Abstract
Purpose: Although extensive research has been carried out on the determinants of mobile or wearable health care technology (mHealth), as well as on its acceptance by patients and other health care providers, very little research has been done on physiotherapists' perspectives on the use of mHealth in their current or future practice. The aims of this study were to (1) explore the attitudes of physiotherapists toward mHealth using a modified technology acceptance model questionnaire, (2) understand the applications and delivery paradigms that are most desirable, and (3) assess the content validity of the questionnaire. Method: The questionnaire was administered online. Participants (n=76) were recruited using snowball and convenience sampling. Data were analyzed using factor analysis and partial least-squares path modelling. Results: Results indicate that perceived usefulness and perceived ease of use were related to early adoptive behaviour among participants. We found no evidence that age, gender, experience, or practice setting influenced early adoptive behaviour. Participants demonstrated favourable attitudes toward mHealth tools in clinical practice. Conclusions: This article provides initial insights into factors that are likely to be significant determinants of adoption of mHealth among physiotherapists. Further work, including qualitative research, will help to identify personal and institutional factors that will improve the acceptance of mHealth.
Key Words: health care surveys, mobile phone, technology assessment, health
Abstract
Objectif : même si des recherches approfondies ont porté sur les déterminants de la technologie de santé mobile et portable (santé mobile) et sur leur acceptation par les patients et les professionnels de la santé, rares sont celles qui traitent des perspectives des physiothérapeutes à l'égard de l'utilisation de la santé mobile dans leur pratique actuelle et future. La présente étude visait à 1) explorer les attitudes des physiothérapeutes à l'égard de la santé mobile au moyen d'un modèle d'acceptation technologique modifié, 2) comprendre les applications et les paradigmes de prestation les plus souhaitables et 3) évaluer la validité du contenu du questionnaire. Méthodologie : les chercheurs ont publié le questionnaire en ligne. Ils ont recruté les sujets (n=76) par échantillonnage en boule de neige et par échantillonnage de commodité. Ils ont analysé les données à l'aide d'une analyse des facteurs et d'un modèle de régression des moindres carrés partiels. Résultats : d'après les résultats, la perception de l'utilité et de la facilité d'utilisation dépend du comportement d'adoption rapide des participants. Aucune donnée n'indique que l'âge, le sexe, l'expérience ou le lieu de pratique influe sur un comportement d'adoption rapide. Les participants avaient des attitudes favorables envers les outils de santé mobile en pratique clinique. Conclusion : le présent article donne des points de vue initiaux sur les facteurs susceptibles d'être des déterminants importants de l'adoption de la santé mobile par les physiothérapeutes. D'autres travaux, y compris des recherches qualitatives, contribueront à déterminer les facteurs personnels et institutionnels qui favoriseront l'acceptation de la santé mobile.
Mots clés : enquêtes sur la santé, évaluation de la technologie, santé, téléphone mobile
The focus of the health care system is shifting from delivering expensive acute care to integrating preventive programmes and complex chronic management into the community.1 At the same time, the development and adoption of consumer-grade technology has been expedited by the increasing computing power and plunging cost of electronics,2 fostering a surge of innovative applications to support functional recovery for patients affected by injury, illness, and disease.3
To date, the most prolific application of mobile or wearable health care technology (mHealth) is in monitoring the type, quantity, and quality of everyday activities.4–9 Inexpensive and unobtrusive wireless sensors, combined with Internet-based communications and sophisticated signal processing and event-detection algorithms, have driven the growth of both clinical and consumer use of tools to monitor fitness and general wellness.4 Clinical providers can harness such technological advances to gather biometric data, engage patients in activity, improve communication, and increase efficiency in their practice.10
There is a natural fit between current, emerging mHealth technologies and the scope of physiotherapy practice.11 Although it is not meant to take the place of therapist-to-patient interaction, mHealth can provide tools to collect reliable outcome measures of vital signs, monitor data outside the clinical visit, provide feedback on posture and body mechanics, supply educational material, and engage patients with motivational prompts.12
Despite a growing body of academic research and considerable commercial interest, physiotherapists have not shown a corresponding integration of mHealth tools into their day-to-day practice.10 Thus, although mHealth, and the increasing use of technology in rehabilitation research, may ultimately improve practice, understanding the barriers to adoption and improving acceptance is needed. Once the technologies are ready for adoption, a thorough investigation of the impact of mHealth on patient outcomes should be carried out. One reason for the slow adoption of mHealth by physiotherapists thus far may be a mismatch between their technological preferences and current offerings. Although some research has been carried out on patient attitudes toward technology in rehabilitation,13 a gap exists in determining the factors that may influence physiotherapists' willingness to use or recommend new technologies. Although there has been some research in this area,14 the literature is often specific to a single application or limited to physicians.15–20 Gaining an insight into the experiences, attitudes, and opinions of physiotherapists may encourage the development of mHealth products and services that are more widely accepted.
Conceptual Model for Understanding Acceptance and Adoption of mHealth
The inconsistent adoption of mHealth21 has highlighted the need for theories that can predict and explain attitudes toward, and the eventual intention to use, technology in health care. The Technology Acceptance Model (TAM)22 is a popular framework that has been used frequently across a range of industries, including health care.23 In the Venkatesh and Davis version, the TAM consisted of perceived usefulness (PU), perceived ease of use (PEOU), behavioural intention to use (BI), and usage behaviour.24 PU and PEOU have been found to be the strongest determinants of BI, and PEOU has a direct effect on PU.25
Several studies have suggested that the TAM needs to be extended to improve its explanation of behaviour relating to the acceptance of technology in health care.18,23–26 In the research reported in this article, we consider a simplified TAM model that examines early adopter (EA) behaviour as a proxy for BI, along with the predictive power of PU and PEOU for EA. This reduced model excludes actual behaviour, as well as any explicit intention to immediately implement technology,27 because of the low levels of current use of mHealth in physiotherapists' daily practice. To clarify and contextualize the assessment instrument, we also examine therapists' willingness to implement mHealth tools in common clinical practice.
Objective of This Article
The research we report in this article seeks to understand the attitudes of physiotherapists toward mHealth and the use of technology in their practice. Our interest was in the possible barriers to adopting mHealth technology, which might explain its relatively slow adoption. To understand attitudes and possible barriers, we developed a questionnaire based on the TAM and administered it to a sample of training and practicing physiotherapists. We then carried out an online survey to evaluate the questionnaire's content validity.
Methods
Questionnaire design
Initial questionnaire items were derived from previously published instruments and modified to suit the context of mHealth technology in physiotherapy practice.18,25,28,29 We identified articles describing previous instruments by means of searches that were conducted in both the PubMed and the Google Scholar databases. Breadth was provided by using a disjunctive search strategy, in which relevant articles were defined as having any one of the following terms: TAM, technology in health, uptake of technology, technology in physical therapy, uptake of technology in physical therapy, or mobile technology in health. Because our goal was not to generate every possibly relevant construct or questionnaire item but rather to include a comprehensive and representative set of measures, we reviewed the titles and abstracts to narrow down the large number of articles returned in the search to a smaller set that listed relevant questionnaire instruments.
The questionnaire's face validity and comprehensibility was evaluated by a panel of experts in fields relevant to technology acceptance, usability research, medicine, and physiotherapy, who filtered and refined the set of questions. The panel consisted of one physician working in neuro-rehabilitation, two physiotherapists working in the neuro-rehabilitation unit of a rehabilitation hospital, one expert in human factors and technology assessment, and one PhD in psychology with expertise in the design of health care environments. The preliminary questionnaire was then pilot tested among 12 physiotherapists at Bridgepoint Active Healthcare, a complex care facility in Toronto. Our research ethics protocol was approved by the institutional review boards at both Mount Sinai Hospital and the University of Toronto. Both quantitative (Cronbach's α and inter-item correlation analysis) and qualitative feedback were used to revise the questionnaire before we sent it out for wider distribution. Domain experts then further reviewed the questionnaire to ensure that it was well structured and that there was no excessive redundancy among the questions.
The final version of the questionnaire, the Physiotherapy Mobile Acceptance Questionnaire (PTMAQ), consists of a set of demographic items relevant to the use of personal technology, along with 30 questions derived using the method described previously. The 30 questions consist of 12 modified TAM items and 18 items relating to clinical variables for which mHealth technology could be recommended (see the Appendix). After consulting with several academically appointed physiotherapy professionals, the clinical variables we used were (1) overall activity level, (2) balance, (3) gait speed, (4) gait quality, (5) cognitive status, and (6) pain level. We chose these clinical variables because they are broad, commonly assessed, and incorporate measures for which various technology solutions have already been found.30–33 Each clinical variable had three variants: one covering measurement at a single point in time, one covering longitudinal measurement, and one dealing with practice items carried out by patients.
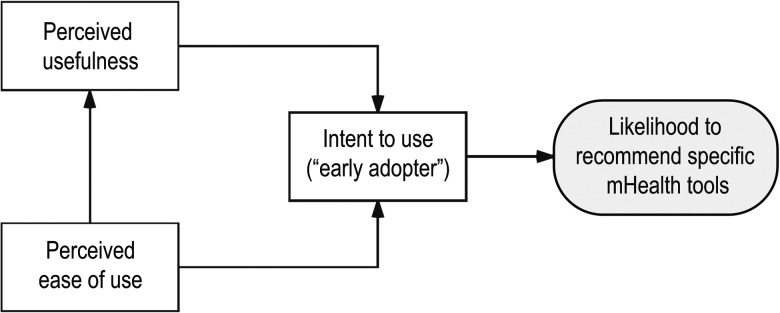
In the PTMAQ's final configuration, the 12 TAM items in the questionnaire were based on a simplified framework, in which we examined the effect of PU and PEOU on EA and the effect of PEOU on PU (see Figure 1). We also examined the extent to which accepting technology in the use of clinical tools could be explained by an individual's EA behaviour.
Figure 1.
Simplified framework for the 12 Technology Acceptance Model items in the questionnaire.
mHealth=mobile or wearable health care technology.
In accordance with previous practice for the TAM, including its formulation by Venkatesh and Davis,24 the PU questions in our questionnaire were framed positively, as were the intention to use–EA questions. However, we chose to frame the PEOU questions negatively so that we could collect a conservative estimate of how usable physical therapists find mobile or wearable technology (MWT) to be.
Participants and conditions
After the questionnaire was refined, it was distributed to practicing physiotherapists and physiotherapy students online using the Typeform platform (Typeform, Barcelona, Spain). The questionnaire link was advertised through e-mail and Twitter, through Ontario's professional physiotherapy organizations, and through the administrative heads of professional physiotherapy degree programmes at Ontario universities. When they completed the questionnaire, all participants were entered into a drawing to win a $50 Amazon gift card. The institutional review board at the University of Toronto approved the protocol. Participants were informed that submitting the survey implied informed consent. In the presentation of the results, the term physical therapist refers both to practicing physical therapists and to students who were training to be physical therapists, unless otherwise noted.
Methods of analysis
Other than the demographic information, data were collected using a five-point Likert scale, on which 1 = strongly disagree, 2=disagree, 3=neutral, 4=agree, and 5=strongly agree. Principal components analysis (PCA) was used to explore the underlying factor structure and reliability, and the internal reliability of the resulting factors was assessed by means of Cronbach's α and inter-item correlation.34 Demographic group differences with respect to the components were evaluated using analyses of variance, and post hoc analysis was carried out using Tukey–Kramer adjustments to account for multiple comparisons. All data analyses were performed using R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
To examine the appropriateness of the modified TAM model for our data set, partial least-squares path modelling (PLS-PM) was used.35 PLS-PM is an approach to structural equation modelling that allows researchers to represent latent constructs, observations, and their relationship in a single statistical model.36–38 Using the PTMAQ, observations were collected on the latent constructs of interest. Factor analysis was then used to create a revised set of constructs based on the correlations among the question items that were observed in our sample. The significance of path coefficients was assessed using two-tailed t-tests; associated p-values were reported along with β values.
Results
Descriptive statistics
A total of 76 completed surveys were submitted. Because we used a network recruitment strategy,39 whereby participants were encouraged to share the survey with colleagues, the exact number of surveys sent out is not known. There were 120 unique visits to the survey website (completion rate=63%; average time to complete=9 min, 20 s). Table 1 shows the demographic attributes of the participants. Although 46 of the participants were physiotherapy students, participants had a relatively broad range of ages and years of experience. All the students had completed at least 1 year of their graduate degree programme and likely had practicum experience in various care settings (as some of them noted; see Table 1).
Table 1.
Demographic Attributes of the Participants
| Category and attribute | No. (%) of participants (n=76) |
| Age, y | |
| <25 | 27 (35.5) |
| 25–34 | 26 (34.2) |
| 35–44 | 13 (17.1) |
| 45–54 | 5 (6.6) |
| ≥55 | 5 (6.6) |
| Gender | |
| Male | 27 (35.5) |
| Female | 48 (63.2) |
| Prefer not to disclose | 1 (1.3) |
| Highest education completed | |
| Undergraduate degree | 32 (42.1) |
| College degree | 6 (7.9) |
| Master's degree | 36 (47.4) |
| PhD | 1 (1.3) |
| Other | 1 (1.3) |
| Years in practice | |
| 0 (student) | 46 (60.5) |
| 1–4 | 4 (5.3) |
| 5–10 | 5 (6.6) |
| 11–20 | 10 (13.2) |
| >20 | 11 (14.5) |
| Primary practice setting | |
| Private | 18 (23.7) |
| Acute care hospital | 5 (6.6) |
| Rehab hospital | 20 (26.3) |
| Home-based care | 2 (2.6) |
| n/a | 31 (40.8) |
| Own a smartphone? | |
| Yes | 75 (98.7) |
| No | 1 (1.3) |
| Wear (or have worn) a wearable tracking device? | |
| Yes | 46 (60.5) |
| No | 30 (39.5) |
| Type of mHealth device used | |
| Activity tracker | 1 (1.3) |
| Pedometer | 4 (5.3) |
| Smartphone (embedded sensors) | 6 (7.9) |
| Watch or band | 30 (39.5) |
| Multiple devices | 7 (9.2) |
n/a=not applicable.
Evaluating the measurement instrument
PCA, followed by orthogonal rotation (varimax), was conducted on the initial items from the non-demographic data to reduce the dimensionality of the data and identify an underlying factor structure. The Kaiser–Meyer–Olkin (KMO) measure verified the sampling adequacy for the analysis: KMO=0.79, whereby a value of more than 0.80 is classified as “meritorious.”40(p.35) Bartlett's test of sphericity (χ2561=2,245.7; p<.001) indicated that correlations between items were sufficiently large for PCA. An initial analysis was run to obtain eigenvalues for each component in the data. After examining the scree plot and eigenvalues, we retained six components, which explained 68% of the total variance. Item reliability analysis was then carried out using Cronbach's α as the criterion; this enabled us to create scales based on the items that loaded on each of the factors.41 For each factor, items were removed if removing them did not reduce Cronbach's α.42
Table 2 shows the factor loadings after item removal and rotation. To assist in interpreting the table, we ordered the components to match the order of the items in the questionnaire as closely as possible. Items were considered relevant to a factor if their loadings were greater than 0.6.43 On the basis of the item loadings, component 1 represents PU (specifically as it relates to usefulness, patient engagement, and communication of progress), component 2 represents EA behaviour and receptiveness to using emerging mHealth, and component 3 represents PEOU (reverse-scored). For the clinical variables, the factor analysis aggregated the clinical measures on three components: Component 4 relates to gait speed, component 5 relates to gait quality and balance, and component 6 relates to the non-biomechanical measures of pain and cognitive status. The reliability of the components, as estimated with Cronbach's α, were all above the recommended 0.7 threshold. The average inter-item correlation ranged from 0.48 to 0.79, suggesting that the internal consistency was high, with the possibility of some redundancy between items.44
Table 2.
Factor Loadings and Reliability Analysis
| Component and item | Loading | |||||
| Usefulness in engagement and communication | ||||||
| PU3 | 0.71* | 0.23 | −0.08 | 0.25 | −0.05 | 0.21 |
| PU1 | 0.69* | 0.16 | 0.24 | −0.12 | 0.10 | 0.06 |
| ACTIVITY3 | 0.63* | 0.03 | 0.05 | 0.07 | 0.53 | 0.23 |
| PU2 | 0.62* | 0.23 | 0.01 | 0.16 | 0.01 | −0.01 |
| Early adoptive behaviour | ||||||
| EA1 | 0.07 | 0.85* | 0.10 | 0.01 | 0.09 | 0.02 |
| EA2 | 0.28 | 0.72* | −0.01 | 0.04 | 0.21 | 0.14 |
| EA3 | 0.42 | 0.64* | 0.05 | 0.23 | −0.09 | 0.02 |
| Perceived difficulty of using MWT | ||||||
| PEOU2† | 0.12 | 0.01 | 0.83* | 0.10 | −0.05 | 0.11 |
| PEOU1† | 0.01 | 0.32 | 0.79* | −0.09 | 0.10 | −0.04 |
| PEOU3† | 0.01 | −0.16 | 0.67* | 0.18 | 0.22 | 0.16 |
| Clinical usefulness: gait speed | ||||||
| GAITSPEED1 | 0.04 | 0.06 | 0.04 | 0.81* | 0.33 | 0.08 |
| GAITSPEED2 | 0.12 | −0.01 | 0.00 | 0.78* | 0.35 | 0.29 |
| GAITSPEED3 | 0.25 | 0.14 | 0.06 | 0.78* | 0.22 | 0.29 |
| Clinical usefulness: balance and gait quality | ||||||
| BALANCE1 | −0.01 | 0.11 | 0.10 | 0.09 | 0.85* | 0.24 |
| BALANCE2 | 0.06 | 0.08 | 0.03 | 0.14 | 0.81* | 0.37 |
| BALANCE3 | 0.17 | 0.31 | 0.04 | 0.12 | 0.67* | 0.33 |
| GAITQUAL1 | 0.09 | 0.06 | 0.04 | 0.30 | 0.81* | 0.18 |
| GAITQUAL2 | 0.14 | 0.02 | 0.07 | 0.23 | 0.81* | 0.23 |
| GAITQUAL3 | 0.17 | 0.11 | 0.07 | 0.28 | 0.75 | 0.25 |
| Clinical usefulness: non-biomechanical measures | ||||||
| COG1 | 0.04 | 0.07 | 0.11 | 0.00 | 0.23 | 0.73* |
| COG2 | 0.01 | 0.08 | −0.01 | 0.07 | 0.31 | 0.75* |
| COG3 | 0.08 | 0.07 | −0.06 | 0.08 | 0.25 | 0.81* |
| PAIN1 | 0.06 | 0.05 | 0.16 | 0.21 | 0.18 | 0.77* |
| PAIN2 | 0.13 | 0.03 | 0.18 | 0.18 | 0.16 | 0.80* |
| PAIN3 | 0.24 | −0.04 | 0.27 | 0.15 | 0.23 | 0.72* |
| Other | ||||||
| Eigenvalue | 4.38 | 2.77 | 2.63 | 3.13 | 5.55 | 4.78 |
| % of variance | 12.9 | 8.1 | 7.7 | 9.2 | 16.3 | 14.0 |
| Cronbach's α | 0.75 | 0.75 | 0.74 | 0.92 | 0.94 | 0.91 |
| Average inter-item correlation | 0.48 | 0.51 | 0.49 | 0.79 | 0.72 | 0.62 |
Note: Items without significant factor loadings have been removed. PTMAQ items are abbreviated to improve readability. Numerals are used to indicate the following: 1 refers to objective measurement of the variable, 2 refers to longitudinal measurement of the variable over time, and 3 refers to encouragement of practice carried out by patients.
Factor loading >0.6.
Item was reverse-scored for analysis.
PU=perceived usefulness; ACTIVITY=activity level; EA=early adopter; MWT=mobile or wearable technology; PEOU=perceived ease of use; COG=cognitive status; PAIN=pain level; PTMAQ=Physiotherapy Mobile Acceptance Questionnaire.
Descriptive statistics for the questionnaire responses (mean, SD, and correlation of the item with the component, corrected for item overlap and scale reliability) are shown in Table 3, ordered by the factors on which they load. Again, the components are ordered as closely as possible to the items in the questionnaire.
Table 3.
Components, Items, and Descriptive Statistics
| Component and item | Mean (SD) | Correlation (rb) | |
| Usefulness in engagement and communication | |||
| PU3 | MWT may help my patients stay engaged during the rehabilitation process (especially outside our face-to-face sessions). | 4.11 (0.73) | 0.78 |
| PU1 | I would find it interesting to use mobile or wearable technology to monitor my patient's progress (e.g., using a FitBit to assess their daily activity levels). | 4.17 (0.79) | 0.91 |
| ACTIVITY3 | I would recommend a MWT to my patient if it encouraged my patient to increase their overall activity level (within or outside our one-on-one sessions). | 4.47 (0.64) | 0.51 |
| PU2 | Mobile or wearable technology may provide me with a way to communicate my patient's progress more clearly. | 4.04 (0.72) | 0.55 |
| Early adoptive behaviour | |||
| EA1 | I would be willing to try out a new MWT in my practice before it had been clinically validated. | 4.17 (0.88) | 0.74 |
| EA2 | In the future, I would be likely to test or try out a new MWT with my patients. | 4.00 (0.80) | 0.70 |
| EA3 | I would be willing to use a new MWT if I received appropriate training. | 4.22 (0.64) | 0.59 |
| Perceived difficulty of using MWT | |||
| PEOU2 | I expect learning how to use a new MWT that was specifically designed for physiotherapy will be quite difficult (e.g., a device that provides feedback on my patient's balance). | 2.30 (0.80) | 0.74 |
| PEOU1 | I expect using a MWT in my practice will take a lot of extra time (e.g., a wearable tool that collects data about my patient's gait). | 2.72 (0.87) | 0.67 |
| PEOU3 | I expect it will take significant additional training before I am comfortable using a MWT in my practice. | 2.43 (1.06) | 0.58 |
| Clinical usefulness: gait speed | |||
| GAITSPEED1 | I would recommend a MWT to my patient if it gave me a more objective measurement of my patient's gait speed. | 4.17 (0.84) | 0.88 |
| GAITSPEED2 | I would recommend a MWT to my patient if it gave me a longitudinal measurement of my patient's gait speed over time. | 4.24 (0.80) | 0.93 |
| GAITSPEED3 | I would recommend a MWT to my patient if it encouraged my patient to practice exercises to improve their gait speed (within or outside our one-on-one sessions). | 4.17 (0.88) | 0.82 |
| Clinical usefulness: balance and gait quality | |||
| BALANCE1 | I would recommend my patient use a [MWT] if it gave me a more objective measurement of my patient's balance. | 4.29 (0.80) | 0.86 |
| BALANCE2 | I would recommend my patient use a [MWT] if it gave me a longitudinal measurement of my patient's balance over time. | 4.29 (0.78) | 0.88 |
| BALANCE3 | I would recommend my patient use a [MWT] if it encouraged my patient to practice exercises to improve their balance (within or outside our one-on-one sessions). | 4.28 (0.93) | 0.78 |
| GAITQUAL1 | I would recommend my patient use a [MWT] if it gave me a more objective measurement of my patient's gait quality. | 4.21 (0.87) | 0.87 |
| GAITQUAL2 | I would recommend my patient use a [MWT] if it gave me a longitudinal measurement of my patient's gait quality over time. | 4.25 (0.84) | 0.90 |
| GAITQUAL3 | I would recommend my patient use a [MWT] if it encouraged my patient to practice exercises to improve their gait quality (within or outside our one-on-one sessions). | 4.26 (0.89) | 0.88 |
| Clinical usefulness: non-biomechanical measures | |||
| COG1 | I would recommend my patient use a [MWT] if it gave me a more objective measurement of my patient's cognitive status (e.g., by having them answer a questionnaire or perform an assessment like the Stroop task). | 3.59 (0.93) | 0.72 |
| COG2 | I would recommend my patient use a [MWT] if it gave me a longitudinal measurement of my patient's cognitive status over time (e.g., by having them repeatedly answer a questionnaire or perform an assessment like the Stroop task). | 3.64 (0.86) | 0.81 |
| COG3 | I would recommend my patient use a [MWT] if it encouraged my patient to practice exercises to improve their cognitive status (e.g., through guided meditation or a cognitive-strengthening game). | 3.72 (0.89) | 0.83 |
| PAIN1 | I would recommend my patient use a [MWT] if it gave me a more objective measurement of my patient's pain level (e.g., by having them answer a questionnaire electronically). | 4.03 (0.93) | 0.83 |
| PAIN2 | I would recommend my patient use a [MWT] if it gave me a longitudinal measurement of my patient's pain level over time. | 4.08 (0.90) | 0.85 |
| PAIN3 | I would recommend my patient use a [MWT] if it encouraged my patient to practice exercises to mediate their pain level. | 4.12 (0.73) | 0.78 |
Note: PTMAQ items are abbreviated to improve readability. Numerals are used to indicate the following: 1 refers to objective measurement of the variable, 2 refers to longitudinal measurement of the variable over time, and 3 refers to encouragement of practice carried out by patients.
rb=correlation of item with mean score for the construct items (as shown in this table); PU=perceived usefulness; MWT=mobile or wearable technology; ACTIVITY=activity level; EA=early adopter; PEOU=perceived ease of use; COG=cognitive status; PAIN=pain level; PTMAQ=Physiotherapy Mobile Acceptance Questionnaire.
Structural model assessment
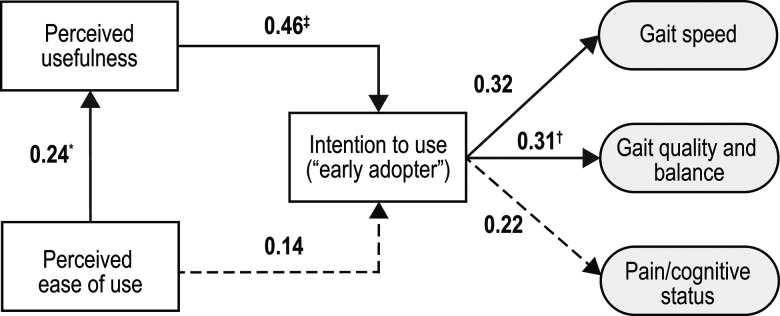
Once we determined that the measurement model was satisfactory, we used the PLS-PM technique to examine the relationships between the components. Figure 2 presents the path coefficients (referring to the structural relationship among the tested variables) that were estimated in the analysis. Note that in the figure, each participant's component score is the mean of the scores obtained for the items listed under that component in Table 3. The data indicate that PU had a reasonably strong effect on EA (β=0.46; p<0.001), but the hypothesis that PEOU had a direct effect on EA was not supported (β=0.14; p=0.20). We did find that PEOU had a moderate effect on PU (β=0.24; p=0.041) and that EA was related to the clinical components identified during factor analysis. The effect of EA on the likelihood of recommending an mHealth device for both gait speed (β=0.32; p=0.006) and gait quality and balance (β = 0.31; p=.008) was statistically significant, and the non-biomechanical construct (pain–cognitive status) exhibited a near-significant trend (β=0.22; p=0.07).
Figure 2.
Path coefficients estimated by the analysis. Dotted line indicates the relationship was non-significant
*p<0.05.
†p<0.01.
‡p<0.001.
The overall quality of the structural model was evaluated by R2, the coefficient of determination; the redundancy index; and GoF. The average redundancy indicated that PU and PEOU explained 31% of the variability in the EA indicators. The average redundancy and R2 values for the three clinical measures were also less than 0.10. However, the average communalities and average variance extracted were all above the recommended 50%.45 GoF was 0.28, indicating the model's reasonable predictive power.
Individual differences in technology acceptance
Composite scores for each component were obtained by averaging the items within the component. Univariate analyses of variance were then carried out to see how the component scores varied across the different levels of the demographic variables. Because this was an exploratory analysis, directional hypotheses were not formulated, and two-tailed tests of significance were used for the post hoc pairwise comparisons (independent-samples z-tests) that were carried out for the components that had a significant relationship with a demographic variable. We used z-tests because the degrees of freedom were greater than 70 for comparison and, with this relatively high number, the t-distribution asymptotes to a z-distribution.
For the post hoc tests, levels of significance were interpreted on the basis of a Bonferroni-adjusted p-value. There was a significant main effect for primary setting of practice on component 6 (pain level–cognitive status; F3,41=5.20; p=0.002). That effect was found to be due to a significant difference between private practice and rehabilitation hospital (z=3.34; p=0.001). Participants in the hospital had a more favourable attitude toward MWT. We also found a significant main effect for owning a wearable tracking device on gait speed (F1,73=6.15; p=0.008), with participants who owned a tracking device being more favourable toward use of MWT. We found no other significant effects of age, gender, experience, student status, practice setting, or personal technology use on any other constructs. Physical therapists rated MWT as a better prospect for use with both gait speed (z=2.7; p=.007) and gait quality and balance (z=3.25; p=.001) than for use with the non-biomechanical (pain level–cognitive status) components.
Discussion
We found no evidence to suggest that age, gender, years of experience, practice setting, or personal technology use were predictors of early adoptive behaviour. This contrasts with the popular notion that acceptance of emerging technology is much greater among the (digital native) generation, who have grown up in the age of ubiquitous Internet and mobile connectivity. In particular, although our sample had a high proportion of students (46 of 76), we found no significant differences between students and non-students on any of the measured constructs or in personal technology use.
Exploratory factor analysis indicated the presence of six factors, which mirrored, to a large extent, the conceptual structure of the questionnaire that we had developed. When variations in the conceptual structure occurred, they involved the clinical variables. “Encouraging overall activity” was grouped with the PU factor, balance and gait quality were combined into a single factor, and cognitive status and pain level were combined into a single factor. Note that the first two activity questions are not reported in Tables 2 or 3 because they were not associated with any of the six derived factors used to formulate the revised components.
As shown in Figure 2, PU was found to be the strongest driver of BI, with the impact of PEOU on BI being largely indirect (as a supplementary effect on PU). PU and PEOU jointly explained 31% of the variability in EA behaviour. Although this indicates that a significant portion of the variability is attributable to the TAM constructs, it is likely that constructs outside the TAM (and not assessed in this study) also influence the physiotherapists' EA behaviour with respect to mHealth and their practice. BI was significantly related to willingness to recommend MWT in the context of gait speed and in the context of gait quality and balance, but the relationship of BI to pain and cognitive status was weaker.
This study has several limitations. First, the transferability of its findings is limited by the small sample of participants, who were predominantly from Ontario. Second, the sample included a relatively large proportion of students. Third, we found limited variation in the data collected, suggesting congruent perspectives among our sample. However, this convergence in opinion may have occurred because participants in an online survey are more likely to be comfortable with using information technology, and have more interest in its use, than physiotherapists in general.
In formulating the 18 items associated with the clinical variables in Section III of the questionnaire, we chose to group the items under the six clinical variables. Alternatively, these 18 items could have been organized so that the six clinical variables were nested under the goals for each clinical variable (more objective measurement, better longitudinal data, more practice). That alternative instrument would have had 6 items on more objective measurement (1 for each of the six clinical variables), 6 items on better longitudinal data, and 6 items on more practice. It may be of interest in future research to examine whether the responses to Section III change, depending on whether questions are grouped by the six clinical variables or by the three goals (more objective measurement, better longitudinal data, more practice).
Future qualitative studies can build on this work to better illuminate the barriers and facilitators that exist in specific environments and institution types. Our experience suggests that future studies should include a demonstration of existing tools or prototypes to better determine technology acceptance factors. Other recommendations for future research are to revise the questionnaire to include other (non-TAM) possible drivers of EA and to administer the revised questionnaire to a broader (e.g., international) and larger sample. The questionnaire could also be modified by researchers developing rehabilitation technologies (by changing the wording appropriately for the rehabilitation technologies of interest) so that they can assess whether potential end users are ready to use their target devices or technologies.
Anyone administering this questionnaire in the future should also consider distributing it using methods other than an online survey to avoid the possibility that the results will be biased toward the views of technology enthusiasts. Alternative data collection strategies might involve distributing the survey within organizations that include a large number of physiotherapists and at physiotherapy conferences.
Conclusions
This study highlights the importance of understanding physiotherapists' attitudes and perceptions of mHealth in clinical practice. As key stakeholders, physiotherapists determine the appropriateness of interventions by combining clinical reasoning with the needs and preferences of their patients.46 Their acceptance of, or resistance to, emerging technologies will have a significant impact on the adoption of these tools, and it will likely have an impact on their potential for success in an evolving health care environment.
We believe this to be the first study to establish the initial face validity and content validity of an mHealth acceptance measure designed specifically for physiotherapists and grounded in a pre-existing theoretical model. Our aim in this work was to develop a measurement tool to identify the predictors of technology adoption among physiotherapists from diverse backgrounds and with different levels of experience. Our findings confirmed that there are good prospects for physiotherapists to implement emerging mHealth technology in their practice, but barriers remain.10
Our research suggests that an important determinant of early adoptive behaviour is how useful the technology appears to be. Physiotherapists, like other clinical professionals,25 are willing to dedicate time and resources to learning and implementing a new tool as long as its added value is well demonstrated. In our sample, the potential for mHealth devices to increase patient engagement and improve communication of progress was rated highly, suggesting that this is an important aspect of how physiotherapists assess utility. This leads to the recommendation that the interface and user experience of mHealth systems should enable key metrics to be efficiently reported and communicated (between clinicians and their patients and among members of a care team).
Although the present results indicate that physical therapists have a mostly positive attitude toward the potential for using MWT in their practice, it is clear that patient satisfaction and adherence, and tangible clinical outcomes, will need to be demonstrated before particular technological solutions will be adopted. One other caveat from this survey is that ratings were generally lower for PU (as can be seen in the lower mean ratings for PEOU items in Table 3) than for the other components. Consequently, suitable training initiatives may need to accompany the rollout of any new MWT tools for physical therapists.
Key Messages
What is already known on this topic
Mobile or wearable technology (MWT) is making significant inroads in the consumer space and advanced research, but it is not necessarily widely implemented in the day-to-day practice of physical therapists. Previous questionnaires and usability studies have provided perspectives on specific technologies, which are useful in that context but limited in scope.
What this study adds
This study demonstrates that a previously validated framework of technology acceptance is appropriate in exploring physiotherapists' attitudes toward incorporating MWT into their practice. Because this study is the first, to the authors' knowledge, to address this literature gap, its aim was to highlight the need for increased dialogue between technology developers and physiotherapists so that tools are developed that are useful and more readily adopted in clinical practice.
Appendix
Physiotherapy Mobile Acceptance Questionnaire (PTMAQ)
Section I—Demographics and Personal Technology Use
-
1.Age
-
□<25
-
□25–34
-
□35–44
-
□45–54
-
□55+
-
□
-
2.Gender
-
□Male
-
□Female
-
□Prefer not to disclose
-
□
-
3.Education level
-
□Undergraduate degree
-
□College degree
-
□Master's degree
-
□PhD
-
□Other _________________
-
□
-
4.Number of years in profession
-
□0 (student)
-
□1–4
-
□5–10
-
□11–20
-
□>20
-
□
-
5.Area of specialization
-
□ ___________________________
-
□
-
6.Primary setting of practice
-
□ Private
-
□ Hospital
-
□ Acute care
-
□ Long-term care
-
□ Home care
-
□
-
7.Do you own a smartphone device? (defined as a mobile phone that has a touch screen, can access the Internet, and can run third-party apps)
-
□ Yes
-
□ No
-
□ If yes, what type (iPhone, Android, etc.)? ______________________________
-
□
-
8.Do you use any third-party applications (that you have downloaded onto your smartphone)?
-
□ Yes
-
□ No
-
□
-
9.Do you wear or have you ever worn a wearable tracking device (pedometer, FitBit, Apple Watch, etc.)?
-
□ Yes
-
□ No
-
□ If yes, what type? ______________________________
-
□
Section II—Modified Technology Acceptance Model (TAM) Items
Response scale: strongly disagree, disagree, neutral, agree, strongly agree. (The component–item abbreviation appears in parentheses after the item.)
Perceived usefulness
-
1.
Mobile or wearable technology can promote engagement between the health care provider and patient or caregiver.
-
2.
Mobile or wearable technology may provide me with a way to communicate my patient's progress more clearly. (PU2)
-
3.
I would find it useful to use mobile or wearable technology to monitor my patient's progress (e.g., using a FitBit to assess their daily activity levels). (PU1)
-
4.
Mobile or wearable technology may help my patients stay engaged during the rehabilitation process (especially outside our face-to-face sessions). (PU3)
Perceived ease of use
-
5.
I expect using a mobile or wearable device in my practice will take a lot of extra time (e.g., a wearable tool that collects data about my patient's gait). (PEOU1)
-
6.
I expect it will take significant additional training before I am comfortable using a mobile or wearable device in my practice. (PEOU3)
-
7.
I expect learning how to use a new mobile or wearable device that was specifically designed for physiotherapy will be quite difficult (e.g., a device that provides feedback on my patient's balance). (PEOU2)
-
8.
I expect it will be difficult to teach or coach my patients on the use of a new mobile or wearable device (e.g., a mobile application that displays instructional videos on exercises and allows the patient to track their progress).
Intention to use/early adopter
-
9.
I would be willing to try out a new mobile or wearable technology in my practice before it had been clinically validated. (EA1)
-
10.
In the future, I would be likely to test or try out new mobile or wearable technology with my patients. (EA2)
-
11.
I would be willing to use a new mobile or wearable technology if I received appropriate training. (EA3)
-
12.
In the future, if my patient wore a sensor that automatically collected information about their health or well-being, I would refer to the data to understand how my patient is responding to treatment.
Section III—Likelihood of Recommending an mHealth Tool for Specific Clinical Purposes
-
13.
I would recommend my patient use a wearable device if it gave me a more objective measurement of my patient's overall activity level.
-
14.
I would recommend my patient use a wearable device if it gave me a longitudinal measurement of my patient's overall activity level over time.
-
15.
I would recommend my patient use a wearable device if it encouraged my patient to increase their overall activity level (within or outside of our one-on-one sessions). (ACTIVITY3)
-
16.
I would recommend my patient use a wearable device if it gave me a more objective measurement of my patient's balance. (BALANCE1)
-
17.
I would recommend my patient use a wearable device if it gave me a longitudinal measurement of my patient's balance over time. (BALANCE2)
-
18.
I would recommend my patient use a wearable device if it encouraged my patient to practice exercises to improve their balance (within or outside of our one-on-one sessions). (BALANCE3)
-
19.
I would recommend my patient use a wearable device if it gave me a more objective measurement of my patient's gait speed. (GAITSPEED1)
-
20.
I would recommend my patient use a wearable device if it gave me a longitudinal measurement of my patient's gait speed over time. (GAITSPEED2)
-
21.
I would recommend my patient use a wearable device if it encouraged my patient to practice exercises to improve their gait speed (within or outside of our one-on-one sessions). (GAITSPEED3)
-
22.
I would recommend my patient use a wearable device if it gave me a more objective measurement of my patient's gait quality. (GAITQUAL1)
-
23.
I would recommend my patient use a wearable device if it gave me a longitudinal measurement of my patient's gait quality over time. (GAITQUAL2)
-
24.
I would recommend my patient use a wearable device if it encouraged my patient to practice exercises to improve their gait quality (within or outside of our one-on-one sessions). (GAITQUAL3)
-
25.
I would recommend my patient use a mobile device if it gave me a more objective measurement of my patient's cognitive status (e.g., by having them answer a questionnaire or perform an assessment like the Stroop task). (COG1)
-
26.
I would recommend my patient use a mobile device if it gave me a longitudinal measurement of my patient's cognitive status over time (e.g., by having them repeatedly answer a questionnaire or perform an assessment like the Stroop task). (COG2)
-
27.
I would recommend my patient use a mobile device if it encouraged my patient to practice exercises to improve their cognitive status (e.g., through guided meditation or a cognitive-strengthening game). (COG3)
-
28.
I would recommend my patient use a mobile device if it gave me a more objective measurement of my patient's pain level (e.g., by having them answer a questionnaire electronically). (PAIN1)
-
29.
I would recommend my patient use a mobile device if it gave me a longitudinal measurement of my patient's pain level over time (e.g., by having them repeatedly answer a questionnaire electronically). (PAIN2)
-
30.
I would recommend my patient use a mobile device if it encouraged my patient to practice exercises to mediate their pain level. (PAIN3)
References
- 1. Majmudar MD, Colucci LA, Landman AB.. The quantified patient of the future: opportunities and challenges. Healthc (Amst). 2015;3(3):153–6. 10.1016/j.hjdsi.2015.02.001. Medline:26384227 [DOI] [PubMed] [Google Scholar]
- 2. Steinhubl SR, Muse ED, Topol EJ.. The emerging field of mobile health. Sci Transl Med. 2015;7(283):283rv3–283rv3. 10.1126/scitranslmed.aaa3487. Medline:25877894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Appelboom G, Yang AH, Christophe BR, et al. The promise of wearable activity sensors to define patient recovery. J Clin Neurosci. 2014;21(7):1089–93. 10.1016/j.jocn.2013.12.003. Medline:24534628 [DOI] [PubMed] [Google Scholar]
- 4. Dobkin BH, Dorsch A.. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25(9):788–98. 10.1177/1545968311425908. Medline:21989632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–92. 10.1093/sleep/26.3.342. Medline:12749557 [DOI] [PubMed] [Google Scholar]
- 6. Mansfield A, Wong JS, Bayley M, et al. Using wireless technology in clinical practice: does feedback of daily walking activity improve walking outcomes of individuals receiving rehabilitation post-stroke? Study protocol for a randomized controlled trial. BMC Neurol. 2013;13:93 10.1186/1471-2377-13-93. Medline:23865593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khusainov R, Azzi D, Achumba IE, et al. Real-time human ambulation, activity, and physiological monitoring: taxonomy of issues, techniques, applications, challenges and limitations. Sensors (Basel). 2013;13(10):12852–902. 10.3390/s131012852. Medline:24072027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evenson KR, Goto MM, Furberg RD.. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 2015;12(1):159 10.1186/s12966-015-0314-1. Medline:26684758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lauritzen J, Muñoz A, Luis Sevillano J, et al. The usefulness of activity trackers in elderly with reduced mobility: a case study. Stud Health Technol Inform. 2013;192(1–2):759–62. 10.3233/978-1-61499-289-9-759. Medline:23920659 [DOI] [PubMed] [Google Scholar]
- 10. Jones J, Norman K, Saunders S. The state of the union: trends and drivers of change in physiotherapy in Ontario in 2014 [Internet]. Toronto: College of Physiotherapists of Ontario; 2014. [cited 2016 Aug 29]. Available from: https://qspace.library.queensu.ca/handle/1974/12616 [Google Scholar]
- 11. American Physical Therapy Association [Internet]. The physical therapist scope of practice. Alexandria (VA); The Association; [cited 2016 August 29]. Available from: http://www.apta.org/ScopeOfPractice/
- 12. Dicianno BE, Parmanto B, Fairman AD, et al. Perspectives on the evolution of mobile (mHealth) technologies and application to rehabilitation. Phys Ther. 2015;95(3):397–405. 10.2522/ptj.20130534. Medline:24925075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laver K, Ratcliffe J, George S, et al. Early rehabilitation management after stroke: what do stroke patients prefer? J Rehabil Med. 2011;43(4):354–8. 10.2340/16501977-0678. Medline:21305229 [DOI] [PubMed] [Google Scholar]
- 14. Van Schaik P, Bettany-Saltikov JA, Warren JG.. Clinical acceptance of a low-cost portable system for postural assessment. Behav Inf Technol. 2002;21(1):47–57. 10.1080/01449290110107236 [DOI] [Google Scholar]
- 15. Lottridge DM, Chignell M, Danicic-Mizdrak R, et al. Group differences in physician responses to handheld presentation of clinical evidence: a verbal protocol analysis. BMC Med Inform Decis Mak. 2007;7(1):22 10.1186/1472-6947-7-22. Medline:17655759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Illiger K, Hupka M, von Jan U, et al. Mobile technologies: expectancy, usage, and acceptance of clinical staff and patients at a university medical center. JMIR Mhealth Uhealth. 2014;2(4):e42 10.2196/mhealth.3799. Medline:25338094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen J, Park Y, Putzer GJ.. An examination of the components that increase acceptance of smartphones among healthcare professionals. Electron J Heal Informatics. 2010;5(2):16 http://www.ejhi.net/ojs/index.php/ejhi/article/view/132 [Google Scholar]
- 18. Gagnon MP, Orruño E, Asua J, et al. Using a modified technology acceptance model to evaluate healthcare professionals' adoption of a new telemonitoring system. Telemed J E Health. 2012;18(1):54–9. 10.1089/tmj.2011.0066. Medline:22082108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wade VA, Eliott JA, Hiller JE.. Clinician acceptance is the key factor for sustainable telehealth services. Qual Health Res. 2014;24(5):682–94. 10.1177/1049732314528809. Medline:24685708 [DOI] [PubMed] [Google Scholar]
- 20. Bergmann JHM, McGregor AH.. Body-worn sensor design: what do patients and clinicians want? Ann Biomed Eng. 2011;39(9):2299–312. 10.1007/s10439-011-0339-9. Medline:21674260 [DOI] [PubMed] [Google Scholar]
- 21. North F, Chaudhry R.. Apple HealthKit and health app: patient uptake and barriers in primary care. Telemed J E Health. 2016;22(7):608–13. 10.1089/tmj.2015.0106. Medline:27172297 [DOI] [PubMed] [Google Scholar]
- 22. Davis FD. Perceived ease of use, and user acceptance of information technology. Manage Inf Syst Q. 1989;13(3):319–40. 10.2307/249008 [DOI] [Google Scholar]
- 23. Holden RJ, Karsh B-T.. The technology acceptance model: its past and its future in health care. J Biomed Inform. 2010;43(1):159–72. 10.1016/j.jbi.2009.07.002. Medline:19615467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Venkatesh V, Davis FD.. A theoretical extension of the technology acceptance model: four longitudinal studies. Manage Sci. 2000;46(2):186–204. 10.1287/mnsc.46.2.186.11926 [DOI] [Google Scholar]
- 25. Wu JH, Wang SC, Lin LM.. Mobile computing acceptance factors in the healthcare industry: a structural equation model. Int J Med Inform. 2007;76(1):66–77. 10.1016/j.ijmedinf.2006.06.006. Medline:16901749 [DOI] [PubMed] [Google Scholar]
- 26. Sellen K, Callum J, Pendergrast J, et al. Does technology acceptance determine attitude towards health information technology? The case of electronic remote blood delivery [Internet]. In: Annual Symposium of the American Medical Informatics Association, Workshop on Interactive Systems in Healthcare; 2011. [cited 2017 Jun 24]; Washington, DC. Available from: http://openresearch.ocadu.ca/id/eprint/1307/1/Sellen_Tech_2011.pdf
- 27. Hu PJ, Chau PYK, Liu Sheng OR, et al. Examining the Technology Acceptance model using physician acceptance of telemedicine technology. J Manage Inf Syst. 1999;16(2):91–112. 10.1080/07421222.1999.11518247 [DOI] [Google Scholar]
- 28. Rai A, Chen L, Pye J, et al. Understanding determinants of consumer mobile health usage intentions, assimilation, and channel preferences. J Med Internet Res. 2013;15(8):e149 10.2196/jmir.2635. Medline:23912839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glegg SMN, Holsti L, Velikonja D, et al. Factors influencing therapists' adoption of virtual reality for brain injury rehabilitation. Cyberpsychol Behav Soc Netw. 2013;16(5):385–401. 10.1089/cyber.2013.1506. Medline:23713844 [DOI] [PubMed] [Google Scholar]
- 30. Allard M, Husky M, Catheline G, et al. Mobile technologies in the early detection of cognitive decline. PLoS One. 2014;9(12):e112197 10.1371/journal.pone.0112197. Medline:25536290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonato P. Advances in wearable technology and applications in physical medicine and rehabilitation. J Neuroeng Rehabil. 2005;2(1):2 10.1186/1743-0003-2-2. Medline:15733322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gebruers N, Vanroy C, Truijen S, et al. Monitoring of physical activity after stroke: a systematic review of accelerometry-based measures. Arch Phys Med Rehabil. 2010;91(2):288–97. 10.1016/j.apmr.2009.10.025. Medline:20159136 [DOI] [PubMed] [Google Scholar]
- 33. Shull PB, Jirattigalachote W, Hunt MA, et al. Quantified self and human movement: a review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture. 2014;40(1):11–9. 10.1016/j.gaitpost.2014.03.189. Medline:24768525 [DOI] [PubMed] [Google Scholar]
- 34. Field A, Miles J, Field Z.. Discovering statistics using R. London: Sage; 2012. [Google Scholar]
- 35. Chin WW. The partial least squares approach to structural equation modeling. In: Marcoulides GA, editor. Modern methods for business research . Mahwah (NJ): Lawrence Erlbaum Associates; 1998. p. 295–336 [Google Scholar]
- 36. Evermann J, Tate M.. Comparing out-of-sample predictive ability of PLS, covariance, and regression models. In: International Conference on Information Systems; 2014 Dec 14–17; Auckland Atlanta (GA): AIS Electronic Library; 2014. [cited 2016 August 29]. Available from: http://aisel.aisnet.org/icis2014/proceedings/GeneralIS/23/ [Google Scholar]
- 37. Vinzi VE, Trinchera L, Amato S.. PLS path modeling: from foundations to recent developments and open issues for model assessment and improvement. In: Vinzi VE, Chin WW, Henseler J, et al., editors. Handbook of partial least squares. Heidelberg, Germany: Springer-Verlag Berlin Heidelberg; 2010. p. 47–82. 10.1007/978-3-540-32827-8_3 [DOI] [Google Scholar]
- 38. Petrarca F, Carpita M, Brentari E, et al. , editors. Advances in latent variables: methods, models and applications. Cham: Springer International Publishing Switzerland; 2015. [Google Scholar]
- 39. Bower P, Brueton V, Gamble C, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials. 2014;15:399 10.1186/1745-6215-15-399. Medline:25322807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaiser HF. An index of factorial simplicity. Psychometrika. 1974;39(1):31–6. 10.1007/BF02291575 [DOI] [Google Scholar]
- 41. Spector PE. Summated rating scale construction: an introduction. Thousand Oaks (CA): Sage Publications; 1992. 10.4135/9781412986038 [DOI] [Google Scholar]
- 42. Zwick WR, Velicer WF.. Comparison of five rules for determining the number of components to retain. Psychol Bull. 1986;99(3):432–42. 10.1037/0033-2909.99.3.432 [DOI] [Google Scholar]
- 43. Matsunaga M. How to factor-analyze your data right: do's, don'ts, and how-to's. Int J Psychol Res (Medellin). 2010;3(1):97–110. https://doi.org/10.21500/20112084.854 [Google Scholar]
- 44. Clark L, Watson D.. Construct validity: basic issues in objective scale development. Psychol Assess. 1995;7(3):309–19. 10.1037/1040-3590.7.3.309 [DOI] [Google Scholar]
- 45. Sanchez G. PLS path modeling with R [Internet]. Berkeley (CA): Trowchez Editions; 2013. [cited 2016 Aug 29]. Available from: http://www.gastonsanchez.com/PLS_Path_Modeling_with_R.pdf [Google Scholar]
- 46. Tatla SK, Shirzad N, Lohse KR, et al. Therapists' perceptions of social media and video game technologies in upper limb rehabilitation. JMIR Serious Games. 2015;3(1):e2 10.2196/games.3401. Medline:25759148 [DOI] [PMC free article] [PubMed] [Google Scholar]