Abstract
Chronic intermittent hypoxia (CIH), main feature of obstructive sleep apnea, produces nitro-oxidative stress, which contributes to potentiate carotid body (CB) chemosensory discharges and sympathetic-adrenal-axis activity, leading to hypertension. The MnSOD enzymatic activity, a key enzyme on oxidative stress control, is reduced by superoxide-induced nitration. However, the effects of CIH-induced nitration on MnSOD enzymatic activity in the CB and adrenal gland are not known. We studied the effects of CIH on MnSOD protein and immunoreactive (MnSOD-ir) levels in the CB, adrenal gland and superior cervical ganglion (SCG), and on 3-nitrotyrosine (3-NT-ir), CuZnSOD (CuZnSOD-ir), MnSOD nitration, and its enzymatic activity in the CB and adrenal gland from male Sprague-Dawley rats exposed to CIH for 7 days. CIH increased 3-NT-ir in CB and adrenal gland, whereas MnSOD-ir increased in the CB and in adrenal cortex, but not in the whole adrenal medulla or SCG. CIH nitrated MnSOD in the CB and adrenal medulla, but its activity decreased in the adrenal gland. CuZnSOD-ir remained unchanged in both tissues. All changes observed were prevented by ascorbic acid treatment. Present results show that CIH for 7 days produced MnSOD nitration, but failed to reduce its activity in the CB, because of the increased protein level.
Keywords: carotid body, intermittent hypoxia, MnSOD, obstructive sleep apnea, oxidative stress
Introduction
Obstructive sleep apnea (OSA), a highly frequent sleep-breathing disorder affecting 9% of adult men and 4% of women, is considered an independent risk factor for develop systemic hypertension.1–3 OSA is characterized by recurrent episodes of partial or complete obstruction of the upper airways during sleep producing chronic intermittent hypoxia (CIH). The hypoxia and hypercapnia elicited by each obstructive episode stimulate the carotid body (CB) chemoreceptors, producing respiratory muscle motor and sympathetic activation, micro-awakenings and finally restoration of the air flow.4 Among these alterations, CIH is considered the main factor for the OSA-driven hypertension.1–4 Oxidative stress, inflammation and sympathetic hyperactivity have been proposed as pathogenic mechanisms involved in the OSA-induced systemic hypertension.1,3–5 Rodents exposed to CIH mimicking the apnea episodes are the gold-standard model to study the mechanisms underlying the hypertension, because comorbidities in OSA patients make difficult to establish the contribution of the proposed mechanisms to the cardiovascular alterations.6–11
Oxidative stress contributes to autonomic cardiovascular alterations in OSA patients2–4 and animal exposed to CIH.11–13 The recurrence of the hypoxic-reoxygenation cycles produces nitro-oxidative stress in the CB, which contributes to enhance the CB chemosensory discharge during normoxia and hypoxia, leading to sympathetic hyperactivity and hypertension.9,11–15 Administration of antioxidants, including superoxide dismutase (SOD) mimetics targeted against superoxide radical (O2−), prevents the CIH-induced CB oxidative stress, the potentiation of the CB chemosensory discharge, the increased sympathetic activity, and the hypertension.10,12,16 Therefore, O2− is involved in the enhanced CB chemosensory discharge induced by CIH.11 It is well known that O2− reacts with nitric oxide (NO) to produce peroxynitrite with a high rate constant of 6.7 × 109 M−1s−1.17 Peroxynitrite radical nitrates tyrosyl groups in proteins altering its function through the generation of 3-nitrotyrosine (3-NT) residues.18 Indeed, CIH increases 3-NT immunoreactivity (3-NT-ir) levels in the rat CB, whereas the administration of ascorbic acid (AA) during CIH prevents the CB chemosensory potentiation, the formation of 3-NT, and the hypertension.16 The levels of O2− are regulated by the mitochondrial (MnSOD) and the cytoplasmic (CuZnSOD) SOD isoforms, playing an important role in the CB chemosensory potentiation induced by CIH. One of the possible targets for nitration induced peroxynitrite is the MnSOD.19–21 Indeed, nitration of MnSOD occurs in hypoxia and ischemia-reperfusion-related diseases.18,22–26 Accordingly, we hypothesized that CIH-induced peroxynitrite may nitrate MnSOD, decreasing its enzymatic activity in the CB and adrenal gland, contributing to the nitro-oxidative stress observed during CIH.10,12,16 To test this hypothesis, we studied the effects of CIH on the MnSOD nitration and its enzymatic activity in the rat CB and the adrenal gland. We measured the MnSOD and CuZnSOD immunoreactivity and protein levels and enzymatic activity as well as 3-NT-ir levels. We administrated AA to the rats exposed to CIH to determine if oxidative stress contributes to the observed changes of MnSOD and CuZnSOD levels in the CB and adrenal gland. In addition, we measured the MnSOD protein and immunoreactive levels in the superior cervical ganglion (SCG), a representative autonomic ganglion that share with the CB and adrenal gland the same embryonic neural crest-derived origin.
Materials and Methods
Animals
Experiments were performed in male Sprague-Dawley rats weighting 250 g fed with standard diet ad libitum and kept on a 12:12 h light–dark cycle and room temperature between 23 and 25C. All the experimental procedures were approved by the Bio-Ethical Committee of the Biological Sciences Faculty, Pontificia Universidad Católica de Chile, Santiago, Chile, and were performed according to the National Institutes of Health guide for the care and use of animals.
Intermittent Hypoxia Exposure and Administration of Antioxidants
Unrestrained, freely moving rats were housed in individual chambers and exposed to cycles of 5% to 6% inspired O2 for 20 sec followed by room air for 280 sec, 12 times per hour for 8 hr (CIH), or to sham air:air conditions (Sham) for 7 days. The O2 levels inside the chambers were continuously monitored using and oxygen analyzer (Ohmeda 5120, BOC Healthcare, Manchester, UK) and the CO2 levels and humidity maintained at low levels by continuous air extraction.6,16 Another group of rats exposed to the same CIH protocol was supplemented with AA (Sigma, St Louis, MO) in the drinking tap water from the first day of CIH exposure (CIH-AA). The AA solution (1.25 g/L) was prepared every day and preserved in dark containers to avoid oxidation.16 All the tissues used in this study were collected the day after the protocol of CIH was completed.
Western Blot
One day after finished sham or CIH exposure, rats were anesthetized with sodium pentobarbitone (40 mg × kg−1) and the tissues (CB, adrenal gland and SCG) were surgically removed and collected in lysis buffer (HEPES 20 mM, EGTA 1 mM, mannitol 210 mM, and 70 mM sucrose at pH 7.2), with 2% protease inhibitor mix (Sigma, St Louis, MO), homogenized by sonication, and maintained at −80C. Protein concentration was determined using DC Protein Assay (BioRad, Hercules, CA), and samples (30 µg) were mixed with LDS sample buffer (Invitrogen, Carlsbad, CA), immersed in boiling water for 5 min, electrophoresed on 12% SDS-poly-acrilamide gels and transferred to PVDF (polyvinylidene difluoride) membranes (iBlot, Invitrogen, Carlsbad, CA). After blocking in 5% bovine serum albumin (BSA) diluted in phosphate buffered saline (PBS) buffer with 0.1% Tween-20 at room temperature, the blots were incubated overnight at 4C with the primary antibodies against (Table 1) MnSOD (1:200, #06-984, Millipore, Temecula, CA), CuZnSOD (1:200, #574597, Calbiochem, Temecula, CA) or 3-NT (1:500, # A21285, Molecular Probes, Eugene, OR), and then incubated with horseradish peroxidase conjugated secondary antibodies (Sigma, St Louis, MO). Membranes were washed and proteins were visualized by ECL (enhanced chemiluminescence).
Table 1.
Antibodies Used in This Study.
| MnSOD | 1/200 IH 1/1000 WB |
Rabbit/Polyclonal | 06-984 | Millipore24,51 |
| CuZnSOD | 1/200 IH 1/1000 WB |
Sheep/Polyclonal | 574597 | Millipore (Calbiochem)44,52 |
| 3-NT | 1/500 | Rabbit/Polyclonal | A21285 | ThermoFisher (Molecular Probes)12,53 |
Abbreviations: WB, Western blot; 3-NT, 3-nitrotyrosine.
Co-immunoprecipitation
For detection of nitrated MnSOD, the adrenal medulla obtained from the adrenal gland of five rats and the CB removed of 12 rats per group were homogenized to generate protein extracts. Then, 400 µg of protein (for adrenal medulla) or 200 µg of protein (for CBs) were incubated with 20 µl of anti-3-NT agarose beads (# 16-163, Millipore, Temecula, CA) in 4C for 12 hr. Samples were centrifuged to 14000 g for 5 min twice. Supernatant was discarded and the pellet was washed 3 times with PBS. Finally, the pellet was resuspended in 50 µl of LDS sample buffer (Invitrogen, Carlsbad, CA) and Western blot was realized against MnSOD as previously described. We also performed the inverse control purifying MnSOD from the tissues and completing the Western blot this time against 3-NT as follows. Dynabeads coupled to protein A (# 10002D, Life Technologies, Carlsbad, CA) were mixed with 200 µl of anti-MnSOD antibody (1:20) prepared in PBS with 0,1% Tween-20. The complex Dynabead-MnSOD antibody was incubated with 200 µg of homogenized proteins obtained from adrenal medullas or CBs to 4C for 12 hr. We collect the Dynabeads coupled to antigen using a magnet, and the pellet was resuspended with elution solution (glycine 50 mM at pH: 2.8). We add 10 µl of 4× LDS sample buffer and put the tubes in boiling water by 10 min. Separated Dynabeads were isolated with the magnet, and supernatant was used to realize the Western blot against 3-NT.
Immunohistochemistry
Immunohistochemistry assays were used to detect MnSOD, CuZnSOD, and 3-NT relative levels and localization in CB, adrenal gland and SCG.16,27 Anesthetized rats were perfused intracardially with PBS at pH 7.4 for 15 min followed by buffered paraformaldehyde (PFA, Sigma, St. Louis, MO). Carotid bifurcations and adrenal glands were dissected and postfixed in the same fixative solution for 12 h to 4C. Then, the samples were dehydrated in ethanol, included in paraffin cut in 5 µm section and mounted on silanized slices. Samples were deparaffinized and submitted to antigen-retrieval protocol (using a microwave, 700W for 4 min in citrate buffer 1 M, pH 6.0). Samples were incubated in 0.3% H2O2 to inhibit endogenous peroxidase and then blocked using normal horse serum solution (ABC, Vectastain kit, Vector, Burlingame, CA) for 1 hr. The samples were then incubated with the same primary antibodies against MnSOD, CuZnSOD, or 3-NT as specified for Western blot assay overnight to 4C (Table 1). Then the immunoreactivity staining was detected using a streptavidin-peroxidase kit (ABC, Vectastain kit, Vector, Burlingame, CA) and revealed at 37C in dark chamber with 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma, St Louis, MO). Samples were counterstained with Harris hematoxylin and mounted with Entellan (Merck, Whitehouse station, NJ). Photomicrographs were taken at 100× or 40× using a CCD camera coupled to an Olympus CX 31 microscope (Olympus Corp. Tokyo, Japan), digitized and analyzed using ImageJ software (NIH, Bethesda, MD). The immunoreactive intensity was averaged from eight-fields for each CB or adrenal medulla and was expressed as optical integrated intensity.
MnSOD Enzymatic Activity
For MnSOD activity measurement, the CB and adrenal medulla were extracted from 12 rats per group in lysis buffer and stored at −80C. Then, MnSOD enzymatic activity was measured with a Superoxide Dismutase Assay Kit (Cayman Chemical Company, Ann Arbor, MI) following the manufacturer instructions, and expressed as units of enzyme per mL (U/ml).
Statistical Data Analysis
Data were expressed as mean ± SEM. Differences between two groups were assessed with Student’s t-test. For analyses between three or more groups, we evaluate the differences between groups using one-way ANOVA, followed by Newman-Keuls post hoc comparisons.
Results
Differential Effects of CIH on MnSOD Levels in the Rat Carotid Body, Adrenal Gland, and Superior Cervical Ganglion
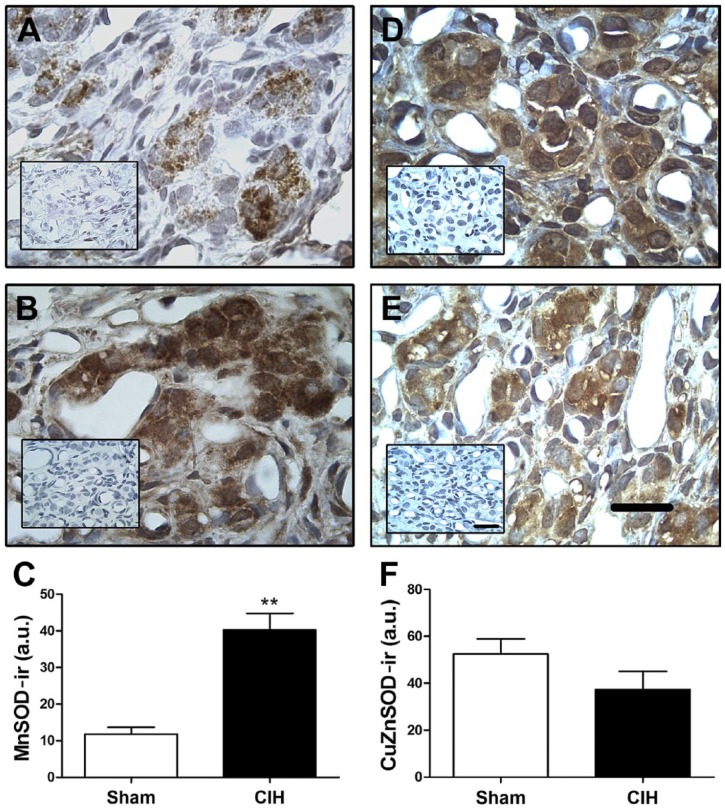
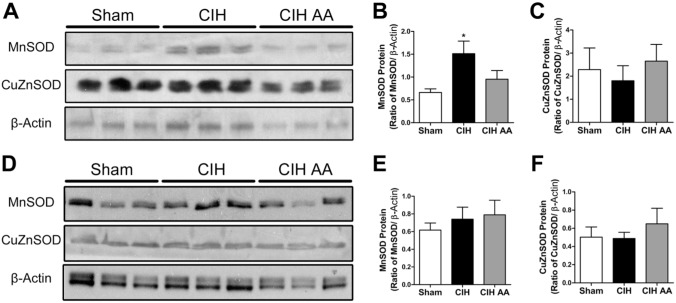
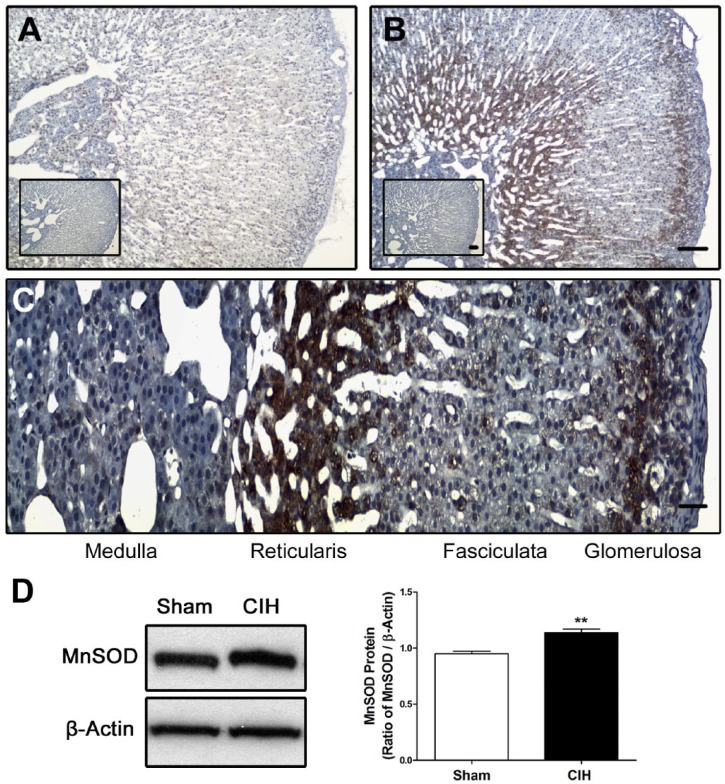
Exposure to CIH for 7 days increased the level of MnSOD-ir in the CB tissue including clusters of chemoreceptor cells (Fig. 1B), as compared with sham animals (Fig. 1A). The mean data reveals a significant increase of MnSOD-ir on CBs from rats exposed to CIH compared with the level measured in the CBs from Sham rats (40.2 ± 4.5 vs. 11.8 ± 1.9 a.u. CIH vs. sham, respectively, Fig. 1C). The Western blot assay confirmed that CIH increased MnSOD levels in CB homogenates compared with sham rats (p<0.01, Fig. 4A and B). On the contrary, CIH did not modify the CuZnSOD-ir in the CB (Fig. 1D–F). This result was confirmed by Western blot whose quantification did not show any statistical difference between the protein levels of CuZnSOD in the CB from sham and CIH-rats (Fig. 4A and C). Then, we studied the MnSOD protein levels in the adrenal gland. The immunohistochemistry performed in the adrenal gland showed that CIH increased MnSOD-ir mainly in the zones glomerulosa and reticularis, and to a lesser extent in the zone fasciculata and the medulla (Fig. 2A–C). The Western blot performed with whole adrenal gland homogenates showed a significant increase of MnSOD protein levels in CIH-rats compared with the sham group (p<0.01, Fig. 2D), indicating that CIH increased MnSOD in the adrenal gland. However, the Western blot analysis performed with homogenates from the isolated adrenal medulla-separated from the adrenal cortex-extracted from CIH and sham rats, did not show any significant differences in MnSOD or CuZnSOD protein levels (p=0.62, Fig. 4D–F).
Figure 1.
Effects of CIH on MnSOD and CuZnSOD levels in rat CB. MnSOD (A–C) and CuZnSOD (D–F) immunoreactivity (MnSOD-ir and CuZnSOD-ir, respectively) in CBs from rats exposed to sham conditions (A and D) and CIH (B and E) for 7 days. Inset, negative control from the same rat but without the primary antibody. Scale bar, 20 µm. Quantification of MnSOD positive staining (C) showed that CIH produced a significant increase of MnSOD-ir, **p<0.01 Student’s t-test, n=6 sham and n=4 CIH-rats. Quantification of CuZnSOD-ir (F) did not show statistical differences. p>0.05, Student’s t-test, n=4 rats per group. Abbreviations: CIH, chronic intermittent hypoxia; CB, carotid body.
Figure 4.
Effects of ascorbic acid on MnSOD and CuZnSOD levels in the carotid body and adrenal medulla from CIH-rats. Western blot assays performed on CB homogenates (A), demonstrate that ascorbic acid prevented the CIH-induced increase in MnSOD levels in rat CB (B), while CuZnSOD did not change either with CIH or the CIH-AA exposure (C) *p<0.05 versus sham group, Bonferroni after one-way ANOVA n=11 rats per group. Western blot realized with adrenal medulla homogenates (D) from sham, CIH or CIH-AA rats did not show statistical differences in MnSOD (E) or CuZnSOD levels (F). One-way ANOVA, n=4–5 rats per group. Abbreviations: CIH, chronic intermittent hypoxia; CB, carotid body.
Figure 2.
Levels of MnSOD in adrenal gland of rats exposed to CIH. Levels of MnSOD-ir in the adrenal gland from a sham (A), and CIH-rat (B). Scale bar 100 µm. High magnification showed MnSOD-ir in different zones of adrenal gland of a CIH-rat (zone glomerulosa, fasciculata, reticularis and medulla) (C). Scale bar 20 µm. The western blot realized with whole adrenal gland reveal an increase of MnSOD in CIH-rats (D). **p<0.01, Student’s t-test, sham n=4 and CIH n=3. Abbreviation: CIH, chronic intermittent hypoxia.
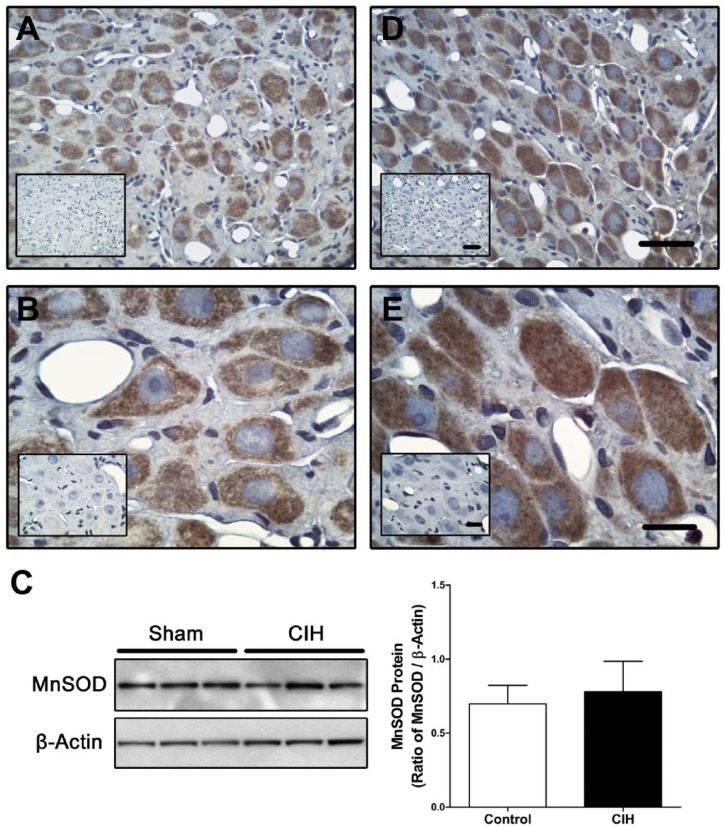
In the sympathetic SCG, MnSOD-ir was present in neurons from sham (Fig. 3A–B) and CIH-rats (Fig. 3D and E). The Western blot assay performed in homogenates from the SCG did not show any differences between Sham and CIH-rats (Fig. 3C).
Figure 3.
The level of MnSOD measured in superior cervical ganglion is not altered by CIH. Immuno-histochemical assay for MnSOD in cervical superior ganglia extracted from a sham (A and B) or a CIH exposed rat (D and E). Scale bar for A and D, 50 µm. Scale bar for B and E, 20 µm. Representative western blot (C) and quantification of immunoblot showed that CIH did not produce changes of MnSOD in homogenates of superior cervical ganglia. Student’s t-test, sham n=4, CIH n=5. Abbreviation: CIH, chronic intermittent hypoxia.
Effect of Ascorbic Acid on MnSOD and CuZnSOD Levels in the CB and Adrenal Gland Following CIH
We studied the effects of the antioxidant AA to determine if oxidative stress due to CIH may induce changes of MnSOD and CuZnSOD protein levels in the CB and adrenal medulla. Exposure to CIH produced a significant 3-fold increase of MnSOD protein levels in CB homogenates, effect that was prevented by AA (Fig. 4A and B). On the contrary, the Western blot analysis performed in homogenized adrenal medulla showed that either CIH or CIH-AA treatment failed to modify the MnSOD levels compared with the Sham group (Fig. 4D and E). The levels of CuZnSOD in the CB (Fig. 4A) or in adrenal medulla homogenates (Fig. 4D) were not affected by CIH exposure or AA supplementation (Fig. 4C and F). The Western blot assays, performed under standard denaturing conditions, showed two bands consistent with the molecular weight of MnSOD. Thus, we cannot preclude that posttranslational modifications may produce these dual bands, as has been reported for phosphorylation of CuZnSOD in eukaryotic cells28,48 and MnSOD in wild type mice liver samples.49,50
CIH Increases the 3-NT Levels in Rat CB and Adrenal Medulla
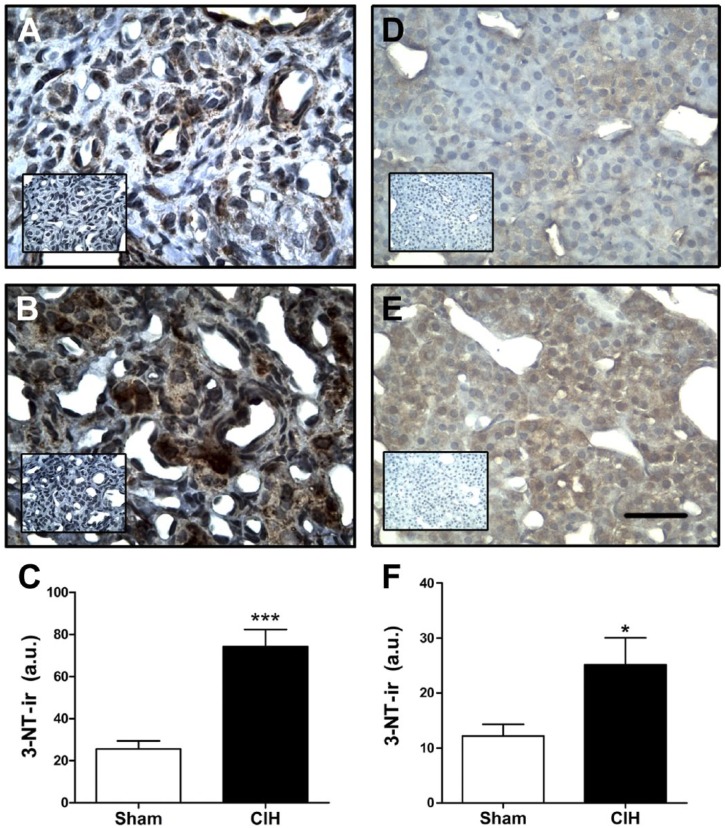
Present results showed that CIH increased MnSOD-ir and protein levels in the CB, in the reticularis and glomerulosa zones of the adrenal gland cortex, but not in the adrenal medulla or the sympathetic SCG. As oxidative stress produced by CIH may trigger post-transductional modifications such as protein nitration,18,26 we measured 3-NT-ir in the CB and adrenal medulla. Exposure to CIH for 7 days increased 3-NT-ir in the CB (Fig. 5A and B) and specifically in the in the chromaffin cells of the adrenal medulla (Fig. 5D and E). The quantification of the 3-NT-ir grouped data showed that CIH produced a significant 3-fold increase of 3-NT in the CB (74.2 ± 8.1 vs. 25.6 ± 3.8 a.u., CIH vs. sham, respectively, p<0.001, Fig. 5C), and a 2-fold increase in the adrenal medulla (25.1 ± 4.9 vs. 12.2 ± 2.1 a.u, CIH vs. sham, respectively, p<0.05 Fig. 5F).
Figure 5.
Effects of CIH on 3-NT levels in rat carotid body and adrenal medulla. Photographs show the positive immunoreactivity for 3-NT (3-NT-ir) in the CB (A and B) and in chromaffin cells from adrenal the medulla (D and E), which correspond to high magnification images selected from adrenal medullas showed in left side of Fig. 2C. A and D, sham rats. B and E, CIH exposed rats. Inset, negative sham realized omitting the primary antibody. Scale bar, 20 µm. The exposure to CIH produced a significant increase in 3-NT-ir levels in the CB (C) and adrenal medulla (F). ***p<0.001, *p<0.05 compared with sham rats. Student’s t-test, sham n=10 and CIH n=4 for CBs; sham n=6, CIH n=7 for adrenal medulla. Abbreviations: CIH, chronic intermittent hypoxia; 3-NT, 3-nitrotyrosine; 3-NT-ir, 3-NT immunoreactivity; CB, carotid body.
Effects of CIH on MnSOD Nitration and Enzymatic Activity y in Rat CBs and Adrenal Medulla
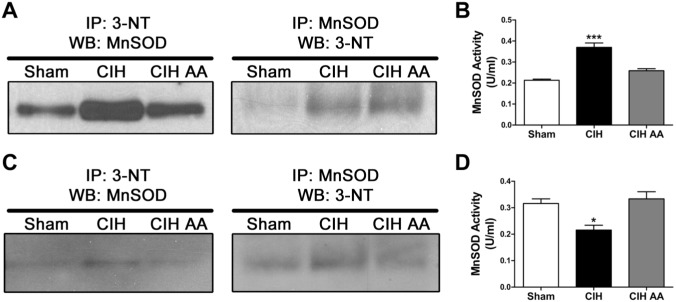
As nitration of MnSOD may affect its enzymatic activity,18,24 we measured nitrated MnSOD in CB and adrenal medulla using immuno-precipitated beads. Nitrated proteins in CB homogenates were pulled-down and a Western blot against MnSOD was performed on those extracts, showing that rats exposed to CIH had higher levels of nitrated MnSOD compared with the data obtained from sham rats (Fig. 6A). The nitration of MnSOD induced by CIH was reduced when the rats were supplemented with AA (Fig. 6A, left panel). The inverse control, by pulling down first the MnSOD from the homogenate and performing the Western blot against 3-NT, showed similar results (Fig. 6A, right panel). As expected, we found that CIH increased the nitrated MnSOD, effect that was prevented by the administration of AA.
Figure 6.
Co-immunoprecipitation using agarose beads reveals that CIH increase the level of nitrated MnSOD in the CBs (A) and adrenal medulla (C), and that this nitration is prevented with administration of ascorbic acid concomitant with CIH (CIH-AA), n=12 rats per lane. MnSOD enzymatic activity was measured in CB homogenates in the three groups using a colorimetry kit. ***p<0.001 versus sham and CIH-AA, *p<0.05 versus sham and CIH-AA. Bonferroni after one-way ANOVA. For CBs n=11–12 rats per group; for adrenal medullas n=7–8 rats per group. Abbreviations: CIH, chronic intermittent hypoxia; CB, carotid body.
The MnSOD enzymatic activity was measured in CB homogenates using a colorimetric assay. Contrary to the expected, the CB extracted from rats exposed to CIH showed an increased MnSOD activity (0.37 ± 0.02 U/ml vs. 0.21 ± 0.01 U/ml, CIH-rats vs. sham, respectively; Fig. 6B). The increased MnSOD activity was prevented in CIH-rats by AA (0.26 ± 0.01 U/ml, Fig. 6B).
The analysis of the co-immunoprecipitation in adrenal medulla homogenates showed that CIH increased nitrated MnSOD compared with the sham group, and this increase was prevented by AA administration (Fig. 6C, left panel). When we pulled-down all the MnSOD protein and perform the Western blot against 3-NT, we also detected an increase in nitrated MnSOD of CIH exposed rats, that was prevented by AA (Fig. 6C, right panel). The quantification of the MnSOD activity in adrenal medulla showed that the activity of MnSOD was reduced in CIH-rats (0.22 ± 0.02 U/ml vs. 0.32 ± 0.02 U/ml, p<0.05, CIH-rats vs. sham, respectively, Fig. 6D). The CIH-induced decrease in MnSOD activity was prevented by AA supplemented during CIH (0.33 ± 0.03 U/m, Fig. 6D).
Discussion
The main findings of this study showed that (1) exposure to CIH for 7 days increased MnSOD but not CuZnSOD levels in the rat CB, while CIH did not increase MnSOD or CuZnSOD in the adrenal medulla; (2) 3-NT levels and MnSOD nitration increased in the CB and adrenal medulla of rats exposed to CIH; (3) CIH increased MnSOD enzymatic activity in the CBs, but decreased its activity in the adrenal medulla; (4) the changes in protein levels and activity of MnSOD induced by CIH in the CB were prevented by AA during CIH.
Present results show that CIH for 7 days increased the level of MnSOD in the cortical areas of the adrenal gland (Fig. 2C). Other studies have found increased levels of MnSOD in the adrenal cortex induced by treatment with adrenocorticotrophic hormone and increased levels of corticosterone.31,32 Remarkably, exposure to CIH also increases the levels of corticosterone33,34 and the peak levels of plasmatic adrenocorticotrophic hormone induced by stress.35 Then, it is possible that the CIH-induced increase of MnSOD in the adrenal cortex was elicited by the activation of the sympathetic-adrenal axis.
The rat CB has an approximate diameter of 400 µm and a weight of 25 µg, which make extremely difficult to perform molecular biology experiments with CB samples.36,37 This reason is why some authors, based on a common embryonic origin, have used other cellular cells to understand the mechanisms occurring in the glomus cells of the CB, including the chromaffin cells of the adrenal medulla, the cervical superior ganglia (CSG) neurons, and PC12 cells.28,29,38,39 We found, using Western blot and immunohistochemistry, that CIH produces an increase of MnSOD in the CB, but not in the adrenal medulla or the CSG cells. Recently, Gao et al. compared the transcriptomes of O2 sensitive glomus and chromaffin cells from the CB and adrenal medulla, respectively, with SCG neurons, which are O2 insensitive. They found a marked differential pattern of expression transcriptomes of several metabolic enzymes, mitochondrial electron transport subunits, and ion channels in those cells, suggesting that cell types with the similar embryological origin-neural crest-derived progenitors, but different responses to acute hypoxia, may have a differential expression in response to hypoxia.29
Several studies emphasized that reactive oxygen species (ROS) and reactive nitrogen species (RNS) are necessary to enhance CB chemosensory discharges and produce sympathetic over-activation following CIH.11,13,17 Wide-range antioxidants, such as AA, Tempol, and SOD mimetics administrated concomitantly with the CIH stimuli, prevented both the enhanced CB chemosensory discharge and the hypertension.10,16,40 Furthermore, we found that the CIH-induced increased the levels of 3-NT in the CB and plasma lipid peroxidation are prevented by AA treatment.16 Moreover, the administration of Ebselen, a specific peroxynitrite scavenger, reverts the CIH-induced hypertension, and prevents both the accumulation of 3-NT in the CB and the enhanced CB chemosensory discharge induced by CIH.12 Thus, enzymes that regulate the ROS and RNS levels seems to be crucial for the modulation of CB chemosensory activity. Prabhakar proposed that CIH changes in the ratio of hypoxia-inducible factors HIF-1α/HIF-2α, increasing the transcription of pro-oxidant enzymes leading to oxidative stress, as well as the down-regulation of antioxidant enzymes like SOD.41 Another possible mechanism that may impair the function of MnSOD is the nitration of the enzyme. Indeed, nitration of MnSOD has been involved in cardiovascular and renal pathologies produced by hypoxia and ischemia-reperfusion.18,24,25,42 Accordingly, we expected that nitration of MnSOD may decrease its enzymatic activity, contributing to produce nitro-oxidative stress in the CB and adrenal gland.10,16 In the adrenal medulla, we found that CIH produced oxidative stress, nitrated MnSOD and decreased its enzymatic activity. On the contrary, our results showed an increase of MnSOD activity in the CB, even when the levels of 3-NT and the specific nitration of MnSOD increased in rats exposed to CIH. Thus, it is plausible that the increase in MnSOD activity in the CB of rats exposed to CIH (Fig. 6B) was due to the increase of MnSOD protein levels following 7 days of CIH (Figs. 1A–C and 3A and B). The increase in MnSOD levels did not occur in the adrenal medulla, while the inhibitory effect of nitration on MnSOD was present, explaining why CIH decreased the MnSOD activity in the rat adrenal medulla (Fig. 6D). Nanduri et al. found that 10 days of CIH decreased MnSOD RNA transcript levels in PC12 cells, the adrenal medulla and the CB, and decreases of MnSOD activity.28,43 Thus, their results suggest that a more prolonged period of intermittent hypoxia reduced the expression on MnSOD, although they did not measure the MnSOD protein levels in the CB.
Other studies have found that hypoxia increased the levels of MnSOD, acting as a compensatory effect to the increased levels of free radical. Indeed, Chou et al. found that exposure to sustained hypoxia for 4 weeks, increased the levels of MnSOD in the rat lung without any change in CuZnSOD, while the mRNA levels of both proteins were not altered,44 supporting the idea that MnSOD changes are originated by posttraductional modifications. Other studies have shown an increase in MnSOD levels in a hypoxia/reperfusion liver model,45 and in cardiomyocytes of rats exposed to CIH.46 Moreover, Balková et al. found that the CIH-increased levels of MnSOD were prevented when the rats were treated with the antioxidant N-acetylcysteine. These results agree with our finding showing that supply of AA during CIH prevented the increase in MnSOD levels (Fig. 4A and B). Nayak et al. demonstrated that ROS stabilizes the HIF-2α in a mechanism that involve the mTOR 2 protein complex.47 This mechanism may help to explain our results showing that antioxidant treatment prevented the CIH-induced increase of MnSOD (Fig. 4B). These observation contrasts with the results of other studies showing that the transcription of MnSOD in the CB is under the control of the HIF-2α,37,43 and that CIH decreased the MnSOD mRNA levels.28,43 Thus, we cannot preclude that prolonged exposition to CIH (10 or more days) may reduce the levels and activity of MnSOD in the rat CB.
In summary, our results show that short-term CIH increased the MnSOD levels and its enzymatic activity in the CB, despite of the fact that the enzyme is nitrated. The nitration in the adrenal medulla of rats exposed to CIH may contribute to decrease the MnSOD activity. In addition, the treatment with the antioxidant AA, prevented the CIH-induced effects in the CB and the adrenal medulla. Present results show that 7 days of CIH increased MnSOD nitration, but failed to reduce its enzymatic activity in the CB, because MnSOD levels increased, suggesting a compensatory increase of MnSOD in the CB in response to CIH, which is not sufficient to prevent the nitro-oxidative stress in the CB.
Acknowledgments
We thank to Dr. Julio Alcayaga (Faculty of Science, University of Chile, Santiago, Chile) for his invaluable help on the design and construction of our intermittent hypoxia-exposure device.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: EAM and RI contributed for the study design; EAM, PA, and RI contributed to the execution of the study and experiment; EAM analyzed the data; and EAM and RI drafted the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from FONDECYT 1150040.
Contributor Information
Esteban A. Moya, Division of Physiology, Department of Medicine, University of California San Diego, La Jolla, California Laboratorio de Neurobiología, Departamento de Fisiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Paulina Arias, Laboratorio de Neurobiología, Departamento de Fisiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Rodrigo Iturriaga, Laboratorio de Neurobiología, Departamento de Fisiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Literature Cited
- 1. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement From the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). J Am Coll Cardiol. 2008;52(8):686-717. https://www.sciencedirect.com/science/article/pii/S0735109708016483?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 2. Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177(4):369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33(5):1195–205. [DOI] [PubMed] [Google Scholar]
- 4. Iturriaga R, Oyarce MP, Dias ACR. Role of carotid body in intermittent hypoxia-related hypertension. Curr Hypertens Rep. 2017;19(5):38. [DOI] [PubMed] [Google Scholar]
- 5. Lavie L. Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev. 2003;7(1):35–51. [DOI] [PubMed] [Google Scholar]
- 6. Iturriaga R, Moya EA, Del Rio R. Carotid body potentiation induced by intermittent hypoxia: implications for cardiorespiratory changes induced by sleep apnoea. Clin Exp Pharmacol Physiol. 2009;36(12):1197–204. [DOI] [PubMed] [Google Scholar]
- 7. Dumitrascu R, Heitmann J, Seeger W, Weissmann N, Schulz R. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid Med Cell Longev. 2013;2013:234631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension. 1992;20(5):612–9. http://www.ncbi.nlm.nih.gov/pubmed/1428112. [DOI] [PubMed] [Google Scholar]
- 9. Fletcher EC, Lesske J, Behm R, Miller CC, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72(5):1978–84. [DOI] [PubMed] [Google Scholar]
- 10. Peng Y-JJ, Overholt JL, Kline DD, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100(17):10073–8. http://www.ncbi.nlm.nih.gov/pubmed/12907705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng Y-J. Prabhakar N-R. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96(3):1236–42. http://jap.physiology.org/cgi/doi/10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- 12. Moya EA, Arias P, Varela C, Oyarce MP, Del Rio R, Iturriaga R. Intermittent hypoxia-induced carotid body chemosensory potentiation and hypertension are critically dependent on peroxynitrite formation. Oxid Med Cell Longev. 2016;2016:9802136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Del Rio R, Andrade DC, Lucero C, Arias P, Iturriaga R. Carotid body ablation abrogates hypertension and autonomic alterations induced by intermittent hypoxia in rats. Hypertension. 2016;68(2):436–45. [DOI] [PubMed] [Google Scholar]
- 14. Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. 2004;560(2):577–86. http://doi.wiley.com/10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol. 2005;32(5–6):447–9. [DOI] [PubMed] [Google Scholar]
- 16. Del Rio R, Moya EA, Iturriaga R. Carotid body and cardiorespiratory alterations in intermittent hypoxia: the oxidative link. Eur Respir J. 2010;36(1):143–50. [DOI] [PubMed] [Google Scholar]
- 17. Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1(1):244–57. 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamakura F, Kawasaki H. Post-translational modifications of superoxide dismutase. Biochim Biophys Acta. 2010;1804(2):318–25. http://linkinghub.elsevier.com/retrieve/pii/S1570963909002994. [DOI] [PubMed] [Google Scholar]
- 19. Castro L, Demicheli V, Tórtora V, Radi R. Mitochondrial protein tyrosine nitration. Free Radic Res. 2011;45(1):37–52. http://www.tandfonline.com/doi/full/10.3109/10715762.2010.516254. [DOI] [PubMed] [Google Scholar]
- 20. Pacher P, Beckman JS, Liaudet L. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75(1):1–45. [DOI] [PubMed] [Google Scholar]
- 21. Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res. 2007;75(2):291–302. [DOI] [PubMed] [Google Scholar]
- 22. Anantharaman M, Tangpong J, Keller JN, Murphy MP, Markesbery WR, Kiningham KK, St Clair DK. β-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer’s disease. Am J Pathol. 2006;168(5):1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med. 2001;31(12):1603–8. [DOI] [PubMed] [Google Scholar]
- 24. MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A. 1996;93(21):11853–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC38148/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gray KD, MacMillan-Crow LA, Simovic MO, Stain SC, May AK. Pulmonary MnSOD is nitrated following hepatic ischemia-reperfusion. Surg Infect. 2004;5(2):166–73. https://www.ncbi.nlm.nih.gov/pubmed/15353113. [DOI] [PubMed] [Google Scholar]
- 26. Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6(8):662–80. http://www.nature.com/doifinder/10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 27. Del Rio R, Moya EA, Parga MJ, Madrid C, Iturriaga R. Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur Respir J. 2012;39(6):1492–500. [DOI] [PubMed] [Google Scholar]
- 28. Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X, Kinsman B, Peng YJ, Kumar GK, Fox AP, Godley LA, Semenza GL, Prabhakar NR. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc Natl Acad Sci U S A. 2012;109(7):2515–20. http://www.pnas.org/cgi/doi/10.1073/pnas.1120600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao L, Bonilla-Henao V, García-Flores P, Arias-Mayenco I, Ortega-Sáenz P, López-Barneo J. Gene expression analyses reveal metabolic specifications in acute O 2-sensing chemoreceptor cells. J Physiol. 2017;595(18):6091–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moya EA, Alcayaga J, Iturriaga R. NO modulation of carotid body chemoreception in health and disease. Respir Physiol Neurobiol. 2012;184(2):158–64. 10.1016/j.resp.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 31. Raza FS, Okamoto M, Takemori H, Vinson GP. Manganese superoxide dismutase activity in the rat adrenal. J Endocrinol. 2005;184(1):77–84. http://www.ncbi.nlm.nih.gov/pubmed/15642785. [DOI] [PubMed] [Google Scholar]
- 32. Suwa T, Mune T, Morita H, Daido H, Saio M, Yasuda K. Role of rat adrenal antioxidant defense systems in the aldosterone turn-off phenomenon. J Steroid Biochem Mol Biol. 2000;73(1–2):71–8. [DOI] [PubMed] [Google Scholar]
- 33. Hwang GS, Chen CC, Chou JC, Chang LL, Kan SF, Lai WH, Lieu FK, Hu S, Wang PS, Wang SW. Stimulatory effect of intermittent hypoxia on the production of corticosterone by zona fasciculata-reticularis cells in rats. Sci Rep. 2017;7(1):9035 http://www.nature.com/articles/s41598-017-07054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zoccal DB, Bonagamba LGH, Antunes-Rodrigues J, Machado BH. Plasma corticosterone levels is elevated in rats submitted to chronic intermittent hypoxia. Auton Neurosci. 2007;134(1–2):115–7. http://www.ncbi.nlm.nih.gov/pubmed/17293169. [DOI] [PubMed] [Google Scholar]
- 35. Ma S, Mifflin SW, Cunningham JT, Morilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitary-adrenal stress reactivity and Fos induction in the rat locus coeruleus in response to subsequent immobilization stress. Neuroscience. 2008;154(4):1639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pardal R, Ludewig U, Garcia-Hirschfeld J, Lopez-Barneo J. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc Natl Acad Sci U S A. 2000;97(5):2361–6. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=15806&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yuan G, Peng YJ, Reddy VD, Makarenko VV, Nanduri J, Khan SA, Garcia JA, Kumar GK, Semenza GL, Prabhakar NR. Mutual antagonism between hypoxia-inducible factors 1α and 2α regulates oxygen sensing and cardio-respiratory homeostasis. Proc Natl Acad Sci U S A. 2013;110(19):E1788–96. http://www.pnas.org/cgi/doi/10.1073/pnas.1305961110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9(9):1391–6. http://www.liebertonline.com/doi/abs/10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- 39. Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575(1):229–39. http://doi.wiley.com/10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol. 2007;293(5):H2971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prabhakar NR. Carotid body chemoreflex: a driver of autonomic abnormalities in sleep apnoea. Exp Physiol. 2016;101(8):975–85. http://doi.wiley.com/10.1113/EP085624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37(6):1613–22. [DOI] [PubMed] [Google Scholar]
- 43. Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A. 2009;106(4):1199–204. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2626608&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chou TF, Ma MC, Tsai CP, Chen CF. Enhancement of superoxide dismutase activity in rat lungs after hypoxic preconditioning. Chin J Physiol. 2009;52(5 Suppl):376–83. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=20359128. [DOI] [PubMed] [Google Scholar]
- 45. Pardo M, Budick-Harmelin N, Tirosh B, Tirosh O. Antioxidant defense in hepatic ischemia-reperfusion injury is regulated by damage-associated molecular pattern signal molecules. Free Radic Biol Med. 2008;45(8):1073–83. [DOI] [PubMed] [Google Scholar]
- 46. Balková P, Hlaváčková M, Milerová M, Neckář J, Kolář F, Novák F, Nováková O. N-acetylcysteine treatment prevents the up-regulation of MnSOD in chronically hypoxic rat hearts. Physiol Res. 2011;60(3):467–74. [DOI] [PubMed] [Google Scholar]
- 47. Nayak BK, Feliers D, Sudarshan S, Friedrichs WE, Day RT, New DD, Fitzgerald JP, Eid A, Denapoli T, Parekh DJ, Gorin Y, Block K. Stabilization of HIF-2α through redox regulation of mTORC2 activation and initiation of mRNA translation. Oncogene. 2013;32(26):3147–55. http://www.nature.com/doifinder/10.1038/onc.2012.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leitch JM, Li CX, Baron JA, Matthews LM, Cao X, Hart J, Culotta VC. Post-translational modification of Cu/Zn superoxide dismutase under anaerobic conditions. Biochemistry. 2013;51(2):677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oktay Y, Dioum E, Matsuzaki S, Ding K, Yan LJ, Haller RG, Szweda LI, Garcia JA. Hypoxia-inducible factor 2α regulates expression of the mitochondrial aconitase chaperone protein frataxin. J Biol Chem. 2007;282(16):11750–6. [DOI] [PubMed] [Google Scholar]
- 50. Candas D, Li JJ. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid Redox Signal. 2014;20(10):1599–617. http://online.liebertpub.com/doi/abs/10.1089/ars.2013.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Laye MJ, Nielsen MB, Hansen LS, Knudsen T, Pedersen BK. Physical activity enhances metabolic fitness independently of cardiorespiratory fitness in marathon runners. Dis Markers. 2015;2015:806418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lien YC, Lin SM, Nithipongvanitch R, Oberley TD, Noel T, Zhao Q, Daosukho C, St Clair DK. Tumor necrosis factor receptor deficiency exacerbated Adriamycin-induced cardiomyocytes apoptosis: an insight into the Fas connection. Mol Cancer Ther. 2006;5(2):261–9. http://www.ncbi.nlm.nih.gov/pubmed/16505099. [DOI] [PubMed] [Google Scholar]
- 53. Reifenberger MS, Arnett KL, Gatto C, Milanick MA. The reactive nitrogen species peroxynitrite is a potent inhibitor of renal Na-K-ATPase activity. Am J Physiol Renal Physiol. 2008;295(4):F1191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]