Abstract
The use of mesenchymal stromal cell (MSC) transplantation to repair the injured spinal cord has shown consistent benefits in preclinical models. However, the low survival rate of grafted MSC is one of the most important problems. In the injured spinal cord, transplanted cells are exposed to hypoxic conditions and exposed to nutritional deficiency caused by poor vascular supply. Also, the transplanted MSCs face cytotoxic stressors that cause cell death. The aim of this study was to compare adipose-derived MSCs (AD-MSCs) and bone marrow-derived MSCs (BM-MSCs) isolated from individual C57BL6/J mice in relation to: (i) cellular characteristics, (ii) tolerance to hypoxia, oxidative stress and serum-free conditions, and (iii) cellular survival rates after transplantation. AD-MSCs and BM-MSCs exhibited a similar cell surface marker profile, but expressed different levels of growth factors and cytokines. To research their relative stress tolerance, both types of stromal cells were incubated at 20.5% O2 or 1.0% O2 for 7 days. Results showed that AD-MSCs were more proliferative with greater culture viability under these hypoxic conditions than BM-MSCs. The MSCs were also incubated under H2O2-induced oxidative stress and in serum-free culture medium to induce stress. AD-MSCs were better able to tolerate these stress conditions than BM-MSCs; similarly when transplanted into the spinal cord injury region in vivo, AD-MSCs demonstrated a higher survival rate post transplantation Furthermore, this increased AD-MSC survival post transplantation was associated with preservation of axons and enhanced vascularization, as delineated by increases in anti-gamma isotype of protein kinase C and CD31 immunoreactivity, compared with the BM-MSC transplanted group. Hence, our results indicate that AD-MSCs are an attractive alternative to BM-MSCs for the treatment of severe spinal cord injury. However, it should be noted that the motor function was equally improved following moderate spinal cord injury in both groups, but with no significant improvement seen unfortunately following severe spinal cord injury in either group.
Keywords: Adipose-derived mesenchymal stromal cell (AD-MSC), bone marrow-derived mesenchymal stromal cell (BM-MSC), spinal cord injury (SCI), treatment
Introduction
Spinal cord injury (SCI) can cause severe neural tissue damage and result in a persistent loss of neurological function. Recent advances of stem cell-based therapy harbor expectations for new treatment options for patients with SCI. Mesenchymal stromal cells (MSCs) isolated from adult tissues have been reported to possess therapeutic activity with potential applications for SCI treatment. MSC transplantation into the injured spinal cord promotes axonal regeneration, spares the loss of neurons within the lesion and improves motor function1,2,3. We have reported that transplantation of bone marrow-derived MSCs (BM-MSCs) into an acute SCI model did not differentiate into glial or neuronal elements, but promoted functional recovery and axonal regeneration through production of neurotrophic factors and cytokines with immune regulation function4. In addition to improvement of motor functions, BM-MSCs also were shown to regulate neuropathic pain after SCI by neuronal protection and repair of the destructed blood–spinal cord barrier5.
Although previous studies showed the benefits of BM-MSC transplants, some problems have been revealed. SCI can cause various degrees of ischemia6 and oxidative stress7. These local tissue reactions to damage trigger the immune-inflammatory cascade and change the cytokine environment in the injured lesion, which is known as the secondary phase of the injury response. The stressful microenvironment and changing cytokine profile causes apoptosis of grafted cells, so one of the most important unresolved problems of future stem cell-based therapies for SCI is the enhancement of cell survival after transplantation. MSCs transplanted into the injured spinal cord are known to disappear in a few weeks8,9. We have reported that a combination therapy of BM-MSC transplants and treatment with anti-interleukin (IL)-6 receptor antibody increased transplanted BM-MSC survival and motor function recovery through anti-apoptotic activity and regulation of inflammatory reactions10. However, improvement of survival rates of transplanted MSCs alone, when they are undergoing severe cell stresses and immune-biological conditions, as is seen in the acute phase after SCI, remains a challenging problem.
Adipose-derived MSCs (AD-MSCs) were first isolated from human subcutaneous fat tissue and have multi-differentiation potential11. Adipose tissue was recently evaluated as an alternative source of cells to bone marrow, which can be harvested using less invasive methods under local anesthesia and in larger quantities than in bone marrow12. Approximately 1 g of adipose tissue yields 5×103 MSCs, which is 500-fold greater than that of bone marrow13. Recent studies have stated that AD-MSCs have the potential to treat SCI14–16. However, the therapeutic mechanisms of AD-MSCs and differences in their activity from BM-MSCs are still to be clarified.
In this study, we compared AD-MSCs and BM-MSCs to reveal differences between the cells derived from these tissue sources; in terms of the cell surface marker presence, mRNA expression of growth factors and cytokines, and stress tolerance in vitro. Furthermore, AD-MSCs and BM-MSCs were transplanted into a contusion model of SCI to examine MSC survival rates and promotion of motor function recovery.
Materials and Methods
Isolation and Culture of AD-MSCs and BM-MSCs
AD-MSCs were isolated from 8–10 week-old male C57BL/6 J mice (n=12, Nippon SLC, Japan) and green fluorescent protein (GFP)-AD-MSCs were isolated from transgenic GFP-positive mice of the same age (n=6; Nippon SLC). The mice were anesthetized and their subcutaneous adipose tissue was collected and minced using a razor blade. The adipose tissue was then enzymatically dissociated for 50 min at 37°C using 0.075% collagenase type I (Sigma-Aldrich, MO, USA). The solution was passed through a 70 μm filter and centrifuged at 250×g for 5 min. The cells were washed three times with phosphate-buffered saline (PBS), the pellet was resuspended and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen). Cultures were maintained at 80–90% confluent levels in a 37.5°C incubator with 5% CO2 and passaged with 0.025% trypsin/ethylenediaminetetraacetic acid (Invitrogen) when required.11 BM-MSCs were also isolated from 8–10 week-old male C57BL/6 J mice or CAG-EGFP mice and maintained under the same conditions as AD-MSCs. The mice were anesthetized and their limbs were amputated followed by removal of skin, muscle and as much connective tissue as possible. The mice were euthanized by CO2 after harvesting of adipose tissue and bone marrow. Bone marrow was harvested from the femur and tibia with 25-gauge needles and passed through a 70 µm filter and centrifuged at 250×g for 5 minutes. The cells were washed with PBS three times and cultured in the same culture medium as AD-MSCs (DMEM with 10% FBS), undergoing routine passaging through trypsinization at 80–90% confluence.
Flow Cytometry
To analyze the expression of specific cell surface proteins on AD-MSCs and BM-MSCs, flow cytometric analysis was performed (n=5 AD-MSCs and n=5 BM-MSCs). The cells from the third passage were trypsinized into single-cell suspensions and labeled with the following anti-mouse antibodies conjugated to fluorochromes: PerCP-Cy5.5-CD11b (0.25 μg/100 μl; BD Biosciences, Bedford, MA, USA), PE-CD14 (0.25 μg/100 μl; BioLegend, CA, USA), PE-CD34 (0.25 μg/100 μl; BioLegend), PE-CD44 (10 μl/100 μl; BioLegend), APC-CD45 (0.25 μg/100 μl; BioLegend), PE-CD49d (0.25 μg/100 μl; BioLegend), PE-CD73 (0.25 μg/100 μl; BioLegend), PE-CD90.2 (0.25 μg/100 μl; BioLegend), PE-CD105 (0.25 μg/100 μl; Abcam, Cambridge, UK), PE-CD106 (0.25 μg/100 μl; BioLegend), APC-CD133 (0.25 μg/100 μl; BioLegend) and PE-Sca-1 (0.5 μg/100 μl; BioLegend). Cells were incubated on ice for 45 minutes with each antibody. Corresponding mouse isotype antibodies were used as controls (1:5; Santa Cruz Biotechnology, TX, USA). The cells were analyzed using the fluorescence-activated cell sorting instrument (FACSCanto II; BD Biosciences) according to the manufacture’s protocol. The percentage of expressed antigen was calculated for 10,000 gated-cell events and the data processed (FACSDiva software; BD Biosciences).
Comparison Analysis of mRNA Expression of AD-MSC and BM-MSC
For assessment of in vitro mRNA expression of AD-MSCs and BM-MSCs, the QuantiGene Plex 2.0 Reagent System (Affymetrix, CA, USA) was used. This system is a sandwich nucleic acid hybridization method that uses branched DNA technology to directly quantify nucleic acid molecules at physiological levels17. Total RNA was isolated from 1×105 stem cells. AD-MSCs and BM-MSCs (n=3; both passage 3) were cultured in DMEM with 10% FBS in a T25 flask for 24 hours in a 37.5°C incubator with 5% CO2, and mRNA was isolated and purified according to the protocol of the manufacture. The mRNA expression was determined using the sets of bead-based oligonucleotide probe specific for mouse cytokines developed by Panomics Inc. (CA, USA), these included; chemokine ligand (CCL)2, CCL5, chemokine (C-X-C motif) ligand (CXCL)10, CXCL12, basic fibroblast growth factor (β-FGF), vascular endothelial growth factor A (VEGF-A), hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1), platelet-derived growth factor β (PDGF-β), brain-derived neurotrophic factor (BDNF), nerve growth factor, and angiopoietin 1 (Ang-1). Samples were analyzed using a Bio-Plex System Array reader with Luminex 100xMAP technology, and data were acquired using Bio-Plex Data Manager software version 5.0 (Bio-Rad Laboratories, CA, USA). Assays were performed according to the manufacturer’s protocol (Panomics Inc.). mRNA expression was normalized to the Hprt housekeeping gene, which was not significantly different between treatment groups.
Cell Culture under Different Oxygen Conditions
For assessment of cell proliferation under the hypoxic condition, AD-MSCs and BM-MSCs (n=3; both passage 3) were seeded into six-well plates at the density of 1×104 cells per well and cultured in DMEM with 10% FBS under the 20.5% O2 and 1.0% O2 conditions for 7 days. The culture medium was changed 1, 3, and 5 days after seeding. The cells were trypsinized and stained with 0.4% trypan blue and counted using a cell counter (Countess Automated Cell Counter; Invitrogen) at days 1, 3, 5, and 7 after seeding.
Quantification of Cell Viability Under Oxidative Stress
To investigate cellular viability and metabolic activity, the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay and calcein/EthD staining were performed. The XTT assay is based on the same principle as the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay, but has higher sensitivity and a higher dynamic range18. AD-MSCs and BM-MSCs (n=3; both passage 3) were seeded into 96-well plates at a density of 3×103 cells per well and cultured for 24 hours in DMEM with 10% FBS in a 37.5°C incubator with 5% CO2 to permit all cells to attach, and then the culture medium was replaced with DMEM with 10% FBS containing 0, 250, 500, 750, and 1000 µM of H2O2 (six replicate wells for the AD-MSCs or BM-MSCs tested) or with serum-free DMEM only (no H2O2 treatment), and incubated for a further 24 hours. After this the wells were washed twice with PBS, and 50 μl of the 0.5mg/ml XTT dye mixture (Cell Proliferation Kit II; Sigma-Aldrich) was added into each well. The plate was incubated in the XTT dye for 5 hours after which absorbance at 475 nm was measured by a UV-visible spectrophotometer microplate reader (VersaMax®; Molecular Devices, CA, USA). Cell viability was also investigated using calcein AM/EthD-1 staining kit (LIVE/DEAD® Viability/Cytotoxicity reagent; Invitrogen). This assay is a mixture of calcein AM and ethidium homodimer-1 (EthD-1). Calcein AM is a membrane-permeable dye that is used for labeling of living cells with impermeant green-wavelength fluorophore. Ethidium homodimer-1 does not permeate live cells, but it can enter into dead cells that have a porous membrane and bind to nucleotide to produce red fluorescence19. AD-MSCs and BM-MSCs were exposed to H2O2 or serum-free conditions for 24 hours, as described above, then the medium was removed and replaced with PBS containing 5 μM ethidium homodimer (EthD-1) and 5 μM calcein AM (LIVE/DEAD staining). After that cells were incubated at 37°C for 40 minutes and the number of living (green) or dead (red) cells were counted manually by inverted fluorescence microscopy, where cells were counted in 10 randomly selected microscopic fields (original magnification ×200) in each well.
Animal Model of SCI
Adult C57BL/6 J male mice 8–10 weeks old (n=128) and adult Thy1-YFP transgenic mice (https://www.jax.org/strain/003709) 8–10 weeks old (n=30) were used to examine the survival and effects of transplanted AD-MSCs and BM-MSCs. Thy-1 protein appears in the cell body, dendrites and in the axons at the terminal phase of axonal growth20,21. Hence, Thy1-YFP transgenic mice, which is the parent line backcrossed to C57BL/6 J, have been used as a means of tracing neuronal axonal growth in several studies22,23. The animals were anesthetized with an isoflurane (Forane®; Abbot Japan, Osaka, Japan) and oxygen mixture (2.5% for induction and 1.5% for maintenance) and underwent T9-T10 total laminectomy under magnification using microscope. A contusion SCI was produced using a commercially available SCI device (Infinite Horizons Impactor; Precision Systems & Instrumentation LLC, VA, USA) with an impact force of 60-kdyne (moderate contusion; n=80) or 80-kdyne (severe contusion; n=68, as previously described24,25,26. The mice received manual bladder expression twice daily until recovery of spontaneous sphincter control and voiding. They were then transferred to a bacteria-free biologically clean room set on a 12-hour light/dark cycle and provided with food and water ad libitum.
Animals and Experimental Groups
At 3 days after the injury, the animals were divided into eight sets of experimental conditions as follows:
moderate contusion model treated with AD-MSC (Mo-AD-MSC group; 1×105 cells/3 µl PBS; C57BL/6 J mice; n=20, Thy1-YFP transgenic mice; n=10)
moderate contusion model treated with BM-MSCs (Mo-BM-MSC group; 1×105 cells/3 µl PBS; C57BL/6 J mice; n=20, Thy1-YFP transgenic mice; n=10)
control group of moderate contusion with 3 µl PBS without cells (Mo-control group; C57BL/6 J mice; n=20, Thy1-YFP transgenic mice; n=10).
severe contusion model treated with AD-MSCs (Se-AD-MSC group; 1×105 cells/3 µl PBS; C57BL/6 J mice; n=10)
severe contusion treated with BM-MSCs (Se-BM-MSC group; 1×105 cells/3 µl PBS; C57BL/6 L; n=10)
control group of severe contusion model with 3 µl PBS without cells (Se-control group; C57BL/6 J mice; n=10).
severe contusion model treated with GFP-AD-MSCs (Se-GFP-AD-MSC group; 1×105 cells/3 µl PBS; C57BL/6 J mice; n=19)
severe contusion model treated with GFP-BM-MSCs (Se-GFP-BM-MSC group; 1×105 cells/3 µl PBS; C57BL/6 J mice; n=19)
We previously reported that the appropriate transplantation time for MSCs to survive in the injured spinal cord was 3 days after injury13. In this study, based on our previous publication, all groups underwent the second surgery for MSC transplantation 3 days after contusion. MSCs (AD-MSCs, BM-MSCs, GFP-positive AD-MSCs, GFP-positive BM-MSCs) in 3 µl PBS were injected into the injury epicenter, which was clearly seen in the central region of the laminectomy area. Control groups were injected with 3 µl PBS without cells. In all groups, the MSC or PBS were injected into the contusion epicenter using a 10-µl Hamilton syringe with a 32-gauge stainless steel needle attached to an automated micropump. The needle remained in place for 5 minutes after injection to ensure that all cells were administered into the spinal cord. After surgery, the mice were maintained in an isothermic cage until recovery.
Assessment of Transplanted Cell Survival
The severe SCI model was used to evaluate the survival rate of transplanted AD-MSCs or BM-MSCs in vivo. The Se-GFP-AD-MSC group and Se-GFP-BM-MSC group animals were examined at 7, 14, 21, and 28 days (1–4 weeks) after transplantation. The GFP-positive cells were considered most likely to be surviving MSCs because, as we have shown previously, they did not differentiate into glial or neuronal elements27 and GFP-positive cells disappear if these are taken up by phagocytic cells. Under anesthesia, mice were intracardially perfused with ice-cold PBS, followed by fixation with 4% paraformaldehyde in PBS. Excised tissue samples were immersed in sucrose for 24 hours, and segments of the spinal cord (T8 to T12) were cut on a cryostat into sagittal frozen sections at 14 μm thick. To count the number of GFP-positive cells present in tissue sections, three midsagittal sections of the injured portion of harvested spinal cords removed from each of four mice in each group were randomly selected and 20 high-magnification (×200) non-overlapping photomicrographs of the injury epicenter were collected using a confocal microscope (TCS SP2; Leica Microsystems, Wetzlar, Germany); these digitized images were then used to count the number of GFP-positive cells using a color image analyzer software (MacSCOPE; Mitani, Japan)10.
Immunohistochemistry and Semi-Quantitative Analysis of Tissue Staining
Once deep anesthesia was achieved, the Mo-AD-MSC group, Mo-BM-MSC group, Mo-control group, Se-GFP-AD-MSC group, and Se-GFP-BM-MSC group of mice were intracardially perfused with 200 ml of ice-cold PBS, followed by fixation with 4% paraformaldehyde in PBS, and the spinal cords were excised and post-fixed with 4% paraformaldehyde for 3 hours. Then, the samples were immersed in 10% sucrose in PBS at 4°C for 24 hours, and 30% sucrose in PBS for next 24 hours. The spinal cord segments (T8 to T12) were prepared for sectioning using optimum cutting temperature compound (Sakura Finetek, Japan) and frozen at −80°C and cut using a cryostat into the axial or sagittal sections at 14 μm thick. The sections were rinsed in PBS three times after fixing with 4% paraformaldehyde in PBS. For immunofluorescence staining with fluorescent antibodies, frozen sections were permeabilized with 0.1 mol/l Tris-HCl buffer (pH 7.6) containing 0.3% Triton X-100 (Sigma-Aldrich). The following primary antibodies were diluted in commercial diluent (Antibody Diluent with Background Reducing Components; Dako Cytomation, Glostrup, Denmark) and applied overnight at 4°C: anti-gamma isotype of protein kinase C (PKCγ; 1:50, rabbit immunoglobulin (Ig)G; Cell Signaling Technology, Inc., MA, USA); anti-pAkt monoclonal antibody (1:100, rabbit IgG; Cell Signaling Technology, Inc.); anti-extracellular signal-regulated protein kinase 1/2 (ERK1/2) monoclonal antibody (1:200, rabbit IgG; Cell Signaling Technology, Inc.); anti-CD31 monoclonal antibody (1:250, rabbit IgG; Abcam). The sections were then incubated for 1 hour at room temperature with Alexa fluor-conjugated 488 or 568 secondary antibodies (1:250; Molecular Probes, OR, USA). Then the sections were rinsed in PBS three times and examined by epifluorescence. Control immunolocalization using the same immunohistochemical methods minus the primary antibodies was performed and this demonstrated an absence of signal. The digitized images of immunofluorescence were collected using a confocal laser scanning microscope (TCS SP2; Leica Microsystems). This time-point was used to evaluate blood vessels (by CD31 immunopositivity) because previous research had shown that angiogenesis both diminished cystic cavity formation at 3–7 days after SCI and that neuronal fiber outgrowth was associated with new blood vessels28. In the spinal cord, PKCγ is an intracellular signaling kinase found in the axons of the corticospinal tract29.
For semi-quantitative analysis of the presence of PKCγ-positive MSCs at 2 and 4 weeks after transplantation in animals used in the moderate contusion model, the following procedure was performed; five axial sections of length ±500 µm from the epicenter were selected randomly, and high-magnification (×200) photomicrographs of the posterior funiculus were taken (TCS SP2; Leica Microsystems), then analyzed using color image analyzer software (MacSCOPE; Mitani). To evaluate the survival response of transplanted MSCs, we assessed by immunopositivity the presence of the prosurvival pAkt and ERK1/2 signaling pathways specifically within these transplanted cells (GFP-labeled), and not in other cells within the spinal cord. The Akt pathway is a signal transduction pathway that promotes cellular survival and growth in response to extracellular signals. Also, ERK1/2 is an important subfamily of mitogen-activated protein kinases that control cellular activities. We reported that MR-16 treatment, which blockades IL-6 signaling, increased the expression Akt and ERK1/2 in transplanted MSCs, with levels of expression strongest at 7 days after transplantation10. To semi-quantify the expression of Akt and ERK1/2, the following procedure was performed using the severe contusion model: three mid-sagittal sections at the injury epicenter were selected at random, and 10 non-overlapping high-magnification photomicrographs (×400) were taken per section (TCS SP2; Leica Microsystems). The numbers of Akt/ERK1/2 positive cells that colocalized with GFP positivity were counted using color image analyzer software (MacSCOPE: Mitani).
Assessment of Motor Function
Behavioral outcome after transplantation of MSCs was evaluated by the Basso Mouse Scale (BMS) for locomotion, in which the scores range from 0 points (no ankle movement) to 9 points (complete functional recovery). BMS scores of each group (n=10 animals per group) were recorded at 7, 14, 21, 28, 35, and 42 days (for 6 weeks) after treatment by two independent examiners blinded to the experimental conditions (HN and SW); the groups tested were the Mo-AD-MSC group, Mo-BM-MSC group, Mo-control group, Se-AD-MSC group, Se-BM-MSC group, and Se-control group. When the scores of the right and left hind limbs were different from each other, the average of the two scores were recorded.
Statistical Analysis
All experiments and analysis were performed on at least three animals per group. Data have been reported as means ± standard deviation (SD). Differences between groups were examined for statistical significance using either the paired Student’s t test or one-way analysis of variance. A p<0.05 denoted the presence of significant difference with Tukey’s post-hoc analysis. All statistical analyses were performed using SPSS 10.0 (SPSS Inc., Chicago, USA).
Results
Cell Surface Markers of AD-MSCs and BM-MSCs
The results of flow cytometric analysis of cell surface markers are shown in Table 1. Flow cytometric analysis demonstrated that AD-MSCs and BM-MSCs were shown to have the same surface maker pattern; positive for CD34 (86.3%±18.0%; 98.2%±2.3%), CD44 (95.0%±6.8%; 99.9%±0.1%), CD73 (47.1%±6.9%; 56.4%±16.1%), CD90.2 (46.5%±1.8%; 56.6%±12.7%)), CD106 (95.3%±2.8%; 88.2%±4.6%) and Sca-1 (97.6%±3.3%; 96.1%±5.2%), but not CD11b (0.8%±0.4%; 0.4%±0.2%), CD14 (0.7%±0.6%; 4.3%±1.0%), CD45 (0.6%±0.6%; 1.7%±0.9%), CD49d (1.1%±1.3%; 0.8%±0.3%) CD105 (5.4%±6.2%; 1.3%±1.3%) and CD133 (0.6%±0.5%; 0.5%±0.4%).
Table 1.
Mean Percentage of Each Cell Surface Markers by Flow Cytometric Analysis.
| CD11b | CD14 | CD34 | CD44 | CD45 | CD49d | CD73 | CD90.2 | CD105 | CD106 | CD133 | Sca-1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD-MSC | 0.8 ± 0.4 | 0.7 ± 0.6 | 86.3 ± 18.0 | 95.0 ± 6.8 | 0.6 ± 0.6 | 1.1 ± 1.3 | 47.1 ± 6.9 | 46.5 ± 1.8 | 5.4 ± 6.2 | 95.3 ± 2.8 | 0.6 ± 0.5 | 97.6 ± 3.3 |
| BM-MSC | 0.4 ± 0.2 | 4.3 ± 1.0 | 98.2 ± 2.3 | 99.9 ± 0.1 | 1.7 ± 0.9 | 0.8 ± 0.3 | 56.4 ± 16.1 | 56.6 ± 12.7 | 1.3 ± 1.3 | 88.2 ± 4.6 | 0.5 ± 0.4 | 96.1 ± 5.2 |
Values are presented as the mean ± SD (%)
No significant difference in both groups
AD-MSC: adipose-derived mesenchymal stromal cell; BM-MSC: bone marrow-derived mesenchymal stromal cell
Comparison Analysis of mRNA Expression of AD-MSCs and BM-MSCs
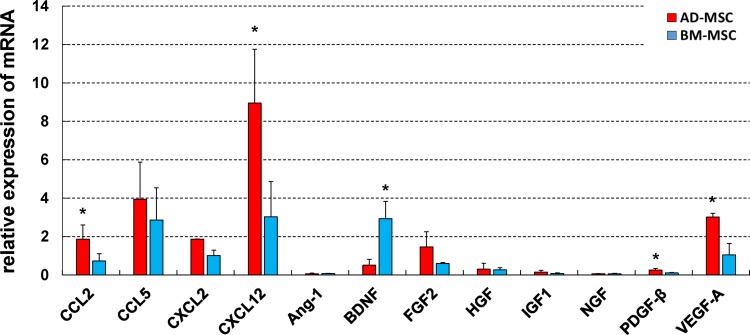
The QuantiGene Plex 2.0 Reagent System (Affymetrix) was used for comparative analysis of cytokine synthesis. The relative mRNA expression of AD-MSCs and BM-MSCs are shown in Fig. 1. Many of the cytokines examined were expressed at similar levels in both cell types; however, there were significant differences in the relative expression of CCL2, CXCL12, PDGF-β, and VEGF-A, with AD-MSCs dominant for each cytokine, and BDNF with BM-MSCs dominant. The data indicated that both MSCs have potential to synthesize cytokine/chemokine, but that they differ from each other.
Fig. 1.
Comparative analysis of mRNA expression of AD-MSCs and BM-MSCs using the QuantiGene Plex 2.0 Reagent System. The data showed that the expression of CCL2, CXCL12, PDGF-β, and VEGF-A in AD-MSCs were significantly higher than their expression in BM-MSCs. In contrast, BM-MSCs expressed significantly higher levels of BDNF than AD-MSCs. Data are shown as mean±SD. n=3 per groups, *p<0.05.
AD-MSC: adipose-derived mesenchymal stromal cell; BDNF: brain-derived neurotrophic factor; BM-MSC: bone marrow-derived mesenchymal stromal cell; CXCL12: chemokine (C-X-C motif) ligand 12; PDGF-β: platelet-derived growth factor β; SD: standard deviation
VEGF: vascular endothelial growth factor.
Cell Proliferation Ability Under Different Oxygen Conditions
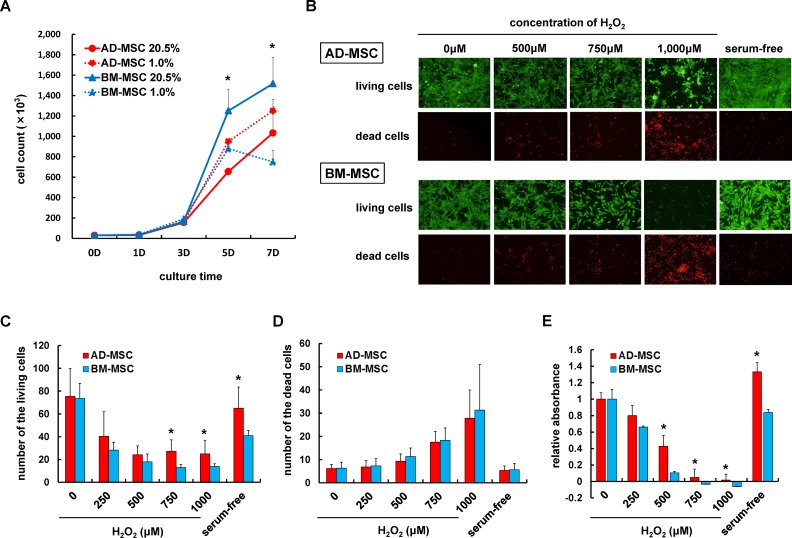
The growth kinetics of AD-MSCs and BM-MSCs were analyzed to determine which cell type was more tolerant to hypoxia. As shown in Fig. 2A, AD-MSCs and BM-MSCs could be culture expanded even under 1.0% O2 conditions by day 5. However, AD-MSCs were more proliferative in 1.0% O2 conditions compared with their growth in 20.5% O2 conditions, whereas the number of BM-MSCs under 1.0% O2 conditions was decreased. There were significant differences in cell numbers recorded at day 5 and 7, for each cell type, suggesting that in the later stages of culture AD-MSCs maintained their proliferation rates in hypoxia, while BM-MSCs decreased their proliferation ability under hypoxia.
Fig. 2.
Assessment of stress tolerance of AD-MSCs and BM-MSCs under hypoxia and oxidative stress. (A) The proliferation rates of AD-MSCs and BM-MSCs under 20.5% O2 or 1.0% O2 culture conditions. At day 5, the number of AD-MSCs in 1.0% was significantly greater than AD-MSCs in 20.5% O2. In contrast, the BM-MSC proliferation rate in 1.0% O2 was significantly decreased at day 7. Data are shown as mean±SD, n=3 per groups, *p<0.05. (B) Representative images of AD-MSCs and BM-MSCs following 24 hours incubation in H2O2 containing DMEM/10% FBS medium or serum-free medium, stained with calcein AM/EthD-1 (LIVE/DEAD). Cells were photographed using a fluorescence microscope at 100× magnification. Living cells (green) were stained with calcein AM, and dead cells (red) were stained with EthD-1. (C, D) The number of the living/dead cells incubated in H2O2 at different concentrations or in serum-free medium. There were significantly greater numbers of viable AD-MSCs than BM-MSCs at the higher concentrations (750 μM, 1000 μM) of H2O2 and in serum-free conditions. Data are shown as mean±SD. n=6 wells per groups, *p<0.05. (E) Cell viability was further assessed using the XTT assay to test for metabolic activity. As shown, metabolic activity in AD-MSCs exposed to high concentrations of H2O2 (500–1000 μM) and in serum-free medium was significantly greater than that of BM-MSCs under the same stress condition. Data are shown as mean±SD. n=6 wells per groups, *p<0.05.
AD-MSC: adipose-derived mesenchymal stromal cell; BM-MSC: bone marrow-derived mesenchymal stromal cell; DMEM: Dulbecco’s modified Eagle’s medium; FBS: fetal bovine serum; XTT: 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide.
Metabolic Activity and Cell Viability Under Oxidative Stress
Oxidative stress or serum-free conditions are known to induce apoptosis in various cells. For quantification of metabolic activity and viability of AD-MSCs and BM-MSCs under stress, calcein AM/EthD staining and XTT assays were performed. After exposure to high H2O2 concentrations and serum-free medium, the numbers of calcein AM-positive (viable) BM-MSCs were markedly decreased. AD-MSCs were also damaged following exposure to oxidative stress or serum-free medium, but in contrast to BM-MSCs, AD-MSCs were more tolerant to these stress exposures as demonstrated by greater numbers of viable cells (Fig. 2B–D). XTT assays showed reduction of metabolic activation under high H2O2 concentrations, however, increased relative absorbance of AD-MSCs compared with BM-MSCs in the 500, 750, 1000 µM H2O2 and serum-free medium (Fig. 2E).
Transplanted MSC Viability Detected by GFP-labeling and Akt/ERK 1/2 Positivity
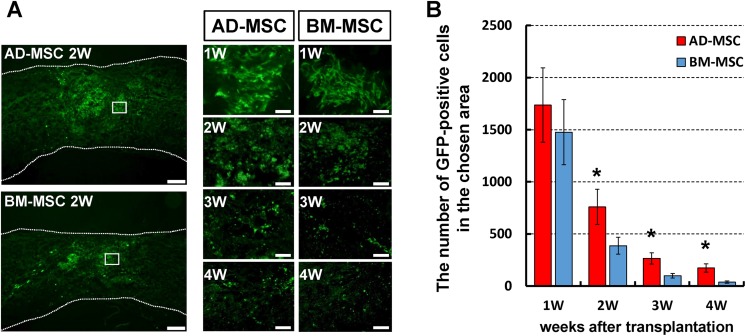
In the severely injured spinal cord, the number of GFP-positive cells gradually decreased in both groups with time. By 4 weeks after GFP-BM-MSC transplantation, very few viable cells were observed; however, in the GFP-AD-MSC group the area of GFP positivity with cell protrusions evident, indicative of a stromal morphology30, remained strongly apparent throughout the time period and was significantly higher than that of the GFP-BM-MSC group at 2, 3, 4 weeks after transplantation (Fig. 3).
Fig. 3.
The survival of AD-MSCs and BM-MSCs after transplantation into the injured spinal cord. (A) Representative images are shown of midsagittal spinal cord sections in mice transplanted with GFP-labeled AD-MSCs and GFP-labeled BM-MSCs in the severe SCI model. High-magnification pictures were taken from respective boxed area showing GFP-MSCs with cell protrusions. Scale bars: 200 µm, 20 μm. (B) The midsagittal GFP-positive area was analyzed after transplantation. There was a significantly higher number of positive cells of GFP-labeled AD-MSCs than GFP-labeled BM-MSCs at 2, 3, and 4 weeks after transplantation. Data shown as means±SD. n=4 per groups, *p<0.05.
AD-MSC: adipose-derived mesenchymal stromal cell; BM-MSC: bone marrow-derived mesenchymal stromal cell; GFP: green fluorescent protein; SCI: spinal cord injury; SD: standard deviation.
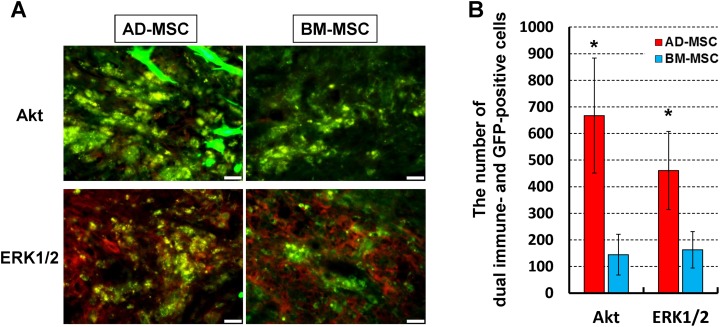
Previously, we found that the prosurvival signaling factors, Akt and ERK 1/2, are mostly upregulated in transplanted MSCs at 7 days after transplantation31. Based on this result, we performed immunofluorescent staining to compare the expression of Akt and ERK 1/2 at 7 days after AD-MSC and BM-MSC transplantation. As shown in Fig. 4, most of the AD-MSCs that were present at this time expressed Akt and relatively stronger GFP (with cell protrusions evident) even in the severe contusion model. In contrast, the appearance of GFP in BM-MSCs was weaker than the AD-MSCs and there were few Akt positive BM-MSCs apparent. Similarly, there was a significant increase in the prevalence of ERK 1/2 immunopositive GFP-AD-MSCs compared with ERK 1/2 GFP-BM-MSCs.
Fig. 4.
Akt and ERK 1/2 immunoreactivity in transplanted AD-MSCs and BM-MSCs. (A) Representative immunofluorescence images show the colocalization of Akt and ERK1/2 (red) GFP-positive MSC (green) with cell protrusions in the spinal cord epicenter on 7 days after transplantation. Scale bars: 20 µm. (B) The number of Akt or ERK1/2 and GFP-positive MSCs. The amount of GFP-AD-MSC colocalization with Akt or ERK1/2 was significantly increased compared with that of GFP-BM-MSCs. Data are shown as mean±SD. n=3 per groups, *p<0.05.
AD-MSC: adipose-derived mesenchymal stromal cell; BM-MSC: bone marrow-derived mesenchymal stromal cell; GFP: green fluorescent protein; MSC: mesenchymal stromal cell.
Immunohistochemistry for Corticospinal Tract Axons and Endothelial Cells
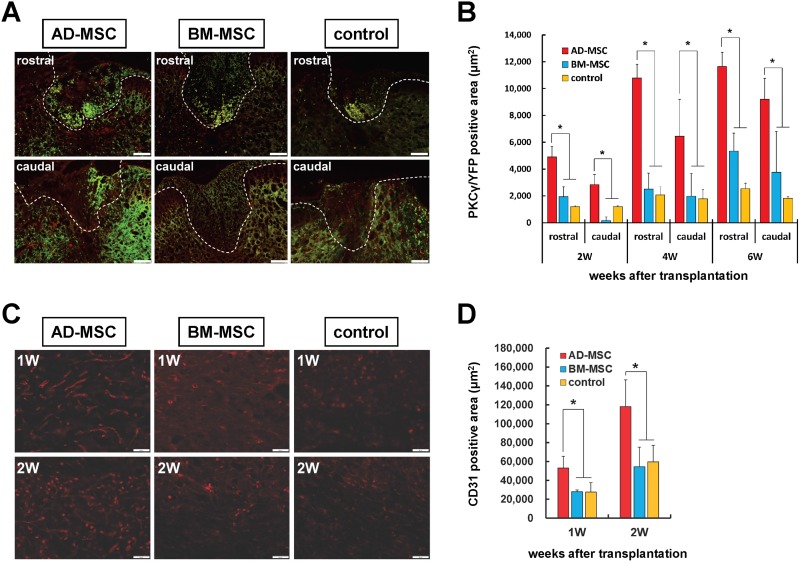
To compare the therapeutic effects of AD-MSCs and BM-MSCs on spinal cord repair, immunofluorescence staining of PKC-positive corticospinal tract axons (YFP transgenic mice) and CD31-positive vascular endothelium was performed in the moderate contusion model. It has already been demonstrated that BM-MSC transplantation promotes axonal regeneration, so here we compared the AD-MSC group and BM-MSC group. Staining for PKCγ levels showed that the area of spared corticospinal tracts in the AD-MSC transplanted group was significantly greater than the BM-MSC transplanted group and control group at 2, 4 and 6 weeks post transplantation (Fig. 5A, B). In addition, a significantly greater CD31-positive area was seen in the AD-MSC group compared with the BM-MSC group and the control group at 1 and 2 weeks post transplantation (Fig. 5C, D).
Fig. 5.
The effects of AD-MSC and BM-MSC transplantation on corticospinal tracts and blood vessel formation in severe SCI. (A) Representative images are shown for each experimental group of the corticospinal tracts ± 500 μm from the injury epicenter at 4 weeks after transplantation. Scale bar: 50 μm. (B) Immunofluorescence positive area of PKCγ (red) merged with axons in YFP-mice at 2, 4, and 6 weeks after AD-MSC and BM-MSC transplantation versus controls (no cells). Data showed that the PKCγ-positive corticospinal tracts were significantly increased at 2, 4, and 6 weeks after transplantation in the AD-MSC transplanted group compared with the BM-MSC transplanted group and control group. (C) Representative images showing CD31 immunofluorescence staining (red) at 1 and 2 weeks after transplantation of AD-MSCs or BM-MSCs versus controls (no cells). CD31 positive blood vessels area and tube formation were increased in the AD-MSC transplanted group. Scale bar: 50 μm). (D) Immunofluorescence positive area of CD31 on 1 and 2 weeks after transplantation. Note the CD31 positive capillary density was significantly increased in AD-MSC transplanted group compared with the BM-MSC transplanted group and control group. Data are shown as mean±SD. n=5 per groups, *p<0.05.
AD-MSC: adipose-derived mesenchymal stromal cell; BM-MSC: bone marrow-derived mesenchymal stromal cell; PKCγ: anti-gamma isotype of protein kinase C; SCI: spinal cord injury; SD: standard deviation.
Changes in Locomotor Function
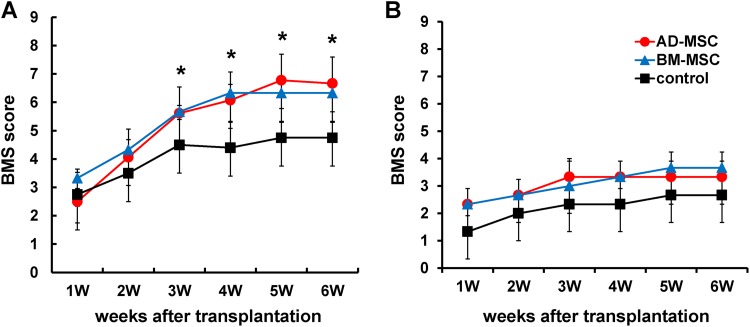
The motor function was assessed for 6 weeks after AD-MSC and BM-MSC transplantation versus control groups. Motor function scores were increased compared with the Mo-control group in both the Mo-AD-MSC group and Mo-BM-MSC group (i.e., in the moderate contusion model), with a significantly improved BMS score in both MSC transplanted groups versus control, but no significant difference between the MSC groups (Fig. 6A). However, there were no significant changes over time or between groups in the Se-AD-MSC, Se-BM-MSC and Se-control groups (i.e. in the severe contusion model; Fig. 6B).
Fig. 6.
The effects of AD-MSCs and BM-MSCs after transplantation on hind limb function. (A) BMS scores after MSC transplantation following mild contusion SCI (60 kdyne contusion). Both MSC treatment groups showed significantly better motor function than the control group. (B) BMS score after MSC transplantation following severe contusion SCI (80 kdyne contusion). There were no significant differences between each of the groups. Data are show as mean±SD. n=10 per groups, *p<0.05.
AD-MSC: adipose-derived mesenchymal stromal cell; BM-MSC: bone marrow-derived mesenchymal stromal cell; BMS: Basso Mouse Scale; MSC: mesenchymal stromal cell; SCI: spinal cord injury; SD: standard deviation.
Discussion
MSC transplantation is a promising therapy for the repair of the damaged nervous system32. We have demonstrated that MSC transplantation regulates inflammatory and immune responses and promotes functional improvement after SCI27,10. Bone marrow is a commonly used source for MSCs, however, BM-MSC transplantation has encountered difficulty including poor cell survival after transplantation. Adipose tissue contains larger numbers of MSCs than bone marrow and is more accessible with less donor site morbidity following harvest12. Hence, the use of AD-MSCs represents an attractive alternative to BM-MSCs. The major findings of our current research were: (1) the surface marker expression of AD-MSCs and BM-MSCs isolated from individual C57BL6/J mice was similar; (2) AD-MSCs showed higher tolerance to cytotoxic factors such as hypoxia, serum deprivation, and oxidative stress than BM-MSCs; (3) AD-MSCs expressed growth factors and cytokines/chemokines with some similarity and some differences when compared with BM-MSCs; (4) the survival rate of AD-MSCs was higher than BM-MSCs after transplantation into a severe SCI model in vivo; (5) AD-MSC transplantation contributed to greater levels of neuronal/vascular protection compared with BM-MSCs and control groups; (6) the motor function was equally improved in both AD-MSCs and BM-MSCs compared with the control group following moderate SCI, but there was no significant improvement following severe SCI in either of the treated groups.
Some investigators have reported that the surface immunophenotype of AD-MSCs resembles that of BM-MSCs33,34,35,16. On the other hand, differences in surface markers expression also have been reported between AD-MSCs and BM-MSCs. For example, CD34 is present on human AD-MSCs early in passage, but has not been found on human BM-MSCs36,37,11, and CD34 expression decreases over time in culture38. The CD markers of murine MSCs are still not be clarified. Pester et al. investigated the character of MSCs isolated from five strains of mice bone marrow and they reported that CD markers varied in the expression of CD3436. Our results showed that AD-MSCs and BM-MSCs isolated from the same C57BL/6 J mice express a similar pattern of CD markers and that the surface markers, including CD34, may not be a useful marker to distinguish between AD-MSCs and BM-MSCs.
Current thinking is that the potential beneficial effects of MSCs in SCI are not related to neuronal or glial differentiation of MSCs, but rather from their secretion of growth factors and/or cytokines. Recent research showed that AD-MSCs and BM-MSCs synthesize many cytokines and growth factors39,40, which may regulate their regenerative activity after transplantation. Wright et al. reported that MSC transplantation may promote axonal regeneration both by stimulating nerve growth via secreted factors and also by reducing the nerve-inhibitory effects of the extracellular molecules present20. In this in vitro study, MSCs acted as “cellular bridges” and also “towed” neurites over nerve-inhibitory substrates. Hence, the morphology of surviving transplanted MSCs, which demonstrated a stromal morphology with cell protrusions, indicated possible similar modes of activity30. Therefore, we performed multiplex bead assays to identify and compare cytokines secreted by murine AD-MSCs and BM-MSCs isolated from the same mice. This technique does not require target gene amplification thereby reducing measurement bias. Our data showed that AD-MSCs express and may synthesize higher levels of CXCL12, PDGF-β, and VEGF-A than BM-MSCs in vitro. VEGF is a one of the major factors of neovascularization41 and AD-MSCs synthesize increased VEGF in hypoxic conditions42. Moreover, VEGF treatment has neuroprotective effects in SCI and improves motor function43,44. Our data showed that VEGF-A expression was greater in AD-MSCs than BM-MSCs even when cultured at 20.5% O2. CXCL12 is expressed in the dorsal corticospinal tract and meningeal cells of the spinal cord during development45. Along with its receptor CXCR4, CXCL12 is a key wound healing factor that promotes axonal outgrowth46, hematopoiesis47, angiogenesis48, and has anti-apoptotic effects48. Liu et al. reported that the CXCL12 pretreated MSC demonstrated increased cell survival and proliferation in vitro. They also showed CXCL12 pretreatment inhibited apoptosis induced by H2O2 and increased the secretion of prosurvival and angiogenic cytokines49. In this study, we found that CXCL12 was expressed to a greater level in AD-MSCs than BM-MSCs. Furthermore, we found increased preservation of the corticospinal tract and vascular endothelium after AD-MSC transplantation compared with BM-MSCs. The trophic factors secreted by AD-MSCs might contribute to these therapeutic effects.
Damage to the spinal cord is thought to be composed of two phases. The first phase of SCI is the initial physical insult to the spinal cord leading directly to cell death and bleeding. The secondary phase consists of multiple pathophysiological processes that cause further tissue loss and dysfunction. Several secondary mechanisms are including hypoxia6, acidosis50, and free radical formation7,31. Transplanted MSCs are exposed not only to cytotoxic conditions due to tissue damage, but to a lack of oxygen and nutrients caused by poor vascular supply. Recent studies indicate that AD-MSCs may have increased stress tolerance to hypoxia and lower nutrition levels compared with BM-MSCs. In generally, living cells need oxygen to survive, but the level of oxygen may affect cell viability and proliferation. Yamamoto et al. reported that hypoxia enhanced the proliferation rate of AD-MSCs51. They showed that the expression of Oct3/4 and Nanog, as a stem cell marker, and the secreted growth factors were increased under the hypoxic condition. Oh et al. reported that neural stem cells co-transplanted with AD-MSCs showed better survival rates than neural stem cells transplanted alone52. Moreover, hypoxic stress also stimulates AD-MSC production of angiogenic cytokines such as VEGF53,41. In another study, AD-MSCs proliferated better in culture medium containing low (2%) levels of serum than BM-MSCs, and secreted higher levels of HGF and VEGF when cultured in low serum compared with high (20%) serum medium34. Therefore, we hypothesized that AD-MSCs have a higher tolerance to hypoxia, oxidative stress, and serum-free conditions than BM-MSCs, and researched cell proliferation and survival under these stressed conditions. Our findings indicated that AD-MSCs cultured under 1.0% O2 showed enhanced proliferation compared with those cells in 20.5% O2, while the proliferation capacity of BM-MSCs decreased under 1.0% O2 compared with 20.5% O2.
Reactive oxygen species (ROS) are produced by damaged cells that cause oxidative stress and tissue damage during SCI. Hydrogen peroxide (H2O2), one of the ROS, produce hydroxyl radicals that cause tissue damage either through direct oxidation or by triggering apoptotic signaling pathways54. We previously reported that transplanted MSCs die by apoptosis10. Here, AD-MSCs had significantly greater viability than BM-MSCs after 24 hours of exposure to high H2O2 concentrations or serum-free conditions, indicating that AD-MSCs are more resistant than BM-MSCs to cytotoxic conditions. Moreover, to compare survival rates of AD-MSCs and BM-MSCs after transplantation, we used a severe contusion model in mice. In this experiment, we observed a greater survival rate of transplanted AD-MSCs compared with BM-MSCs; these cells exhibited cell protrusions, which is indicative of a stromal morphology. Furthermore, the transplanted AD-MSCs expressed Akt and ERK1/2 more strongly than transplanted BM-MSCs. Akt and ERK1/2 are key factors that regulate cell survival and the activation of these pathways promotes cell survival and inhibits apoptosis4,55. Our findings support those of Ertaş et al. who reported that AD-MSCs are more resistant than BM-MSCs to low oxygen levels and serum deprivation56 and suggested that Akt and ERK1/2 play important roles in this protective effect. Our findings also suggest that the stronger tolerance of AD-MSCs to cytotoxic stress-induced cell death is important in their improved survival rates after transplantation.
AD-MSCs, as the alternative cell source for SCI treatment, may have different characteristics and mechanisms of treatment effect compared with BM-MSCs. We examined the angiogenic effects of AD-MSCs because we found evidence of increased mRNA expression for angiogenic factors in AD-MSCs compared with BM-MSCs in vitro, specifically VEGF and CXCL12. Using immunofluorescence staining, we showed that the prevalence of CD31-positive vascular endothelial cells was significantly increased in the AD-MSC transplanted group compared with either the BM-MSC transplanted group or the control group at 7 days and 14 days after treatment. Angiogenesis is regulated by numerous cytokines, including VEGF, which is one of the most important angiogenetic factors. VEGF binds to tyrosine kinase receptors and stimulates angiogenesis57. In addition to angiogenesis, VEGF as well as CXCL12 can also stimulate the development, protection and proliferation of neurons58. Our findings also showed that PKCγ-positive corticospinal tract axonal staining was increased in the AD-MSC transplanted group compared with the BM-MSC group at 4 weeks after treatment.
The main purpose of MSC transplantation for SCI treatment is functional improvement. The result showed both AD-MSC and BM-MSC transplantation significantly improve motor function compared with the control group in a moderate contusion (60 kdyne) model. However, no statistically significant differences were detected between either treatment groups or the control group in a severe contusion model (80 kdyne). The results suggest that there may be a limitation to evaluating subtle changes in locomotor movements using this rodent model and that there is a need to further optimize the efficacy of MSC transplants.
Conclusion
In this study, we demonstrated that AD-MSCs have higher tolerance to hypoxia, serum-free conditions and oxidative stress compared with BM-MSCs in vitro. Moreover, AD-MSCs showed higher survival rates than BM-MSCs when transplanted into sites of severe SCI in vivo. AD-MSCs synthesized trophic factors and cytokines/chemokines that relate to neuronal survival, axonal regeneration, vascularization, and increased cell survival. Increased prevalence of CD31-positive vascular endothelium and PKC-positive corticospinal tract axons indicated increased neuroprotection and healing in the AD-MSC transplanted mice compared with BM-MSC transplanted mice, with functional improvement after transplants of either MSC type in moderate contusion SCI. Hence, our findings suggest AD-MSCs are a particularly attractive cell therapeutic option for severe SCI due to their enhanced stress resistance and secreted factor profile.
Acknowledgements
Thy1-YFP transgenic mice (strain B6.Cg-Tg(Thy1-YFP)16Jrs/J) were kindly given to us by S. Ito (Department of Medical Chemistry, Kansai Medical University, Osaka, Japan), and this study was technically supported by K. Suyama (Department of Orthopedic Surgery, Surgical Science, Tokai University School of Medicine, Kanagawa, Japan). One of the coauthors, Kenzo Uchida, who made a great contribution to this study, passed away in October 2015. We pray his soul may rest in peace.
Footnotes
Ethical Approval: The experimental protocol was approved by the Ethics Committee for Animal Experimentation of Fukui University, Japan.
Statement of Human and Animal Rights: This article does not contain studies with human subjects. Animal experiments were reviewed and approved by Life Science Research Laboratory, University of Fukui, Division of Bioresearch, Japan.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by Grants-in-Aid to A. T., H. N. and K. U. for General Scientific Research from the Ministry of Education, Science and Culture of Japan (#24390351, #25462290, #16K20043, and #16K10817).
References
- 1. Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int. 2013;2013:786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40(7):609–619. [DOI] [PubMed] [Google Scholar]
- 3. Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28(8):1611–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58(11):621–631. [DOI] [PubMed] [Google Scholar]
- 5. Watanabe S, Uchida K, Nakajima H, Matsuo H, Sugita D, Yoshida A, Honjoh K, Johnson WE, Baba H. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells. 2015;33(6):1902–1914. [DOI] [PubMed] [Google Scholar]
- 6. Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41(7):369–378. [DOI] [PubMed] [Google Scholar]
- 7. Liu D, Liu J, Wen J. Elevation of hydrogen peroxide after spinal cord injury detected by using the Fenton reaction. Free Radic Biol Med. 1999;27(3-4):478–482. [DOI] [PubMed] [Google Scholar]
- 8. Parr AM, Kulbatski I, Wang XH, Keating A, Tator CH. Fate of transplanted adult neural stem/progenitor cells and bone marrow-derived mesenchymal stromal cells in the injured adult rat spinal cord and impact on functional recovery. Surg Neurol. 2008;70(6):600–607. [DOI] [PubMed] [Google Scholar]
- 9. Swanger SA, Neuhuber B, Himes BT, Bakshi A, Fischer I. Analysis of allogeneic and syngeneic bone marrow stromal cell graft survival in the spinal cord. Cell Transplant. 2005;14(10):775–786. [DOI] [PubMed] [Google Scholar]
- 10. Tan Y, Uchida K, Nakajima H, Guerrero AR, Watanabe S, Hirai T, Takeura N, Liu SY, Johnson WE, Baba H. Blockade of interleukin 6 signaling improves the survival rate of transplanted bone marrow stromal cells and increases locomotor function in mice with spinal cord injury. J Neuropathol Exp Neurol. 2013;72(10):980–993. [DOI] [PubMed] [Google Scholar]
- 11. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011;11(57):160–170. [PubMed] [Google Scholar]
- 13. Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009;76(2):56–66. [DOI] [PubMed] [Google Scholar]
- 14. Kang SK, Shin MJ, Jung JS, Kim YG, Kim CH. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006;15(4):583–594. [DOI] [PubMed] [Google Scholar]
- 15. Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, Kim WH, Kang KS, Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10(4):273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou Z, Chen Y, Zhang H, Min S, Yu B, He B, Jin A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy. 2013;15(4):434–448. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong LE, Driscoll MV, Donepudi AC, Xu J, Baker A, Aleksunes LM, Richardson JR, Slitt AL. Effects of developmental deltamethrin exposure on white adipose tissue gene expression. J Biochem Mol Toxicol. 2013;27(2):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48(17):4827–4833. [PubMed] [Google Scholar]
- 19. Uchida K, Nakajima H, Hirai T, Yayama T, Chen KB, Kobayashi S, Roberts S, Johnson WE, Baba H. Microarray analysis of expression of cell death-associated genes in rat spinal cord cells exposed to cyclic tensile stresses in vitro. BMC Neurosci. 2010;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wright KT, El Masri W, Osman A, Roberts S, Chamberlain G, Ashton BA, Johnson WE. Bone marrow stromal cells stimulate neurite outgrowth over neural proteoglycans (CSPG), myelin associated glycoprotein and Nogo-A. Biochem Biophys Res Commun. 2007;354(2):559–566. [DOI] [PubMed] [Google Scholar]
- 21. Xue GP, Rivero BP, Morris RJ. The surface glycoprotein Thy-1 is excluded from growing axons during development: a study of the expression of Thy-1 during axogenesis in hippocampus and hindbrain. Development. 1991;112(1):161–176. [DOI] [PubMed] [Google Scholar]
- 22. Carter LM, Starkey ML, Akrimi SF, Davies M, McMahon SB, Bradbury EJ. The yellow fluorescent protein (YFP-H) mouse reveals neuroprotection as a novel mechanism underlying chondroitinase ABC-mediated repair after spinal cord injury. J Neurosci. 2008;28(52):14107–14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Unezaki S, Yoshii S, Mabuchi T, Saito A, Ito S. Effects of neurotrophic factors on nerve regeneration monitored by in vivo imaging in Thy1-YFP transgenic mice. J Neurosci Methods. 2009;178(2):308–315. [DOI] [PubMed] [Google Scholar]
- 24. Ghasemlou N, Kerr BJ, David S. Tissue displacement and impact force are important contributors to outcome after spinal cord contusion injury. Exp Neurol. 2005;196(1):9–17. [DOI] [PubMed] [Google Scholar]
- 25. Guerrero AR, Uchida K, Nakajima H, Watanabe S, Nakamura M, Johnson WE, Baba H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation. 2012;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Forgione N, Chamankhah M, Fehlings MG. A mouse model of bilateral cervical contusion-compression spinal cord injury. J Neurotrauma. 2017;34(6):1227–1239. [DOI] [PubMed] [Google Scholar]
- 27. Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2011;29(8):1614–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445(4):308–324. [DOI] [PubMed] [Google Scholar]
- 29. Lieu A, Tenorio G, Kerr BJ. Protein kinase C gamma (PKCγ) as a novel marker to assess the functional status of the corticospinal tract in experimental autoimmune encephalomyelitis (EAE). J Neuroimmunol. 2013;256(1-2):43–48. [DOI] [PubMed] [Google Scholar]
- 30. Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suyama K, Watanabe M, Sakabe K, Otomo A, Okada Y, Terayama H, Imai T, Mochida J. GRP78 suppresses lipid peroxidation and promotes cellular antioxidant levels in glial cells following hydrogen peroxide exposure. PLoS One. 2014;9(1):e86951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011;24(1):59–64. [DOI] [PubMed] [Google Scholar]
- 33. De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, Benhaim P, Hedrick MH, Fraser JK. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89(2-3):267–270. [DOI] [PubMed] [Google Scholar]
- 34. Katsuno T, Ozaki T, Saka Y, Furuhashi K, Kim H, Yasuda K, Yamamoto T, Sato W, Tsuboi N, Mizuno M, Ito Y, Imai E, Matsuo S, Maruyama S. Low serum cultured adipose tissue-derived stromal cells ameliorate acute kidney injury in rats. Cell Transplant. 2013;22(2):287–297. [DOI] [PubMed] [Google Scholar]
- 35. Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23(3):412–423. [DOI] [PubMed] [Google Scholar]
- 36. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103(5):1662–1668. [DOI] [PubMed] [Google Scholar]
- 37. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 38. Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208(1):64–76. [DOI] [PubMed] [Google Scholar]
- 39. Blaber SP, Webster RA, Hill CJ, Breen EJ, Kuah D, Vesey G, Herbert BR. Analysis of in vitro secretion profiles from adipose-derived cell populations. J Transl Med. 2012;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YD, Cheatham B, Storms RW, Gimble JM. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212(3):702–709. [DOI] [PubMed] [Google Scholar]
- 41. Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. [DOI] [PubMed] [Google Scholar]
- 42. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. [DOI] [PubMed] [Google Scholar]
- 43. Sundberg LM, Herrera JJ, Narayana PA. Effect of vascular endothelial growth factor treatment in experimental traumatic spinal cord injury: in vivo longitudinal assessment. J Neurotrauma. 2011;28(4):565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zachary I. Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals. 2005;14(5):207–221. [DOI] [PubMed] [Google Scholar]
- 45. Jaerve A, Müller HW. Chemokines in CNS injury and repair. Cell Tissue Res. 2012;349(1):229–248. [DOI] [PubMed] [Google Scholar]
- 46. Jaerve A, Bosse F, Müller HW. SDF-1/CXCL12: its role in spinal cord injury. Int J Biochem Cell Biol. 2012;44(3):452–456. [DOI] [PubMed] [Google Scholar]
- 47. Nakao N, Nakayama T, Yahata T, Muguruma Y, Saito S, Miyata Y, Yamamoto K, Naoe T. Adipose tissue-derived mesenchymal stem cells facilitate hematopoiesis in vitro and in vivo: advantages over bone marrow-derived mesenchymal stem cells. Am J Pathol. 2010;177(2):547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ho TK, Tsui J, Xu S, Leoni P, Abraham DJ, Baker DM. Angiogenic effects of stromal cell-derived factor-1 (SDF-1/CXCL12) variants in vitro and the in vivo expressions of CXCL12 variants and CXCR4 in human critical leg ischemia. J Vasc Surg. 2010;51(3):689–699. [DOI] [PubMed] [Google Scholar]
- 49. Liu X, Duan B, Cheng Z, Jia X, Mao L, Fu H, Che Y, Ou L, Liu L, Kong D. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011;2(10):845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Staub F, Mackert B, Kempski O, Peters J, Baethmann A. Swelling and death of neuronal cells by lactic acid. J Neurol Sci. 1993;119(1):79–84. [DOI] [PubMed] [Google Scholar]
- 51. Yamamoto Y, Fujita M, Tanaka Y, Kojima I, Kanatani Y, Ishihara M, Tachibana S. Low oxygen tension enhances proliferation and maintains stemness of adipose tissue-derived stromal cells. Biores Open Access. 2013;2(3):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oh JS, Kim KN, An SS, Pennant WA, Kim HJ, Gwak SJ, Yoon DH, Lim MH, Choi BH, Ha Y. Cotransplantation of mouse neural stem cells (mNSCs) with adipose tissue-derived mesenchymal stem cells improves mNSC survival in a rat spinal cord injury model. Cell Transplant. 2011;20(6):837–849. [DOI] [PubMed] [Google Scholar]
- 53. Hsiao ST, Lokmic Z, Peshavariya H, Abberton KM, Dusting GJ, Lim SY, Dilley RJ. Hypoxic conditioning enhances the angiogenic paracrine activity of human adipose-derived stem cells. Stem Cells Dev. 2013;22(10):1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu D, Bao F. Hydrogen peroxide administered into the rat spinal cord at the level elevated by contusion spinal cord injury oxidizes proteins, DNA and membrane phospholipids, and induces cell death: attenuation by a metalloporphyrin. Neuroscience. 2015;285:81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–2927. [DOI] [PubMed] [Google Scholar]
- 56. Ertaş G, Ural E, Ural D, Aksoy A, Kozdağ G, Gacar G, Karaöz E. Comparative analysis of apoptotic resistance of mesenchymal stem cells isolated from human bone marrow and adipose tissue. ScientificWorldJournal. 2012;2012:105698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–580. [DOI] [PubMed] [Google Scholar]
- 58. Nesic O, Sundberg LM, Herrera JJ, Mokkapati VU, Lee J, Narayana PA. Vascular endothelial growth factor and spinal cord injury pain. J Neurotrauma. 2010;27(10):1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]