Abstract
Tryptophan is an essential plant-derived amino acid that is needed for the in vivo biosynthesis of proteins. After consumption, it is metabolically transformed to bioactive metabolites, including serotonin, melatonin, kynurenine, and the vitamin niacin (nicotinamide). This brief integrated overview surveys and interprets our current knowledge of the reported multiple analytical methods for free and protein-bound tryptophan in pure proteins, protein-containing foods, and in human fluids and tissues, the nutritional significance of l-tryptophan and its isomer d-tryptophan in fortified infant foods and corn tortillas as well the possible function of tryptophan in the diagnosis and mitigation of multiple human diseases. Analytical methods include the use of acid ninhydrin, near-infrared reflectance spectroscopy, colorimetry, basic hydrolysis; acid hydrolysis of S-pyridylethylated proteins, and high-performance liquid and gas chromatography-mass spectrometry. Also covered are the nutritional values of tryptophan-fortified infant formulas and corn-based tortillas, safety of tryptophan for human consumption and the analysis of maize (corn), rice, and soybean plants that have been successfully genetically engineered to produce increasing tryptophan. Dietary tryptophan and its metabolites seem to have the potential to contribute to the therapy of autism, cardiovascular disease, cognitive function, chronic kidney disease, depression, inflammatory bowel disease, multiple sclerosis, sleep, social function, and microbial infections. Tryptophan can also facilitate the diagnosis of certain conditions such as human cataracts, colon neoplasms, renal cell carcinoma, and the prognosis of diabetic nephropathy. The described findings are not only of fundamental scientific interest but also have practical implications for agriculture, food processing, food safety, nutrition, and animal and human health. The collated information and suggested research need will hopefully facilitate and guide further studies needed to optimize the use of free and protein-bound tryptophan and metabolites to help improve animal and human nutrition and health.
Keywords: l-tryptophan, d-tryptophan, proteins, tryptophan metabolites, analysis, plant genetic engineering, high-tryptophan proteins, fortified infant foods, fortified tortillas, absorption, nutrition, safety, health benefits, research needs
Introduction
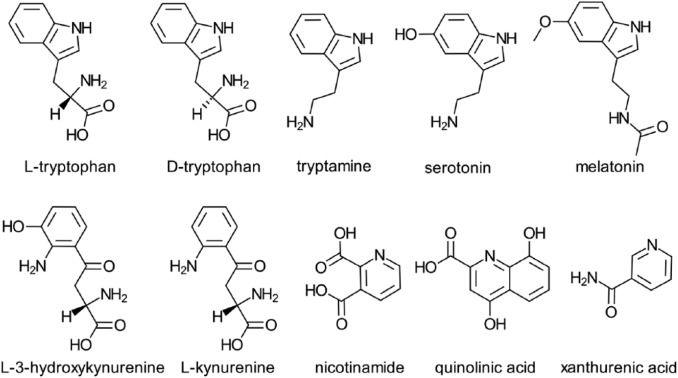
A total of 20 naturally occurring amino acids play a fundamental role in animal and human nutrition and health.1 Tryptophan is an essential amino acid required for normal growth and it serves as an in vivo precursor for several bioactive compounds including nicotinamide (vitamin B6), serotonin, melatonin, tryptamine, kynurenine, 3-hydroxykynurenine, and quinolinic and xanthurenic acids (Figure 1). Its role in animal and human health can therefore impact on many diseases and conditions.
Figure 1.
Structures of l- and d-tryptophan and 8 bioactive metabolites.
Because the indole ring of tryptophan is degraded under the acid condition used for protein hydrolysis, tryptophan cannot be analyzed by standard amino acid analysis methods. There is, therefore, a need for alternate quantitative methods to assess the multiple functions of tryptophan in proteins, foods including dietary supplements, and in animal and human fluids and tissues (reviewed in the works by Friedman and Finley,2 Finley et al,3 Friedman and Cuq,4 Cuq and Friedman,5 Molnár-Perl6,7).
This overview consists of the following 3 parts: (a) the evaluation of selected reported analytical methods used to determine the tryptophan content of pure proteins and protein-containing foods, including high-tryptophan corn, rice, and soybean varieties being developed using plant genetic engineering methods; (b) the human nutritional function and safety of dietary tryptophan and the assessment of the replacement of d-tryptophan produced during food processing for the l-isomer in mice; and (c) brief descriptions of some of the reported health-promoting aspects of tryptophan, tryptophan-containing foods, and foods with added (supplemented) tryptophan. Here, tryptophan refers to l-tryptophan and the isomeric form produced during food processing and by microbes is designated as d-tryptophan.
The Analysis of Tryptophan in Food, Body Fluids, and Tissues
Numerous methods have been proposed to combat the issue that tryptophan is destroyed during the acid hydrolysis of a protein by 6N HCl at high temperature that precedes the analysis of the liberated amino acids by chromatography. Here, we present brief overview of some of the promising techniques for the analysis of free and protein-bound tryptophan in food matrices and in body fluids and tissues that is designed to overcome this problem.
Acid ninhydrin method
One of the methods for the determination of protein-bound tryptophan discussed here is that proposed by Gaitonde and Dovey8 in which they proposed a method in which the reaction with ninhydrin in a mixture of formic and hydrochloric acid for 10 minutes at 100°C transforms tryptophan to a yellow product (λmax = 390 nm; ε = 7120). Zahnley and Davis9 in this laboratory subsequently modified this method by correcting for tyrosine absorption.
To help quantify this assay further, we compared the results from 6 acid hydrolytic procedures and the modified nonhydrolytic acid ninhydrin method for 3 proteins (casein, lysozyme, and soybean protein) and 10 carbohydrate-containing widely consumed foods (see the works by Friedman et al10 and Friedman11). The results in Tables 1 and 2 show that for carbohydrate-containing foods, the thioglycolic and organic acid methods appear ineffective, basic hydrolysis by NaOH or Ba(OH)2 might be preferable over hydrolysis by organic acids, and because the acid ninhydrin assay was reproducible with a wide range of commonly use food products, it seems useful for complex foods.
Table 1.
| Analytical method | Lysozyme | Casein | Milk powder | Soy protein | Soy flour |
|---|---|---|---|---|---|
| Acid hydrolysis | |||||
| Thioglycolic | 5.84 | 1.27 | nd | 0.33 | nd |
| Mercaptoethane-sulfonic | 6.69 | 1.26 | 0.09 | 1.24 | 0.31 |
| Methanesulfonic | 5.72 | 0.71 | nd | 0.65 | nd |
| p-toluenesulfonic | 6.64 | 1.07 | nd | 1.01 | nd |
| Basic hydrolysis | |||||
| Sodium hydroxide | 5.65 | 1.01 | 1.26 | 1.01 | 1.07 |
| Barium hydroxide | — | 1.30 | 1.25 | 1.81 | 0.81 |
| Colorimetry | |||||
| Acid ninhydrinc | 7.66 | 1.70 | 1.73 | 1.36 | 1.91 |
Values are averages from 3 separate determinations except for a single assay by the acid ninhydrin procedure; nd, not detected.
Nitrogen content (~): lysozyme, 16.2; casein, 13.6; milk powder, 5.32; soybean protein, 14.0; soybean flour, 8.32.
The tryptophan content of lysozyme was calculated to be 7.66 g/16 g N from its known amino acid sequence and determined nitrogen content. Observed values were corrected for contributions of tyrosine to the absorbance at 385 nm by the following formula9: tryptophan (observed)/tryptophan expected = 1.013 + 0.034 (tyrosine/tryptophan).
Table 2.
Tryptophan content of proteins and flours determined by an acid ninhydrin assay without hydrolysis.a
| Protein source | N (%) | Tryptophan (g/16 g N) |
|---|---|---|
| Proteins | ||
| Casein | 13.6 | 1.70 |
| Lysozyme | 16.2 | 7.66 |
| Soy protein | 14.0 | 1.36 |
| Foods | ||
| Barley flour | 1.26 | 1.55 (1.27)b |
| Beef, minced | 13.6 | 1.25 (1.40) |
| Corn flour | 1.46 | 1.85 (1.28) |
| Cottonseed flour | 10.0 | 1.37 |
| Lima bean flour | 3.32 | 1.42 (1.31) |
| Oat flour | 2.56 | 1.68 (1.33) |
| Rice flour | 0.98 | 1.72 (1.37) |
| Soybean flour | 8.32 | 1.43 (1.33) |
Reports of the use of the acid ninhydrin method in practice include that by Sodek et al13 to measure the tryptophan content of beans, maize, and wheat and those of Molnár-Perl and Pintér-Szakács14 and Pintér-Szakács and Molnár-Perl15 to determine tryptophan content of pure proteins, feeds, and foods.
Improved tryptophan-cysteine assay
Because the SH group of cysteine seems to favor the degradation of tryptophan, Inglis16 developed a procedure for the complete analysis of the amino acid composition of a protein-containing cysteine, cystine, and tryptophan on a single run of the amino acid analyzer by first modifying the SH groups with 4-vinylapyridine to the acid-stable 4-S-pyridylethyl-l-cysteine (4-PEC) side chain17–19 and using tryptamine to protect tryptophan against degradation during the acid hydrolysis of the modified protein. The method was used effectively with β-lactoglobulin and ovalbumin. The method was further modified by Yamada et al20 who reported high recoveries of both cysteine and tryptophan by vapor-phase S-pyridylethylation before hydrolysis with 2.5 M mercaptoethanesulfonic acid vapor at 176°C for 12.5 minutes.21 The vapor phase method was applied to lysozyme and myoglobin.
High-performance liquid chromatography method for free tryptophan in cereals and legumes
Çevikkalp et al22 developed a simple procedure for the determination tryptophan in cereals and legume grown in Turkey. In this method, following alkaline hydrolysis in 5 N NaOH solution (120°C for 12 hours), the hydrolysates were filtered through ashless filter paper, the pH was adjusted with HCl to 6.3, and the hydrolysates were separated within 10 minutes on a high-performance liquid chromatography (HPLC) column with fluorescence detection. The method was used to determine the following concentrations of tryptophan (in mg/100 g) in 6 food products: durum wheat, barley, 165; rye, 125; kidney beans, 240; chickpeas, 220; and red lentils, 129. The high values in kidney beans and chickpeas are noteworthy.
Rapid colorimetric method for free tryptophan
Wu et al23 developed a method for free tryptophan based on a tryptophanase-derived tryptophan-to-indole conversion followed by the reaction with hydroxylamine to form a pink product with a λmax value of 530 nm. A 96-well throughput assay was then used to quantify free tryptophan, protein-bound tryptophan, and tryptophan in biological samples.
Near-infrared reflectance spectroscopy for tryptophan
Because the chemical analysis of free and protein-bound amino acids is complicated, Fontaine et al24 describe the use of near-infrared reflectance spectroscopy (NIRS) to measure tryptophan, other essential amino acids, and protein content of 1100 food samples of global origin collected during a 5-year period. The NIRS was validated with 98 wheat and 78 corn samples and compared with amino acid predictions using linear crude protein. Readers should consult the cited manuscript for detailed descriptions of NIRS sample measurements and calibrations. The authors conclude that the developed NIRS calibrations enable fast and accurate predictions of essential amino acids in cereals.
Zhang et al25 used NIRS to measure the tryptophan content of 272 milled rice samples. The method was less reliable for brown than for white rice. The authors suggest that the method could be used to select rice seeds with high tryptophan content and for quality control during rice processing.
Analysis of tryptophan metabolites in human plasma
Because tryptophan catabolism via the kynurenine pathway seems to link inflammation to cancer, cardiovascular diseases, depression, and multiple sclerosis, Arnhard et al26 validated an HPLC/MS method for the simultaneous quantitation of tryptophan and the metabolites kynurenine, kynurenic acid, and quinolinic acids. The method with stable isotope-labeled analogues as internal standards was used to determine the concentrations of the metabolites in 100 human plasma samples.
Isotope-labeled and unlabeled method for tryptophan and metabolites
Another assay developed for use in animal systems was developed by Sano et al27 who devised a method for the detection of 15N2-labeled tryptophan, l-kynurenine, serotonin, and quinolinic acid in rat plasma. These compounds were first transformed to acylated derivatives using pentafluoropropionic anhydride and pentafluoro-1-propanol. The derivatives were then analyzed using gas chromatography/mass spectrometry (GC/MS). The method was used to determine the isotope enrichment in plasma tryptophan over the course of a continuous infusion in pregnant rats. The authors suggest that the method has the potential to facilitate our understanding of the 4 tryptophan metabolism pathways in animals and humans.
Analysis of high-tryptophan transgenic seeds
Analytical methods have also been developed and used for determining free and protein-bound tryptophan in new varieties of maize, rice, and soybeans. Indeed, the production of novel nutritionally enhanced major food crops via genetic engineering might help meet the worldwide need for inexpensive better-quality foods.
Maize
Rosales et al28 and Sarika et al29 used the nonhydrolytic NIRS method to determine the protein, tryptophan, and lysine content of 266 samples of quality protein maize that has on average twice the tryptophan content of normal maize. The authors suggest that the efficient NIRS method is preferable to wet chemistry methods for the analysis of large numbers of samples generated in plant breeding programs. Sarika et al29 found that despite the nutritional superiority of Opaque-16, a high-tryptophan and high-lysine maize mutant, the physico-biochemical characteristics of its endosperm are not affected.
Rice
In an analysis of rice, Wakasa et al30 found the amount of free tryptophan in a transgenic rice variety was about twice that in seeds in wild-type plants. The protein-bound tryptophan level was also enhanced. This observation led the authors to suggest that the tryptophan content of rice seeds could be increased transgenetically to improve the nutritional value of the human diet and also animal feeds. By contrast, Dubouzet et al31 described a metabolic engineering method used to promote the transformation of tryptophan to serotonin and to serotonin-derived indole compounds in rice calli, suggesting that the method provides a novel approach for the production of tryptophan-derived bioactive compounds.
Soybeans
Kita et al32 discovered that transgenic soybean plants were found to accumulate free tryptophan to levels as high as 3.8 to 4.8 mg/g dry weight of seed flour, up to a 12-fold increase compared with tryptophan levels in nontransgenic seeds. For analysis, free tryptophan and other amino acids were extracted with sulfosalicylic acid and analyzed by the ninhydrin method using an automated amino acid analyzer. The high-tryptophan soybeans can be used to increase the tryptophan content of mixed diets.
Analysis of d-tryptophan
As reviewed in detail elsewhere,33 because all amino acids residues in a protein undergo racemization simultaneously, but at different rates,34 assessment of the extent of racemization in a protein requires quantitative measurement of ~40 l- and d-optical isomers. A discussion of reported methods used to analyze the isomeric forms of amino acids simultaneously is beyond the scope of this article.
By contributing to food security, a major worldwide challenge, these selected results, and related ongoing studies suggest that the production of novel nutritionally enhanced major food crops via genetic engineering might help meet the worldwide need for inexpensive better-quality foods.35
Tryptophan in Nutrition: Absorption, Availability, Sources, and Safety
There have been many studies on the utilization of tryptophan from different sources by humans and animals and some of these reports are included here to highlight the potential for tryptophan supplementation (fortification).
Various studies have investigated the absorption of tryptophan into the human body. For example, the effect of the vegetable (V-8) juice containing 40 g of α-lactalbumin (a protein rich in tryptophan) on plasma tryptophan levels in fasting males (n = 6) was investigated. The α-lactalbumin produced an increase in plasma tryptophan of up to 3-fold over fasting values within 90 minutes, declining rapidly by 240 minutes.36 In the same study, a gluten-containing beverage raised the plasma tryptophan level by 25%, whereas a zein-containing drink lowered the level to about 50% of the fasting value. The authors suggest that negative effect of the corn protein zein probably reflects its poor digestibility, resulting in slower absorption of the amino acids from the gut.
The effects of dietary tryptophan on the immune system have also been investigated. Tryptophan catabolites can have immunomodulatory functions, such as via the kynurenine pathway. The dietary role of tryptophan in the immune system has therefore been the subject of study. For example, it has been reported that the dietary supplementation of tryptophan to healthy adults (n = 35) for the study period did not result in significant changes in serum cytokine concentrations for the entire study or in male and female subgroups, suggesting that depletion of central nervous system 5-HT via dietary tryptophan depletion does not seem to affect the immune system.37 The results suggest that depletion of central nervous system serotonin (5-HT) via dietary tryptophan depletion does not seem to affect the immune system in the short term.
Brain serotonin, derived from tryptophan, is known to influence affective events, such as mood disorders. In a study to investigate the effects of different forms of dietary tryptophan, Markus et al38 tested whether hydrolyzed protein had greater effects on the plasma tryptophan/large neutral amino acid ratio and mood than intact protein in healthy volunteers (n = 18). They observed significant faster increases and longer-lasting improvement in the ratio with the hydrolyzed tryptophan source versus the intact or pure tryptophan. In a related study, Markus et al39 found that consumption of a tryptophan-rich egg protein hydrolysate by 17 participants with high and 18 with low chronic stress resulted in an increase in plasma tryptophan uptake into the brain and in improved mood and performance under acute stress exposure, suggesting the therapeutic value of the hydrolysate. Similarly, Mitchell et al40 investigated, using a double-blind crossover design, the dose-dependent effects of a tryptophan-rich egg protein hydrolysate on brain tryptophan availability. The results suggest that the hydrolysate is a useful food ingredient that can increase tryptophan availability.
The availability of tryptophan in animal feed has also been investigated. For example, the results of a study by Iji et al41 designed to determine tryptophan transport in broiler chicks show that (a) the rate of tryptophan uptake declined with age, (b) tryptophan uptake occurred mainly in the ileum and less in the jejunum, (c) the Na+-independent uptake of tryptophan into jejunal vesicles was lower in the presence of d-tryptophan, and (d) the uptake dependent on the concentrations of other dietary amino acids as well as other factors in the diet. These facts should be taken into account in the design of nutritionally improved poultry feed diets supplemented with tryptophan.
Reichl42 has provided a comprehensive discussion of the kinetics of the absorption and metabolism of amino acids in tissues from the gut into the bloodstream in rodents, cows, pigs, and sheep. The described results indicate that although tryptophan is well absorbed, its absorption is reduced by the presence of other amino acids.
Infant nutrition
The following observations indicate that tryptophan has been shown to be of paramount importance in infant nutrition. For example, Huang et al43 discuss the tryptophan requirement of infants in the first month of life. Shibui et al44 reported that the high-tryptophan protein α-lactalbumin, a whey protein that constitutes 22% of the proteins in human milk and 3.5% in bovine milk, plays an important function in milk production and in infant nutrition, including as a supplement in infant milk formulas. A study by Dowlati et al45 revealed that dietary supplementation with 2 or 4 g tryptophan or 20 or 40 g high-tryptophan α-lactalbumin had no measurable effect on total tryptophan levels of breast milk.
This observation is reinforced by a study with infants breast-fed or fed with a tryptophan-fortified formula.46 The concentration of total plasma tryptophan was significantly higher in the breast-fed group than in the group fed tryptophan-unfortified formula. By contrast, no significant difference was apparent between the breast-fed group and the group fed the tryptophan-fortified formula, suggesting the need for tryptophan fortification to achieve plasma tryptophan levels similar to those in breast-fed infants. Another study reported that cereals fortified with tryptophan and other nutrients improved the sleep of 30 infants aged 8 to 16 months with sleep disorder involving excessive nocturnal waking.47 The authors suggest that these results support the concept of chrononutrition via dietary effects on the sleep/wake rhythm of infants. Finally, Draher and White48 validated a method for determining the tryptophan content of infant and adult/pediatric formulae that uses enzymatic (instead of alkaline) protein hydrolysis that releases intact tryptophan with mean recoveries ranging from 93.8% to 104.9%.
Tryptophan-fortified corn tortillas
According to Bressani et al,49 in most Central American countries, lime-treated corn provides 31% of the total protein and 45% of the energy intake and beans 24% of the protein and 12% of the calories. These diets are low in protein quality and quantity and in energy. To overcome these deficiencies, corn can be supplemented either with 2 limiting amino acids tryptophan and lysine or high-quality proteins such as soybean flour. Serna-Saldivar50 note that the consumption of tortillas (flat cakes baked from lime-treated corn) without supplementation with high-quality protein foods can lead to the Kwashiorkor disease in infants. This is due to the lack of 2 mentioned essential amino acids. These facts stimulated interest in exploring the nutritional potential of widely consumed corn flour-based tortillas fortified with lysine, tryptophan, and high-quality protein flours. Here, we briefly mention some of the reported studies,
Tovar and Carpenter51 found that it appears that the higher protein efficiency ratio, a measure of protein nutritional quality in rats, for corn with added tryptophan as the limiting amino acid as compared with added lysine was due to lower ad libitum food intake with the same weight gain.
Waliszewski et al52 showed that addition of lysine and tryptophan to corn flour at concentrations of 83%, 100%, or 150% that correspond to the values recommended by the Food and Agriculture Organization (FAO) of the United Nations did not seem to significantly affect the sensory properties of the prepared tortillas.
An evaluation of the effect of consumption of corn tortillas fortified with lysine and tryptophan on the growth of Mexican malnourished preschool children for 12 months found that the fortification resulted in better weight gain as compared with the group consuming unfortified tortillas.53
Amaya-Guerra et al54 found that soybean fortification of corn tortillas with high-tryptophan soy flour improved brain development of rats, suggesting that the fortification has the potential to enhance the nutrition quality of widely consumed corn tortillas.
The cited studies suggest that fortifications of tortillas with tryptophan or with high-tryptophan soy proteins55 have the potential to enhance the nutritional quality and health benefits of corn tortillas. It is also worth noting that Delgado et al56 found that tortillas prepared from high-anthocyanin pigmented maize have lower levels of potentially toxic acrylamide compared with those prepared from nonpigmented corn kernels, suggesting that the phenolic compounds are most likely responsible for the beneficial effect.
Safety of tryptophan
Because tryptophan is widely used a dietary supplement for perceived benefits including sleep and mood regulation, there is a need for assessing its safety. On the basis of research presented at the 9th Amino Acid Assessment Workshop,57 a No Observable Adverse Effect Level (NOAEL) for diet-added tryptophan of 4.5 g/d has been proposed for young adults. This value is generally reinforced by the following experimental observations on the safety of tryptophan in young women and rats.
Two studies by Hiratsuka et al58,59 showed that oral administration of tryptophan to 17 healthy Japanese young women at concentrations ranging from 1.0 to 5.0 g/d for 21 days did not affect food intake, body weight, general biomarkers, and amino acid composition in blood and urine or a profile of mood states. The urinary excretion of nicotinamide and several other metabolites was directly related to consumed tryptophan levels. The authors suggest that because 3-hydroxykinurenine was the most characteristic excreted urinary metabolite, it could serve as a surrogate biomarker for excess intake of tryptophan. These observations imply that use of tryptophan as a human food or animal feed dietary supplement might not have adverse effects. Shibui et al44 examined the safety of tryptophan in rats. Feeding an experimental diet with added tryptophan at doses 0 (basal diet), 1.25%, 2.5%, and 5.0% for 13 weeks resulted in no toxicologic changes in clinical signs, ophthalmology, urinalysis, hematology, organ weight, and histopathology. However, body weight gain and food consumption significantly decreased in men in the 2.5% group and in both sexes in the 5.0% group, accompanied by decrease in serum glucose in women in the 5.0% group. These adverse effects were not observed after a 5-week recovery period, suggesting reversibility of the adverse effects. The no-observed-adverse-effect level was 1.25% for men and 2.5% for women. The authors concluded that tryptophan has a low toxicity profile in rats.
Nutritional and safety aspects of d-tryptophan
l-amino acid residues in food proteins may be transformed to d-isomers during food processing under the influence of high pH and heat. Although racemization rates of the 18 different l-amino acid residues in a protein vary, the relative rates in different proteins are similar. Because the formation d-peptide bonds and cross-linked amino acids such as lanthionine and lysinoalanine can impair digestibility and nutritional quality, there is need to develop a better understanding of these events in order minimize adverse effects on protein nutritional quality and safety.
As part of a program to evaluate the chemistry and the nutritional and toxicologic aspects of novel amino acids formed during food processing, including d-amino acids, we compared the weight gain in mice fed with free amino acid diets in which d-tryptophan was substituted for l-tryptophan (reviewed by Friedman and Levin60,61). The results indicate that the mice can utilize supplied d-tryptophan in the absence of l-tryptophan; ie, the mice must meet the entire need for l-tryptophan from the d-isomer. The relative nutritional potency of d-tryptophan compared with l-tryptophan in mice is strongly dose dependent. It is inversely related to dietary concentration and ranges from 29% to 64%. The maximum growth (weight gain) of the mice with l-tryptophan was at concentrations of 0.174% in the diet, whereas the concentration of d-tryptophan up to 0.52% resulted in a maximum weight gain of 82% of that observed with l-tryptophan. Thus, the maximum growth of the mice when supplied with d-tryptophan was not achieved until its concentration was 2.5-fold higher than the corresponding level of l-tryptophan (Figure 2).
Figure 2.
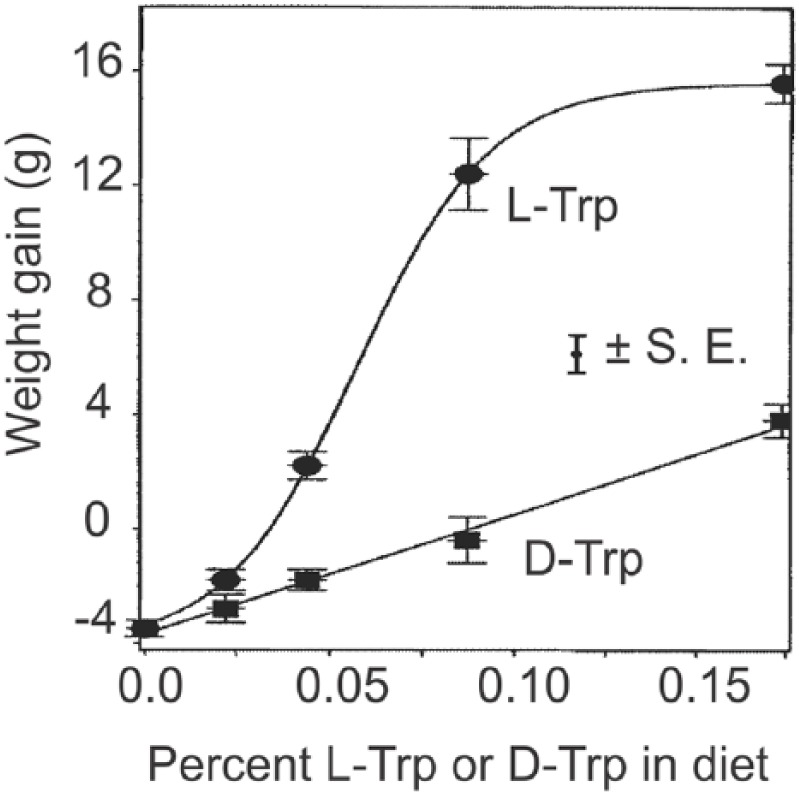
Relationship of weight gains to percent of l- and d-tryptophan isomers in amino acid diets fed to mice for 14 days.
Adapted using methods described in Friedman and Gumbmann62 and Friedman and Levin.60,61
Using mostly methods of supplementing food proteins with free-d-tryptophan, Baker63 describes variable outcomes for the utilization of d-tryptophan by different animal species. The relative potency for chicks was 20% of that of l-tryptophan. d-tryptophan is well utilized in growing pigs. Czarnecki and Baker64 reported that the potency of d-tryptophan relative to l-tryptophan for dogs was 35%.
Another study reported that feeding male rats test diets supplemented with several concentrations of d-tryptophan ranging from 0% to 0.5% for 21 days induced adverse effects of weight gain and in urinary metabolites, suggesting a safe level of <0.2% of dietary d-tryptophan.65 These observations imply that humans and rodents might not show the same susceptibility to possible adverse effects of tryptophan and possibly also tryptophan metabolites. In humans, the biological utilization of d-tryptophan in the male infant seems to be low,66 suggesting the need for further exploration of the nutritional utilization in humans of pure d-tryptophan and of d-tryptophan–containing peptides and food proteins. There is also a need to find out how the biological and nutritional effects of d-tryptophan vary, depending on whether they are consumed in the free state or as part of a peptide or food protein.
Niacin source
An investigation in weanling rats of the efficiency of transformation of d-tryptophan to the vitamin niacin (nicotinamide) showed that the availability of d-tryptophan was almost the same as that of l-tryptophan and was 1/6 as active as niacin.67–69 Other researchers reported that the 2 oxidative enzymes, tryptophan 2,3-dioxygenase and indoleamine 2,3-doxygenase, contribute equally to the biosynthesis of nicotinamide from both d-tryptophan and l-tryptophan.70
Williams and Hill71 propose a biochemical mechanism that operates through evolution, whereby high nicotinamide in the diet switches off the “de novo” pathway stopping in-house production of nicotinamide from tryptophan through kynurenine and causing immune intolerance, including of the fetus and dietary-induced infertility. Based on persuasive evidence, the authors suggest that fertility drops to below replacement levels owing to a high meat diet and that reducing variance in meat consumption might help stabilize the world population growth, a possible major challenge for future generations. It is noteworthy that we found that the tryptophan content of minced beef is somewhat lower than in several widely consumed plant foods (Table 2).
Possible Roles of Tryptophan in the Diagnosis and Mitigation of Human Diseases
Tryptophan has been implicated in a plethora of diseases and conditions because of its fundamental role as a precursor to many bioactive metabolites, leading to its consideration in the improvement of health and nutrition, and as a diagnostic tool.
Diagnostic aspects
The properties of tryptophan and its involvement in metabolic processes make it a suitable candidate for its investigation as a biomarker for diagnosis, examples of which are provided below.
Cataract diagnosis at the molecular level
Cataracts remain a global health issue and their sensitive detection is important. Gakamsky et al72 found using MS and fluorescence steady-state and lifetime spectroscopy that fluorescence of tryptophan and its derivatives in the postsurgical human and porcine lens samples correlates strongly with cataract grade and age, suggesting that the method can be used to diagnose cataracts at the molecular level.
Biomarker for diabetic nephropathy
A prospective study by Chou et al73 on the correlation of serum metabolites and the annual change of estimated glomerular filtration in patients with diabetic nephropathy, a leading cause of end-stage renal disease, revealed that the serum concentration of tryptophan <44.20 µmol/L is a potential surrogate prognostic marker for the disease.
Imaging of neoplasms of the human colon
Using a photo-type multispectral imaging system optimized for the macroscopic imaging of tissues, Baner et al74 found that tryptophan fluorescence of surgical specimens of colonic neoplasms and normal mucosa after resection could be useful in differentiating normal and cancerous cells because there is an increase in emission intensity from cancerous cells. They also reported that in tissues tryptophan autofluorescence images corrected using green reflectance images might also be useful for displaying neoplasms.
A relevant study by Albani et al75 revealed that the fluorescence of the protein β-lactoglobulin, a single polypeptide consisting of 162 amino acid residues with 2 tryptophan residues, is associated exclusively with the tryptophan at position 19 and not at all at position 61 of the protein chain, suggesting that the structural microenvironment of protein-bound tryptophan residues seem to affect fluorescence emission. Whether this is generally the case with other proteins merits study.
Biomarker for clear cell renal cell carcinoma
Based on an examination of serum-free amino acid and plasma-free amino acid profiles in 484 samples from 124 healthy controls and 56 patients with clear cell renal cell carcinoma, Lee et al76 discovered that a combination of serum histidine and plasma tryptophan may be a useful biomarker to detect the renal carcinoma.
Breath test for tryptophan metabolism
A study by Teraishi et al77 showed that the oral administration of 13C-tryptophan to human volunteers having a major depressive disorder and controls correlated negatively with exhaled maximum 13CO2 levels, suggesting that the 13C-tryptophan breath test could serve as a novel biomarker for detecting a subgroup of patients with altered (increased) tryptophan-kynurenine metabolism.
Mitigation of the adverse effects of human diseases and conditions
The use of tryptophan to help combat diseases and conditions has also been extensively investigated in a variety of clinical areas.
Autism spectrum disorder in children
To help clarify the potential relationship between tryptophan impairment and the severity of autistic symptoms, Kałużna-Czaplińska et al78 examined the retention status of supplemented tryptophan and the correlation between the supplementation of the diet with B vitamins and magnesium and the level of the excreted (excess) tryptophan in patients with autism spectrum disorder. Statistical correlations were observed between tryptophan levels and the severity of symptoms in different groups of patients. The authors conclude that the tryptophan level is critical and that intake of B vitamins and magnesium with the diet might influence its metabolic homeostasis.
Cardiovascular disease
Murr et al79 found in a study with 1106 patients with coronary artery disease that low serum tryptophan is associated with immune activation and indicates reduced life expectancy. Mangge et al80 hypothesize that disturbed tryptophan metabolism (breakdown) triggered by pro-inflammatory cascades in obese individuals seems to be associated with cardiovascular disease. This results in an increased serum kynurenine to tryptophan ratios, which can be measured for a better understanding of cardiovascular disease. In addition, the depletion of tryptophan limits protein synthesis, including hemoglobin production that may be associated with a likelihood of fatal cardiovascular events through the reduction in oxygen supply causing anemia. Because the breakdown of tryptophan is accelerated by exercise, the authors suggest that obese individuals should strive for a balance between food consumption and physical activity. It is worth mentioning that we previously suggested that acrylamide, a reactive molecule that is present in numerous processed plant foods that modifies hemoglobin after consumption, could also adversely affect oxygen transport to tissues.81
An epidemiology study by Yu et al82 that examined 231 cardiovascular disease cases shows that (a) interferon γ–mediated inflammation accelerates degradation of tryptophan into downstream metabolites, (b) increases in tryptophan consumption after 1 year were associated with a lower risk of the disease, (c) the baseline levels of the tryptophan metabolite kynurenic acid were associated with a higher risk of myocardial infarction and coronary artery disease death but not stroke, and (d) adjustment for changes in plasma tryptophan attenuated the inverse association between a Mediterranean diet and the cardiovascular disease. The authors conclude that an increase in the plasma tryptophan level was significantly associated with a decreased risk of cardiovascular disease and that the Mediterranean diet consisting of extra-virgin olive oil, nuts, fruits, vegetables, and cereals might counteract the deleterious effect of a high kynurenine risk score.
Chronic kidney disease
Schulman83 describes the molecular events that take place during the inhibition of intestinal absorption of a tryptophan derivative by the drug AST-120 that is expected to slow the progression of the chronic kidney disease in humans. The author mentions a phase 3 trial in progress with about 2000 subjects designed to confirm the preliminary data.
Cognitive decline
Because a tryptophan-enriched diet is reported to prevent the age-induced decline of hippocampal serotonin (5-HT) production that may contribute to age-related cognitive decline, Musumeci et al84 further investigated the effect of tryptophan diets on these cellular events and associated multiple biomarkers in rats. They found that a high tryptophan diet improved passive avoidance impairment of aged rats and partially rescued the age-induced inhibition of transcription factors involved in synaptic plasticity and memory. The authors suggest that the results indicate that enhanced tryptophan intake and the potential increases in 5-HT neurotransmission might help prevent age-related detrimental aspect by inhibiting hippocampal apoptosis.
Because excess tryptophan inhibits the 2 enzymes involved in serotonin synthesis, and increased cerebral levels of neuroactive kynurenine, Badawy85 hypothesizes that moderate use of tryptophan and decreased anxiety associated with exercise could explain behavioral effects of androgenic anabolic steroids associated with tryptophan metabolism. In a related study, Badawy86 discusses the utilization and function of tryptophan in pregnancy.
Diabetes
Because the available evidence indicates that a high-protein/low-carbohydrate diet increases the expenditure of glucose energy and is therefore beneficial in patients with type-2 diabetes, Inubushi et al87 examined the effect of tryptophan on glucose absorption in the small rat intestine. They found that tryptophan suppressed both serum glucose and insulin levels and inhibited glucose absorption from the intestine, suggesting that the amino acid suppressed the elevation of the blood glucose levels and reduced the adverse effects associated with insulin secretion from the β-cell pancreatic cells.
Chen et al88 assessed the possible association of tryptophan with the development of type 2 diabetes in 213 individuals in China, 51 with diabetes and 163 who remained healthy during a 10-year period. They determined tryptophan levels using ultra-performance liquid chromatography triple quadrupole MS and found that (a) the serum tryptophan level was positively and independently associated with the onset risk of diabetes; (b) patients with higher tryptophan levels had a higher degree of insulin resistance, secretion of triglycerides, and blood pressure; and (c) the addition of tryptophan seems to enhance the value of existing acid predictors, suggesting that tryptophan might represent a new biomarker associated with diabetes risk, but this awaits validation in other and larger populations.
In the consideration of diabetes, it is also worth mentioning that Imahori et al89 isolated 2 known compounds (4-quinlylaldoxime and indole-3-aldehyde) and 2 novel compounds formed during the in vitro reaction of tryptophan and glucose at physiological temperature and pH. One of the novel compounds, indole-3-aldehyde, was mutagenic in the Salmonella Typhimurium assay. Although 4-quinlylaldoxime was detected in rat diabetes extracts, the isolated new compounds were not detected in rat plasma. The authors suggest that genotoxic amino-carbonyl reaction products may be formed under diabetic conditions that can induce genetic damage to tissues.
Depression and other affective disorders
Strasser et al90 discuss the bioanalytical procedures for the determination of the concentrations of tryptophan and phenylalanine and their respective first stable intermediates kynurenine and tyrosine. The authors suggest that these immunometabolic parameters, along with other biomarkers, should be monitored in studies of the mechanisms of progression of inflammation-associated with depression and potential therapy.
Lindseth et al91 found that the consumption of a high-tryptophan diet by 25 healthy young adults for 4 days indicated significantly greater positive affect than those on a low-tryptophan diet, suggesting that tryptophan consumption has the potential to reduce depressive symptoms and anxiety.
Inflammatory bowel disease and Crohn disease
Nikolaus et al92 reported that the serum levels of tryptophan were significantly lower in patients with inflammatory bowel disease (IBD) than in controls, with a stronger reduction in levels in patients with Crohn disease. These observations and a detailed examination of associated biomarkers and metabolites, especially quinolinic acid, show a high activity of tryptophan degradation in patients with IBD, suggesting that tryptophan deficiency could contribute to the development or aggravation of the disease. Administration of high doses of metabolites (nicotinamide, indole-3-aldehyde) might modify the microbiome and shunt tryptophan metabolism toward anti-inflammatory pathways.
HIV and microbial infections
Because the oxidation of tryptophan owing to immune induction of the enzyme indoleamine 2,3 dioxygenase is considered the main cause of tryptophan depletion in patients with HIV, Bipath et al93 examined plasma tryptophan levels in 105 low-income sub-Saharan HIV-infected patients and 60 HIV-negative controls. The results show that the plasma tryptophan levels of the South African patients were 44.1% lower than in the HIV-free controls. The decrease in tryptophan levels in patients with HIV from developed countries was much lower, 18.8% compared with controls. Tryptophan levels correlated with the pro-inflammatory indicators neopterin, interleukin-6, and C-reactive protein. The authors suggest that the most probable causes in the lower tryptophan levels are food insecurity and higher levels of inflammatory activity and that inflammation-induced tryptophan depletion in the patients with HIV forms a much wider effect of pro-inflammatory activity on the nutritional profile of HIV-infected patients. Will dietary tryptophan supplementation help mitigate the course of the HIV infection?
Strasser et al94 found that daily administration of a probiotic formulation for 12 weeks to highly trained athletes (n = 17) resulted in a significant (11%) reduction in serum tryptophan levels compared with the placebo control group (n = 16) that remained unchanged. The data also show that the ratio of subjects taking the placebo who experienced upper respiratory tract infections was increased 2.2-fold compared with the individuals on probiotics, suggesting that the daily consumption of probiotics limited exercise-induced reductions in tryptophan levels and reduced the incidence of the infection without affecting athletic performance. It seems that catabolism of tryptophan might contribute to the function of the immune system that helps protect against infections. Also worth noting are related studies on the complex mechanistic relationship between tryptophan metabolism, exercise, weight loss, and inflammation-associated depression.95,96
Chacko et al97 discovered that the greater sensitivity of the human Chlamydia pneumoniae pathogenic microorganism than animal strains to tryptophan availability appears to be an adaptation that reflects the chronic nature of the infection in the human host. The human strain has sensitivity to tryptophan deficiency and can adapt accordingly. This observation suggests the possibility that tryptophan might help in the treatment of individuals having pneumonia.
Multiple sclerosis
An investigation of the effect of dietary tryptophan enrichment (0.03-0.04 g of tryptophan/kg body weight) of whey protein on affective and cognitive functions in patients with multiple sclerosis showed that the tryptophan-fortified diet enhanced memory processes without improving the mood states.98
Sleep
Lieberman et al99 used available data for 29 687 US adults to determine the effect of the average daily intake of 826 mg/d of tryptophan on liver and kidney function, depression, and sleep outcomes. The authors conclude that the high intake of tryptophan does not seem to affect liver and kidney function or carbohydrate metabolism but was inversely associated with the self-reported level of depression and positively associated with sleep duration. In a related investigation, Bravo et al100 analyzed whether consumption of cereals enriched with tryptophan might facilitate the reconsolidation of the sleep/wake cycle and counteract depression and anxiety. In the study by Wenefrida et al,35 middle-aged/elderly individuals consumed standard cereals with a tryptophan content of 22.5 mg/30 g at breakfast and dinner in the control and cereals with a tryptophan content of 60 mg/30 g in the treatment week. The results show that consumption of the higher tryptophan cereals increased sleep efficiency and sleep time and improved anxiety and depression symptoms. The authors suggest that high-tryptophan cereals might be useful as a chrononutrition treatment that can alter the age-related sleep/wake cycle.
Social behavior
A review by Steenbergen et al101 concludes that tryptophan supplementation seems to improve control over social behavior in individuals having disorders associated with dysfunctions in serotogenic functioning, presumably by affecting 5-HT brain levels, whereas it seems to promote social behavior in healthy individuals. The authors suggest that tryptophan could be a promising tool for modulating social behavior.
Conclusions and Future Research
The nutritionally essential amino acid tryptophan contributes to protein synthesis and the regulation of numerous physiological mechanisms. These include serving as a precursor for the neurotransmitter serotonin and the vitamin niacin. It is therefore important to be able to analyze tryptophan levels accurately and sensitively but unfortunately this is not straightforward; protein-bound tryptophan is degraded in the acid hydrolysis used for analysis of all amino acids. A number of methods have therefore been developed that can overcome this problem, including the nonhydrolytic acid ninhydrin method that might be preferable over basic hydrolysis by sodium or barium hydroxide as well as the nonhydrolytic NIRS assay, widely used to analyze large numbers of samples from plant breeding and plant engineering programs.
Because tryptophan and its metabolites seem safe to consume, and they have potential health benefits, a major challenge is to foster the further development via plant molecular genetic engineering methods, of high-tryptophan, as well as high-lysine and high-methionine cereals and legumes. The protein quality of such transgenic foods might approach that of the much more expensive meat, so they have the potential to alleviate malnutrition at an affordable cost. Moreover, because, after consumption, tryptophan is reported to mitigate the course of multiple chronic diseases, there is an urgent need for additional clinical studies designed to investigate the possible therapeutic potential of inexpensive, high-tryptophan foods. Such studies should include the evaluation of the functions of tryptophan metabolites resulting from tryptophan-gut-microbiota interactions on the causes and prevention of human diseases.102–106
This review will hopefully help catalyze the progress in the research of tryptophan in agronomy, food science, human nutrition, and health and the interaction of plant, food, biomedical, and medical scientists to help meet in these challenges.
Acknowledgments
I thank Carol E Levin for creative contributions to the improvement of the manuscript.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Mendel Friedman conceived and wrote the manuscript.
ORCID iD: Mendel Friedman  https://orcid.org/0000-0003-2582-7517
https://orcid.org/0000-0003-2582-7517
References
- 1. Mercer LP, Dodds SJ, Smith DI. Dispensable, indispensable, and conditionally indispensable amino acid ratios in the diet. In: Friedman M, ed. Absorption and Utilization of Amino Acids (vol. 1). Boca Raton, FL: CRC Press; 1989:1–13. [Google Scholar]
- 2. Friedman M, Finley JW. Methods of tryptophan analysis. J Agric Food Chem. 1971;19:626–631. [DOI] [PubMed] [Google Scholar]
- 3. Finley JW, Johnston PH, Friedman M. A potential improved tryptophan analysis of food proteins in the presence of carbohydrates. In: Friedman M, ed. Protein Nutritional Quality of Foods and Feeds (vol. 1). New York, NY: Marcel Dekker; 1975:453–462. [Google Scholar]
- 4. Friedman M, Cuq JL. Chemistry, analysis, nutritional value, and toxicology of tryptophan in food. A review. J Agric Food Chem. 1988;36:1079–1093. [Google Scholar]
- 5. Cuq JL, Friedman M. Effect of heat on tryptophan in food: chemistry, toxicology, and nutritional consequences. In: Friedman M, ed. Absorption and Utilization of Amino Acids (vol. 3). Boca Raton, FL: CRC Press; 1989:103–128. [Google Scholar]
- 6. Molnár-Perl I. Tryptophan analysis in peptides and proteins, mainly by liquid chromatography. J Chromatogr. 1997;763:1–10. [Google Scholar]
- 7. Molnár-Perl I. Advances in the analysis of tryptophan and its related compounds by chromatography. In: Huether G, Kochen W, Simat TJ, Steinhart H, eds. Tryptophan, Serotonin, and Melatonin. Advances in Experimental Medicine and Biology (vol. 467). Boston, MA: Springer; 1999:801–816. [DOI] [PubMed] [Google Scholar]
- 8. Gaitonde MK, Dovey T. A rapid and direct method for the quantitative determination of tryptophan in the intact protein. Biochem J. 1970;117:907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zahnley JC, Davis JG. Effect of high tyrosine content on the determination of tryptophan in protein by the acidic ninhydrin method. Application to chicken ovoinhibitor. Biochem J. 1973;135:59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedman M, Levin CE, Noma AT, Montague WC, Jr, Zahnley JC. Comparison of tryptophan assays for food proteins. In: Schlossberger HG, ed. Progress in Tryptophan and Serotonin Research. Berlin, Germany: Walter de Gruyter; 1984:119–123. [Google Scholar]
- 11. Friedman M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J Agric Food Chem. 2004;52:385–406. [DOI] [PubMed] [Google Scholar]
- 12. Concon JM. Rapid and simple method for the determination of tryptophan in cereal grains. Anal Biochem. 1975;67:206–219. [DOI] [PubMed] [Google Scholar]
- 13. Sodek L, Vecchia PTD, Lima ML. Rapid determination of tryptophan in beans (Phaseolus vulgaris) by the acid ninhydrin method. J Agric Food Chem. 1975;23:1147–1150. [DOI] [PubMed] [Google Scholar]
- 14. Molnár-Perl I, Pintér-Szakács M. Spectrophotometric determination of tryptophan in intact proteins by the acid ninhydrin method. Anal Biochem. 1989;177:16–19. [DOI] [PubMed] [Google Scholar]
- 15. Pintér-Szakács M, Molnár-Perl I. Determination of tryptophan in unhydrolyzed food and feedstuffs by the acid ninhydrin method. J Agric Food Chem. 1990;38:720–726. [Google Scholar]
- 16. Inglis AS. Single hydrolysis method for all amino acids, including cysteine and tryptophan. In: Hirs CHW, Timasheff SN, eds. Methods in Enzymology: Enzyme Structure Part I (vol. 91). New York, NY: Elsevier; 1983:26–36. [DOI] [PubMed] [Google Scholar]
- 17. Mak AS, Jones BL. Application of S-pyridylethylation of cysteine to the sequence analysis of proteins. Anal Biochem. 1978;84:432–440. [DOI] [PubMed] [Google Scholar]
- 18. Friedman M, Brandon DL. Nutritional and health benefits of soy proteins. J Agric Food Chem. 2001;49:1069–1086. [DOI] [PubMed] [Google Scholar]
- 19. Friedman M. Application of the S-pyridylethylation reaction to the elucidation of the structures and functions of proteins. J Protein Chem. 2001;20:431–453. [DOI] [PubMed] [Google Scholar]
- 20. Yamada H, Moriya H, Tsugita A. Development of an acid hydrolysis method with high recoveries of tryptophan and cysteine for microquantities of protein. Anal Biochem. 1991;198:1–5. [DOI] [PubMed] [Google Scholar]
- 21. Maeda K, Scheffler JJ, Tsugita A. Tryptophan micro-scale determinations by rapid hydrolysis. Hoppe-Seyler’s Z Physiol Chem. 1984;365:1183–1186. [DOI] [PubMed] [Google Scholar]
- 22. Çevikkalp SA, Löker GB, Yaman M, Amoutzopoulos B. A simplified HPLC method for determination of tryptophan in some cereals and legumes. Food Chem. 2016;193:26–29. [DOI] [PubMed] [Google Scholar]
- 23. Wu Y, Wang T, Zhang C, Xing XH. A rapid and specific colorimetric method for free tryptophan quantification. Talanta. 2018;176:604–609. [DOI] [PubMed] [Google Scholar]
- 24. Fontaine J, Schirmer B, Horr J. Near-infrared reflectance spectroscopy (NIRS) enables the fast and accurate prediction of essential amino acid contents. 2. Results for Wheat, Barley, Corn, Triticale, Wheat Bran/middlings, Rice Bran, and Sorghum. J Agric Food Chem. 2002;50:3902–3911. [DOI] [PubMed] [Google Scholar]
- 25. Zhang B, Zhang DP, Zhang WB, et al. Study on the global and local calibration methods of tryptophan content in rice by near infrared reflectance spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi. 2011;31:73–76. [PubMed] [Google Scholar]
- 26. Arnhard K, Pitterl F, Sperner-Unterweger B, Fuchs D, Koal T, Oberacher H. A validated liquid chromatography-high resolution-tandem mass spectrometry method for the simultaneous quantitation of tryptophan, kynurenine, kynurenic acid, and quinolinic acid in human plasma. Electrophoresis. 2018;39:1171–1180. [DOI] [PubMed] [Google Scholar]
- 27. Sano M, Ferchaud-Roucher V, Nael C, et al. Simultaneous detection of stable isotope-labeled and unlabeled L-tryptophan and of its main metabolites, L-kynurenine, serotonin and quinolinic acid, by gas chromatography/negative ion chemical ionization mass spectrometry. J Mass Spectrom. 2014;49:128–135. [DOI] [PubMed] [Google Scholar]
- 28. Rosales A, Galicia L, Oviedo E, Islas C, Palacios-Rojas N. Near-infrared reflectance spectroscopy (NIRS) for protein, tryptophan, and lysine evaluation in quality protein maize (QPM) breeding programs. J Agric Food Chem. 2011;59:10781–10786. [DOI] [PubMed] [Google Scholar]
- 29. Sarika K, Hossain F, Muthusamy V, et al. Opaque16, a high lysine and tryptophan mutant, does not influence the key physico-biochemical characteristics in maize kernel. PLoS ONE. 2018;13:e0190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wakasa K, Hasegawa H, Nemoto H, et al. High-level tryptophan accumulation in seeds of transgenic rice and its limited effects on agronomic traits and seed metabolite profile. J Exp Bot. 2006;57:3069–3078. [DOI] [PubMed] [Google Scholar]
- 31. Dubouzet JG, Matsuda F, Ishihara A, Miyagawa H, Wakasa K. Production of indole alkaloids by metabolic engineering of the tryptophan pathway in rice. Plant Biotechnol J. 2013;11:1103–1111. [DOI] [PubMed] [Google Scholar]
- 32. Kita Y, Nakamoto Y, Takahashi M, Kitamura K, Wakasa K, Ishimoto M. Manipulation of amino acid composition in soybean seeds by the combination of deregulated tryptophan biosynthesis and storage protein deficiency. Plant Cell Rep. 2009;29:87–95. [DOI] [PubMed] [Google Scholar]
- 33. Friedman M. Dietary significance of processing-induced D-amino acids in food. In: Stadler RH, Lineback D, eds. Process-Induced Food Toxicants: Occurrence, Formation, Mitigation, and Health Risks. Hoboken, NJ: John Wiley & Sons, Inc.; 2009:509–537. [Google Scholar]
- 34. Friedman M, Liardon R. Racemization kinetics of amino acid residues in alkali-treated soybean proteins. J Agric Food Chem. 1985;33:666–672. [Google Scholar]
- 35. Wenefrida I, Utomo HS, Linscombe SD. Mutational breeding and genetic engineering in the development of high grain protein content. J Agric Food Chem. 2013;61:11702–11710. [DOI] [PubMed] [Google Scholar]
- 36. Fernstrom JD, Langham KA, Marcelino LM, Irvine ZLE, Fernstrom MH, Kaye WH. The ingestion of different dietary proteins by humans induces large changes in the plasma tryptophan ratio, a predictor of brain tryptophan uptake and serotonin synthesis. Clin Nutr. 2013;32:1073–1076. [DOI] [PubMed] [Google Scholar]
- 37. Hildebrandt CS, Helmbold K, Linden M, et al. No detectable effects of acute tryptophan depletion on short-term immune system cytokine levels in healthy adults. World J Biol Psychiatry. 2018:1–8. doi: 10.1080/15622975.2018.1428357 [DOI] [PubMed] [Google Scholar]
- 38. Markus CR, Firk C, Gerhardt C, Kloek J, Smolders GF. Effect of different tryptophan sources on amino acids availability to the brain and mood in healthy volunteers. Psychopharmacology. 2008;201:107–114. [DOI] [PubMed] [Google Scholar]
- 39. Markus CR, Verschoor E, Firk C, Kloek J, Gerhardt CC. Effect of tryptophan-rich egg protein hydrolysate on brain tryptophan availability, stress and performance. Clin Nutr. 2010;29:610–616. [DOI] [PubMed] [Google Scholar]
- 40. Mitchell ES, Slettenaar M, Quadt F, et al. Effect of hydrolysed egg protein on brain tryptophan availability. Br J Nutr. 2011;105:611–617. [DOI] [PubMed] [Google Scholar]
- 41. Iji PA, Saki A, Tivey DR. Body and intestinal growth of broiler chicks on a commercial starter diet. 3. development and characteristics of tryptophan transport. Br Poult Sci. 2001;42:523–529. [DOI] [PubMed] [Google Scholar]
- 42. Reichl JR. Absorption and metabolism of amino acids studied in vitro, in vivo, and with computer simulations. In: Friedman M, ed. Absorption and Utilization of Amino Acids (vol. 1). Boca Raton, FL: CRC Press; 1989:93–156. [Google Scholar]
- 43. Huang L, Hogewind-Schoonenboom JE, Zhu L, et al. Tryptophan requirement of the enterally fed term infant in the first month of life. J Pediatr Gastroenterol Nutr. 2014;59:374–379. [DOI] [PubMed] [Google Scholar]
- 44. Shibui Y, Matsumoto H, Masuzawa Y, et al. Thirteen week toxicity study of dietary L-tryptophan in rats with a recovery period of 5 weeks. J Appl Toxicol. 2018;38:552–563. [DOI] [PubMed] [Google Scholar]
- 45. Dowlati Y, Ravindran AV, Maheux M, Steiner M, Stewart DE, Meyer JH. No effect of oral L-tryptophan or alpha-lactalbumin on total tryptophan levels in breast milk. Eur Neuropsychopharmacol. 2015;25:779–787. [DOI] [PubMed] [Google Scholar]
- 46. Fazzolari-Nesci A, Domianello D, Sotera V, Räihä NCR. Tryptophan fortification of adapted formula increases plasma tryptophan concentrations to levels not different from those found in breast-fed infants. J Pediatr Gastroenterol Nutr. 1992;14:456–459. [DOI] [PubMed] [Google Scholar]
- 47. Cubero J, Chanclón B, Sánchez S, Rivero M, Rodríguez AB, Barriga C. Improving the quality of infant sleep through the inclusion at supper of cereals enriched with tryptophan, adenosine-5′-phosphate, and uridine-5′-phosphate. Nutr Neurosci. 2009;12:272–280. [DOI] [PubMed] [Google Scholar]
- 48. Draher J, White N. HPLC determination of total tryptophan in infant formula and adult/pediatric nutritional formula following enzymatic hydrolysis: single-laboratory validation, first action 2017.03. J AOAC Int. 2018;101:824–830. [DOI] [PubMed] [Google Scholar]
- 49. Bressani R, Elías LG, Braham JE. Improvement of the protein quality of corn with soybean protein. Adv Exp Med Biol. 1978;105:29–65. [DOI] [PubMed] [Google Scholar]
- 50. Serna-Saldivar SO. Nutrition and fortification of corn and wheat tortillas. In: Rooney LW, Serna-Saldivar SO, eds. Tortillas: Wheat Flour and Corn Products. St. Paul, MN: AACC International, Inc; 2015:29–63. [Google Scholar]
- 51. Tovar LR, Carpenter KJ. The effects of alkali-cooking of corn and supplementation with amaranth seed on its deficiencies in lysine and tryptophan. Arch Latinoam Nutr. 1982;32:961–972. [PubMed] [Google Scholar]
- 52. Waliszewski KN, Estrada Y, Pardio V. Sensory properties changes of fortified nixtamalized corn flour with lysine and tryptophan during storage. Plant Foods Hum Nutr. 2004;59:51–54. [DOI] [PubMed] [Google Scholar]
- 53. Ramón Canul LG, Chel Guerrero LA, Betancur Ancona DA, Castellanos Ruelas AF. Feeding malnourished children with corn tortilla fortified with amino acids in Yucatán, México. Nutr Clin Diet Hosp. 2012;32:36–42. [Google Scholar]
- 54. Amaya-Guerra C, Serna Saldívar SO, Alanis-Guzman MG. Soyabean fortification and enrichment of regular and quality protein maize tortillas affects brain development and maze performance of rats. Br J Nutr. 2006;96:161–168. [DOI] [PubMed] [Google Scholar]
- 55. de Dios Figueroa Cárdenas J, Acero Godínez MG, Vasco Méndez NL, Lozano Guzmán A, Flores Acosta LM. Nutritional quality of nixtamal tortillas fortified with vitamins and soy proteins. Int J Food Sci Nutr. 2003;54:189–200. [DOI] [PubMed] [Google Scholar]
- 56. Delgado RM, Aràmbula-Villa G, Luna-Bàrcenas G, et al. Acrylamide content in tortilla chips prepared from pigmented maize kernels. Rev Mex Ing Quim. 2016;15:69–78. [Google Scholar]
- 57. Cynober L, Bier DM, Kadowaki M, Morris SM, Jr, Elango R, Smriga M. Proposals for upper limits of safe intake for arginine and tryptophan in young adults and an upper limit of safe intake for leucine in the elderly. J Nutr. 2016;146:2652S–2654S. [DOI] [PubMed] [Google Scholar]
- 58. Hiratsuka C, Fukuwatari T, Sano M, Saito K, Sasaki S, Shibata K. Supplementing healthy women with up to 5.0 g/d of L-tryptophan has no adverse effects. J Nutr. 2013;143:859–866. [DOI] [PubMed] [Google Scholar]
- 59. Hiratsuka C, Sano M, Fukuwatari T, Shibata K. Time-dependent effects of L-tryptophan administration on urinary excretion of L-tryptophan metabolites. J Nutr Sci Vitaminol. 2014;60:255–260. [DOI] [PubMed] [Google Scholar]
- 60. Friedman M, Levin CE. Nutritional value of D-amino acids, D-peptides, and amino acid derivatives in mice. In: Pollegioni L, Servi S, eds. Unnatural Amino Acids: Methods and Protocols (vol. 794). New York, NY: Humana Press; 2012:337–353. [DOI] [PubMed] [Google Scholar]
- 61. Friedman M, Levin CE. Nutritional and medicinal aspects of D-amino acids. Amino Acids. 2012;42:1553–1582. [DOI] [PubMed] [Google Scholar]
- 62. Friedman M, Gumbmann MR. The nutritive value and safety of D-phenylalanine and D-tyrosine in mice. J Nutr. 1984;114:2089–2096. [DOI] [PubMed] [Google Scholar]
- 63. Baker DH. Utilization of isomers and analogs of amino acids and other sulfur-containing compounds. Prog Food Nutr Sci. 1986;10:133–178. [PubMed] [Google Scholar]
- 64. Czarnecki GL, Baker DH. Utilization of D- and L-tryptophan by the growing dog. J Anim Sci. 1982;55:1405–1410. [DOI] [PubMed] [Google Scholar]
- 65. Shibata K, Ohno T, Sano M, Fukuwatari T. The urinary ratio of 3-hydroxykynurenine/3-hydroxyanthranilic acid is an index to predicting the adverse effects of D-tryptophan in rats. J Nutr Sci Vitaminol. 2014;60:261–268. [DOI] [PubMed] [Google Scholar]
- 66. Albanese AA, Snyderman SE, Lein M, Smetak EM, Vestal B. The biological value of corn and wheat proteins in the male infant, with a note on the utilization of D-tryptophan. J Nutr. 1949;38:215–224. [DOI] [PubMed] [Google Scholar]
- 67. Shibata K, Sawabe M, Fukuwatari T, Sugimoto E. Efficiency of D-tryptophan as niacin in rats. Biosci Biotechnol Biochem. 2000;64:206–209. [DOI] [PubMed] [Google Scholar]
- 68. Shibata K, Shimada H, Kondo T. Effects of feeding tryptophan-limiting diets on the conversion ratio of tryptophan to niacin in rats. Biosci Biotechnol Biochem. 1996;60:1660–1666. [DOI] [PubMed] [Google Scholar]
- 69. Shibata K, Fukuwatari T, Kawamura T. Conversion percentage of tryptophan to nicotinamide is higher in rice protein diet than in wheat protein diet in rats. Int J Tryptophan Res. 2015;8:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maeta A, Sano M, Fukuwatari T, Funakoshi H, Nakamura T, Shibata K. Contributions of tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase to the conversion of D-tryptophan to nicotinamide analyzed by using tryptophan 2,3-dioxygenase-knockout mice. Biosci Biotechnol Biochem. 2014;78:878–881. [DOI] [PubMed] [Google Scholar]
- 71. Williams AC, Hill LJ. Meat and nicotinamide: a causal role in human evolution, history, and demographics [published online ahead of print May 2, 2017]. Int J Tryptophan Res. doi: 10.1177/1178646917704661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gakamsky A, Duncan RR, Howarth NM, et al. Tryptophan and non-tryptophan fluorescence of the eye lens proteins provides diagnostics of cataract at the molecular level. Sci Rep. 2017;7:40375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chou CA, Lin CN, Chiu DT, Chen IW, Chen ST. Tryptophan as a surrogate prognostic marker for diabetic nephropathy. J Diabetes Invest. 2018;9:366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Baner A, Brandsch J, Franz R, Piringer O. The application of a predictive migration model for evaluating the compliance of plastic materials with European food regulations. Food Addit Contam. 1996;13:587–601. [DOI] [PubMed] [Google Scholar]
- 75. Albani JR, Vogelaer J, Bretesche L, Kmiecik D. Tryptophan 19 residue is the origin of bovine β-lactoglobulin fluorescence. J Pharm Biomed Anal. 2014;91:144–150. [DOI] [PubMed] [Google Scholar]
- 76. Lee HO, Uzzo RG, Kister D, Kruger WD. Combination of serum histidine and plasma tryptophan as a potential biomarker to detect clear cell renal cell carcinoma. J Transl Med. 2017;15:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Teraishi T, Hori H, Sasayama D, et al. 13C-tryptophan breath test detects increased catabolic turnover of tryptophan along the kynurenine pathway in patients with major depressive disorder. Sci Rep. 2015;5:15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kałużna-Czaplińska J, Jóźwik-Pruska J, Chirumbolo S, Bjørklund G. Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metab Brain Dis. 2017;32:1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Murr C, Grammer TB, Kleber ME, Meinitzer A, März W, Fuchs D. Low serum tryptophan predicts higher mortality in cardiovascular disease. Eur J Clin Invest. 2015;45:247–254. [DOI] [PubMed] [Google Scholar]
- 80. Mangge H, Stelzer I, Reininghaus EZ, Weghuber D, Postolache TT, Fuchs D. Disturbed tryptophan metabolism in cardiovascular disease. Curr Med Chem. 2014;21:1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Friedman M. Acrylamide: inhibition of formation in processed food and mitigation of toxicity in cells, animals, and humans. Food Funct. 2015;6:1752–1772. [DOI] [PubMed] [Google Scholar]
- 82. Yu E, Ruiz-Canela M, Guasch-Ferré M, et al. Increases in plasma tryptophan are inversely associated with incident cardiovascular disease in the Prevención con Dieta Mediterránea (PREDIMED) study. J Nutr. 2017;147:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schulman G. A nexus of progression of chronic kidney disease: tryptophan, profibrotic cytokines, and charcoal. J Ren Nutr. 2012;22:107–113. [DOI] [PubMed] [Google Scholar]
- 84. Musumeci G, Castrogiovanni P, Szychlinska MA, et al. Protective effects of high tryptophan diet on aging-induced passive avoidance impairment and hippocampal apoptosis. Brain Res Bull. 2017;128:76–82. [DOI] [PubMed] [Google Scholar]
- 85. Badawy AAB. Modulation of tryptophan and serotonin metabolism as a biochemical basis of the behavioral effects of use and withdrawal of androgenic-anabolic steroids and other image- and performance-enhancing agents [published online ahead of print February 19, 2018]. Int J Tryptophan Res. doi: 10.1177/1178646917753422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Badawy AAB. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep. 2015;35:e00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Inubushi T, Kamemura N, Oda M, et al. L-tryptophan suppresses rise in blood glucose and preserves insulin secretion in type-2 diabetes mellitus rats. J Nutr Sci Vitaminol (Tokyo). 2012;58:415–422. [DOI] [PubMed] [Google Scholar]
- 88. Chen T, Zheng X, Ma X, et al. Tryptophan predicts the risk for future type 2 diabetes. PLoS ONE. 2016;11:e0162192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Imahori D, Matsumoto T, Kojima N, et al. Chemical structures of novel Maillard reaction products under hyperglycemic conditions. Chem Pharm Bull. 2018;66:363–367. [DOI] [PubMed] [Google Scholar]
- 90. Strasser B, Sperner-Unterweger B, Fuchs D, Gostner JM. Mechanisms of inflammation-associated depression: immune influences on tryptophan and phenylalanine metabolisms. In: Dantzer R, Capuron L, eds. Inflammation-Associated Depression: Evidence, Mechanisms and Implications. Current Topics in Behavioral Neurosciences (vol. 31). London, England: Springer; 2017:95–115. [DOI] [PubMed] [Google Scholar]
- 91. Lindseth G, Helland B, Caspers J. The effects of dietary tryptophan on affective disorders. Arch Psychiatr Nurs. 2015;29:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nikolaus S, Schulte B, Al-Massad N, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017;153:1504.e2–1516.e2. [DOI] [PubMed] [Google Scholar]
- 93. Bipath P, Levay PF, Viljoen M. Tryptophan depletion in context of the inflammatory and general nutritional status of a low-income South African HIV-infected population. J Health Popul Nutr. 2016;35:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Strasser B, Geiger D, Schauer M, et al. Probiotic supplements beneficially affect tryptophan-kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, double-blinded, placebo-controlled trial. Nutrients. 2016;8:E752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Strasser B, Fuchs D. Diet versus exercise in weight loss and maintenance: focus on tryptophan. Int J Tryptophan Res. 2016;9:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Strasser B, Berger K, Fuchs D. Effects of a caloric restriction weight loss diet on tryptophan metabolism and inflammatory biomarkers in overweight adults. Eur J Nutr. 2015;54:101–107. [DOI] [PubMed] [Google Scholar]
- 97. Chacko A, Barker CJ, Beagley KW, et al. Increased sensitivity to tryptophan bioavailability is a positive adaptation by the human strains of Chlamydia pneumoniae. Mol Microbiol. 2014;93:797–813. [DOI] [PubMed] [Google Scholar]
- 98. Lieben CK, Blokland A, Deutz NE, Jansen W, Han G, Hupperts RM. Intake of tryptophan-enriched whey protein acutely enhances recall of positive loaded words in patients with multiple sclerosis. Clin Nutr. 2018;37:321–328. [DOI] [PubMed] [Google Scholar]
- 99. Lieberman HR, Agarwal S, Fulgoni VL., III Tryptophan intake in the US adult population is not related to liver or kidney function but is associated with depression and sleep outcomes. J Nutr. 2016;146:2609S–2615S. [DOI] [PubMed] [Google Scholar]
- 100. Bravo R, Matito S, Cubero J, et al. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age. 2013;35:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Steenbergen L, Jongkees BJ, Sellaro R, Colzato LS. Tryptophan supplementation modulates social behavior: a review. Neurosci Biobehav Rev. 2016;64:346–358. [DOI] [PubMed] [Google Scholar]
- 102. Gao J, Xu K, Liu H, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hoel H, Hove-Skovsgaard M, Hov JR, et al. Impact of HIV and type 2 diabetes on gut microbiota diversity, tryptophan catabolism and endothelial dysfunction. Sci Rep. 2018;8:6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kepert I, Fonseca J, Muller C, et al. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J Allergy Clin Immunol. 2017;139:1525–1535. [DOI] [PubMed] [Google Scholar]
- 105. Konopelski P, Ufnal M. Indoles—gut bacteria metabolites of tryptophan with pharmacotherapeutic potential [published online ahead of print April 27, 2018]. Curr Drug Metab. doi: 10.2174/1389200219666180427164731. [DOI] [PubMed] [Google Scholar]
- 106. Krishnan S, Ding Y, Saedi N, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23:1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]