Abstract
Background:
Extracellular vesicles (EVs) released from mesenchymal stem/stromal cells (MSCs) mediate their paracrine effect, but their efficacy to protect the microcirculation of the kidney is unknown. Using a novel swine model of unilateral renovascular disease (RVD) complicated by metabolic syndrome (MetS), we tested the hypothesis that EVs would attenuate renal microvascular loss.
Methods:
Four groups of pigs (n = 7 each) were studied after 16 weeks of diet-induced MetS and RVD (MetS+RVD), MetS+RVD treated 4 weeks earlier with a single intra-renal delivery of EVs harvested from autologous adipose tissue-derived MSCs, and Lean and MetS Sham controls. Stenotic-kidney renal blood flow (RBF) and glomerular filtration rate (GFR) were measured in-vivo (fast CT), whereas EV characteristics, renal microvascular architecture (micro-CT), and injury pathways were studied ex-vivo.
Results:
mRNA sequencing and proteomic analysis revealed that EVs are packed with several pro-angiogenic genes and proteins, such as vascular endothelial growth factor. Labeled EVs were detected in the stenotic kidney 4 weeks after injection internalized by tubular and endothelial cells. EVs restored renal expression of angiogenic factors and improved cortical microvascular and peritubular capillary density. Renal apoptosis, oxidative stress, tubular injury, and fibrosis were also attenuated in EV-treated pigs. RBF and GFR decreased in MetS+RVD compared with MetS, but normalized in MetS+RVD+EVs.
Conclusions:
Intra-renal delivery of MSC-derived EVs bearing pro-angiogenic properties restored the renal microcirculation and in turn hemodynamics and function in chronic experimental MetS+RVD. Our study suggests a novel therapeutic potential for MSC-derived EVs in restoring renal hemodynamics in experimental MetS+RVD.
Keywords: metabolic syndrome, renovascular disease, mesenchymal stem cells, extracellular vesicles, microcirculation
Introduction
Metabolic syndrome (MetS) is a cluster of cardiovascular disease-related risk factors that is frequently associated with chronic kidney disease (CKD), and increases its progression toward end-stage renal disease1,2. Renovascular disease (RVD) produces chronic underperfusion of the renal parenchyma, leading to progressive loss of renal mass and function3. Coexisting MetS and RVD are linked to poorer outcomes after revascularization4, underscoring the need for targeted interventions capable of preserving the post-stenotic kidney in subjects with MetS.
Mesenchymal stem/stromal cells (MSCs) are fibroblast-like multipotent adult cells present in multiple tissues with the ability to differentiate into several cell types. These cells possess unique vasculoprotective properties5, and their exogenous delivery has proven to be effective in restoring renal structure and function in several animal models of renal disease6. We have previously shown in swine RVD that intra-renal delivery of MSCs improved renal function and structure and reduced tissue injury beyond the stenotic lesion7. Furthermore, MSC delivery in conjuction with renal artery revascularization improves renal function and structure, and reduced oxidative stress, apoptosis, fibrosis, and microvascular remodeling in the stenotic kidney8,9. Importantly, with the increasing clinical translation testing the safety and efficacy of MSCs in patients, it is imperative to elucidate the mechanisms underlying their beneficial effects.
A large body of evidence indicates that an important mechanism by which MSCs confer protection is the release of extracellular vesicles (EVs), small membrane particles that express characteristics of their parental cells, can be internalized into recipient renal cells, and activate a proliferative program by delivering protein, mRNA, and micro-RNA (miR) content10–12. In line with this, we have recently shown that MSC-derived EVs are selectively enriched for pro-angiogenic genes and proteins, and miRNA that regulate angiogenesis13,14. Among them are the potent angiogenic factor vascular endothelial growth factor (VEGF), as well as activators and effectors of the Notch signaling pathway, an evolutionarily conserved signaling system that regulates vascular remodeling and arterial fate of endothelial cells. However, the implications of these observations on the renal microvasculature, and whether delivery of MSC-derived EVs preserves the structure and function of the renal microcirculation, remain to be elucidated. Therefore, using a novel swine model of MetS+RVD, we tested the hypothesis that EVs would attenuate microvascular loss in MetS+RVD.
Materials and Methods
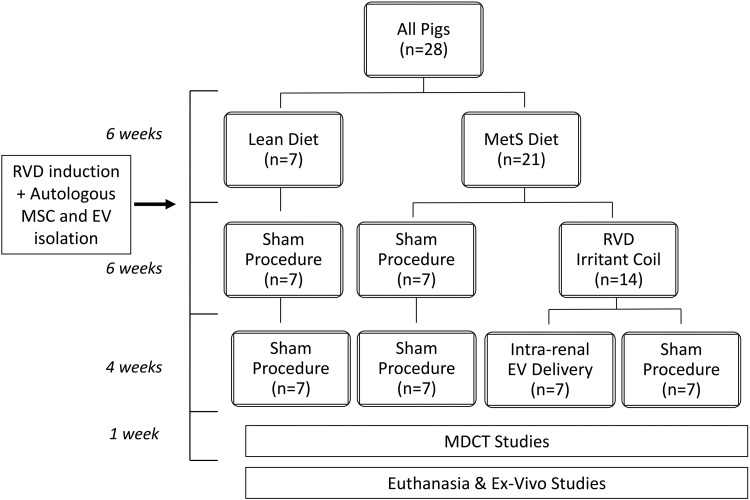
All animal procedures were approved by the Institutional Animal Care and Use Committee (approval case number: A00003694-18). Twenty-eight domestic female pigs were studied for 16 weeks (Fig. 1). At baseline, 21 pigs started a high-cholesterol/high-fructose diet (MetS)15 for the entire course of the study, whereas the other seven were fed regular pig chow (Lean).
Figure 1.
Schematic of the experimental protocol. At baseline, pigs were fed either a MetS (n = 21) or Lean diet (n = 7). Six weeks later, RVD was induced in 14 MetS pigs, whereas 7 Lean and 7 MetS pigs underwent a sham procedure. Six weeks after induction of RVD, MetS+RVD pigs received a single intra-renal infusion of either autologous MSC-derived EVs or vehicle (n = 7 each). Other MetS and Lean pigs underwent sham procedures (n = 7 each). Four weeks later, pigs were studied in-vivo and ex-vivo.
Six weeks after baseline, pigs were anesthetized with 0.25 g of IM tiletamine hydrochloride/zolazepam hydrochloride (Telazol®, Zoetis, INC, Kalamazoo, MI, USA) and 0.5 g of xylazine (Xylamed, VetOne, Manufacturer is Bimeda,-MTC Animal Health, Cambridge, ON, Canada), and maintained with intravenous ketamine (0.2 mg/kg/min, [Ketaset, Distributed by Zoetis, INC, Kalamazoo, MI, USA]) and xylazine (0.03 mg/kg/min). Unilateral RVD was induced in 14 MetS pigs by placing a local-irritant coil in the main renal artery16, whereas 7 Lean and 7 MetS pigs underwent a sham procedure. In all animals randomized to receive EVs, fat tissue was collected at that time, and subsequently used to harvest autologous MSCs and isolate their EVs.
Six weeks after induction of RVD, the degree of stenosis in each animal was determined using renal angiography. In addition, MetS+RVD pigs received a single infusion of either autologous EVs (labeled with the red fluorescence dye PKH26, Sigma) or vehicle into the stenotic kidney over 5 min (n = 7 each). Two other groups of MetS and Lean pigs (n = 7 each) that underwent only sham procedures (angiography, saline infusion) served as controls.
Systemic blood samples were collected 4 weeks later for cholesterol fractions, isoprostanes (enzyme immunoassay kit), and plasma renin activity (PRA, GammaCoat kit; DiaSorin) levels. Fasting glucose and insulin levels were measured by standard procedures, and insulin resistance calculated by homeostasis model assessment of insulin resistance (HOMA-IR)15. In addition, single-kidney hemodynamics and function were determined using multi-detector computed tomography (MDCT). Arterial blood pressure was measured with an intra-arterial catheter during MDCT studies.
Pigs were euthanized with an intravenous bolus of 100 mg/kg of sodium pentobarbital (Sleepaway, Fort Dodge Inc., Fort Dodge, IA, USA) a few days after MDCT studies17. Kidneys were removed, dissected, and sections frozen in liquid nitrogen (and maintained at –80°C) or preserved in formalin for histology and ex-vivo studies. In addition, a lobe of kidney tissue was perfused and prepared for micro-CT studies.
In-Vivo Studies
MDCT (Somatom Sensation-128, Siemens Medical Solution, Forchheim, Germany) scanning was performed to calculate renal volume, renal blood flow (RBF), and glomerular filtration rate (GFR), as previously shown18–20. Briefly, 140 consecutive scans (330 ms each) were acquired following an intra-superior vena cava bolus of iohexol (350 mg/ml over 2 seconds, [GE Healthcare, Inc. Marlborough, MA, USA]). Analyze™ (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) was used to trace cortical and medullary regions of interest, which were then used to calculate single kidney regional perfusion using MATLAB 7.10 (MathWorks). Renal volume was calculated using planimetric methods, RBF by summing cortical perfusion times cortical volume and medullary perfusion times medullary volume, and GFR from the cortical curve slope21.
Ex-Vivo Studies
MSC and EV Isolation, Characterization, and Culture
MSCs were isolated from abdominal subcutaneous adipose tissue (5–10 g) using collagenase with standard protocol. Cells were cultured with advanced MEM medium (Gibco/Invitrogen) supplemented with 5% platelet lysate (Mayo Clinic Transfusion Medicine) in 37°/5% CO2 22, and kept in cell recovery medium at –80°C for characterization. MSCs were characterized by the expression of common MSC markers (CD44, CD90, and CD105)8,13, and their potential to differentiate into adipocytes, chondrocytes, and osteocytes was assessed as previously described8,23,24.
EVs were isolated from supernatants of MSCs (10×106), cultured in advanced MEM medium without supplements, and ultra-centrifuged twice, as previously described13. Briefly, after two initial centrifugations at 2000 g and 100,000 g (Beckman Coulter Optima L-90 K) for 1 h at 4°C, EVs were washed in serum-free medium 199 containing HEPES 25 mM, and underwent a final ultra-centrifugation. Lastly, pellets were suspended and protein content quantified (Bradford method, BioRad). Limulus testing was performed to rule out endotoxin contamination (Charles River Lab.), and EVs stored at –80°C25 until delivery.
Transmission electron microscopy was performed to investigate size and structure of MSC-derived EVs using digital electron microscopy (JEOL 1200 EXII, Mayo Clinic’s electron microscopy core)26. EVs were then characterized based on the expression of EV (CD40, ß1, CD9, and CD81), and MSC (MHC-class I and CD44) surface markers using fluorescence-activated cell sorting.
EV mRNA and Protein Cargo
mRNA was isolated from MSC-derived EVs using the mirVana PARIS total RNA isolation kit (Life Technologies) according to the manufacturer’s protocol. mRNA sequencing was performed at the Mayo Clinic Bioinformatic Core, as previously described13. Samples were sequenced on an Illumina HiSeq 2000 using TruSeq SBS kit version 3 and HCS v2.0.12 data collection software and data analyzed using the MAPRSeq v.1.2.1 system and the Bioinformatics Core standard tool, which includes alignment with TopHat 2.0.627,28 and gene counts with the featureCounts software29. Normalized expression values for each gene were calculated as reads per kilobase per million (RPKM).
In addition, liquid chromatography mass spectrometry (LC-MS/MS) proteomic analysis was performed as previously described30,31. EV pellets were solubilized and lysed, and protein samples denatured. Aliquots were resolubilized in reducing sample buffer and samples electrophoresed. Gel sections were digested with trypsin31, and peptides extracted and transferred onto a PicoFrit column 9 (NewObjective), self-packed with Agilent Poroshell 120 S 2.7 µm EC-C18 stationary phase, using a Dionex UltiMate 3000 RSLC LC system (Thermo-Fisher Scientific). Peptides were separated and eluting peptides analyzed using a QExactive mass spectrometer (Thermo-Fisher Scientific).
EV Tracking
Labeled EVs were tracked and localized in frozen 5 μm sections of the stenotic kidneys by immunofluorescence staining with CD31, the distal tubular marker peanut agglutinin (PA, Vector Lab), and the proximal tubular marker Phaseolus vulgaris erythroagglutinin (PHA-E, Vector Lab).
Microvascular Density and Angiogenesis
Renal microvascular architecture was assessed using micro-CT. Renal segments were flushed with an intravascular contrast agent through a cannula ligated in a branch of each renal artery. Samples were prepared and scanned, and images analyzed as previously described20. Spatial density of cortical microvessels and microvascular tortuosity were calculated using Analyze™. Peritubular capillaries were counted in hemotoxylin and eosin stained slides at ×100 magnification, and the ratio of capillary number to tubules calculated9. Angiogenic activity was also assessed by renal VEGF immunoreactivity and immunofluorescence staining for the NOTCH family proteins Notch-1 and the endothelial Notch ligand delta-like-4 (DLL4, Abcam)32.
Renal Injury Pathways
Apoptosis was assessed in 5-μm mid-hilar cross-sections of the kidney stained with caspase-333, with positive cells manually counted in 15–20 fields under fluorescence microscopy (ZEN® 2012 blue edition, Carl ZEISS). Renal endothelial cell apoptosis was assessed by double immunofluorescence staining with terminal deoxynucleotidyl-transferase dUTP nick-end-labeling (TUNEL) and the endothelial marker CD31. Renal oxidative stress was evaluated by the in-situ production of superoxide anion (dihydroethidium, DHE)34, and renal endothelial cell oxidative stress by double staining with nitrotyrosine and CD31. Tubular injury was assessed in sections stained with periodic acid-Schiff (PAS), as described35, whereas tubulo-interstitial fibrosis and glomerular score (% of sclerotic out of 100 glomeruli) were assessed in trichrome stained slides.
Statistical Methods
Results were expressed as mean±SD. Parametric (ANOVA/Student t-test) and nonparametric (Wilcoxon/Kruskal–Wallis) tests were used as appropriate. All analyses were performed in JMP software package version 10.0 (SAS Institute Inc.) and significance was accepted for p ≤ 0.05.
Results
The systemic characteristics in all pigs at the end of the study are summarized in Table 1. Body weight and blood pressure were comparably higher in all MetS groups compared with Lean. Pigs with RVD showed moderate, but significant stenoses (p > 0.05 ANOVA). Total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride levels were similarly elevated in all MetS groups compared with Lean. Fasting glucose levels were similar among the groups, yet fasting insulin and HOMA-IR levels were higher in MetS, indicating early pre-diabetic MetS15.
Table 1.
Systemic Characteristics and Single-Kidney Function in Study Groups at 16 Weeks.
| Lean | MetS | MetS+RVD | MetS+RVD+EVs | |
|---|---|---|---|---|
| Body weight (kg) | 72.7 ± 4.6 | 93.8 ± 0.9* | 90.7 ± 1.8* | 88.9 ± 2.9* |
| Mean arterial pressure (mmHg) | 103.8 ± 4.1 | 125.5 ± 4.6* | 131.7 ± 7.1* | 122.2 ± 2.9* |
| Degree of stenosis (%) | 0 | 0 | 65.0 ± 8.0* | 66.7 ± 3.3* |
| Total cholesterol (mg/dl) | 81.4 ± 7.8 | 390.3 ± 109.5* | 404.2 ± 52.2* | 402.2 ± 135.3* |
| HDL cholesterol (mg/dl) | 49.0 ± 5.5 | 120.3 ± 19.5* | 114.2 ± 29.1* | 126.7 ± 26.8* |
| LDL cholesterol (mg/dl) | 35.1 ± 5.8 | 358.0 ± 124.0* | 303.7 ± 81.2* | 346.8 ± 82.6* |
| Triglycerides (mg/dl) | 7.4 ± 2.1 | 18.3 ± 9.9* | 18.8 ± 11.7* | 12.9 ± 5.1* |
| 8-isoprostane (pg/ml) | 84.1 ± 10.6 | 139.9 ± 23.2* | 259.0 ± 92.1*† | 135.5 ± 49.7*‡ |
| Fasting glucose (mg/dl) | 132.5 ± 43.9 | 118.8 ± 25.3 | 114.0 ± 29.8 | 102.6 ± 30.5 |
| Fasting insulin (µU/ml) | 0.40 ± 0.12 | 0.75 ± 0.06* | 0.72 ± 0.08* | 0.71 ± 0.07* |
| HOMA-IR score | 0.7 ± 0.1 | 1.8 ± 0.2* | 1.8 ± 0.1* | 1.9 ± 0.1* |
| Renal volume (ml) | 134.6 ± 7.0 | 221.6 ± 7.6* | 177.8 ± 8.9*† | 228.9 ± 20.7*‡ |
| RBF (ml/min) | 515.8 ± 28.1 | 860.5 ± 89.0* | 618.0 ± 30.0*† | 861.1 ± 101.4*‡ |
| GFR (ml/min) | 79.2 ± 2.8 | 146.8 ± 7.1* | 95.0 ± 5.5*† | 134.4 ± 14.5*‡ |
| Plasma renin activity (ng/ml/h) | 0.16 ± 0.08 | 0.17 ± 0.06 | 0.17 ± 0.11 | 0.16 ± 0.11 |
HDL: high-density lipoprotein; LDL: low-density lipoprotein; HOMA-IR: homeostasis model assessment of insulin resistance; RBF: renal blood flow; GFR: glomerular filtration rate. *p < 0.05 vs. Lean; †p < 0.05 vs. MetS; ‡p < 0.05 vs. MetS+RVD.
EV Characterization
Isolated and cultured MSC expressed CD44, CD90, and CD105, and transdifferentiated into osteocytes, chondrocytes, and adipocytes, as previously shown8,23,24.
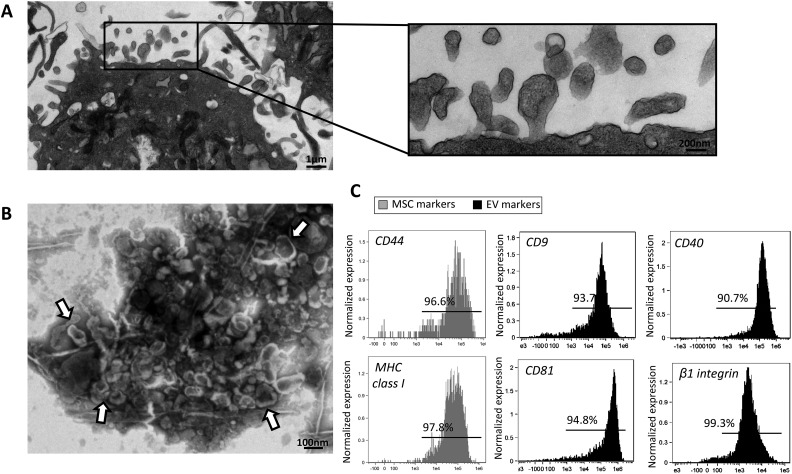
Transmission electron microscopy showed that MSCs release large amounts of EVs that exhibit the classic “cup-shape” morphology on negative staining (Fig. 2A-B), and express common EV and MSC markers (Fig. 2C).
Figure 2.
A: Transmission electron microscopy showing EVs released from MSCs. B: EVs exhibit a classic morphology on negative staining. C: Fluorescence-activated cell sorting reveals that EVs express common EV and MSC markers.
EV Content and Engraftment
Next-generation mRNA sequencing and proteomic analysis revealed that EVs contain several pro-angiogenic genes and proteins (Tables 2 and 3). Among them are growth factors and receptors, adhesion molecules, proteases, inhibitors and matrix proteins, transcription factors, and other factors involved in angiogenesis, suggesting important pro-angiogenic potential of MSC-derived EVs.
Table 2.
Angiogenic genes packed in MSC-derived EVs.
| Official gene symbol | Gene name | Expression (RPKM) | SD |
|---|---|---|---|
| Growth factors and receptors: | |||
| VEGFA | Vascular Endothelial Growth Factor A | 43.56 | 8.84 |
| VEGFC | Vascular Endothelial Growth Factor C | 14.28 | 2.81 |
| FLT1 | Fms Related Tyrosine Kinase 1 | 0.39 | 0.05 |
| KDR | Kinase Insert Domain Receptor | 0.01 | 0.001 |
| EREG | Epiregulin | 8.34 | 1.34 |
| FGF2 | Fibroblast Growth Factor 2 | 18.63 | 1.95 |
| JAG1 | Jagged 1 | 1.22 | 0.23 |
| PGF | Placental Growth Factor | 6.59 | 1.78 |
| Adhesion molecules: | |||
| LAMA5 | Laminin Subunit Alpha 5 | 0.32 | 0.05 |
| NRP1 | Neuropilin 1 | 0.11 | 0.01 |
| STAB1 | Stabilin 1 | 0.15 | 0.02 |
| COL4A3BP | Collagen Type IV Alpha 3 Binding Protein | 1.46 | 0.22 |
| IL8 | C-X-C Motif Chemokine Ligand 8 | 0.17 | 0.04 |
| Proteases, inhibitors, and matrix proteins: | |||
| ANGPTL4 | Angiopoietin Like 4 | 0.21 | 0.04 |
| PECAM1 | Platelet And Endothelial Cell Adhesion Molecule 1 | 0.13 | 0.03 |
| PF4 | Platelet Factor 4 | 0.82 | 0.25 |
| Transcription factors: | |||
| HAND2 | Heart And Neural Crest Derivatives Expressed 2 | 0.06 | 0.01 |
| SPHK1 | Sphingosine Kinase 1 | 2.76 | 0.46 |
| TRIM39 | Tripartite Motif Containing 39 | 0.41 | 0.05 |
| Other factors involved in angiogenesis: | |||
| TGFBR1 | Transforming Growth Factor Beta Receptor 1 | 3.48 | 0.54 |
| CCL11 | C-C Motif Chemokine Ligand 11 | 0.18 | 0.04 |
| ENG | Endoglin | 0.31 | 0.06 |
| EPHB4 | EPH Receptor B4 | 2.21 | 0.37 |
| FGFR3 | Fibroblast Growth Factor Receptor 3 | 0.18 | 0.03 |
| HGF | Hepatocyte Growth Factor | 7.57 | 1.09 |
| IL6 | Interleukin 6 | 23.66 | 4.98 |
| PDGFA | Platelet Derived Growth Factor Subunit A | 0.40 | 0.08 |
| PTGS1 | Prostaglandin-Endoperoxide Synthase 1 | 16.56 | 2.75 |
| TGFB2 | Transforming Growth Factor Beta 2 | 13.62 | 2.54 |
| THBS2 | Thrombospondin 2 | 94.93 | 19.26 |
| TIMP1 | TIMP Metallopeptidase Inhibitor 1 | 67.15 | 12.24 |
| TIMP3 | TIMP Metallopeptidase Inhibitor 3 | 148.30 | 26.36 |
| WNT2 | Wnt Family Member 2 | 1.04 | 0.24 |
| WNT7B | Wnt Family Member 7B | 2.21 | 0.35 |
Table 3.
Angiogenic Proteins Packed in MSC-derived EVs.
| Uniprot ID | Protein name | Expression (arbitrary units) | SD |
|---|---|---|---|
| Growth factors and receptors: | |||
| Q8SPZ9_PIG | Vascular Endothelial Growth Factor A | 11.0 | 2.0 |
| F1RYW8_PIG | Epidermal Growth Factor Receptor Binding | 19.0 | 1.6 |
| Q7YRN5_PIG | Fibroblast Growth Factor | 27.6 | 0.3 |
| Adhesion molecules: | |||
| Q5RLQ5_PIG | Neuropilin 2 | 16.1 | 2.1 |
| F1S2I3_PIG | Collagen Type IV Alpha 3 Binding Protein | 15.5 | 2.0 |
| IL8_PIG | Interleukin 8 | 26.3 | 0.2 |
| K7GLN9_PIG | Neuropilin 1 | 27.2 | 0.4 |
| Proteases, inhibitors, and matrix proteins: | |||
| K7GPI3_PIG | Platelet And Endothelial Cell Adhesion Molecule 1 | 3.7 | 1.5 |
| ANGL4_PIG | Angiopoietin Like 4 | 11.2 | 2.0 |
| ANGP1_PIG | Angiopoietin 1 | 21.4 | 1.8 |
| K7GLK2_PIG | Vascular Cell Adhesion Molecule 1 | 28.9 | 0.2 |
| F6PUK1_PIG | Serpin Family F Member 1 | 27.8 | 0.1 |
| Other factors involved in angiogenesis: | |||
| F1SRY9_PIG | Angio Associated Migratory Cell Protein | 12.9 | 2.4 |
| F1SJD8_PIG | Wnt Family Member 2 | 3.8 | 1.6 |
| I3LVH1_PIG | Wnt Family Member 7B | 11.0 | 2.0 |
| F1RMC2_PIG | Docking Protein 2 | 11.3 | 2.1 |
| D3K5N3_PIG | Midkine (Neurite Growth-Promoting Factor 2) | 15.2 | 2.0 |
| B0LDS8_PIG | Ephrin B2 | 11.8 | 2.2 |
| I3LL31_PIG | Platelet Derived Growth Factor C | 12.3 | 2.3 |
| TGFB2_PIG | Transforming Growth Factor Beta 2 | 16.5 | 2.1 |
| I3L972_PIG | Notch 3 | 20.6 | 1.7 |
| AMC2_PIG | C-X-C Motif Chemokine Ligand 6 | 24.8 | 0.2 |
| K7GQM6_PIG | TIMP Metallopeptidase Inhibitor 1 | 24.4 | 0.3 |
| F1SB93_PIG | Hepatocyte Growth Factor | 16.2 | 2.1 |
| CCL2_PIG | C-C Motif Chemokine Ligand 2 | 26.1 | 0.2 |
| Q8MKE5_PIG | Interleukin 6 | 26.8 | 0.1 |
| I3LVI7_PIG | Notch 2 | 26.5 | 0.2 |
| EGLN_PIG | Endoglin | 27.1 | 0.3 |
| F1SLQ6_PIG | Prostaglandin-Endoperoxide Synthase 1 | 30.1 | 0.2 |
| PLMN_PIG | Plasminogen | 27.1 | 0.5 |
| Q9TTB7_PIG | TIMP Metallopeptidase Inhibitor 3 | 29.3 | 0.2 |
Notably, EVs also contained genes and proteins that regulate apoptosis (Tables 4 and 5) and oxidative stress (Tables 6 and 7), underscoring the potential of EVs to modulate these pathways.
Table 4.
Antiapoptotic Genes Packed in MSC-derived EVs.
| Official gene symbol | Gene name | Expression (RPKM) | SD |
|---|---|---|---|
| BNIP3 | BCL2 Interacting Protein 3 | 0.02 | 0.00 |
| CD27 | CD27 Molecule | 0.03 | 0.00 |
| CD40LG | CD40 Ligand | 0.68 | 0.15 |
| DFFA | DNA Fragmentation Factor Subunit Alpha | 1.96 | 0.30 |
| FAS | Fas Cell Surface Death Receptor | 0.01 | 0.00 |
| IGF1R | Insulin Like Growth Factor 1 Receptor | 1.96 | 0.29 |
| MCL1 | MCL1, BCL2 Family Apoptosis Regulator | 18.52 | 3.32 |
| NOD1 | Nucleotide Binding Oligomerization Domain Containing 1 | 0.06 | 0.01 |
| NOL3 | Nucleotide Binding Oligomerization Domain Containing 3 | 1.43 | 0.20 |
| RIPK2 | Receptor Interacting Serine/Threonine Kinase 2 | 1.11 | 0.18 |
| XIAP | X-Linked Inhibitor Of Apoptosis | 2.77 | 0.53 |
Table 5.
Angiogenic Proteins Packed in MSC-derived EVs.
| Uniprot ID | Protein name | Expression (arbitrary units) | SD |
|---|---|---|---|
| C1PIG3_PIG | AKT Serine/Threonine Kinase 1 | 20.2 | 1.7 |
| A0A0B8RTW8_PIG | BCL2 Associated Athanogene 3 | 13.3 | 2.4 |
| F1RZR2_PIG | BCL2 Antagonist/Killer 1 | 26.2 | 0.3 |
| A0A0B8S0B5_PIG | BCL2 Like 1 | 18.9 | 1.6 |
| F1S402_PIG | Baculoviral IAP Repeat Containing 6 | 26.0 | 0.3 |
| F1SJY7_PIG | BCL2 Interacting Protein 1 | 24.8 | 0.4 |
| F1S071_PIG | BCL2 Interacting Protein 2 | 24.9 | 0.1 |
| F1RJT5_PIG | BCL2 Interacting Protein 3 Like | 12.8 | 2.3 |
| F1RHS7_PIG | DNA Fragmentation Factor Subunit Alpha | 11.9 | 2.2 |
| TNR6_PIG | Fas Cell Surface Death Receptor | 16.5 | 2.1 |
| F1SRY1_PIG | Insulin Like Growth Factor 1 Receptor | 26.3 | 0.2 |
| K9IWB2_PIG | MCL1, BCL2 Family Apoptosis Regulator | 18.6 | 1.6 |
| K7GT21_PIG | X-Linked Inhibitor Of Apoptosis | 12.2 | 2.2 |
Table 6.
Antioxidant genes packed in MSC-derived EVs.
| Official gene symbol | Gene name | Expression (RPKM) | SD |
|---|---|---|---|
| Glutathione Peroxidases: | |||
| GPX1 | Glutathione Peroxidase 1 | 24.64 | 4.92 |
| GPX2 | Glutathione Peroxidase 2 | 0.01 | 0.00 |
| GPX4 | Glutathione Peroxidase 4 | 25.76 | 4.87 |
| GPX6 | Glutathione Peroxidase 6 | 0.004 | 0.00 |
| GPX8 | Glutathione Peroxidase 8 | 10.27 | 1.65 |
| GSTZ1 | Glutathione S-Transferase Zeta 1 | 0.03 | 0.00 |
| Superoxide Dismutases: | |||
| SOD1 | Superoxide Dismutase 1 | 2.57 | 0.42 |
| SOD2 | Superoxide Dismutase 2 | 13.83 | 2.04 |
| SOD3 | Superoxide Dismutase 3 | 3.02 | 0.66 |
| Peroxiredoxins: | |||
| PRDX1 | Peroxiredoxin 1 | 29.96 | 4.44 |
| PRDX5 | Peroxiredoxin 5 | 0.01 | 0.00 |
| PRDX6 | Peroxiredoxin 6 | 0.39 | 0.06 |
| Other Peroxidases: | |||
| CSDE1 | Cold Shock Domain Containing E1 | 24.51 | 3.19 |
| CYGB | Cytoglobin | 7.78 | 1.72 |
| DUOX1 | Dual Oxidase 1 | 0.001 | 0.00 |
| EPX | Eosinophil Peroxidase | 0.01 | 0.00 |
| PTGS1 | Prostaglandin-Endoperoxide Synthase 1 | 16.56 | 2.75 |
| PTGS2 | Prostaglandin-Endoperoxide Synthase 2 | 0.75 | 0.17 |
| LPO | Lactoperoxidase | 0.01 | 0.00 |
| TPO | Thyroid Peroxidase | 0.005 | 0.00 |
| Other Antioxidants: | |||
| GSR | Glutathione-Disulfide Reductase | 2.32 | 0.38 |
Table 7.
Antioxidant proteins packed in MSC-derived EVs.
| Uniprot ID | Protein name | Expression (arbitrary units) | SD |
|---|---|---|---|
| Glutathione Peroxidases: | |||
| O77732_PIG | Glutathione Peroxidase 1 | 29.67 | 0.27 |
| GPX4_PIG | Glutathione Peroxidase 4 | 29.52 | 0.24 |
| J9JIK0_PIG | Glutathione Peroxidase 6 | 12.36 | 2.26 |
| I3L856_PIG | Glutathione Peroxidase 7 | 29.29 | 0.27 |
| K7GN85_PIG | Glutathione S-Transferase Zeta 1 | 26.95 | 0.26 |
| Superoxide Dismutases: | |||
| Q95ME5_PIG | Superoxide Dismutase 1 | 29.65 | 0.37 |
| Q95JF1_PIG | Superoxide Dismutase 2 | 30.91 | 0.23 |
| I3LUD1_PIG | Superoxide Dismutase 3 | 24.96 | 0.27 |
| Peroxiredoxins: | |||
| F1S3U9_PIG | Peroxiredoxin 1 | 32.19 | 0.23 |
| F1SDX9_PIG | Peroxiredoxin 2 | 31.36 | 0.29 |
| F1S418_PIG | Peroxiredoxin 3 | 31.62 | 0.22 |
| A0A0B8RTC3_PIG | Peroxiredoxin 4 | 31.31 | 0.19 |
| Q9GLW8_PIG | Peroxiredoxin 5 | 27.78 | 0.30 |
| PRDX6_PIG | Peroxiredoxin 6 | 32.78 | 0.29 |
| Other Peroxidases: | |||
| CATA_PIG | Catalase | 31.35 | 0.28 |
| F1SBS1_PIG | Cold Shock Domain Containing E1 | 27.73 | 0.40 |
| F1RWP3_PIG | Cytoglobin | 16.66 | 2.18 |
| Q2EN77_PIG | Microsomal Glutathione S-Transferase 3 | 26.78 | 0.30 |
| F1SLQ6_PIG | Prostaglandin-Endoperoxide Synthase 1 | 30.15 | 0.20 |
| F1S9J3_PIG | Peroxidasin | 30.61 | 0.27 |
| Other Antioxidants: | |||
| ALBU_PIG | Albumin | 34.64 | 0.19 |
| APOE_PIG | Apolipoprotein E | 25.75 | 0.50 |
| F1RX66_PIG | Glutathione-Disulfide Reductase | 28.54 | 0.30 |
| F1SG38_PIG | Thioredoxin Reductase 1 | 28.01 | 0.28 |
| F1RHN4_PIG | Thioredoxin Reductase 2 | 21.66 | 1.79 |
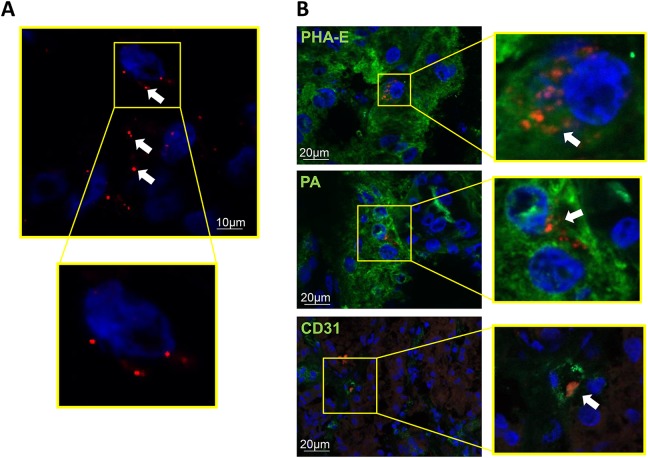
MSC-derived EVs were detected in the post-stenotic swine kidney 4 weeks after intra-renal administration (Fig. 3A), co-localizing with proximal (PHA-E positive) and distal (PA positive) tubular cells, and endothelial cells (CD31) (Fig. 3B).
Figure 3.
A: EV clusters were detected in the swine kidney 4 weeks after intra-renal delivery (arrows). B: Immunofluorescence co-staining with Phaseolus vulgaris erythroagglutinin (PHA-E), peanut agglutinin (PA), and CD31, shows EV engraftment in proximal and distal tubules, and endothelial cells, respectively.
EVs Improved the Renal Microcirculation
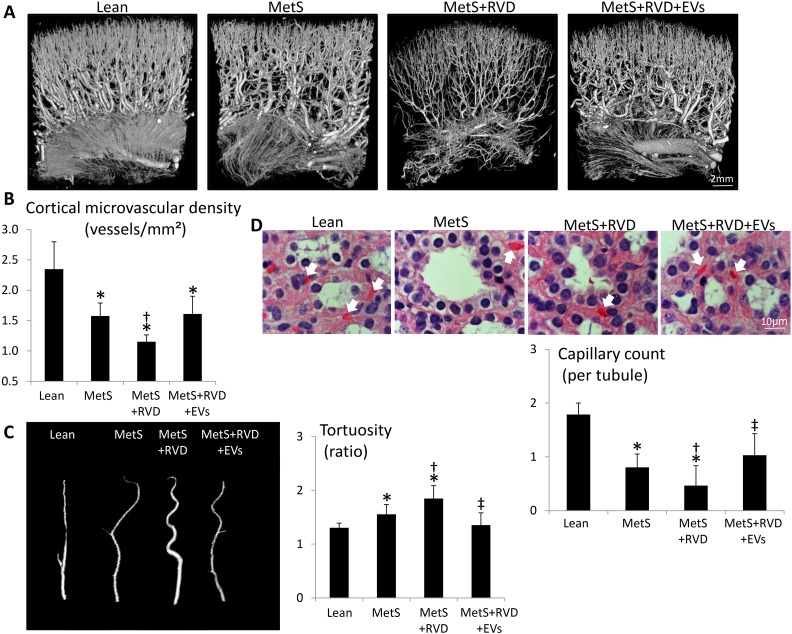
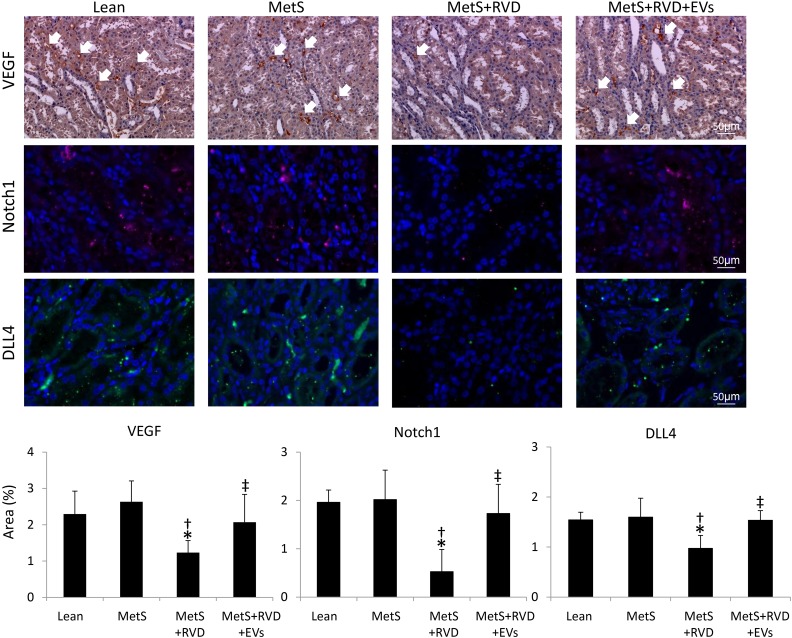
Spatial density of cortical microvessels was measurably diminished in MetS and further reduced in MetS+RVD, but improved (although not fully normalized) in MetS+RVD+EVs (Fig. 4A-B). Microvascular tortuosity (indicating immaturity) was higher in MetS compared with normal and further increased in MetS+RVD, but normalized in EV-treated pigs (Fig. 4C). Peritubular capillary density decreased in MetS compared with Lean, decreased further in MetS+RVD, but improved in EV-treated pigs (Fig. 4D). Immunoreactivity of the pro-angiogenic factors VEGF, Notch-1, and DLL4 was downregulated in MetS+RVD, but EVs restored their expression (Fig. 5).
Figure 4.
EVs improve microvascular architecture in MetS+RVD. A: Representative 3D micro-computed tomography images of the pig kidney showing improved microvascular architecture in EV-treated pigs. B: Quantification of spatial density of renal cortical microvessels (left) and microvascular tortuosity (right). D: Representative renal hemotoxylin and eosin (H&E) staining and quantification of peritubular capillary density. *p < 0.05 vs. Lean; †p < 0.05 vs. MetS; ‡p < 0.05 vs. MetS+RVD.
Figure 5.
EVs improve angiogenic signaling in MetS+RVD. Representative stenotic kidney staining (×40) for the pro-angiogenic factors VEGF, Notch-1, and Notch ligand delta-like-4 (DLL4), and their quantification. *p < 0.05 vs. Lean; †p < 0.05 vs. MetS; ‡p < 0.05 vs. MetS+RVD.
EVs Ameliorated Renal Injury
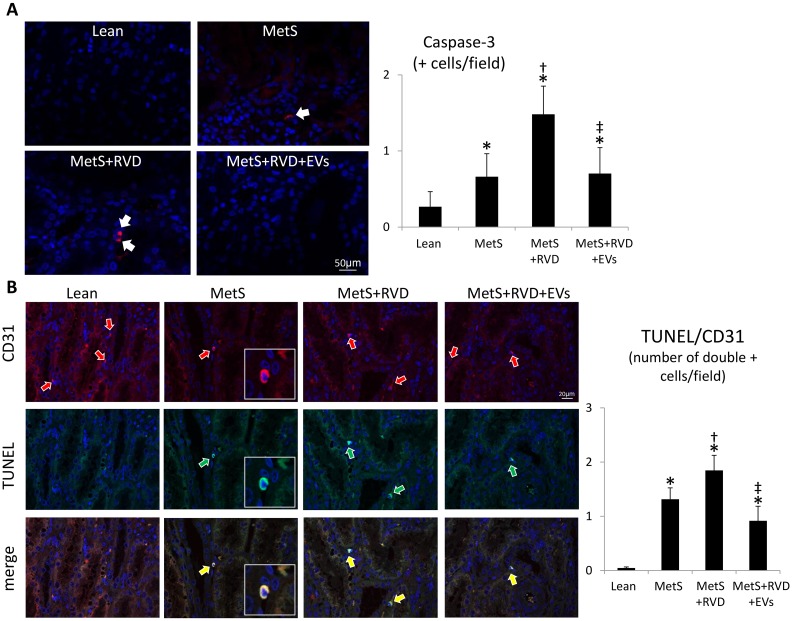
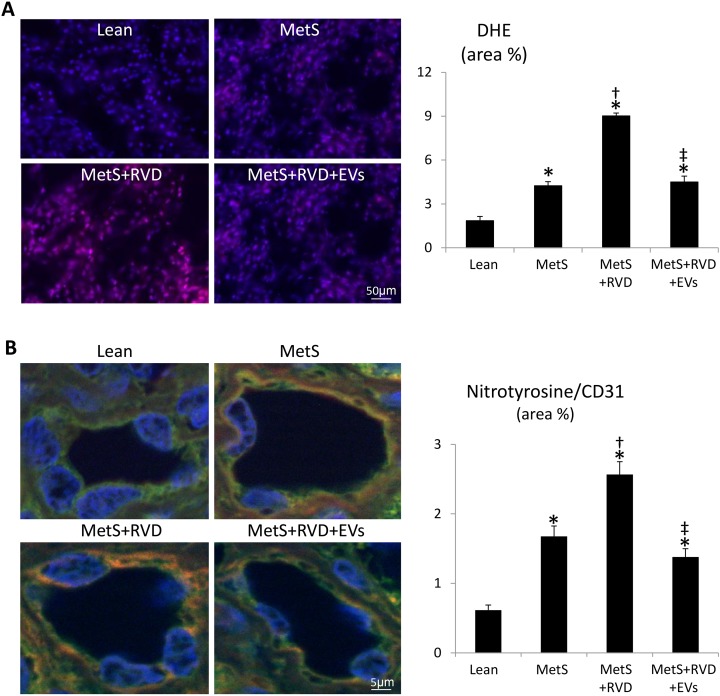
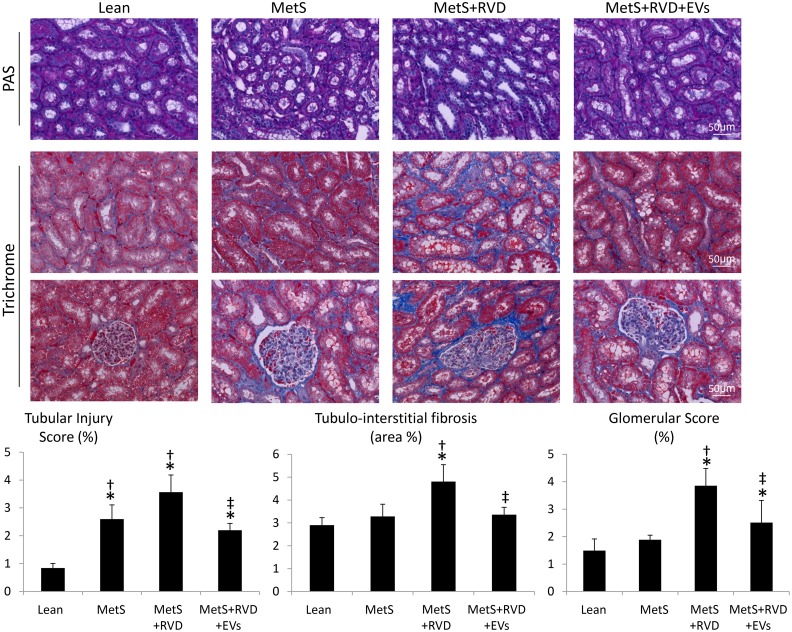
The number of caspase-3+ apoptotic cells increased in MetS kidneys, further increased in MetS+RVD, but decreased in MetS+RVD+EVs (Fig. 6A), as did the number of TUNEL+/CD31+ cells (Fig. 6B), suggesting endothelial cell apoptosis. Renal production of superoxide anion (DHE) was elevated in all MetS compared with Lean, increased further in MetS+RVD, but decreased to levels comparable to MetS in MetS+RVD+EVs (Fig. 7A), as did levels of circulating isoprostanes (Table 1). Double immunoreactivity of nitrotyrosine and CD31 increased in MetS and MetS+RVD, but slightly decreased in EV-treated pigs (Fig. 7B), indicating vascular oxidative stress. Tubular injury score increased in MetS+RVD compared with Lean and MetS, but decreased in MetS+RVD+EVs, as did tubulo-interstitial fibrosis and glomerular score (Fig. 8).
Figure 6.
EVs decreased endothelial cell apoptosis in MetS+RVD. A: Fluorescent renal staining (40×) and quantification of caspase-3. B: Double renal fluorescence staining with terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL, green arrows) and CD31 (green arrows). Yellow arrows indicated double + cells. *p < 0.05 vs. Lean; †p < 0.05 vs. MetS; ‡p < 0.05 vs. MetS+RVD.
Figure 7.
EVs decreased vascular oxidative stress in MetS+RVD. A: Fluorescent renal staining (40×) and quantification of dihydroethidium (DHE). Double renal fluorescence staining with nitrotyrosine (red arrows) and CD31 (green arrows) shows endothelial-cell-specific oxidative stress. Yellow arrows indicate double + cells. *p < 0.05 vs. Lean; †p < 0.05 vs. MetS; ‡p < 0.05 vs. MetS+RVD.
Figure 8.
Representative kidney periodic acid-Schiff (PAS) and trichrome staining (40×), and quantifications of tubular injury, tubulo-interstitial fibrosis, and glomerular score. *p < 0.05 vs. Lean; †p < 0.05 vs. MetS; ‡p < 0.05 vs. MetS+RVD.
MSC-derived EVs Restored Renal Function
Single-kidney volume, RBF, and GFR were higher in all MetS compared with Lean, decreased in MetS+RVD, but increased to MetS levels in MetS+RVD+EVs (Table 1). Yet, PRA levels did not differ among the groups.
Discussion
This study demonstrates that MSC-derived EVs preserve the microcirculation of the post-stenotic kidney in coexisting experimental MetS and RVD. A single intra-renal delivery of autologous EVs restored intra-renal expression of angiogenic factors, reduced microvascular remodeling and loss in the stenotic RVD kidney, and in turn stenotic-kidney tissue injury. Importantly, the protective effects of EVs might be attributed partly to their cargo of pro-angiogenic genes and proteins, and their capacity to improve microvascular disease, an important determinant of renal function beyond a stenotic lesion. Therefore, our observations may shed light into the mechanisms underlying the vasculo-reparative properties of MSCs, and suggest a novel cell-free refinement of MSC therapy to treat MetS+RVD.
The prevalence of MetS approaches 50% in patients presenting with symptomatic RVD, and their coexistence aggravates renal functional outcomes after revascularization4. MetS directly impacts on the vascular system, amplifying vessel thickness and stiffness36, and favoring development of atherosclerosis and thrombosis37. Previous studies in swine RVD have demonstrated that microvascular remodeling, damage, or loss play a critical role in the progression of renal injury. Impaired microvascular structure or function leads to tubular injury, matrix accumulation, interstitial fibrosis, and renal dysfunction38. Importantly, superimposition of MetS aggravates renal microvascular damage in swine RVD39 and blunt renal recovery in patients with RVD4, underscoring the need for effective therapeutic strategies to preserve the renal microcirculation.
Stem cells have shown a tremendous potential to attenuate both acute and chronic renal microvascular injury. We have previously shown in chronic swine RVD that intra-renal delivery of autologous adipose tissue-derived MSCs improves renal function7,8. These effects may be mediated by the release of EVs, which shuffle genes and proteins that mediate the paracrine activity of MSCs. Importantly, the cargo of MSC-derived EVs appears to be selected to activate endogenous repair mechanisms in recipient cells. We have previously shown that EVs are preferentially enriched for genes encoding transcription factors that modulate pro-angiogenic pathways, whereas genes encoding for mitochondrial, calcium signaling, and cytoskeletal proteins were selectively excluded13. Similarly, proteins enriched in MSC-derived EVs are linked to angiogenesis, whereas those depleted are primarily involved in nucleotide binding and RNA splicing14. More recently, we found that interactions among the mRNAs and microRNAs enriched in MSC-derived EVs regulate transcription factor activity in EVs and recipient cells40. These observations suggest that EVs have a selectively enriched cargo with a specific biological signature that promotes angiogenesis and facilitates tissue repair.
The important role of EVs as paracrine mediators of MSCs is underscored by experimental studies showing that MSCs and EVs exhibit a comparable and potentially additive effect on reducing renal injury and dysfunction41, and might in fact confer additional renoprotective effects. Indeed, delivery of combined MSCs and MSC-derived EVs is superior to either one alone in improving renal function42. In agreement with this, we have recently found that MSC-derived EVs attenuate renal inflammation and improve function in coexisting MetS and RVD43, mimicking the effects of MSCs in non-atherosclerotic and atherosclerotic swine RAS7,8,24. Likewise, a recent pilot study demonstrated that systemic administration of umbilical cord MSC-derived EVs improves kidney function in patients with CKD44. However, whether improvement in renal function by EV delivery is associated with preservation of the renal microvascular architecture in RVD remains unknown.
The current study used an innovative animal model integrating both MetS15 and RVD16, recapitulating features of human disease. We found that a single intra-renal delivery of adipose-tissue MSC-derived EVs provides a means to preserve microvascular architecture and recover the function of the MetS+RVD kidney. For example, the spatial density of cortical microvessels, major determinants of GFR in the stenotic kidney34, decreased in MetS+RVD, but slightly improved in MetS+RVD+EVs. Furthermore, intra-renal delivery of EVs improved vascular maturity, reflected in decreased vessel tortuosity45, and improved the number of peritubular capillaries, which maintain tubular integrity46.
The angiogenic potency of MSC-derived EVs, which carry protein-encoding mRNAs that stimulate vascular development (e.g. VEGF-A, VEGF-C, VEGF receptors, etc.) and proteins (e.g. VEGF, Angiopoietin Like 4, Hepatocyte Growth Factor, etc.), might have contributed to the improved renal microvasculature, as renal expression of the pro-angiogenic proteins VEGF, Notch-1, and DLL4 improved in MetS+RVD+EVs. Indeed, VEGF-induced gene expression of Notch1 and DLL4 in human arterial endothelial cells triggers arteriogenesis and angiogenesis, establishing a functional linkage between these two angiogenic signaling pathways47. Speculatively, the uptake of EVs carrying a pro-angiogenic cargo by CD31+ cells might have contributed to improved renal angiogenesis and microvasculature.
Notably, restoration of renal angiogenesis and microvascular architecture in EV-treated pigs was associated with decreased microvascular oxidative stress and apoptosis. We have previously shown that renal microvascular remodeling and loss in the stenotic kidney are associated with increased oxidative stress, and that antioxidant intervention improves RBF and decreases renal fibrosis48. Likewise, acute and chronic treatments with compounds that prevent mitochondrial-dependent apoptosis restore microvascular architecture in RVD, suggesting that apoptosis may also contribute to loss of vascular cells33,35. Consistent with these observations, we found that renal superoxide anion and circulating isoprostanes increased in MetS+RVD, but decreased in EV-treated pigs, whereas the number of caspase-3+ apoptotic cells decreased in MetS+RVD+EV kidneys, extending previous in-vitro studies employing EVs25. In addition, we used nitrotyrosine staining, a footmark of peroxynitrate-mediated protein damage, as an index for increased abundance of superoxide anion, and thus oxidative stress49. Indeed, renal endothelial cell apoptosis and oxidative stress increased in MetS+RVD, but decreased in EV-treated pigs. Notably, restoration of the renal microvasculature might have partly contributed to attenuate oxidative stress and apoptosis by preserving delivery of oxygen. Nevertheless, this might have been a direct antioxidant or anti-apoptotic effect of EVs, which carry genes that modulate apoptosis and oxidative stress, and incorporate into proximal and distal tubular cells and endothelial cells. For example, EVs are packed with numerous antiapoptotic genes, such as several BCL2 family apoptosis regulators and the X-linked inhibitor of apoptosis. Likewise, EVs contained several genes and proteins with important antioxidant properties, including glutathione peroxidases, superoxide dismutases, and peroxiredoxins. Overall, EV-induced microvascular proliferation likely contributed to improve RBF and renal function (GFR and serum creatinine levels), and attenuate tubular injury and fibrosis, underscoring important reno-protective properties of this intervention.
MSC-derived EVs may offer several advantages over their parent cells in repairing the post-stenotic kidney. Being acellular, EVs cannot proliferate in the recipient tissue and are thus exempted from adverse effects associated with delivery of live replicating MSCs, such as maldifferentiation or malignant transformation50. Although renal retention rate is similar between MSCs and their daughter EVs, their small size might allow EV penetration into deeper regions of the kidney upon injection into the renal artery, extending their reparative effects. In addition, EVs are more stable than MSCs and for practical aspects can be cryopreserved and stored for a long time, allowing their use as “off the shelf” products. Taken together, these advantages suggest that delivery of MSC-derived EVs is a useful regenerative strategy to improve the damaged kidney. On the other hand, it is possible that the ability of MSCs to survive, proliferate, and release EVs after engraftment would prolong the beneficial effects of cell-based therapy, but this postulation remains to be tested.
Our study has some limitations including the use of young animals and the short duration of MetS and RVD. Nevertheless, our MetS animals developed obesity, hypertension, hyperlipidemia, and insulin resistance, which impart subtle changes on kidney structure and function, such as hyperfiltration, microvascular loss, apoptosis, oxidative stress, and tubular injury. Moreover, as observed in human subjects4, superimposition of RVD markedly aggravated MetS-induced renal injury, contributing to post-stenotic dysfunction and fibrosis. We have previously shown that retention of EVs in the stenotic kidney peaked at 2 days (∼9% of injected amount) and decreased thereafter, remaining at 2% by 4 weeks after injection43. While EVs were clearly detected, co-localizing with proximal and distal tubular cells, and endothelial cells, the mechanisms regulating EV engraftment remain to be determined and evaluated in future studies.
In summary, our study suggests a novel therapeutic role for MSC-derived EVs in promoting angiogenesis and vascular repair, and thereby improving renal function in chronic experimental MetS+RVD. Intra-renal administration of EVs normalized renal expression of pro-angiogenic factors, improved microvascular architecture, and decreased tissue injury in the post-stenotic kidney. These observations therefore reveal that EVs are endowed with pro-angiogenic potential to repair the damaged kidney in coexisting MetS+RVD. Further studies are needed to examine how these beneficial effects compare with MSCs, and whether they persist in individuals suffering from this prevalent disease.
Footnotes
Ethical Approval: This study was approved by our institutional animal care and use committee (IACUC) (approval case number: A00003694-18).
Statement of Human and Animal Rights: All animal procedures in this study were approved by our institutional animal care and use committee (IACUC) (approval case number: A00003694-18).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partly supported by NIH grant numbers DK73608, DK104273, HL123160, DK102325, DK106427, the Central Society for Clinical and Translational Research, the Mayo Clinic Center for Regenerative Medicine, and the Mayo Clinic Foundation: Mary Kathryn and Michael B. Panitch Career Development Award.
References
- 1. Martins D, Ani C, Pan D, Ogunyemi O, Norris K. Renal dysfunction, metabolic syndrome and cardiovascular disease mortality. J Nutr Metab. 2010;2010:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferraro PM, Lupo A, Yabarek T, Graziani MS, Bonfante L, Abaterusso C, Gambaro G, Incipe Study G. Metabolic syndrome, cardiovascular disease, and risk for chronic kidney disease in an italian cohort: Analysis of the incipe study. Metab Syndr Relat Disord. 2011;9(5):381–388. [DOI] [PubMed] [Google Scholar]
- 3. Lerman L, Textor SC. Pathophysiology of ischemic nephropathy. Urol Clin North Am. 2001;28(4):793–803, ix. [DOI] [PubMed] [Google Scholar]
- 4. Davies MG, Saad WE, Bismuth J, Naoum JJ, Peden EK, Lumsden AB. Impact of metabolic syndrome on the outcomes of percutaneous renal angioplasty and stenting. J Vasc Surg. 2010;51(4):926–932. [DOI] [PubMed] [Google Scholar]
- 5. Zou X, Gu D, Xing X, Cheng Z, Gong D, Zhang G, Zhu Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res. 2016;8(10):4289–4299. [PMC free article] [PubMed] [Google Scholar]
- 6. Eirin A, Lerman LO. Mesenchymal stem cell treatment for chronic renal failure. Stem Cell Res Ther. 2014;5(4):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eirin A, Zhang X, Zhu XY, Tang H, Jordan KL, Grande JP, Dietz AB, Lerman A, Textor SC, Lerman LO. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrol Dial Transplant. 2014;29(2):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30(5):1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ebrahimi B, Eirin A, Li Z, Zhu XY, Zhang X, Lerman A, Textor SC, Lerman LO. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One. 2013;8(7):e67474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7(3):e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A. Transfer of growth factor receptor mrna via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22(5):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nargesi AA, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for renal repair. Curr Gene Ther. 2017;17(1):29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O’Brien D, van Wijnen AJ, Lerman LO. Microrna and mrna cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, van Wijnen AJ, Lerman LO. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci Rep. 2016;6:36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pawar AS, Zhu XY, Eirin A, Tang H, Jordan KL, Woollard JR, Lerman A, Lerman LO. Adipose tissue remodeling in a novel domestic porcine model of diet-induced obesity. Obesity (Silver Spring). 2015;23(2):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol. 1999;10(7):1455–1465. [DOI] [PubMed] [Google Scholar]
- 17. Eirin A, Williams BJ, Ebrahimi B, Zhang X, Crane JA, Lerman A, Textor SC, Lerman LO. Mitochondrial targeted peptides attenuate residual myocardial damage after reversal of experimental renovascular hypertension. J Hypertens. 2014;32(1):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eirin A, Ebrahimi B, Zhang X, Zhu XY, Tang H, Crane JA, Lerman A, Textor SC, Lerman LO. Changes in glomerular filtration rate after renal revascularization correlate with microvascular hemodynamics and inflammation in swine renal artery stenosis. Circ Cardiovasc Interv. 2012;5(5):720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119(4):547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney: Role of increased oxidative stress. Arterioscler Throm Vasc Biol. 2004;24(10):1854–1859. [DOI] [PubMed] [Google Scholar]
- 21. Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol. 2001;281(4):F630–F638. [DOI] [PubMed] [Google Scholar]
- 22. Blande IS, Bassaneze V, Lavini-Ramos C, Fae KC, Kalil J, Miyakawa AA, Schettert IT, Krieger JE. Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate. Transfusion (Paris). 2009;49(12):2680–2685. [DOI] [PubMed] [Google Scholar]
- 23. Eirin A, Zhu XY, Ferguson CM, Riester SM, van Wijnen AJ, Lerman A, Lerman LO. Intra-renal delivery of mesenchymal stem cells attenuates myocardial injury after reversal of hypertension in porcine renovascular disease. Stem Cell Res Ther. 2015;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. 2013;31(1):117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, van Wijnen AJ, Lerman A, Lerman LO. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci Rep. 2016;6:36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalari KR, Nair AA, Bhavsar JD, O’Brien DR, Davila JI, Bockol MA, Nie J, Tang X, Baheti S, Doughty JB, Middha S, Sicotte H, Thompson AE, Asmann YW, Kocher JP. Map-rseq: Mayo analysis pipeline for rna sequencing. BMC Bioinformatics. 2014;15(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. Tophat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liao Y, Smyth GK, Shi W. Featurecounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. [DOI] [PubMed] [Google Scholar]
- 30. Hogan MC, Johnson KL, Zenka RM, Charlesworth MC, Madden BJ, Mahoney DW, Oberg AL, Huang BQ, Leontovich AA, Nesbitt LL, Bakeberg JL, McCormick DJ, Bergen HR, Ward CJ. Subfractionation, characterization, and in-depth proteomic analysis of glomerular membrane vesicles in human urine. Kidney Int. 2014;85(5):1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hogan MC, Bakeberg JL, Gainullin VG, Irazabal MV, Harmon AJ, Lieske JC, Charlesworth MC, Johnson KL, Madden BJ, Zenka RM, McCormick DJ, Sundsbak JL, Heyer CM, Torres VE, Harris PC, Ward CJ. Identification of biomarkers for pkd1 using urinary exosomes. J Am Soc Nephrol. 2015;26(7):1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riahi R, Sun J, Wang S, Long M, Zhang DD, Wong PK. Notch1-dll4 signalling and mechanical force regulate leader cell formation during collective cell migration. Nat Commun. 2015;6:6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res. 2014;103(4):461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eirin A, Zhu XY, Urbieta-Caceres VH, Grande JP, Lerman A, Textor SC, Lerman LO. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol. 2011;300(6):F1394–F1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eirin A, Li Z, Zhang X, Krier JD, Woollard JR, Zhu XY, Tang H, Herrmann SM, Lerman A, Textor SC, Lerman LO. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension. 2012;60(5):1242–1249. [DOI] [PubMed] [Google Scholar]
- 36. Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43(8):1388–1395. [DOI] [PubMed] [Google Scholar]
- 37. Palomo I, Alarcon M, Moore-Carrasco R, Argiles JM. Hemostasis alterations in metabolic syndrome (review). Int J Mol Med. 2006;18(5):969–974. [PubMed] [Google Scholar]
- 38. Eirin A, Lerman LO. Darkness at the end of the tunnel: Poststenotic kidney injury. Physiology (Bethesda). 2013;28(4):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang X, Li ZL, Woollard JR, Eirin A, Ebrahimi B, Crane JA, Zhu XY, Pawar AS, Krier JD, Jordan KL, Tang H, Textor SC, Lerman A, Lerman LO. Obesity-metabolic derangement preserves hemodynamics but promotes intrarenal adiposity and macrophage infiltration in swine renovascular disease. Am J Physiol Renal Physiol. 2013;305(3):F265–F276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, van Wijnen AJ, Lerman LO. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS One. 2017;12(3):e0174303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He J, Wang Y, Lu X, Zhu B, Pei X, Wu J, Zhao W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microrna-dependent repairing. Nephrology (Carlton). 2015;20(9):591–600. [DOI] [PubMed] [Google Scholar]
- 42. Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang CC, Sun CK, Kao GS, Chen SY, Chai HT, Chang CL, Chen CH, Lee MS. Combination of adipose-derived mesenchymal stem cells (admsc) and admsc-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–185. [DOI] [PubMed] [Google Scholar]
- 43. Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92(1):114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W, Adel H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gerwins P, Skoldenberg E, Claesson-Welsh L. Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Crit Rev Oncol Hematol. 2000;34(3):185–194. [DOI] [PubMed] [Google Scholar]
- 46. Rabelink TJ, Wijewickrama DC, de Koning EJ. Peritubular endothelium: The achilles heel of the kidney? Kidney Int. 2007;72(8):926–930. [DOI] [PubMed] [Google Scholar]
- 47. Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of notch1 and dll4 by vascular endothelial growth factor in arterial endothelial cells: Implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. 2004. Cortical microvascular remodeling in the stenotic kidney: Role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 24(10):1854–1859. [DOI] [PubMed] [Google Scholar]
- 49. Ahsan H. 3-nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum Immunol. 2013;74(10):1392–1399. [DOI] [PubMed] [Google Scholar]
- 50. Kunter U, Rong S, Boor P, Eitner F, Muller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007;18(6):1754–1764. [DOI] [PubMed] [Google Scholar]