Abstract
Tau deposits have distinct biochemical characteristics and vary morphologically based on identification with tau antibodies and several chemical dyes. Here, we report 4′,6-diamidino-2-phenylindole (DAPI)-positivity of tau deposits. Furthermore, we investigated the cause for this positivity. DAPI was positive in 3R/4R (3-repeat/4-repeat) tau deposits in Alzheimer’s disease, myotonic dystrophy, and neurodegeneration with brain iron accumulation, and in 4R tau deposits in corticobasal degeneration, but negative in 4R tau deposits in frontotemporal dementia with parkinsonism-17 and progressive supranuclear palsy. The peak emission wavelength of DAPI after binding to a tau deposit was similar to that after binding to a nucleus. This DAPI-positivity was conspicuous at the optimum concentration of 2 μg/ml. DAPI-positivity was diminished after formic acid treatment, but preserved after nucleic acid elimination and phosphate moiety blocking. Our results suggest that staining with 2 μg/ml DAPI is a common but useful tool to differentially detect tau deposits in various tauopathies.
Keywords: 4′,6-diamidino-2-phenylindole; neurofibrillary tangle; Phos-tag; tauopathies
Introduction
Tauopathies are a class of neurodegenerative disorders characterized pathologically by the presence of abnormally aggregated tau proteins in neuronal or glial cells.1 Such processes accompanied by altered proportions of tau isoforms2 cause morphological changes in tau deposits as indicated by flame-shaped tau deposits consisting of 3R/4R (3-repeat/4-repeat) tau isoforms in Alzheimer’s disease (AD), and globose-shaped tau deposits consisting of 4R tau isoforms in progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and frontotemporal dementia (FTD).3 A common feature of both morphologies is positivity for Gallyas silver stain.4–9 In comparison, Pick bodies as typical 3R tau isoform tau deposits have been reported to be unstainable by Gallyas silver stain, although present with globose-shaped morphology.10 In addition, electron microscopy can be used to distinguish flame- and globose-shaped tau deposits11–13 based on their composition of paired helical filaments and straight filaments.11,14,15 Because tau deposits show such complicated characteristics, it is necessary to develop a simple staining method for identifying the distinct array of tau deposits.
The DNA-binding reagent, 4′,6-diamidino-2-phenylindole (DAPI), is a common nuclear dye16 with diverse binding sites such as adenine-thymine base pairs in nucleic acids,17,18 polyphosphate moieties,19–21 and tubulin.22 Such binding increases the fluorescence intensity or the peak shift of fluorescence.
In this study, we first investigated the DAPI-positivity of tau deposits characterized by tau isoform-specific antibodies and a modified Gallyas silver stain. We then examined the concentration dependence of DAPI to determine the optimal concentration. DAPI-positivity of tau deposits was also examined after formic acid pretreatment, nucleic acid elimination, and phosphate moiety blocking.
Materials and Methods
Subjects
Brain tissue specimens were obtained from five cases of AD, a case of myotonic dystrophy (MyD), neurodegeneration with brain iron accumulation (NBIA) and frontotemporal dementia with parkinsonism-17 (FTDP-17), two cases of PSP, and one case of CBD. Our use of human materials abided by the ethical guidelines of the Hiroshima University Graduate School of Biomedical and Health Sciences. The clinical profiles, Aβ phase, Braak neurofibrillary tangle (NFT) stages, Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), and AD neuropathological changes of all cases are listed in Table 1.
Table 1.
Patient Characteristics.
| Diagnosis | Sex | Age | Brain Area | Aβ Phase | NFT Stage | CERAD | AD Neuropathologic Change |
|---|---|---|---|---|---|---|---|
| AD | F | 72 | CA1, Entorhinal cortex | 5 | 5 | Frequent | High |
| AD | F | 85 | CA1, Entorhinal cortex | 5 | 5 | Frequent | High |
| AD | F | 75 | CA1, Entorhinal cortex | 5 | 6 | Frequent | High |
| AD | M | 73 | CA1, Entorhinal cortex | 5 | 6 | Frequent | High |
| AD | M | 88 | CA1, Entorhinal cortex, Perirhinal cortex, Presubiculum |
5 | 5 | Frequent | High |
| MyD | F | 59 | Entorhinal cortex | 2 | 2 | Spare | Low |
| NBIA | F | 57 | CA1, Entorhinal cortex | 1 | 5 | Spare | Low |
| FTDP-17 | F | 64 | Midbrain | NA | NA | NA | NA |
| PSP | F | 71 | Entorhinal cortex | 1 | 2 | Spare | Low |
| PSP | F | 60 | Medulla oblongata | NA | NA | NA | NA |
| CBD | F | 75 | Midbrain, Frontal lobe | 3 | 3 | Frequent | Intermediate |
| Normal | F | 58 | Hippocampus | NA | NA | NA | NA |
Abbreviations: Age, age at death; NFT, neurofibrillary tangle; CERAD, The Consortium to Establish a Registry for Alzheimer’s Disease; AD, Alzheimer’s disease; F, female; M, male; MyD, myotonic dystrophy; NBIA, neurodegeneration with brain iron accumulation; FTDP-17, frontotemporal dementia with parkinsonism-17; PSP, progressive supranuclear palsy; CBD, corticobasal degeneration.
Brain Section Preparation
Autopsies were performed within 24 hr of death, and brain tissue specimens were fixed in 10% (v/v) formalin for 3 weeks and embedded in paraffin blocks. The paraffin blocks were cut into 7-µm thick sections for subsequent procedures. Previous studies have shown significant reduction in autofluorescence in paraffin-fixed sections by UV treatment for 48 hr. Therefore, the autofluorescence of lipofuscin in brain sections was reduced through photobleaching by a UV polymerizer (TUV-200, Dosaka EM Co., Kyoto, Japan) with a long wavelength UV lamp (360 nm, 2 × 8 W) for 48 hr before immunohistochemical staining procedures unless otherwise indicated.23–25 We used a 10× wide field microscope eyepiece and a Zeiss LD A-Plan 63×/1.40 oil immersion objective lens to obtain a 630× magnification. The autofluorescence of lipofuscin was detected by the excitation wavelengths, 405, 488, and 561 nm, with filters for DAPI, FITC, and Cy3, respectively. Paraffin sections were deparaffinized, boiled for 10 min in distilled water in a microwave for antigen retrieval, and then washed with phosphate-buffered saline (PBS) three times for 3 min each time after cooling down to room temperature (RT). After blocking in normal goat serum for 1 hr at RT, sections were incubated with primary antibody overnight at 4C. The primary antibodies are listed in Table 2. The sections were then washed with PBS and incubated with the secondary antibody, Alexa 568-conjugated goat antirabbit IgG or Alexa 568-conjugated goat antimouse IgG (Thermo Fisher Scientific, Eugene, OR), for 2 hr at RT. Subsequently, sections were incubated in 0.0125% thioflavin-S (ThS) solution (Sigma-Aldrich, St. Louis, MO) for 10 min and dipped in 50% alcohol for 4 min for color differentiation. ThS was excited at 405 nm, and emissions were collected in the 505 to 530 nm range. To assess DAPI-positivity, we stained brain sections with 2 μg/ml DAPI solution (Thermo Fisher Scientific) at RT for 30 min before excitation at 405 nm, and then the emissions were collected in the 420 to 480 nm range. All labeled sections were analyzed with an LSM510 confocal laser scanning microscope (Carl Zeiss AG, Oberkochen, Germany).
Table 2.
Tau-specific Antibodies.
| Antibody | Tau Epitope | Species/Subclass | Source | Dilution |
|---|---|---|---|---|
| Phosphor-dependent epitopes | ||||
| AT8 | pSer202+Thr205 | Mouse/IgG | Innogenetics | 1:800 |
| Tau isoform-related epitopes | ||||
| RD3 | 3-Repeat isoform | Mouse/IgG | Millipore | 1:300 |
| RD4 | 4-Repeat isoform | Mouse/IgG | Millipore | 1:100 |
Silver Staining Techniques of Globose-shaped Tau Deposits
Brain sections obtained from PSP and CBD were stained using a modified Gallyas stain and DAPI. Because silver staining affects the imaging of DAPI fluorescence, stainings were performed on serial sections. The detailed silver staining method is as follows.26,27 Deparaffinized brain sections were incubated in 0.25% potassium permanganate solution until they darkened, then de-colored in 2% oxalic acid solution for 3 min, and then incubated in 0.4% lanthanum nitrate solution for 30 min. After washing with distilled H2O (dH2O), sections were incubated in alkali silver iodide solution (0.035% silver nitrate, 10% potassium iodide, 4% sodium hydroxide, pH 13.7) for 2 min, washed with 0.5% acetic acid for 3 min, and developed in a physical developer solution (1 mg/ml silver nitrate, 1 mg/ml ammonium nitrate, 5 mg/ml tungstosilicic acid, 0.09% formaldehyde, and 25 mg/ml anhydrous sodium carbonate) until the sections’ appearance turned brown. The stained brain sections were then incubated sequentially with 0.5% acetic acid, 1% gold chloride and 1% sodium thiosulfate, and then stained with eosin.
Spectral Pattern of DAPI in Tau Deposits and Nuclei
To investigate the DAPI spectral pattern, the fluorescence spectra of a tau deposit and/or a nucleus was recorded using a Lambda stack program by scanning twelve 5-nm wavebands from 400 to 565 nm and twelve 5-nm wavebands from 400 to 555 nm using confocal laser microscopy (Olympus; FV1000-D). In addition, we stained brain sections with ThS without DAPI and tried to obtain an emission spectra of ThS using the laser for illumination of DAPI (excitation 405 nm, emission 420–480 nm) within a tau deposit identified at excitation wavelength (405 nm) and emission wavelength (505–530 nm), showing that the DAPI images were not simply a bleed-through of ThS.
Examination of the DAPI Concentration Dependence
Serial dilutions of DAPI (1, 2, 6, 12, 24, and 30 μg/ml) were prepared to examine the concentration-dependent specificity of tau deposits. The fluorescence intensity of DAPI at each focused laser spot was obtained under laser exposure for 1.2 sec with a fluorescence microscope (BZ-9000, Keyence, Osaka, Japan). The laser for illumination was equipped with a DAPI-BP filter (excitation 360/40 nm, emission 460/50 nm) and a GFP-BP filter (excitation 470/40 nm, emission 535/50 nm). ThS staining was used to identify the tau deposits, and the DAPI and ThS colocalization was assessed by correlation analysis.
Formic Acid Pretreatment
According to previous reports,28 the conversion of α-helix to β-sheet requires dissociation and reformation of hydrogen bonds, which can be achieved by simple organic acid treatment, of which formic acid is the most common. Maintaining formic acid at a high concentration and a low temperature is required for this effect, as a low concentration and higher temperature can reverse this effect. To assess the effect of formic acid on the DAPI staining of tau deposits, deparaffinized sections were incubated with or without 98% formic acid solution (Wako Pure Chemical, Osaka, Japan) at RT for 3 min.29,30 After rinsing with PBS, the sections were blocked with normal goat serum, and then stained with 2 μg/ml DAPI.
Colocalization Analysis
Images were analyzed for colocalization as previously described.31–34 Briefly, regions of interest corresponding to tau deposits that stained positively for ThS and DAPI were created by thresholding (auto-threshold). Among several methods for auto-thresholding in ImageJ, we used the Default and B&W switches to minimize background noise and convert the original grayscale image to binary. Correlation coefficients were measured using WCIF-ImageJ version 1.37a. Pearson’s correlation coefficient (PCC), Mander’s overlap coefficient (MOC), and intensity correlation quotient (ICQ) were calculated using the Mander’s Coefficients plugin. The overlapping in each scatterplot panel was used to demonstrate the pool of colocalized ThS and DAPI.
Proteinase K Pretreatment
The brain sections were pretreated with or without a proteinase K solution35 (20 μg/ml proteinase K, 48.75 mM Tris base, 0.98 mM EDTA, 4.88 mM CaCl2, 0.49% Triton X-100, and 2.5% glycerol) for 10 min at 37°C before double staining with a phospho-tau (Ser202, Thr205) monoclonal antibody (AT8) and DAPI.
Elimination of Nucleic Acids
To investigate the nuclease effect, the deparaffinized sections were incubated in RNase-free DNase I solution (400 U/ml, Sigma-Aldrich), DNase-free RNase A solution (200 μg/ml, Sigma-Aldrich), or a combination of DNase I and RNase A for 3.5 hr,36 and the efficacy of nucleic acid elimination was validated by staining with 2 μg/ml DAPI and 10 μg/ml ethidium bromide (EtBr; Nippon Gene, Tokyo, Japan). Briefly, after nuclease pretreatment, the brain sections were incubated with 10 mg/ml EtBr in Tris-acetate-EDTA (TAE) buffer (a mixture of 40 mM Tris-HCl, 20 mM acetic acid, and 1 mM EDTA) at RT for 10 min, and then washed with PBS. Subsequently, all sections were incubated with DAPI at RT for 30 min and the nuclease efficacy was validated by examining the loss of nucleolus and nucleoplasm.
To investigate Nissl structures after nuclease pretreatment, the brain sections were incubated in 100% alcohol twice for 3 min each time, and then incubated in 0.1% cresyl violet solution (Chroma, 1A 396) at 37C for 15 min. Subsequently, the stained sections were quickly rinsed with tap water to remove excess stain, and then sequentially placed in 70% and 95% alcohol. Finally, acetic acid was added to the sections to differentiate colors between Nissl structures and other intracellular components.
To investigate the DAPI-positivity of tau deposits, the brain sections were pretreated with nuclease and double-stained with ThS and DAPI. ThS was used to identify tau deposits. In addition, to discriminate the fluorescence of DAPI from that of ThS, brain sections pretreated with nuclease were stained with DAPI alone. Furthermore, because of the possibility of ThS fluorophore bleed-through, and the subsequent false positivity of DAPI in tau deposits, we stained brain sections with DAPI alone.
Blocking Free Phosphate With Phos-tag
Blocking free-phosphate was performed using Phos-tag biotin BTL-111, which shows superior performance among Phos-tag systems in western blotting with enhanced chemiluminescence systems.37,38 Paraffin sections were deparaffinized, boiled for 10 min in distilled water in a microwave for antigen retrieval. After cooling, sections were washed with Tris-buffered saline (TBS) three times for 5 min each time. Then, the sections were incubated, without skim milk blocking, with 1 mM Phos-tag complex solution for 2 hr at RT. The sections were then washed with TBS three times for 5 min each and incubated with Tyramide Signal Amplification (TSA, Alexa Fluor 568 Tyramide, Thermo Fisher Scientific) to fluorescently label the HRP terminal of the Phos-tag complex for observation under a fluorescence microscope.
Results
DAPI Staining of Tau Deposits Depends on the Tau Isoform Composition
We used DAPI to stain brain sections from AD, MyD, and NBIA cases, harboring 3R/4R tau isoforms (exhibiting flame-shaped tau deposits), and from FTDP-17, PSP, and CBD, harboring 4R tau isoforms (exhibiting globose-shaped tau deposits). DAPI was positive in all flame-shaped tau deposits from AD (Fig. 1A and B), MyD (Fig. 1C and D), and NBIA (Fig. 1E and F). DAPI was also positive in globose-shaped tau deposits from CBD (Fig. 1G), but negative in globose-shaped deposits from FTDP-17 (Fig. 1H) and PSP (Fig. 1I).
Figure 1.
DAPI-positivity of different isoform tau deposits. Tau isoform fluorescence staining is shown in AD (A and B), MyD (C and D), NBIA (E and F), CBD (G), FTDP-17 (H), and PSP (I). The 3R/4R isoform tau deposits were detected in the CA1 of AD, the entorhinal cortex of MyD, and the CA1 of NBIA. The 4R isoform tau deposits were present in the CA1 of CBD, the midbrain of FTDP-17, and the CA1 of PSP. DAPI-positivity was present in AD, MyD, NBIA, and CBD, but not in FTDP-17 and PSP. Images show RD3 (red), RD4 (red), and DAPI (blue). Calibration bars = 10 µm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; AD, Alzheimer’s disease; MyD, myotonic dystrophy; NBIA, neurodegeneration with brain iron accumulation; CBD, corticobasal degeneration; FTDP-17, frontotemporal dementia with parkinsonism-17; PSP, progressive supranuclear palsy.
Because globose-shaped tau deposits showed diverse silver staining patterns,39 we selectively characterized tau deposits from AD and PSP/CBD by modified Gallyas silver staining. All morphological types of tau deposits, including globose-shaped tau deposits, dystrophic neurites, neuropil threads, tufted astrocytes, thorny astrocytes, and coiled bodies, were visualized by the silver staining. Furthermore, globose-shaped tau deposits from the midbrain of CBD, dystrophic neurites from the perirhinal cortex, and neuropil threads from the presubiculum of AD were DAPI positive, while tufted astrocytes and thorny astrocytes in the gray matter, and coiled bodies in the white matter of CBD were DAPI negative, indicating that DAPI-positivity depends on the type of tau deposits (Fig. S1).
Spectral Pattern of DAPI in Tau Deposits and Nuclei
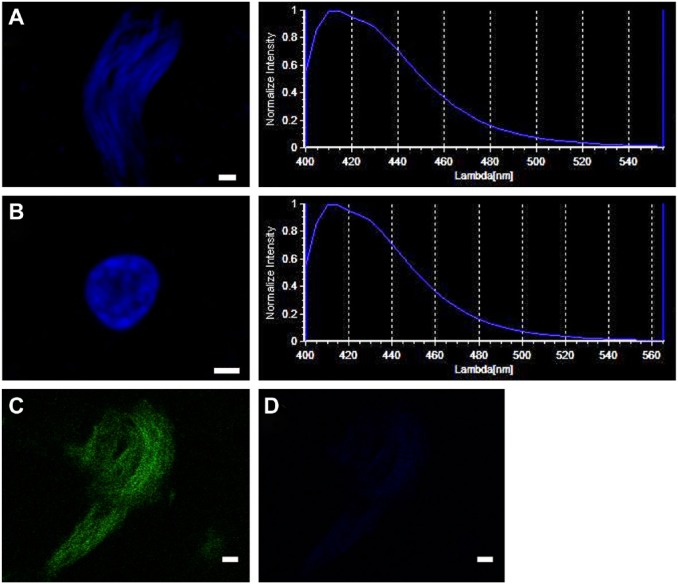
Because DAPI is commonly used to stain nuclei, we investigated the difference in the peak emission wavelength of DAPI in tau deposits and nuclei by lambda scanning. We found that the peak emission wavelength of DAPI after binding to a tau deposit or a nucleus did not show obvious differences (Fig. 2A and B). In addition, we failed to obtain the emission spectra of ThS-tau deposits (Fig. 2C) at the range of excitation wavelength for DAPI illumination (Fig. 2D).
Figure 2.
Spectral pattern of DAPI in tau deposits and nuclei. A similar pattern of emission wavelength of DAPI was detected at sites of tau deposits and nuclei. DAPI-positive tau deposit (A, upper left), emission wavelength of DAPI-tau deposit (A, upper right), DAPI-positive nucleus (B, middle left), and emission wavelength of DAPI-nucleus (B, middle right). (C) A tau deposit was identified with ThS (D) and no ThS-tau deposit was detected at the excitation wavelength of DAPI. Calibration bars = 2 µm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ThS, thioflavin-S.
Concentration-dependent DAPI-positivity of Tau Deposits
ThS and DAPI-double-positivity of tau deposits in AD sections was examined by serially diluting DAPI, displaying it in scatter plots and assessing it using PCC, MOC, and ICQ. With DAPI concentrations of 1, 2, 6, 12, 24, and 30 μg/ml, the PCC values were 0.418, 0.621, −0.044, 0.165, 0.383, and −0.287; the MOC values were 0.792, 0.764, 0.395, 0.428, 0.563, and 0.203; and the ICQ values were 0.326, 0.302, 0.177, 0.145, 0.163, and 0.139, respectively. Both PCC and MOC are independent of gain,34 but MOC is significantly sensitive to background,26 therefore the PCC value is more stable with change in background, which changed with the DAPI concentration. The colocalization analysis with PCC after staining with 1 or 2 μg/ml DAPI was more suitable for the specific detection of tau deposits (Fig. 3).
Figure 3.
Concentration-dependent specificity of DAPI in tau deposits. Serial dilutions of DAPI (blue) at 1, 2, 6, 12, 24, and 30 µg/ml were used to stain brain sections. In parallel, tau deposits were visualized with ThS (green) to show colocalization of these two stains, as shown in scatter plots. The colocalization was assessed using PCC, MOC, and ICQ values. The optimal concentration of DAPI was 2 µg/ml. Calibration bars = 20 µm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ThS, thioflavin-S; PCC, Pearson’s correlation coefficient; MOC, Mander’s overlap coefficient; ICQ, intensity correlation quotient.
Pretreatment With Formic Acid Reduces the DAPI-positivity of Tau Deposits
We compared the DAPI-positivity of tau deposits after pretreatment with or without 2 μg/ml formic acid and found that formic acid pretreatment decreased the DAPI-positivity (Fig. 4). This decreased positivity was shown by correlation analysis between DAPI and ThS, where the correlation values of PCC, MOC, and ICQ were reduced from 0.621, 0.764, and 0.302 to 0.102, 0.587, and 0.217, respectively.
Figure 4.
DAPI-positivity of tau deposits is reduced by pretreatment with formic acid. Brain sections were stained with 2 µg/ml DAPI (blue), with or without pretreatment with formic acid, and tau deposits were visualized using ThS (green). (A) Formic acid (–) and (B) formic acid (+). The colocalization of these two stains is shown in scatter plots and was assessed using PCC, MOC, and ICQ values. Calibration bars = 20 µm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ThS, thioflavin-S; PCC, Pearson’s correlation coefficient; MOC, Mander’s overlap coefficient; ICQ, intensity correlation quotient.
Autofluorescence of Lipofuscin
Fluorescent lipofuscin accumulates with age in neuronal cells. Because of the broad fluorescence spectra of lipofuscin, we attempted to reduce lipofuscin autofluorescence to avoid confusing it with DAPI-positivity. We pretreated brain sections with or without UV for 48 hr and found that the autofluorescence intensity of lipofuscin decreased after UV pretreatment (Fig. S2). Because proteinase K pretreatment can also remove lipofuscin,40 we pretreated with proteinase K and found that DAPI still stained tau deposits, even after the successful elimination of lipofuscin (Fig. 5).
Figure 5.
DAPI-positivity of tau deposits is preserved even after pretreatment with proteinase K. Proteinase K can be used to eliminate lipofuscin. DAPI-positivity was preserved even after elimination of lipofuscin. The images show AT8 (green) and DAPI (blue) in tau deposits pretreated with proteinase K. (A) Proteinase K (–) and (B) proteinase K (+). The tau deposits (arrows), lipofuscin (arrowhead) and faint outline of nuclei (asterisks) are shown. Calibration bars = 50 µm. Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole.
DAPI-positivity of Tau Deposits Is Not Associated With Nucleic Acids
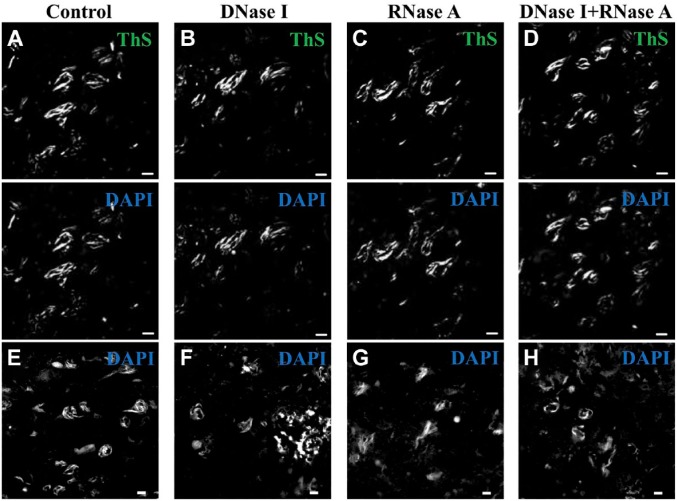
Because DAPI is a fluorescent dye used for DNA staining, DAPI-positivity of tau deposits may indicate an unrecognized presence of nucleic acids. Based on this assumption, we performed nucleic acid elimination prior to DAPI staining. The efficacy of DNase I treatment was validated by the loss of DAPI staining of the nucleoplasm, and the efficacy of RNase A treatment was validated by the loss of EtBr staining of the nucleolus (Fig. S3). In addition, we used cresyl violet to investigate the efficacy of nuclease on Nissl substance enriched with ribosomal RNA in nerve tissues.41 We found that the cresyl violet-positivity of Nissl substance and nuclei was preserved in the sections pretreated with DNase I (–)/RNase A (–) or DNase I (+)/RNase A (–), but significantly decreased in the Nissl substance after pretreatment with DNase I (–)/RNase A (+) or DNase I (+)/RNase A (+) (Fig. S4). Furthermore, in AD sections pretreated with or without nuclease, and then stained with 2 μg/ml DAPI the tau deposits were positively stained by DAPI regardless of the nuclease pretreatment (Fig. 6).
Figure 6.
DAPI-positivity of tau deposits is not affected by RNase A and/or DNase I treatments. (A and E) DNase I (–)/RNase A (–); (B and F) DNase I (+)/RNase A (–); (C and G) DNase I (–)/RNase A (+); (D and H) DNase I (+)/RNase A (+). (A–D) All sections were double-stained with ThS (green) and DAPI (blue). (E–H) All sections were stained with DAPI (blue) alone. Calibration bars = 10 µm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ThS, thioflavin-S.
DAPI-positivity of Tau Deposits Is Independent of Phosphate Moieties
Because phosphate moieties are a DAPI-binding target,19–21 we investigated whether the phosphate moieties of tau deposits are a binding site of DAPI. Samples were incubated with HRP-tagged Phos-tag biotin, which binds and masks the phosphate moieties of tau protein via a metal-ion and can be visualized by TSA staining. However, masking phosphate moieties did not diminish the DAPI-positivity of tau deposits (Fig. 7), suggesting that this positivity is not associated with phosphate moieties.
Figure 7.
Distribution of Phos-tag and DAPI in NFT-like tau deposits in an NBIA case. Confocal images showing TSA (red) and DAPI (blue). Phos-tag binding was imaged with TSA. (A) No Phos-tag blocking. (B) Phos-tag blocking (thick arrows). Calibration bars = 10 µm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; NFT, neurofibrillary tangle; NBIA, neurodegeneration with brain iron accumulation; TSA, Tyramide Signal Amplification.
Discussion
The morphological features of tau deposits are commonly associated with biochemical composition and tau isoform content, including flame- and globose-shapes, and tufted- and thorny-shapes, and the morphological appearance can be assessed microscopically after Gallyas and tau immunohistochemical staining.42 Flame-shaped tau deposits harboring 3R/4R tau isoforms, mostly composed of PHFs, are commonly present in AD, whereas globose-shaped tau deposits harboring 4R tau isoforms, mostly composed of SFs, are predominant in FTDP-17, PSP, and CBD. These tau deposits are all stained by Gallyas silver.4–9 Therefore, we studied the distinct DAPI-positivity of tau deposits characterized by tau isoform antibodies and Gallyas silver staining. DAPI-positivity was present in tau deposits from AD, MyD and NBIA cases (containing 3R/4R tau isoforms) and in CBD cases (containing 4R tau isoforms), but not in FTDP-17 and PSP cases (containing 4R tau isoforms). Although, both the heterodimer of 3R and 4R tau isoform and the homodimer of 3R or 4R tau isoform can form amyloid-like tau fibrils, the minor variation in β-sheet structure may lead to varying results of staining with some structurally similar compounds,43 for example, THK5117-positive globose-shaped tau deposits in PSP, but THK523-negative globose-shaped tau deposits in PSP.44 It is difficult to differentially stain tau deposits in both PSP and CBD with a single chemical compound. However, ultrastructural studies have shown that the tau deposits in CBD contain both straight and twisted filaments, whereas the tau deposits in PSP consist primarily of straight filaments.45,46 In addition, western blotting results have shown distinct trypsin-resistant band patterns of tau protein in both cases.47 These differences may explain the different positivity of DAPI in both cases. However, we cannot exclude the possibility that other materials cause the DAPI-positivity in the CBD brain. In addition to the DAPI-positivity of tau deposits as described above, we examined DAPI-positivity of other morphological tau deposits including dystrophic neurites, neuropil threads, tufted astrocytes, thorny astrocytes and coiled bodies. The dystrophic neurites and neuropil threads derived from neuronal cells were DAPI positive, while tufted astrocytes, thorny astrocytes and coiled bodies derived from glial cells were DAPI negative, indicating that DAPI-positivity depends on the cellular source of the tau deposits. These findings suggest that DAPI can be used for screening tau deposits.
The strict control of DAPI concentration is of prime importance for the specific staining of tau deposits. A previous study has reported that staining with DAPI at concentrations ranging from 0.01–5 µg/ml differentiated between the nucleus and cytoplasm.48 Similarly, to distinguish tau deposits by DAPI staining, we used serial dilutions of DAPI in combination with ThS, and their colocalization was analyzed by PCC, MOC, and ICQ. Both PCC and MOC are independent of gain,34 and MOC is sensitive to the image background. Therefore, PCC is more suitable for correlation analysis, so we used PCC to determine the optimal concentration. Using this method, we found that the optimal concentration of DAPI for the detection of tau deposits is 2 μg/ml.
DAPI is a commonly used nucleic acid dye, but it also binds to dimeric tubulin because of its hydrophobicity.22,49 In addition, polyphosphate groups provide binding sites that interact with DAPI.19–21 Thus, it is necessary to investigate whether the presence of tubulin, phosphate moieties or nucleic acids in tau deposits are the cause of DAPI-positivity. As the absence of tubulin in tau deposits has been reported previously,50,51 we examined the other two candidates only by nucleic acid elimination with a nuclease and blocking phosphate moieties with Phos-tag. Phos-tag is a novel phosphate-binding tag used to identify and mask phosphate moieties. The advantage of masking phosphate moieties with Phos-tag is the visualization of masked sites. To improve the masking effect, we used Phos-tag biotin BTL-111 instead of Phos-tag biotin BTL-104 because of its higher sensitivity.37 Because the broad excitation and emission spectra of lipofuscin may cause false positive staining, we investigated whether the DAPI-positivity of tau deposits was because of lipofuscin and found that the DAPI-positivity was preserved even after the removal of lipofuscin by proteinase K40 and UV treatment.
Recently, pyridinyl-butadienyl-benzothiazole derivatives such as PBB3, and arylquinoline derivatives such as BF158 and 170, have been developed as tau PET tracers.52 DAPI shares molecular similarity with these compounds in that they all have structures consisting of aromatic chromophores and polar functional groups on the side-chain that forms hydrogen bonds, resulting in hydrophobic interactions with tau deposits. In addition, such compounds have similar staining properties to DAPI, which is reflected in the comparison of their positivity in flame- and globose-shaped tau deposits.53,54 Lansoprazole, a proton-pump inhibitor, is considered a potential PET imaging agent for diagnosing tauopathies54 and it has a structural similarity to DAPI. In addition, THK523, a newly developed arylquinoline-derived tau PET ligand, stained tau deposits in AD, but not in CBD, PSP, or Pick disease.53,55 Because the affinity of tau radiotracers to β-sheet structures decreased after pretreatment with formic acid,36,37 we compared DAPI-positivity with and without formic acid pretreatment. A decrease in the DAPI-positivity of tau deposits following pretreatment with formic acid indicated that the DAPI-binding sites in the tau deposits may be associated with the presence of β-sheet structures.
In conclusion, DAPI was positive in all 3R/4R isoform tau deposits and partially positive in 4R isoform tau deposits. In addition, DAPI was negative in glial cell-derived tau deposits. This DAPI-positivity of tau deposits was independent of nucleic acids and phosphate moieties, and may depend on the presence of β-sheet structures. Our results suggest that staining with 2 μg/ml DAPI is a common but useful tool for differentially detecting tau deposits in various tauopathies.
Supplemental Material
Supplemental material, DS_10.1369_0022155418793600 for 4′,6-Diamidino-2-Phenylindole Distinctly Labels Tau Deposits by Chengyu Li, Tetsuya Takahashi, Tejashwi Shrestha, Eiji Kinoshita, Tomoyasu Matsubara, Masayasu Matsumoto and Hirofumi Maruyama in Journal of Histochemistry & Cytochemistry
Acknowledgments
We thank Ms. M. Sasanishi and Ms. Y. Furuno for their excellent technical support, and the Analysis Center of Life Science, Hiroshima University, Hiroshima, Japan, for the use of its facilities. We are grateful to Dr. Y. Ohko (Advanced Industrial Science and Technology) for advice. This work was supported by grants from Merck Sharp & Dohme (K.K.), Tokyo, Japan (T.T.) and the Smoking Research Foundation, Tokyo, Japan (T.T.). We thank J. Ludovic Croxford, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: CL and TT conceived the experiments; CL performed the experiments and wrote the manuscript; TT and TS revised the manuscript; TT, TS, TM, MM, and HM made suggestions for manuscript improvements; and EK provided the Phos-tag. All authors have read and approved the final manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: H Maruyama  https://orcid.org/0000-0001-7613-8717
https://orcid.org/0000-0001-7613-8717
Contributor Information
Chengyu Li, Department of Clinical Neuroscience and Therapeutics, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan.
Tetsuya Takahashi, Department of Clinical Neuroscience and Therapeutics, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan.
Tejashwi Shrestha, Department of Clinical Neuroscience and Therapeutics, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan.
Eiji Kinoshita, Department of Functional Molecular Science, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan.
Tomoyasu Matsubara, Department of Clinical Neuroscience and Therapeutics, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan; Department of Neuropathology, Tokyo Metropolitan Institute of Gerontology, Tokyo, Japan.
Masayasu Matsumoto, Department of Clinical Neuroscience and Therapeutics, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan; Sakai City Medical Center, Sakai, Japan.
Hirofumi Maruyama, Department of Clinical Neuroscience and Therapeutics, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan.
Literature Cited
- 1. Ferrer I, López-González I, Carmona M, et al. Glial and neuronal tau pathology in tauopathies: characterization of disease-specific phenotypes and tau pathology progression. J Neuropathol Exp Neurol. 2014;73(1):81–97. [DOI] [PubMed] [Google Scholar]
- 2. Adams SJ, DeTure MA, McBride M, et al. Three repeat isoforms of tau inhibit assembly of four repeat tau filaments. PLoS ONE. 2010;5(5):e10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mackenzie IRA. Neuropathology of atypical parkinsonian disorder. In: Litvan I, editor. Atypical parkinsonian disorders: clinical and research aspects. Current clinical neurology. Totowa, NJ: Humana Press; 2005:47–77. [Google Scholar]
- 4. Ludolph AC, Kassubek J, Landwehrmeyer BG, et al. Tauopathies with parkinsonism: clinical spectrum, neuropathologic basis, biological markers, and treatment options. Eur J Neurol. 2009;16(3):297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buée L, Delacourte A. Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathol. 1999;9(4):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hara M, Hirokawa K, Kamei S, et al. Isoform transition from four-repeat to three-repeat tau underlies dendrosomatic and regional progression of neurofibrillary pathology. Acta Neuropathol. 2013;125(4):565–79. [DOI] [PubMed] [Google Scholar]
- 7. Dickson DW, Kouri N, Murray ME, et al. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci. 2011;45(3):384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghatak NR, Nochlin D, Hadfield MG. Neurofibrillary pathology in progressive supranuclear palsy. Acta Neuropathol. 1980;52(1):73–6. [DOI] [PubMed] [Google Scholar]
- 9. Taipa R, Pinho J, Melo-Pires M. Clinico-pathological correlations of the most common neurodegenerative dementias. Front Neurol. 2012;3(68):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bronner IF, Ter Meulen BC, Azmani A, Severijnen LA, Willemsen R, Kamphorst W, Ravid R, Heutink P, van Swieten JC. Hereditary Pick’s disease with the G272V tau mutation shows predominant three-repeat tau pathology. Brain. 2005;128(11):2645–53. [DOI] [PubMed] [Google Scholar]
- 11. Tomonaga M. Ultrastructure of neurofibrillary tangles in progressive supranuclear palsy. Acta Neuropathol. 1977;37(2):177–81. [DOI] [PubMed] [Google Scholar]
- 12. Hanger DP, Wray S. Tau cleavage and tau aggregation in neurodegenerative disease. Biochem Soc Trans. 2010;38(4):1016–20. [DOI] [PubMed] [Google Scholar]
- 13. Taniguchi-Watanabe S, Arai T, Kametani F, Nonaka T, Masuda-Suzukake M, Tarutani A, Murayama S, Saito Y, Arima K, Yoshida M, Akiyama H, Robinson A, Mann DMA, Iwatsubo T, Hasegawa M. Biochemical classification of tauopathies by immunoblot, protein sequence and mass spectrometric analyses of sarkosyl-insoluble and trypsin-resistant tau. Acta Neuropathol. 2016;131(2):267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, III, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flemming KD, Jones LK. Mayo clinic neurology board review: clinical neurology for initial certification and MOC. Oxford: Oxford University Press; 2015. doi: 10.1093/med/9780190244927.003.0026. [DOI] [Google Scholar]
- 16. Roukos V, Pegoraro G, Voss TC, Misteli T. Cell cycle staging of individual cells by fluorescence microscopy. Nat Protoc. 2015;10(2):334–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson WD, Tanious FA, Barton HJ, Jones RL, Fox K, Wydra RL, Strekowski L. DNA sequence dependent binding modes of 4’,6-diamidino-2-phenylindole (DAPI). Biochemistry. 1990;29(36):8452–61. [DOI] [PubMed] [Google Scholar]
- 18. Katouzian-Safadi M, Cremet JY, Charlier M. Limitation of DNA-4’,6-diamidine-2-phenylindole assay in the presence of an excess of tRNA. Anal Biochem. 1989;176(2):416–9. [DOI] [PubMed] [Google Scholar]
- 19. Stotz SC, Scott LO, Drummond-Main C, Avchalumov Y, Girotto F, Davidsen J, Gómez-Gárcia MR, Rho JM, Pavlov EV, Colicos MA. Inorganic polyphosphate regulates neuronal excitability through modulation of voltage-gated channels. Mol Brain. 2014;7(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gomes FM, Ramos IB, Wendt C, Girard-Dias W, De Souza W, Machado EA, Miranda K. New insights into the in situ microscopic visualization and quantification of inorganic polyphosphate stores by 4’,6-diamidino-2-phenylindole (DAPI)-staining. Eur J Histochem. 2013;57(4):227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Omelon S, Georgiou J, Habraken W. A cautionary (spectral) tail: red-shifted fluorescence by DAPI–DAPI interactions. Biochem Soc Trans. 2016;44(1):46–9. [DOI] [PubMed] [Google Scholar]
- 22. Arbildua JJ, Brunet JE, Jameson DM, López M, Nova E, Lagos R, Monasterio O. Fluorescence resonance energy transfer and molecular modeling studies on 4’,6-diamidino-2-phenylindole (DAPI) complexes with tubulin. Protein Sci. 2006;15(3):410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neumann M, Gabel D. Simple method for reduction of autofluorescence in fluorescence microscopy. J Histochem Cytochem. 2002;50(3):437–9. [DOI] [PubMed] [Google Scholar]
- 24. Davis AS, Richter A, Becker S, Moyer JE, Sandouk A, Skinner J, Taubenberger JK. Characterizing and diminishing autofluorescence in formalin-fixed paraffin-embedded human respiratory tissue. J Histochem Cytochem. 2014;62(6):405–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang J, Yang F, Campos LS, Mansfield W, Skelton H, Hooks Y, Liu P. Quenching autofluorescence in tissue immunofluorescence. Wellcome Open Res. 2017;2:79–92. [Google Scholar]
- 26. Uchihara T. Silver diagnosis in neuropathology: principles, practice and revised interpretation. Acta Neuropathol. 2007;113(5):483–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuninaka N, Kawaguchi M, Ogawa M, Sato A, Arima K, Murayama S, Saito Y. Simplification of the modified Gallyas method. Neuropathology. 2015;35(1):10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang CT, Wang Y, Frank CW, Chang YC. Chemoresponsive surface-tethered polypeptide brushes based on switchable secondary conformations. RSC Adv. 2015;5(105):86113–9. [Google Scholar]
- 29. Harada R, Okamura N, Furumoto S, Furukawa K, Ishiki A, Tomita N, Hiraoka K, Watanuki S, Shidahara M, Miyake M, Ishikawa Y, Matsuda R, Inami A, Yoshikawa T, Tago T, Funaki Y, Iwata R, Tashiro M, Yanai K, Arai H, Kudo Y. [(18)F]THK-5117 pet for assessing neurofibrillary pathology in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2015;42(7):1052–61. [DOI] [PubMed] [Google Scholar]
- 30. Okamura N, Suemoto T, Furumoto S, Suzuki M, Shimadzu H, Akatsu H, Yamamoto T, Fujiwara H, Nemoto M, Maruyama M, Arai H, Yanai K, Sawada T, Kudo Y. Quinoline and benzimidazole derivatives: candidate probes for in vivo imaging of tau pathology in Alzheimer’s disease. J Neurosci. 2005;25(47):10857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blum FC, Chen C, Kroken AR, Barbieri JT. Tetanus toxin and botulinum toxin a utilize unique mechanisms to enter neurons of the central nervous system. Infect Immun. 2012;80(5):1662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adler J, Parmryd I. Colocalization analysis in fluorescence microscopy. Methods Mol Biol. 2012;931:97–109. [DOI] [PubMed] [Google Scholar]
- 33. Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol. 2011;300(4):C723–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adler J, Parmryd I. Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytometry A. 2010;77(8):733–42. [DOI] [PubMed] [Google Scholar]
- 35. Ramos-Vara JA, Beissenherz ME. Optimization of immunohistochemical methods using two different antigen retrieval methods on formalin-fixed paraffin-embedded tissues: experience with 63 markers. J Vet Diagn Invest. 2000;12(4):307–11. [DOI] [PubMed] [Google Scholar]
- 36. Pinon-Lataillade G, Masson C, Bernardino-Sgherri J, Henriot V, Mauffrey P, Frobert Y, Araneda S, Angulo JF. KIN17 encodes an RNA-binding protein and is expressed during mouse spermatogenesis. J Cell Sci. 2004;117(16):3691–702. [DOI] [PubMed] [Google Scholar]
- 37. Kinoshita E, Kinoshita-Kikuta E, Koike T. Phos-tag-based microarray techniques advance phosphoproteomics. J Proteomics Bioinform. 2013;S6:2–9. [Google Scholar]
- 38. Kinoshita E, Kinoshita-Kikuta E, Sugiyama Y, Fukada Y, Ozeki T, Koike T. Highly sensitive detection of protein phosphorylation by using improved Phos-tag Biotin. Proteomics. 2012;12(7):932–7. [DOI] [PubMed] [Google Scholar]
- 39. Castellani RJ, Alexiev BA, Phillips D, Perry G, Smith MA. Microscopic investigations in neurodegenerative diseases. In: Méndez-Vilas A, Diaz J, editors. Modern research and educational topics in microscopy: applications in physical/chemical sciences, techniques. Applications in Biology and Medicine. Formatex Research Center; 2007:171–82. [Google Scholar]
- 40. Ng KP, Gugiu B, Renganathan K, Davies MW, Gu X, Crabb JS, Kim SR, Rózanowska MB, Bonilha VL, Rayborn ME, Salomon RG, Sparrow JR, Boulton ME, Hollyfield JG, Crabb JW. Retinal pigment epithelium lipofuscin proteomics. Mol Cell Proteomics. 2008;7(7):1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vogel E, Gbureck A, Kiefer W. Vibrational spectroscopic studies on the dyes cresyl violet and coumarin 152. J Mol Struct. 2000;550:177–90. [Google Scholar]
- 42. Takahashi M, Weidenheim KM, Dickson DW, Ksiezak-Reding H. Morphological and biochemical correlations of abnormal tau filaments in progressive supranuclear palsy. J Neuropathol Exp Neurol. 2002;61(1):33–45. [DOI] [PubMed] [Google Scholar]
- 43. Schmidt ML, Schuck T, Sheridan S, Kung MP, Kung H, Zhuang ZP, Bergeron C, Lamarche JS, Skovronsky D, Giasson BI, Lee VM, Trojanowski JQ. The fluorescent Congo red derivative, (trans, trans)−1-bromo-2,5-bis-(3-hydroxycarbonyl-4-hydroxy)styrylbenzene (BSB), labels diverse β-pleated sheet structures in postmortem human neurodegenerative disease brains. Am J Pathol. 2001;159(3):937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harada R, Okamura N, Furumoto S, Tago T, Yanai K, Arai H, Kudo Y. Characteristics of tau and its ligands in PET imaging. Biomolecules. 2016;6(1):7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ksiezak-Reding H, Morgan K, Mattiace LA, Davies P, Liu WK, Yen SH, Weidenheim K, Dickson DW. Ultrastructure and biochemical composition of paired helical filaments in corticobasal degeneration. Am J Pathol. 1994;145(6):1496–508. [PMC free article] [PubMed] [Google Scholar]
- 46. Murray ME, Kouri N, Lin WL, Jack CR, Jr, Dickson DW, Vemuri P. Clinicopathologic assessment and imaging of tauopathies in neurodegenerative dementias. Alzheimers Res Ther. 2014;6(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hasegawa M. Molecular mechanisms in the pathogenesis of Alzheimer’s disease and tauopathies-prion-like seeded aggregation and phosphorylation. Biomolecules. 2016;6(2):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsumoto K. Improved staining method of DAPI for evaluating DNA content in phytoplankton nucleus. Bull Fac Bioresources. 1993;11:145–53. [Google Scholar]
- 49. Bonne D, Heusele C, Simon C, Pantaloni D. 4’,6-diamidino-2-phenylindole, a fluorescent probe for tubulin and microtubules. J Biol Chem. 1985;260(5):2819–25. [PubMed] [Google Scholar]
- 50. Zhang F, Su B, Wang C, Siedlak SL, Mondragon-Rodriguez S, Lee HG, Wang X, Perry G, Zhu X. Posttranslational modifications of α-tubulin in alzheimer disease. Transl Neurodegener. 2015;4(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alonso ADC, Li B, Grundke-Iqbal I, Iqbal K. Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci U S A. 2006;103(23):8864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matsumura K, Ono M, Kitada A, Watanabe H, Yoshimura M, Iikuni S, Kimura H, Okamoto Y, Ihara M, Saji H. Structure-activity relationship study of heterocyclic phenylethenyl and pyridinylethenyl derivatives as tau-imaging agents that selectively detect neurofibrillary tangles in Alzheimer’s disease brains. J Med Chem. 2015;58(18):7241–57. [DOI] [PubMed] [Google Scholar]
- 53. Fodero-Tavoletti MT, Furumoto S, Taylor L, McLean CA, Mulligan RS, Birchall I, Harada R, Masters CL, Yanai K, Kudo Y, Rowe CC, Okamura N, Villemagne VL. Assessing THK523 selectivity for tau deposits in Alzheimer’s disease and non-Alzheimer’s disease tauopathies. Alzheimers Res Ther. 2014;6(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol. 2015;14(1):114–24. [DOI] [PubMed] [Google Scholar]
- 55. Fodero-Tavoletti MT, Okamura N, Furumoto S, Mulligan RS, Connor AR, McLean CA, Cao D, Rigopoulos A, Cartwright GA, O’Keefe G, Gong S, Adlard PA, Barnham KJ, Rowe CC, Masters CL, Kudo Y, Cappai R, Yanai K, Villemagne VL. 18F-THK523: a novel in vivo tau imaging ligand for Alzheimer’s disease. Brain. 2011;134(4):1089–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1369_0022155418793600 for 4′,6-Diamidino-2-Phenylindole Distinctly Labels Tau Deposits by Chengyu Li, Tetsuya Takahashi, Tejashwi Shrestha, Eiji Kinoshita, Tomoyasu Matsubara, Masayasu Matsumoto and Hirofumi Maruyama in Journal of Histochemistry & Cytochemistry