11/13: clones 10, 11, and 13 of the induced pluripotent stem cell line no. 194 of myoblastoid origin; ESC P27: embryonal stem cell line after the 27th passage. Values are given as means ± SD; *p < 0.05, **p < 0.01, ***p < 0.001. ACTB: β-actin; c-MYC: cellular c-Myc oncogene product; ESCs: embryonic stem cells; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; iPSCs: induced pluripotent stem cells; MyoD: myogenic differentiation 1; OCT4: octamer-binding transcription factor 4; SOX2: sex determining region Y - box 2.
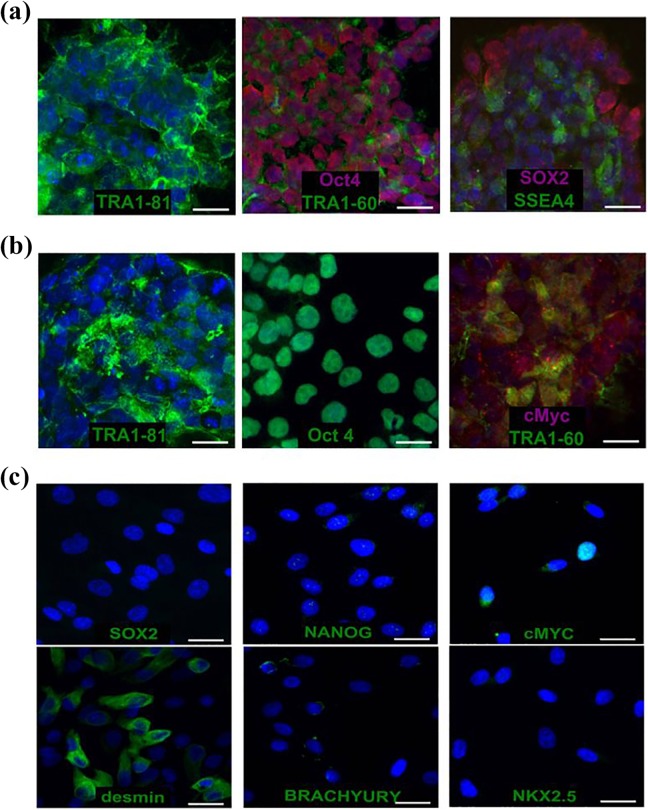
In turn, at the protein level in the SMiPSC line, the immunofluorescence staining revealed adequate levels of the nuclear markers for pluripotency, namely, OCT4 and SOX2, and the typical surface antigens SSEA-4, TRA1-60, and TRA1-81 (Figure 2(a)). In the control ESC P27 cell line, pluripotency markers (Figure 2 (b)), surface TRA1-60 and TRA1-81 antigens, and nuclear OCT4 and c-MYC were detected. In contrast, skeletal myoblasts did not express SOX2 and NANOG, proteins typical of pluripotent cells (Figure 2(c)). Nevertheless, the individual cells revealed a low intensity signal for c-MYC, which was consistent with the qPCR analysis. As expected, myoblast desmin was detected, whereas BRACHYURY, a mesoderm marker, and NKX2.5, a cardiac progenitor marker, were not present in stained cells.
Figure 2.
Immunostaining of: (a) SMiPSCs with the pluripotency markers located in nuclei (OCT4 and SOX2) and on the surface (TRA1-60, TRA-81, and SSEA-4 antigens); (b) ESC (P27) line with the nuclear markers OCT4 and c-MYC and the surface markers TRA1-60 and TRA-81; (c) human myoblasts as a primary cell suspension with SOX2, NANOG, and c-MYC (pluripotency markers), desmin (muscle marker), BRACHYURY (mesoderm indicator), and NKX2-5 (cardiac marker). Scale bar: 50 μm.
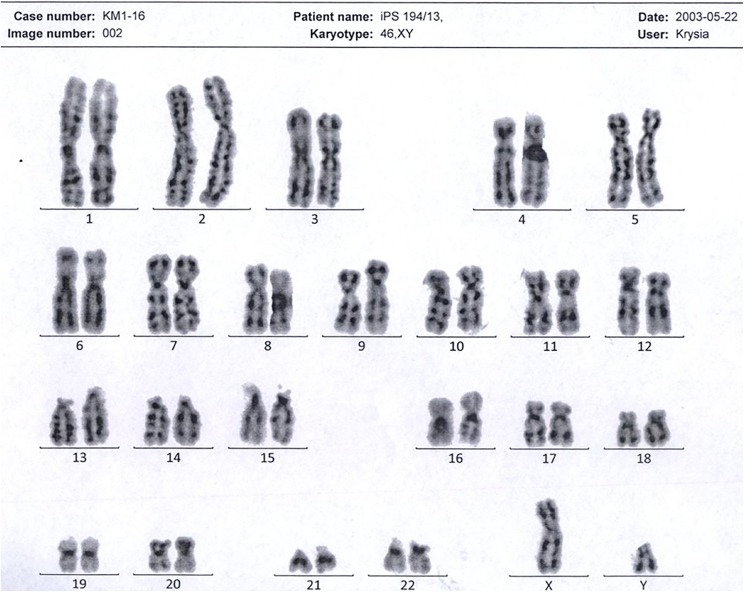
To preclude chromosomal aberrations and proceed with cell differentiation, the generated SMiPSC clones were karyotyped. From the cell pellet, 20 metaphase cells were analyzed (Figure 3). Staining with Giemsa verified the normal male 46, XY karyotype. Cell examination at 300 bands ruled out aneuploidy and/or extensive structural aberrations. However, the resolution of the stained specimen did not allow detection of small irregularities.
Figure 3.
A normal male karyotype was obtained from the generated SMiPSC 194 line.
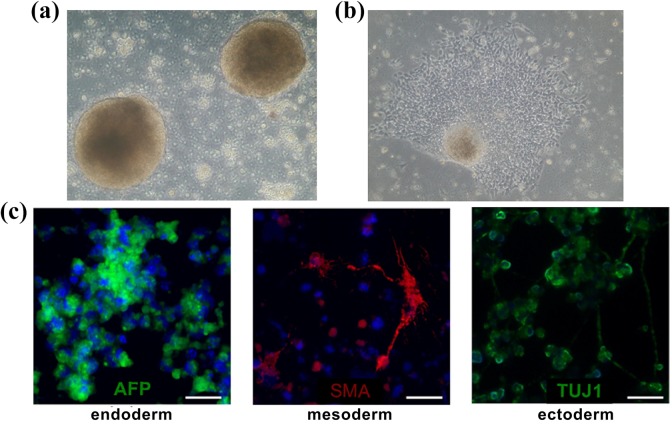
The next assay to verify pluripotency concerned EB formation. In suspension culture, the EBs appeared after a couple of days. Plating the EBs onto adherent dishes induced EB outgrowth that was directed into all cell lineages. After 14 days of in vitro EB culture, they were collected, fixed, and immunostained, and α-fetoprotein (AFP) (endodermal lineage), smooth muscle actin (SMA) (mesodermal derivative), and β-tubulin (TUJ-1) (originated from ectoderm) were detected (Figure 4).
Figure 4.
Images taken from spontaneous in vitro differentiation via embryoid bodies: (a) SMiPSC-derived embryoid bodies on day 5 of in vitro suspension culture; (b) outgrowing embryoid body in adherent cell culture; (c) immunolabeled EBs demonstrated α-fetoprotein (AFP), smooth muscle actin (SMA), and neural class III β-tubulin (TUJ-1) expression. Magnification of (a) and (b) pictures at 10×. Scale is 50 μm.
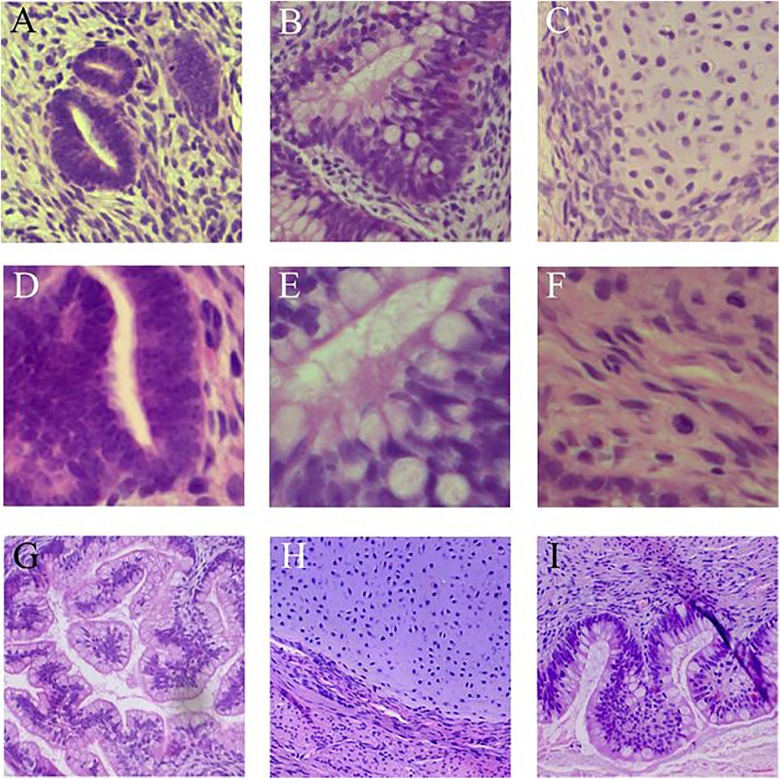
The most stringent functional assay of pluripotency is teratoma formation. SMiPSC line 194 was subcutaneously injected into immunocompromised mice, and after 3 months, formed tumors were fixed, sliced, and analyzed for histological structure examination. The cross-section analysis revealed the formation of cell-like structures with non-uniform tissue histology between void cavities (Supplementary Figure 4). Within the teratoma, the isolated areas were arranged in neural tubes and rosettes as ectoderm derivatives, secretory cells of the endoderm and in cells specifically connected to mesenchymal stem cell differentiation, including chondroid tissue-like structures and smooth muscle-like areas (Figure 5(a) to (i)).
Figure 5.
Tissue-like structures specific for derivatives of three germ layers in SMiPSC-derived teratoma sections: (a) and (d): neural tube; (b), (e), (g), (i): endodermal originated secretory cells; (c), (f), (h): mesodermal derivatives: chondroid tissue (c), connective tissue (f), and chondroid tissue surrounded by smooth muscle cells (h). Images acquired at 40× ((a) to (c)), 63× ((d) to (f), and 20× ((g) to (i)) magnification with a Leica DMi8 fluorescence microscope.
Cardiac Differentiation of Skeletal Myoblast-Derived iPSCs
The high quality-verified SMiPSC line 194 was further differentiated using two methods based on monolayer culture. The first protocol, developed by Kadari et al.29, applied BMP-4 protein and the chemical modulator CHIR99021 to induce the WNT pathway and IWR-1 for subsequent inhibition of the pathway. Some modifications, such as insulin withdrawal in the first 48 hours of culture, were introduced during semi-quantitative optimization (Supplementary Table 5).
The second applied method of cardiomyocyte differentiation was based on a PSC Cardiomyocyte Differentiation Kit (Thermo Fisher Scientific, Waltham, USA), and a 4-day metabolic selection with lactate was added to the procedure. The first spontaneous contractions were noticed beginning on days 7–8 of differentiation, and after a few days, the cells formed a surface with a consistent, synchronized beating frequency (Supplementary Movie 1). The approach had higher cardiomyocyte generation efficiency and better reproducibility than the other technique, which settled the question of its choice for further studies.
Samples were collected at two time points and checked for cardiomyocyte content via flow cytometry. The percentage of the TNNT2-positive population predominantly reached approximately 70% but in some samples was 90% (Supplementary Figure 6).
Development of a Contractile Apparatus in SM-iPSC-Derived CMs in In Vitro Cell Culture
Cardiomyocyte immunostaining at 20 and 40 days of in vitro culture revealed the expression of the following marker proteins: NKX2.5, cardiac troponin T, heavy myosin chain α, and connexin 43 (Figure 6(a) to (d)). On day 20, the level of NKX2.5 and connexin 43 proteins was similar for both time points, wherein the CX43 signal was located within the perinuclear region. In turn, TNNT2 and α-MHC expression was characterized by a relatively higher density in 40-day SMiPSC-CMs.
Figure 6.
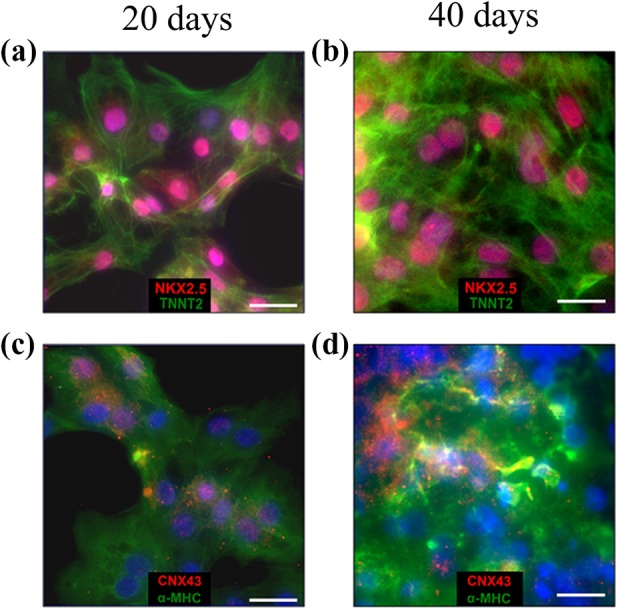
Immunostaining on days 20 and 40 of cardiac differentiation in in vitro culture of NKX2.5 (an early cardiac differentiation marker), TNNT2 (cardiac troponin) ((a) and (b)) and α-MHC (myosin heavy chain α) (cardiac-specific marker), CNX43 (intercellular junction marker) ((c) and (d)). Scale bar is 50 μm.
The length of developed sarcomeres within the contractile apparatus was tested by staining cells with α-actinin (Figure 7(a)). On day 40, a marked improvement was detected in sarcomere organization, which was evidenced by higher density and more visible striation alignment across the cells (Figure 7(c) and (d)). In turn, 20-day cardiomyocytes had irregular subcellular organization and lower myofibril density. Further evidence of a more mature SMiPSC-CM phenotype was a statistically significant elongation of sarcomere length, from 1.31±0.03 μm on day 20 to 1.66±0.03 μm on day 40 of cell differentiation (Figure 7(b)).
Figure 7.

Developing sarcomeres in SMiPSC-CMs: (a) Sarcomere measurements using LAS X software from a DMi8 fluorescence microscope. (b) The results of sarcomere length measurements for 55 cardiac cells on days 20 and 40 of in vitro differentiation. (c) and (d) α-actinin immunostaining at two analyzed cardiac differentiation time points. Plots: mean value + SEM. Scale bar is 50 μm.
Immunofluorescence images enabled the quantification of morphological differences occurring within 40 days of SMiPSC-CM in vitro culture (Figure 8). Although the roundness index did not change considerably (insignificant decrease from 0.57±0.02 to 0.54±0.02), the mean cell perimeter increased from 152.5±10.4 μm on day 20 to 182.7±10.59 μm on day 40 (p<0.05). Likewise, the cell area increased significantly from 1146±87 μm2 on day 20 of differentiation in in vitro cell culture to 1752±164 μm2 on day 40.
Figure 8.
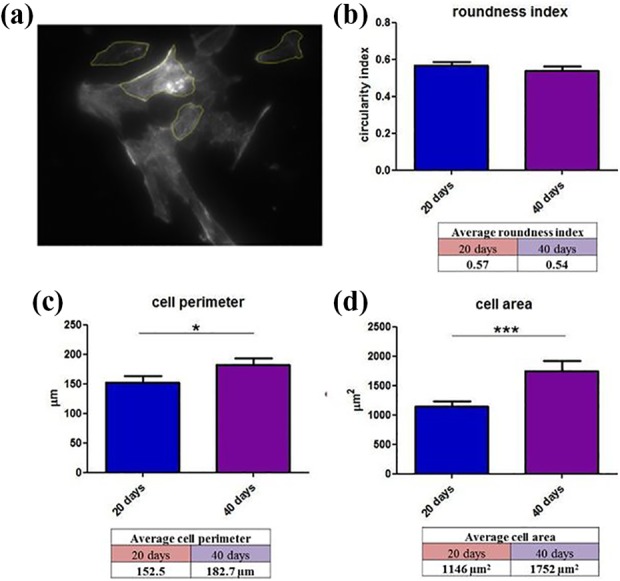
Morphological parameters measured in cardiac myocytes (n=70) in in vitro cell differentiation culture: (a) Image included for measurement with LAS X software. Calculations were given as follows: (b) roundness index, (c) cell perimeter, and (d) cell area. Plots: mean value + SEM.
During maturation, cardiomyocytes gradually lose their proliferative capacity, and thus, DNA replication is no longer associated with cell division (cytokinesis). As a consequence, more cardiac cells become multinuclear and are connected by gap junctions, forming functional syncytia. This was observed with in vitro differentiation of SMiPSC-CMs, in which the contribution of binuclear cardiac cells rose considerably from 16% on day 20 to 29% on day 40 (p<0.001) (Figure 9).
Figure 9.
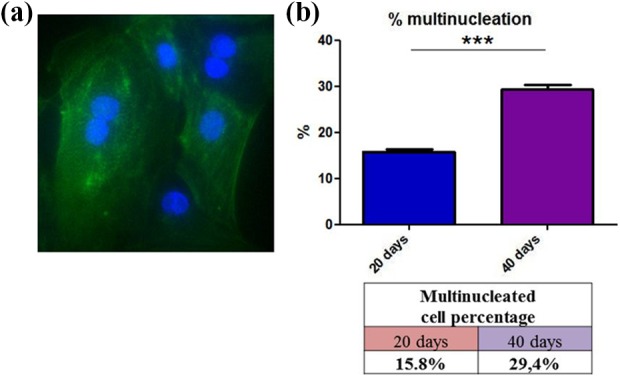
The binucleated cell content in differentiated cardiomyocytes on days 20 and 40 of in vitro culture: (a) Image showing α-actinin staining on day 40. (b) Percentage of multinucleated cells in the 200 cells counted for each analyzed time point.
Assessment of Mitochondrial Abundance and Function in In Vitro Cardiac Cell Differentiation Culture
Examination of mitochondrial morphology and function was performed using MitoTracker Green and JC-1 staining. The first dye enabled detection of the total pool of mitochondria in the cell, and the second distinguished functional organelles exhibiting good mitochondrial membrane potential.
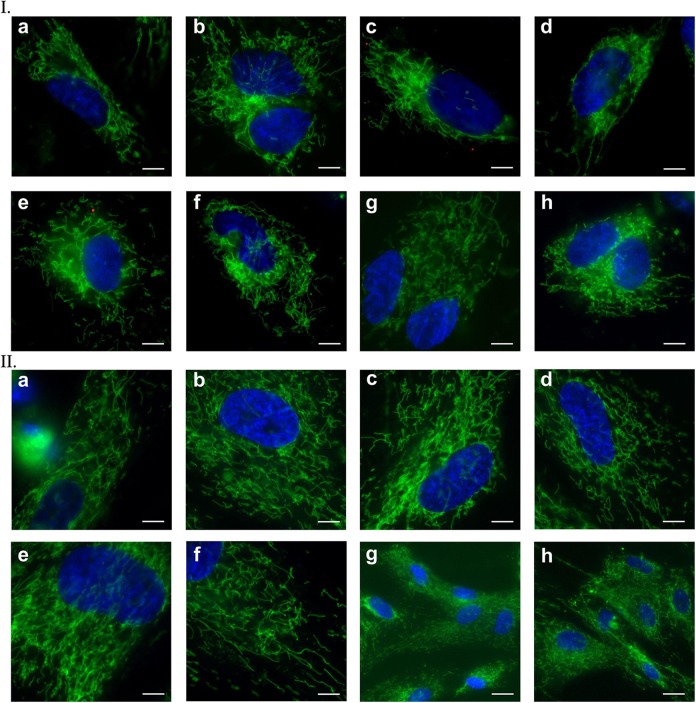
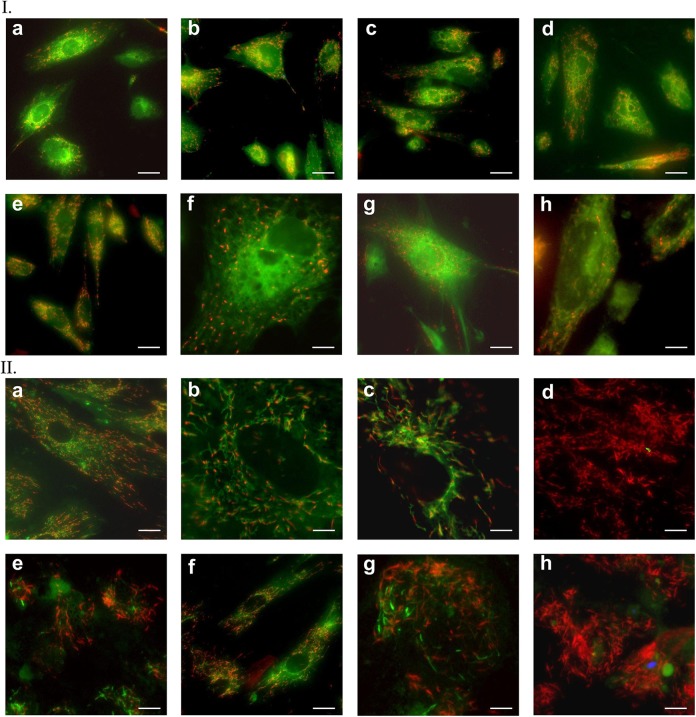
MitoTracker staining revealed changes occurring in differentiation in vitro cultures. Most mitochondria in 20-day SMiPSC-CMs were centered around the nucleus (Figure 10 I (a) to (h)). With increasing cell hypertrophy, the mitochondrial network spread evenly in cells to form a system of interconnected channels in 40-day SMiPSC-CMs (Figure 10 II (a) to (h)). In addition, the mitochondrial density became greater in 40-day-old SMiPSC-CMs. In earlier cardiac myocytes (day 20 of in vitro differentiation culture), the potential was identified mainly within mitochondria with a granular structure (Figure 11 I (a) to (h)). JC-1 staining indicated a greater number of functional mitochondria at day 40 of in vitro culture, and simultaneously, they became more elongated in shape and larger in size (Figure 11 II (a) to (h)).
Figure 10.
Mitochondrial staining with MitoTracker Green after days 20 (I) and 40 (II) of cardiomyocyte differentiation in vitro. (a) to (h) Pictures of both analyzed time points refer to selected stained areas of in vitro cell culture. Scale bar: 50 μm and 150 μm for II(g) and II(h) images, respectively.
Figure 11.
Mitochondrial staining with JC-1 dye on the 20th (I) and 40th (II) day of SMiPSC-CM in vitro differentiation culture. (a) to (h) Pictures of both analyzed time points refer to selected stained areas of in vitro cell culture. Scale bar: 50 μm and 150 μm for II(g) and II(h) images, respectively.
Gene Expression Analysis Reveals Isoform Switch of Cardiac Markers
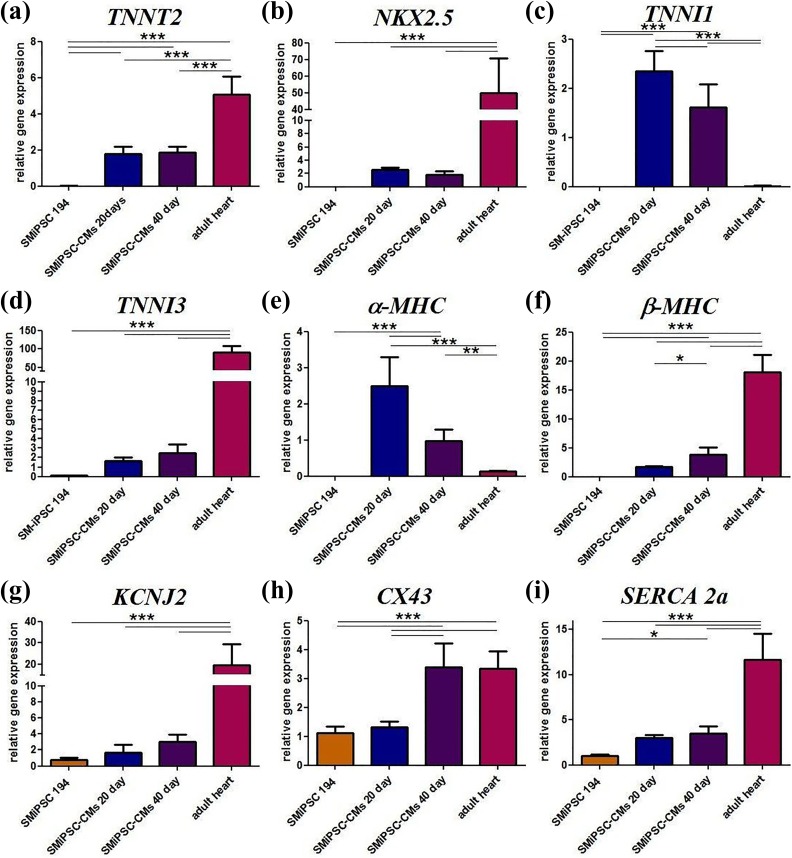
Cardiac troponin T (TNNT2) levels were similar for both the analyzed time points in all the in vitro differentiation cell samples. This indicates an equal content of cardiac cells in the analyzed samples, which then allows proper comparison of the expression of other genes. TNNT2 is essential for spontaneous beating, and its gene expression in adult human ventricular cells was two-fold higher than in SMiPSC-CMs (p<0.001) (Figure 12(a)).
Figure 12.
Cardiac gene expression in generated SMiPSC-CMs on days 20 and 40 of in vitro culture: (a) TNNT2, (b) NKX2.5, (c) TNNI1, (d) TNNI3, (e) α-MHC, (f) β-MHC, (g) KCNJ2, (h) CX43, and (i) SERCA 2a. ACTB and GAPDH gene expression was used to normalize the examined gene expression levels. Samples: SMiPSC 194: 194 line of induced pluripotent stem cells of skeletal myoblast origin as a negative control; SMiPSC-CMs 20/40 day: differentiated SMiPSC-derived cardiomyocytes on days 20 and 40 of in vitro cell differentiation culture; adult heart: sample collected from ventricular heart muscle as a positive reference. Plot: mean value + SD.
There were no significant changes in the expression of the late cardiac progenitor cell marker NKX2.5. However, it was detected in the adult heart sample (positive control) and exceeded 20-fold the level observed in in vitro cultured differentiated cardiac cells (p<0.001) (Figure 12(b)).
A statistically significant decrease in subtype 1 of troponin I was observed after another 20 days of in vitro culture, and there was a nearly marginal level of TNNI1 in the adult heart (p<0.001) (Figure 12(c)). In contrast, a specific cardiac marker for adult heart, subtype 3 of troponin I, was increased (although insignificantly) within 20 days of extended cardiomyocyte in vitro culture (Figure 12(d)). Furthermore, adult cardiac cells expressed 30-fold more TNNI3 troponin than SMiPSC-CMs (p<0.001). qPCR showed that troponin I isoforms may switch over time.
The highest expression level of the gene encoding the fast isoform of cardiac α-myosin heavy chain was detected on day 20 of SMiPSC-CMs in vitro culture (Figure 12(e)). Extended in vitro culture resulted in a statistically significant two-fold decrease in α-MHC expression (p<0.001). An even lower, nearly 20-fold, decrease in α-MHC expression was found in adult heart tissue samples (p<0.001) compared with SMiPSC-CMs on day 20.
Quite the opposite phenomenon appeared with respect to the gene expression results for the isoform of cardiac β myosin heavy chain (Figure 12(f)). The strongest β-MHC expression was detected for adult cardiac ventricular myocytes and was more than four-fold higher than that for 40-day cardiomyocytes (p<0.001). The gene expression in 20-day SMiPSC-CMs was found to be two-fold lower (p<0.05) than that in cell suspension cultured in vitro for 40 days.
A growth in gene expression encoding the inwardly rectifying potassium channel Kir2.1 (KCNJ2) was subtly observed during in vitro cell differentiation culture but exhibited no statistical significance (Figure 12(g)). As expected, the highest expression was measured in the adult heart sample, whereas in 40-day SMiPSC-CMs, expression was six-fold lower (p<0.001), and this value seemed to be inversely proportional to the degree of cardiac differentiation. KCNJ2 was also expressed in control SMiPSC suspension, but at a minimal level.
Intriguingly, SMiPSCs and early 20-day cardiomyocytes had almost equal levels of CX43 gene expression, while the expression for both 40-day CMs and cells from the adult heart ventricle was several-fold higher (p<0.001) (Figure 12(h)). These data may suggest that after roughly a month of cardiac differentiation, connexin 43 in SMiPSC-CMs increased nearly two-fold and levelled up to the expression found in adult ventricular cardiomyocytes.
A similar expression pattern was observed for the gene encoding the sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA 2a), which is responsible for calcium uptake preceding diastole (Figure 12(i)). In fact, SERCA 2a expression in adult heart exceeded three-fold the level detected in in vitro differentiated cardiomyocytes (p<0.001). There were no significant differences in SMiPSC-CMs in vitro differentiation cultures. Apart from this, SMiPSCs also expressed the SERCA 2a gene, but at a three-fold lower level than 20-day SMiPSC-CM suspension.
In addition, generated cardiomyocytes were tested for tendency to possible tumorigenesis. In comparison to the cell source, SM-iPSCs, expression of pluripotent genes, namely, OCT4, SOX2, and NANOG, was several-fold lower (p<0.001), and we have found them to be at a negligible level (Supplementary Figure 7). Nevertheless, the c-MYC level detected in iPSC-CMs was similar to that of the pluripotent cells, and only at 40 days did iPSC-CMs show significantly lower c-MYC transcription (p<0.05).
Imaging of Intracellular Calcium Flow with FURA-2 dye Facilitates Assessment of Cardiomyocyte Contractile Performance
Calcium-induced calcium release initiates mechanical contraction in cardiac myocyte cells. In the aftermath of Ca2+ influx through the L-type calcium channels, a robust calcium release from the SR via RyR channels has been observed. Diastolic calcium uptake from the cytosol occurs mainly through SR calcium ATPase (SERCA) but also through other gates such as sodium/calcium exchangers or mitochondrial uniports. Identification of the Ca2+ flux pattern of cardiomyocytes allowed validation of changes in the functional state of key calcium components responsible for generating spontaneous contractions between days 20 and 40 of in vitro cardiac differentiation. The analysis was performed based on the fluorescence of FURA-2, a ratiometric dye, by investigating its intensity and using a fluorescence ratio (F ratio) reflecting contractile performance (Supplementary Movie 2)32.
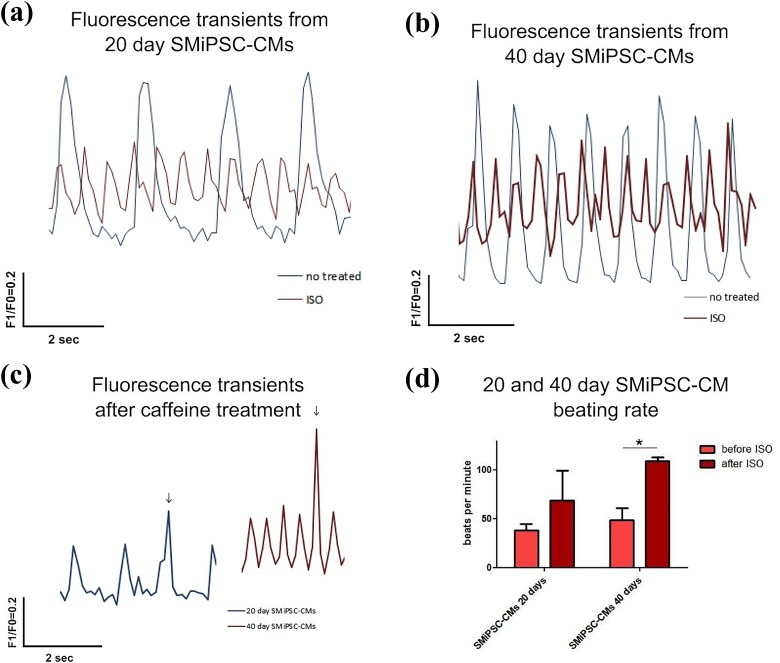
For 20-day SMiPSC-CMs, 38 beats per minute on average were observed, while 40-day cardiomyocytes exhibited 49 beats per minute (Figure 13(a) and (b)). In both cases, addition of 10 μM isoproterenol, a beta-adrenergic agonist, resulted in a pronounced response that was stronger in 40-day cardiomyocytes. Stimulated 20-day SMiPSC-CMs contracted on average 69 times per minute. However, more mature 40-day SMiPSC-CMs showed greater excitability, provoking a gathering pace of approximately 109 beats per minute (p<0.05; Figure 13(d)). Thus, there was a more than two-fold difference between the two compared in vitro culture time points.
Figure 13.
Calcium transients recorded before and after isoproterenol administration on days 20 and 40 of cardiac in vitro differentiation: (a) Isoproterenol treatment of 20-day cardiomyocytes reduced the amplitude of intracellular Ca2+ concentration [Ca2+]i transients, which at an increased rate of [Ca2+]i transients may be the cause of the decrease in voltage-gated L-type Ca2+ current (ICaL) or systolic SR Ca2+ content (i.e., impaired RyR functioning). (b) 40-day cardiomyocytes had a higher basal [Ca2+]i amplitude level than did 20-day cells in in vitro differentiation culture. (c) The fluorescence peak in caffeine-treated 20-day and 40-day SMiPSC-CM suspensions quickly reverted to the previous calcium transient rate, suggesting improperly activated RyR receptors. The time resolution of the signal collected with a Leica DMi8 fluorescence microscope was approx. 10 times per second. F1/F0 represents the normalized FURA-2 emission fluorescence ratio from excitation at 340 nm and 380 nm. (d) The beating rate of 20- and 40-day SMiPSC-CMs before and after isoproterenol treatment. The beating frequency of SMIPSC-CMs was measured as an average value from three cell culture wells (triplicate); five beating areas were considered within each well. * Statistically significant increase of the beating rate after ISO administration on day 40 (p<0.05)
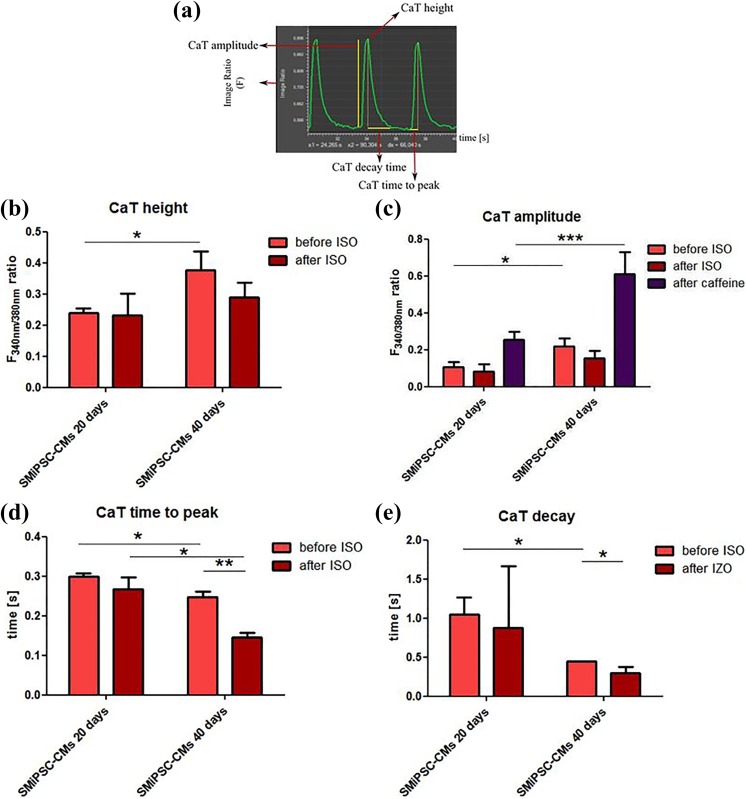
Calcium transients, including beating characteristics, were evaluated by measuring changes in the fluorescence intensity kinetics parameters (Figure 14(a)). The rise in the F ratio for CaT height transients was significantly different for SMiPSC-CMs at analysed time points (0.24±0.01 on day 20 vs. 0.38±0.06 F/F0 on day 40, p<0.01) (Figure 14(b)). A more than two-fold higher F ratio of CaT peak amplitude in 40-day cardiomyocytes was detected (0.11±0.03 vs. 0.22±0.05, p<0.05), and even after isoproterenol stimulation of 40-day CMs, the F ratio did not drop below the level observed in day 20 SMiPSC-CMs (Figure 14(c)). To some extent, this may suggest more abundant and better functioning calcium channels located in the SR and higher capacity of calcium stores in more mature cardiac myoblasts, resulting in stronger contractile force33. However, isoproterenol administration did not cause statistically relevant changes in either diastolic CaT height (0.23±0.07 vs. 0.29±0.05) or CaT amplitude of fluorescence (0.08±0.04 vs. 0.16±0.04) on days 20 and 40 of SMiPSC-CM in vitro differentiation (Figure 14(a) and (b)).
Figure 14.
Parameters of transient calcium turnover in the 194 cell line of SMiPSC-derived cardiomyocytes after 20 and 40 days of cell differentiation in vitro. (a) Demonstration of calcium parameters: CaT height is the maximum value of the F340 nm/F380 nm ratio at which Ca2+ triggers cell contraction. At this point, the calcium quantity in the cytoplasm reaches the highest level, and on the graph, the CaT height is denominated in a peak point; the CaT amplitude of the F1/F0 ratio refers to the magnitude of fluorescence changes from diastole (calcium residing in SR stores) until systole (triggered by calcium ions released to the cytoplasm); CaT time to peak is the time to reach the maximal F1/F0 ratio after Ca2+ release from the SR; CaT decay is the time needed to take up Ca2+ from the cytoplasm prior to diastole. (b) Average height of the fluorescence signal (F340nm/F380 nm ratio) refers to the intracellular calcium pool during contraction and before and after isoproterenol (ISO) administration. (c) Amplitude of fluorescence changes during SMiPSC-CM contraction, before and after isoproterenol administration and after caffeine treatment (activating RyR receptor and preventing Ca ion uptake into SR stores). (d) Time-to-peak F340nm/F380 nm ratio shortening before and after ISO addition. (e) The time of calcium decay before and after isoproterenol administration. The calcium measurements were taken in three cell culture areas, and the mean values originated from five repeats for each area examined.
Caffeine addition hampered calcium uptake when activating non-selective voltage-dependent RyR channels during diastole and enabled detection of a two-fold higher F1/F0 ratio in 40-day cardiac myocytes than in younger 20-day cells, characterized by diminished CaT amplitude (0.25±0.05 vs. 0.61±0.12, p<0.001) (Figure 14(c)). A rapid spike of F1/F0 ratio following caffeine treatment, however, immediately decreased and returned to basal rhythm at both observed time points (Figure 13(c)), implying still ineffective activation of RyRs.
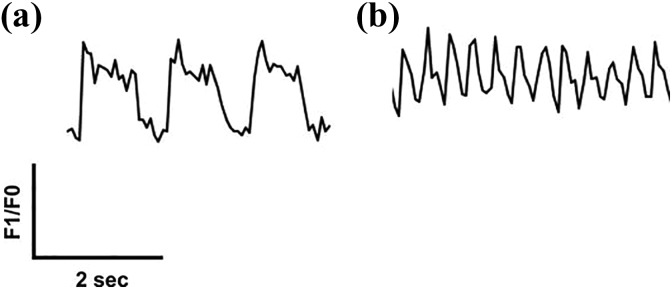
The maximal F1/F0 ratio, and thereby calcium content in the cytoplasm, was reached faster in 40-day SMiPSC-CMs (p<0.05) (Figure 14(d)) than in 20-day SMiPSC-CMs. Calcium transient time to peak [Ca2+]i diminished from an average of 299±2 ms on day 20 to 247±2 ms on day 40 of cardiac differentiation. Isoproterenol-treated cardiac cells had faster Ca2+-transient decay, from 274±2 ms on day 20 to 146±2 ms on day 40 (p<0.01) of in vitro differentiated cells (Figure 14(e)). More mature, 40-day CMs seemed to possess greater excitability for external beta-agonists. Similarly, calcium decay in the cytoplasm evoking diastole was faster in extended cardiomyocyte cell in vitro culture – the time was significantly shortened from 1043±229 ms for 20-day CMs to 449±2 ms on day 40 of CM differentiation (p<0.05). For better-developed 40-day culture SMiPSC-CMs, the addition of isoproterenol reduced the time of Ca2+ reuptake kinetics to only 292±82 ms (p<0.05) (Figure 14(e)). In this respect, 20-day SMiPSC-CMs appeared to be more heterogeneous, as in some in vitro culture areas the intracellular calcium uptake was delayed, which clearly moderated the heart beating rate (Figure 15).
Figure 15.
Comparison of diverse transient kinetics of Ca2+ in 20-day SMiPSC-CMs under isoproterenol treatment. (a) Demonstration of a faulty calcium turnover pattern in 20-day iPSC-derived cardiomyocytes after isoproterenol stimulation in two analyzed cell culture areas. Clusters with slowed contractions (36 beats per minute on average) and disturbed calcium flow, including its uptake from the cytoplasm, a long plateau phase (lasting up to 1 second), and a slower calcium decay time reflect impairment of the contractile machinery. (b) The normal course of calcium transient morphology for another fragment of the examined cell culture surface. Measurements were conducted within 1 minute of fluorescent signal registration.
Discussion
This study revealed that extended in vitro culture of SMiPSC-derived cardiomyocytes, up to 40 days, gradually increased cardiomyocyte maturation in terms of morphology, structure, and function of the contractile apparatus, expression of cardiac marker genes and proteins, and the mitochondrial network.
Fibroblasts have been commonly used in iPSC technology for pluripotency induction34. However, in this study, human skeletal muscle myoblasts were selected as a source cell type. They have similar embryogenesis pathways and thus may show better efficiency for myocardial development and in respect to myocardial cardiomyocyte electrophysiology. Such similarity can influence SMiPSC-CM performance based on the reported evidence of an epigenetic memory similar to that of cardiomyocytes35,36. A possible additional advantage is the non-completely differentiated status of skeletal myoblasts.
Until now, there have been few reports regarding iPSC lines derived from human skeletal muscle progenitor cells subsequently differentiated towards cardiomyocytes. Previous studies have predominantly concentrated on mouse and rat cells and showed the positive impact of transplanted differentiated iPSCs of myogenic origin on myocardial regeneration37–39.
The obtained SMiPSC 194 cell line clones successfully passed the pluripotency tests. SMiPSCs expressed genes and marker proteins typical of ESCs. Genetic reprogramming with Sendai virus vector did not cause chromosomal aberrations. The EBs exhibited three germ layer derivatives, and the SMiPSC line proved its pluripotency through teratoma generation in an immunodeficient mouse. Eventually, the SMiPSC line differentiated towards CM-like cells via two applied culture differentiation protocols.
Undoubtedly, the obtained cardiac myocytes revealed a fetal phenotype, as described elsewhere.10,40 Nevertheless, changes were evident after just 40 days of in vitro cell differentiation. Extended in vitro cardiomyocyte culture revealed progressive hypertrophy in terms of cell perimeter and area, as has been previously documented for cardiomyocytes10. In the long run, an anisotropic cylindrical and more compact morphology might be expected41. Similarly to cardiac muscle cells in the first months after birth, SMiPSC-derived CMs exhibited gradual growth of multinucleated cardiac cells, evidencing a decline in proliferation and the formation of functional syncytia, as repeatedly described42.
In addition, the mitochondrial network was transformed over time in in vitro culture. With enlarging cells, slender and thread mitochondria were distributed more regularly and densely throughout the entire cell volume, which may support the high energy demands in spontaneously beating CMs. These organelles formed a reticulum of channels with generated membrane potentials, and they seemed to arrange along the contractile myofilaments. Although perinuclear mitochondrial clustering was not detected under a fluorescence microscope43, heterogeneity of mitochondrial morphology and membrane potential was more conspicuous in 40-day CMs.
After in vitro cell culture extended by an additional 20 days, cardiac myocyte cells randomly arranged into muscle fibers and formed more regular striatum in accordance with adjacent myofibers. Improved contractile performance was confirmed by elongation of sarcomere length by more than 20%. This measurement is comparable with other in vitro maturation studies10. It is worth noting that it is necessary to trigger stronger contractile force and cell deformation under systole. Furthermore, shifts identified on the molecular level should influence contractile apparatus ability. A specific fetal to adult isoform switch of myosin heavy chains (beta to alpha-MHC ratio) and troponin I (slow-twitch TNNI1 to TNNI3, exclusively expressed in adult heart) was also documented. Nevertheless, substantial differences can be distinguished between the gene expression of SMiPSC-CMs and that of mature myocardium, including TNNT2 and NKX2.5 gene expression, which demonstrated developmental (ontogenic) distance of differentiated in vitro CMs in laboratory conditions and their native counterparts. This finding is consistent with other studies, but in some reports, the α-MHC expression level increased throughout the differentiation period10,44, as opposed to the results obtained in our study. Discrepancies between gene expression observed in SMiPSC-CMs and in adult heart myocytes, as well as acceleration of cardiac maturation, could certainly be achieved by better reconstruction of physiological conditions by applying extra factors, including, for example, tri-iodo-L-thyronine supplementation26, proper electrical stimulation21, 3D coculture systems of tissue engineering and mechanical stress conditioning,45 or cyclic stretching46.
Elevated levels of the β-MHC (MYH7) gene on day 40 of in vitro differentiation hints at a ventricular subtype of cardiomyocytes, as this gene is preferentially expressed in heart ventricles47 and has previously been observed in longer maintenance of in vitro differentiated cells (β-MHC level was dependent on cardiomyocyte subtype)44. However, higher expression of the gene encoding a slow MHC isoform was not sufficient to slow down the beating of SMiPSC-CMs, which is the case in the post-natal period. In contrast, acceleration of pacing was observed. As a matter of fact, other groups have reported contradictory results for iPSC- and ESC-derived CMs in respect to this issue48, and it is thought that the origin of reprogrammed cells and in vitro culture conditions may affect this process. Moreover, in post-infarcted heart model animal studies, an explanation of the MHC transition significance was hindered due to the lack of this process in rodents and thus requires follow-up studies in large animal models, such as rabbits or pigs49.
Satisfactory results were obtained for connexin 43 expression, which was similar for both 40-day SMiPSC-CMs and adult cardiac ventricular cells. In Kamakura’s group, the same level was reached as late as 180 days into in vitro differentiation of CMs.44 However, CX43 protein concentrations in intercalated discs were not observed. Connexin 43 was distributed irregularly but predominantly located in the perinuclear region. This gap junction GJA1 protein supports better contraction propagation throughout the myocardium50 and promotes electrical cell-to-cell coupling of cardiac cell grafts with host infarcted heart, which prevents post-transplantation arrhythmias51.
Elementary calcium measurements with ratiometric FURA-2 disclosed temporal changes in SMiPSC-CM functioning. The kinetics of calcium transients in analyzed time points suggests a rise in the intracellular Ca2+ pool, triggering more powerful systole. An increased amplitude of the F ratio was not reported in other studies10, and here the issue was not firmly proven. Calcium transient changes after caffeine treatment suggest that mature CMs have a more abundant pool of intracellular calcium in the SR, and the mechanism of its handling is better developed. However, when using a Leica DMi8 system, it is not possible to electrically pre-stimulate CMs to stabilize the SR calcium stores. Calcium release was short in time and amplitude, and then, F1/F0 returned to its primordial pace, which might denote ineffectively activated RyR receptors. Thus, the results are not conclusive. In addition, the isoprenaline excitability yielded a greater chronotropic and lusitropic effect in 40-day SMiPSC-CMs, but a simultaneously lower calcium transient amplitude implicated shorter and weaker contractions. In another study, PSC-CMs stimulated by this beta-adrenergic agonist did not develop relevant isometric force during spontaneous beating, which is evidence of immature SR52. Faster decay time at lower calcium transient amplitude is characteristic for cardiomyocytes with reduced CASQ2 expression, which makes RyR receptors prone to premature reactivation53. The obtained data did not demonstrate significant changes in SERCA 2a expression, as we expected.
It is worth noting that in 20-day SMiPSC-derived cardiac myocytes, disorders in normal intracellular calcium turnover after isoproterenol stimulation emerged, which may be a side effect of an underdeveloped contractile apparatus, including calcium channels and adrenergic receptor malfunction (see Figure 15). Primarily, it shows the heterogeneity of differentiated cardiomyocytes in terms of contractile performance in the early phase of in vitro cell culture and may hinder unambiguous interpretation of results.
The safety of cellular therapy applying SMiPSC-CMs in in vivo conditions should be further discussed. Additional tests for pluripotency in 20-day and 40-day cardiomyocytes demonstrated a significant decline of OCT4, SOX2, and NANOG expression, which was then profoundly confirmed in preliminary data originated from transplanting SMiPSC-CMs to the post-infarcted heart in an immunocompromised mouse model (data not shown). Three months following the cell administration, no signs of tumorigenesis were noticed, and even enhanced myocardial performance was revealed. In turn, an elevated c-MYC level in SMiPSC-CMs may be associated with multiple functions of this transcription factor. c-MYC has been involved in cardiac hypertrophy (presented in this study), cell cycle re-entry leading to higher DNA synthesis and increased nuclei number per myocyte54, regulation of substrate metabolism promoting free fatty acid oxidation55, mitochondrial biogenesis, and possibly augmented recovery from ischemia56. However, during cardiac maturation under normal conditions, c-MYC expression gradually ceases. Thus, c-MYC expression per se in cardiomyocytes cannot be perceived only in terms of tumorigenesis risk57.
In conclusion, taking into account the unaltered KCNJ2 level, the presumable improvement in calcium turnover at 40 days of in vitro cell culture is just the beginning of proper formation of all contractile apparatus elements. Nonetheless, with regard to the field of cellular therapies for infarcted heart regeneration, the question of which is the best developmental stage for transplanted iPSC-CMs is far from being answered. According to recent reports, perhaps underdeveloped donor re- and preprogrammed CMs have good flexibility, and the best way to develop them is to adapt signals from mature-phenotype cardiomyocytes of the recipient heart50,58. This was preliminarily confirmed in our initial animal studies using immunocompromised mice (n=9), when the 40-day SMiPSC-CMs intervention to post-infarction heart provided hemodynamic improvement of 10% ejection fraction on average (data not shown).
There are obvious limitations in the studies conducted here. First, only a few clones of the iPSC 194 line were tested, and therefore, additional research using multiple cell lines and a variety of pro-maturing factors could extend the presented findings. Likewise, other functional studies may also be considered, that is, assessment of calcium quantity at the protein level, capture ion channel flows, contractility strength, or electrophysiological activity of SMiPSC-CMs. Moreover, further in vivo animal studies are essential for in vitro obtained cardiomyocytes to indicate their efficacy in supporting the myocardium in the failing heart model.
Supplementary Material
Footnotes
Ethical Approval: The Local Ethical Comittee, Poznan University of Medical Sciences, permission No. 818/13 approved utilization of human tissues while the Local Bioethical Committee for Animal Research in Poznan approved the protocol for experiments performed in the mice post-infarction heart model and for teratoma formation (Permission No. 13/2017).
Statement of Human and Animal Rights: This study does not involve experimentation on human subjects while animal rights were protected according to the requirements by Europen Directive No. 2010/63/UE.
Statement of Informed Consent: Written informed consent was obtained doe to utilization of post-operative tissues.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by National Science Centre, Poland, grant number 2011/01/N/NZ1/04454 and National Centre for Research and Development, Poland, grant number STRATEGMED1/233624/5/NCBR/2014.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int J Cardiol. 2013;168(2):934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28(2):208–16. [DOI] [PubMed] [Google Scholar]
- 3. Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Bellamy V, Rücker-Martin C, Barbry P, Bel A, Bruneval P, Cowan C, Pouly J, Mitalipov S, Gouadon E, Binder P, Hagège A, Desnos M, Renaud J-F, Menasché P, Pucéat M. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120(4):1125–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. [DOI] [PubMed] [Google Scholar]
- 5. Thomson JA, Shapiro SS, Waknitz MA, Marshall VS. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. [DOI] [PubMed] [Google Scholar]
- 6. Burridge PW, Gold JD, Keller G, Wu JC. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10(1):16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lim SY, Sivakumaran P, Crombie DE, Dusting GJ, Pébay A, Dilley RJ. Trichostatin A enhances differentiation of human induced pluripotent stem cells to cardiogenic cells for cardiac tissue engineering. Stem Cells Transl Med. 2013;2(9):715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao N, Liu Z, Chen Z, Wang J, Chen T, Zhao X, Ma Y, Qin L, Kang J, Wei B, Wang L, Jin Y, Yang HT. Ascorbic acid enhances the cardiac differentiation of induced pluripotent stem cells through promoting the proliferation of cardiac progenitor cells. Cell Res. 2012;22(1):219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation. 1994;90:713–25. [DOI] [PubMed] [Google Scholar]
- 10. Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22(14):1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Földes G, Mioulane M, Wright JS, Liu AQ, Novak P, Merkely B, Gorelik J, Schneider MD, Ali NN, Harding SE. Modulation of human embryonic stem cell-derived cardiomyocyte growth: A testbed for studying human cardiac hypertrophy? J Mol Cell Cardiol. 2011;50(2):367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285(6):H2355–H2363. [DOI] [PubMed] [Google Scholar]
- 13. Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: A molecular and electrophysiological approach. Stem Cells. 2007;25(5):1136–44. [DOI] [PubMed] [Google Scholar]
- 14. Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, Delgado SM, Wakatsuki T, Crone WC, Pruitt BL, Palecek SP. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol. 2012;2012:508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kadota S, Minami I, Morone N, Heuser JE, Agladze K, Nakatsuji N. Development of a reentrant arrhythmia model in human pluripotent stem cell-derived cardiac cell sheets. Eur Heart J. 2013;34(15):1147–56. [DOI] [PubMed] [Google Scholar]
- 16. Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95(7):3479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang PY, Yu J, Lin JH, Tsai WB. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011;7(9):3285–93. [DOI] [PubMed] [Google Scholar]
- 18. Heidi Au HT, Cui B, Chu ZE, Veres T, Radisic M. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip. 2009;9(4):564–75. [DOI] [PubMed] [Google Scholar]
- 19. McDevitt TC, Angello JC, Whitney ML, Reinecke H, Hauschka SD, Murry CE, Stayton PS. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J Biomed Mater Res. 2002;60(3):472–9. [DOI] [PubMed] [Google Scholar]
- 20. Sathaye A, Bursac N, Sheehy S, Tung L. Electrical pacing counteracts intrinsic shortening of action potential duration of neonatal rat ventricular cells in culture. J Mol Cell Cardiol. 2006;41(4):633–41. [DOI] [PubMed] [Google Scholar]
- 21. Eng G, Lee BW, Protas L, Gagliardi M, Brown K, Kass RS, Keller G, Robinson RB, Vunjak-novakovic G. Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nat Commun. 2016;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denning C, Borgdorff V, Crutchley J, Firth KSA, George V, Kalra S, Kondrashov A, Hoang MD, Mosqueira D, Patel A, Prodanov L, Rajamohan D, Skarnes WC, Smith JGW, Young LE. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim Biophys Acta. 2016;1863(7 Pt B):1728–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fu JD, Rushing SN, Lieu DK, Chan CW, Kong CW, Geng L, Wilson KD, Chiamvimonvat N, Boheler KR, Wu JC, Keller G, Hajjar RJ, Li RA. Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2011;6(11):e27417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zimmermann WH, Schneiderbanger K, Schubert P, Didié M, Münzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90(2):223–30. [DOI] [PubMed] [Google Scholar]
- 25. Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL, Thornburg KL. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J. 2012;26(1):397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang X, Rodriguez M, Pabon L, Fischer KA, Reinecke H, Regnier M, Sniadecki NJ, Ruohola-Baker H, Murry CE. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J Mol Cell Cardiol. 2014;72:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Engels MC, Rajarajan K, Feistritzer R, Sharma A, Nielsen UB, Schalij MJ, De Vries AAF, Pijnappels DA, Wu SM. Insulin-like growth factor promotes cardiac lineage induction in vitro by selective expansion of early mesoderm. Stem Cells. 2014;32(6):1493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rozwadowska N, Fiszer D, Siminiak T, Kalawski R, Kurpisz M. Evaluation of in vitro culture of human myoblasts for tissue autotransplants to the post-infarcted heart. Pol Heart J. 2002;57:233–7. [Google Scholar]
- 29. Kadari A, Mekala S, Wagner N, Malan D, Köth J, Doll K, Stappert L, Eckert D, Peitz M, Matthes J, Sasse P, Herzig S, Brüstle O, Ergün S, Edenhofer F. Robust generation of cardiomyocytes from human iPS cells requires precise modulation of BMP and WNT signaling. Stem Cell Rev. 2015;11(4):560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhattacharya S, Burridge PW, Kropp EM, Chuppa SL, Kwok WM, Wu JC, Boheler KR, Gundry RL. High efficiency differentiation of human pluripotent stem cells to cardiomyocytes and characterization by flow cytometry. J Vis Exp. 2014;52010(91):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–50. [PubMed] [Google Scholar]
- 33. Li S, Chen G, Li RA. Calcium signalling of human pluripotent stem cell-derived cardiomyocytes. J Physiol. 2013;591(Pt 21):5279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. [DOI] [PubMed] [Google Scholar]
- 35. Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, Yu J, Hebrok M, Hochedlinger K, Costello JF, Song JS, Ramalho-Santos M. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat Cell Biol. 2011;13(5):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahmed RPH, Haider HK, Buccini S, Li L, Jiang S, Ashraf M. Reprogramming of skeletal myoblasts for induction of pluripotency for tumor-free cardiomyogenesis in the infarcted heart. Circ Res. 2011;109:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pasha Z, Haider HK, Ashraf M. Efficient non-viral reprogramming of myoblasts to stemness with a single small molecule to generate cardiac progenitor cells. PLoS One. 2011;6:e23667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singla DK, Long X, Glass C, Singla RD, Yan B. Induced pluripotent stem (iPS) cells repair and regenerate infarcted myocardium. Mol Pharm. 2011;8:1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34(23):5813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Louch WE, Sheehan KA, Wolska BM. Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol. 2011;51(3):288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rodriguez ML, Graham BT, Pabon LM, Han SJ, Murry CE, Sniadecki NJ. Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. J Biomech Eng. 2014;136(5):51005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kuznetsov AV, Troppmair J, Sucher R, Hermann M, Saks V, Margreiter R. Mitochondrial subpopulations and heterogeneity revealed by confocal imaging: Possible physiological role? Biochim Biophys Acta. 2006;1757(5–6):686–691. [DOI] [PubMed] [Google Scholar]
- 44. Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, Yamanaka S, Kimura T. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J. 2013;77(5):1307–14. [DOI] [PubMed] [Google Scholar]
- 45. Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mihic A, Li J, Miyagi Y, Gagliardi M, Li SH, Zu J, Weisel RD, Keller G, Li RK. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials. 2014;35(9):2798–808. [DOI] [PubMed] [Google Scholar]
- 47. Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol Rev. 1996;76(2):371–423. [DOI] [PubMed] [Google Scholar]
- 48. Robertson C, Tran DD, George SC. Concise review: Maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31(5):829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krenz M, Robbins J. Impact of beta-myosin heavy chain expression on cardiac function during stress. J Am Coll Cardiol. 2004;44(12):2390–7. [DOI] [PubMed] [Google Scholar]
- 50. Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538(7625):388–91. [DOI] [PubMed] [Google Scholar]
- 51. Gwizdala A, Rozwadowska N, Kolanowski TJ, Malcher A, Cieplucha A, Perek B, Seniuk W, Straburzynska-Migaj E, Oko-Sarnowska Z, Cholewinski W, Michalak M, Grajek S, Kurpisz M. Safety, feasibility and effectiveness of first in-human administration of muscle-derived stem/progenitor cells modified with connexin-43 gene for treatment of advanced chronic heart failure. Eur J Heart Fail. 2017;19(1):148–57. [DOI] [PubMed] [Google Scholar]
- 52. Yang X, Pabon L, Murry CE. Engineering adolescence: Maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114(3):511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Posnack NG, Idrees R, Ding H, Jaimes R, 3rd, Stybayeva G, Karabekian Z, Laflamme MA, Sarvazyan N. Exposure of human cardiomyocytes to phthalates affects calcium handling and intercellular connectivity. PLoS One. 2014;10(3):e0121927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xiao G, Mao S, Baumgarten G, Serrano J, Jordan MC, Roos KP, Fishbein MC, MacLellan WR. Inducible activation of c-Myc in adult myocardium in vivo provokes cardiac myocyte hypertrophy and reactivation of DNA synthesis. Circ Res. 2001;89(12):1122–9. [DOI] [PubMed] [Google Scholar]
- 55. Olson AK, Ledee D, Iwamoto K, Kajimoto M, O’Kelly Priddy C, Isern N, Portman MA. C-Myc induced compensated cardiac hypertrophy increases free fatty acid utilization for the citric acid cycle. J Mol Cell Cardiol. 2013;55:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ahuja P, Zhao P, Angelis E, Ruan H, Korge P, Olson A, Wang Y, Jin ES, Jeffrey FM, Portman M, MacLellan WR. Myc controls transcriptional regulation of cardiac metabolism and mitochondrial biogenesis in response to pathological stress in mice. J Clin Invest. 2010;120:1494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Masumura Y, Higo S, Asano Y, Kato H, Yan Y, Ishino S, Tsukamoto O, Kioka H, Hayashi T, Shintani Y, Yamazaki S, Minamino T, Kitakaze M, Komuro I, Takashima S, Sakata Y. Btg2 is a negative regulator of cardiomyocyte hypertrophy through a decrease in cytosolic RNA. Sci Rep. 2016;6:28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Funakoshi S, Miki K, Takaki T, Okubo C, Hatani T, Chonabayashi K, Nishikawa M, Takei I, Oishi A, Narita M, Hoshijima M, Kimura T, Yamanaka S, Yoshida Y. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci Rep. 2016;6:19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.