Abstract
Increased consumption and improper disposal of prescription medication, such as beta (β)-blockers, contribute to their introduction into waterways and may pose threats to non-target aquatic organisms. There has been rising concern about the impacts of these prescription drugs on coastal ecosystems, especially because wastewater treatment plants are not designed to eliminate them from the discharge. Few studies have characterized the sublethal effects of β-blocker exposures in marine invertebrates. The overall aim of our research is to identify cellular responses of two commercially important filter-feeding marine bivalves, hard clams (Mercenaria mercenaria) and Eastern oysters (Crassostrea virginica), upon exposures to two β-blocker drugs, propranolol and metoprolol. In vitro exposures with bivalve digestive gland and gill tissues were conducted where tissues were separately exposed to each drug for 24 h. Tissue samples were analyzed for cellular damage (lysosomal membrane destabilization and lipid peroxidation), total antioxidant capacity, and glutathione-s-transferase activity. Elevated damage and changes in enzyme activities were noted in the exposed tissues at environmentally relevant concentrations. Differences in species and tissue sensitivities and responses to exposures were also observed. These studies enhance our understanding of the potential impacts of prescription medication on coastal organisms. Additionally, this work demonstrates that filter-feeders may serve as good model organisms to examine the effects of unintended environmental exposures to β-blockers. These studies are part of our ongoing work aimed at evaluation of sublethal biomarkers of pharmaceutical exposures and identification of key events that can contribute to the development of adverse outcome pathways (AOPs).
Keywords: Bivalves, oysters, clams, beta blockers, pharmaceuticals, cellular biomarkers
1. Introduction
Widespread, continuous use of pharmaceuticals and poor removal efficiencies from wastewater treatment plants (WWTPs) pose a risk to aquatic organisms. One such class of widely prescribed drugs that are not effectively removed from WWTPs and enter waterways is beta (β) blockers. Beta blockers are used for medical treatments related to cardiovascular diseases, such as hypertension, myocardial infarction, angina and arrhythmias (Böhm and Maack 2000, Baker, Hill et al. 2011). They prevent binding of endogenous catecholamines, such as epinephrine (E) and norepinephrine (NE), to β-adrenoceptors (also called β-adrenergic receptors). Beta-adrenoceptors are transmembrane receptor proteins which mediate catecholamine induced activation of adenylate cyclase through the action of G-stimulatory proteins and ultimately enhance the production of the secondary messenger signaling molecule cAMP (Cole and Sood 2012). Beta-blockers prevent this activation and inhibit sympathetic effects of stress hormones (E and NE) such as elevated heart rate. First generation β-blockers, such as propranolol, non-selectively bind to β1 and β2- adrenoceptors; second generation blockers, such as metoprolol, are relatively cardioselective in their binding properties and display selectivity for the β1 over the β2 receptor (Bristow 1997, Smith and Teitler 1999).
Beta-blockers have been identified in wastewater effluent, surface waters, and hospital discharges (Huggett, Khan et al. 2003, Gómez, Petrović et al. 2006, Cantwell, Katz et al. 2017). As the concern around the occurrence of these pharmaceuticals in aquatic ecosystems increases, studies on their environmental fate and biological effects are also emerging (Cleuvers 2005, Fent, Weston et al. 2006, Triebskorn, Casper et al. 2007, Ramil, El Aref et al. 2010). However, current toxicity data for β-blocker exposures to marine organisms are relatively scarce (Gaw, Thomas et al. 2014, Fabbri and Franzellitti 2016). Marine bivalves are especially vulnerable to low-dose contamination due to their filtering capacities and constant exposure to dissolved pollutants in surface waters (Massarsky, Trudeau et al. 2011). Additionally, these organisms are ecologically and commercially important, with oysters having the unique characteristic of being consumed raw by humans.
Evolutionary conserved pathways and molecular similarities between human and invertebrate adrenoceptors increase the potential for adverse cellular and physiological impacts on these non-target organisms (Lacoste, Malham et al. 2001, Massarsky, Trudeau et al. 2011). Early work with hard clams and propranolol shows effects on contractility of the heart (Mann and Gautieri 1984). It has also been reported that adrenoreceptors mediate metamorphosis in bivalve larvae (Coon and Bonar 1987, Wang, Liu et al. 2006, Yang, Li et al. 2014). The bulk of recent information on the effects of β- blockers on marine bivalves is derived from studies with mussels (Gaw, Thomas et al. 2014, Fabbri 2015). Knowledge gaps in our understanding of β-blocker toxicity in marine biota include comparisons among multiple species and identification of reliable biomarkers of such pharmaceutical exposures. Biomarker research can help characterize outcomes of exposures to β-blocking pharmaceuticals in aquatic environments and contribute towards development of adverse outcome pathway (AOPs) for these emerging contaminants. AOPs are a conceptual framework aimed at improving environmental risk assessment by facilitating linkages of existing information about biological events at many levels of biological organization (Ankley, Bennett et al. 2010). Identification of cellular and molecular biomarkers provides information on putative key events at lower levels of organization in an AOP.
Studies with propranolol in Mytilus galloprovincialis have shown effects on cAMP signaling and oxidative stress (Franzellitti, Buratti et al. 2011). However, the role and mechanisms of oxidative stress associated with β-blocker exposures in bivalves are not completely understood. Cellular oxidative stress is associated with elevated oxyradical production and damage to proteins, lipids, and DNA (Kelly, Havrilla et al. 1998, Moore, Allen et al. 2006). Lysosomal membrane integrity, also vulnerable to oxidative stress, is a robust marker of contaminant exposures in bivalves and represents overall cellular health status (Ringwood, Conners et al. 1998, Moore, Allen et al. 2006, Terman, Kurz et al. 2006). Lysosomal membrane destabilization is considered an early-warning sign and a tier 1 screening biomarker (Viarengo, Lowe et al. 2007), and is useful in initial damage assessments for emerging contaminants such as β-blockers. Lysosomes can sequester xenobiotics, especially in bivalve digestive gland cells, that can damage lysosomal membrane structure, and cause lysosomal swelling, autophagy, and eventually cell death (Moore, Lowe et al. 2004, Moore, Viarengo et al. 2012). Cellular responses to prooxidant contaminants often include induction of antioxidants and detoxification enzymes (Viarengo, Lowe et al. 2007). One such group of enzymes belong to the glutathione-s-transferase (GST) superfamily which plays a vital role in detoxification as well as in antioxidant defense and has been shown to participate in bivalve cellular responses to β-blocker exposures (Contardo-Jara, Pflugmacher et al. 2010, Franzellitti, Buratti et al. 2011). In addition to their detoxification properties, members of GST superfamily are also involved in signal transduction associated with cellular stress pathways (Tew and Townsend 2012). Understanding the cross-talk between signaling pathways that shape the overall organismal response to chemical exposures is a valuable step towards investigation of subsequent physiological consequences. Additionally, tissue-specific biomarker information can advance our existing knowledge of stress signaling responses. This interconnectivity of molecular and cellular endpoints provides guidance for the development of AOPs (Ankley, Bennett et al. 2010). The goal of our study was to evaluate tissue-specific responses of two filter-feeding bivalve species to β-blocker exposures. Our approach was to conduct short in vitro exposures with two β-blocking agents to identify suitable biomarkers for further testing.
2. Methods and materials
2.1. Experimental design
Mercenaria mercenaria (hard clams) and Crassostrea virginica (Eastern oysters) were obtained from Narragansett Bay, RI, USA, and Matunuck Oyster Farm, RI, respectively, and were acclimated in the laboratory. They were maintained at 21 °C and fed a mixed algal diet consisting of Dunaliella, Tetraselmis and Skeletonema. Clams were acclimated with sediment (> 60% was coarse sand ranging 500-1000 μm in grain size) which had been washed, muffled, press sieved to remove macrobiota, and stored at 4 °C in the dark before use. Pharmaceutical secondary standards (certified reference material grade) for metoprolol tartrate, (±)1-(Isopropylamino)-3-[p-β-methoxyethyl) phenoxy]-2-propanol (+)-tartrate, (CAS Number 56392-17-7), and propranolol hydrochloride, (±)-1-Isopropylamino-3-(1-naphthyloxy)-2-propanol hydrochloride, (CAS Number 318-98-9), were purchased from Sigma-Aldrich. Oysters (30) and clams (30) were sacrificed in two experiments each (as described below) to harvest digestive gland (DG) and gills tissues. For each experiment, tissues from 15 bivalves for each species were separately exposed to metoprolol in test concentrations ranging from 2 to 2000 ng/L and propranolol ranging from 1 to 1000 ng/L. Three replicates of gill and digestive gland (DG) tissues were exposed to each test concentration. The experiments were repeated once to obtain sufficient tissue for multiple biomarker analyses. Exposures were conducted in six-well plates and each test tissue was bathed in 5 ml of exposure media per well. The exposure media was prepared by mixing 30 %0 natural seawater with an antibiotic mix of streptomycin and penicillin (Sigma-Aldrich), Leibovitz L15 nutrient media, and the test drug. The exposure plates were shielded from light and maintained at 20 ± 2 °C on an orbital shaker for 24 h. At the end of the 24 h exposure, tissues were removed from the plates and a subsample was used for a lysosomal assay as described below in section 3.1. Samples were also frozen at −80 °C for biochemical analyses as described below. All commercial toxicity testing kits were purchased from Sigma-Aldrich and optimized per manufacturer’s instructions. Control charts were maintained documenting blank absorbances and standard curves for each assay run. Spectrophotometric assessments were performed using the Biotek Synergy HTX multimode microplate reader.
2.2. Water chemistry
Primary solutions (1 mg/L propranolol and 2 mg/L metoprolol) were prepared by dissolving propranolol hydrochloride and metoprolol tartrate salts in 30 % 0.22μm filtered seawater. Salt weights were adjusted to obtain desired test concentrations of the active test drugs, propranolol and metoprolol. Primary solutions were serially diluted to prepare working stock solutions which were further used to prepare the exposure media. Working stock solution samples were used for LC-MS/MS measurements to confirm β-blocker concentrations, as described below, before they were added to the tissue exposure wells.
2.2.1. Sample collection and extraction
The sample extraction methods were adapted from EPA Method 1694 (Cantwell, Katz et al. 2016) and used Oasis HLB solid phase extraction (SPE) cartridges (6 cc, 200 mg, Waters Corp., Milford, MA). Briefly, 9 mL of the working stock solutions were collected and stored in test tubes prior to extraction. Samples were adjusted to pH 2 using hydrochloric acid (6N) and spiked with 100 ng of isotopically labeled metoprolol and propranolol (Table S1). Samples were loaded on SPE cartridges (after conditioning with 6 ml each of methanol, Milli-Q water and pH 2 Milli-Q water sequentially) using vacuum suction to maintain a loading rate of 5-10 mL/min. Subsequently, SPEs were washed with 6 ml pH 2 Milli-Q water, dried and eluted into test tubes with methanol. Extracts were evaporated to dryness by a gentle stream of nitrogen, reconstituted using a mixture of Milli-Q water (80%) and methanol (20%), vortexed, transferred to LC vials and stored at 4 °C until analysis.
2.2.2. LC-MS/MS Analysis
Analytical separation of the compounds was performed on a Waters Acquity ultraperformance liquid chromatograph, using a Waters Xevo TQD MS/MS operated in electrospray ionization (ESI) mode for compound identification. For each injection, 10 μL of extract was loaded onto the analytical column (Acquity BEH C18, 2.1 mm × 50 mm, 1.7 μm pore size, Waters Corp). Chromatographic separation of compounds occurred using a ternary gradient of acidic mobile phase mixture consisting of Milli-Q water, acetonitrile and methanol, all of which contained 0.1 % formic acid (Table S2). Following elution, compounds were detected by MS/MS with ionization conditions of the source set to 0.5 kV in ESI+, source temperature 150 °C, desolvation temperature 500 °C, desolvation flow 900 L/h, and cone flow 30 L/h. Compounds were calibrated using a 12-point curve ranging from 0.25 ng/mL to 500 ng/mL and each standard level contained 100 ng/mL of labeled analogs. Frequent checks were conducted using standards to verify instrumental performance over the course of the analytical run (average recoveries between 100.7-107.4 %).
2.3. Cellular damage assays
Cellular damage was measured in DG tissues of oysters and clams using two biomarkers of stress. Membrane permeabilization in lysosomes and lipid peroxidation (i.e., reactive oxygen species (ROS) induced oxidative damage to unsaturated fatty acids) were evaluated as described below. The DG tissue was chosen for these damage assessments because of its role in detoxification, presence of high number of lysosomes, and high lipid content.
2.3.1. Lysosomal destabilization assay
Lysosomal destabilization was measured using a neutral red retention microscopic assay (Ringwood, Hoguet et al. 2003). Digestive gland cells were isolated using Ca2+/Mg2+ free saline and 1 mg/ml trypsin. Primary cell preparations were incubated with neutral red dye (0.04 mg/ml) for an hour, protected from light at room temperature. At the end of the incubation period, cells were scored as stabilized or destabilized. Cells with intact stained lysosomes were categorized as stabilized and those with neutral red dye spilled into the cytosol were categorized as destabilized. Sample scoring was performed blindly to reduce bias. A percent lysosomal destabilization rate was calculated by dividing the number of destabilized cells by total number of cells scored and multiplying by 100.
2.3.2. Lipid peroxidation assay
Lipid peroxidation is a marker of oxidative damage and the assay we used quantifies malondialdehyde, a secondary oxidation product of polyunsaturated fatty acids (Kelly, Havrilla et al. 1998). A 25-45 mg sample of tissue was homogenized on ice in a lysis buffer. Homogenates were centrifuged at 13,000 g for 10 min at 4 °C, followed by addition of 600 μL thiobarbituric acid to 100 μL of supernatant. Samples were incubated in a water bath at 95 °C for an hour, allowed to cool, and centrifuged again for 5 min at room temperature. The lipid peroxidation (MDA) assay kit (Sigma-Aldrich ® MAK085) uses the reaction of malondialdehyde in the sample with thiobarbituric acid to form a colored adduct which is measured colorimetrically at 532 nm. The amount of malondialdehyde detected represents relative oxidative damage levels in tissue samples.
2.4. Total antioxidant capacity assay
The total antioxidant capacity assay provides on overall scan of cellular antioxidant status and is a measure of the amount of reactive oxygen species (ROS) scavenged by non-enzyme antioxidants such as thiols, vitamins, ascorbate, bilirubin, and albumin. A total antioxidant capacity assay kit (Sigma-Aldrich ® MAK187) was used to measure reduction of Cu2+ to Cu+ to assess total antioxidant capacity of gill and DG tissues using trolox as an antioxidant standard. Tissue samples (about 25-45 mg) were homogenized on ice in a lysis buffer. Following centrifugation at 13,000 g for 10 min at 4 °C, samples were diluted 1:100 and mixed with the Cu2+ solution in a 1:1 ratio in a microplate. The plate was incubated for 90 min away from light. Antioxidant capacity was measured based on absorbance readings at 570 nm.
2.5. Glutathione-s-transferase (GST) activity assay
The activity of phase II detoxification enzyme GST was measured using a glutathione-s-transferase (GST) assay kit (Sigma-Aldrich® CS0410). The spectrophotometric kinetic assay utilizes 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate for GST in the presence of reduced glutathione. Tissue samples (about 25-45 mg) were homogenized on ice in a lysis buffer, centrifuged at 10,000 g for 15 min at 4 °C, and diluted 1:5 before the addition of the reaction mix containing phosphate buffered saline, 200 mM reduced L-glutathione, and 100 mM CDNB. The thiol group of glutathione is conjugated to CDNB by GST and the conjugation leads to a measurable change in absorbance at 340 nm. Six kinetic readings, each separated by 1 min, were measured and the rate of change in absorbance was measured. Enzyme activity was measured using the following equation,
where, Ar = Rate of Change in Absorbance VR = Reaction Volume in ml DF = Dilution Factor Ke = Extinction coefficient for CDNB conjugate mM−1cm−1 VE = Enzyme Test Volume in ml
2.6. Statistical analyses
All statistical analyses were performed using SigmaPlot 13.0 statistical software. Analysis of variance (ANOVA) tests were used to evaluate differences between means. Normality and equal variance assumptions were confirmed for all analyses and p values ≤ 0.05 were used to determine significance for ANOVA and post-hoc tests. Analyses with significant ANOVA p-values were followed by Student-Newman-Keuls (SNK) pairwise multiple comparison method to determine differences between test groups. All significant differences resulting from the SNK method are denoted by different letters in the figures. Unless otherwise noted, values are reported as the mean ± standard deviation.
3. Results
3.1. Water chemistry
Working stock solutions of propranolol and metoprolol were quantified for active drug concentrations as listed in Table 1. All working solution recoveries were above 80%.
Table 1.
Nominal and measured concentrations of working stock solutions used for preparing β-blocker exposure mixtures.
| Test Drug | Nominal Conc. | Measured Conc. | Recoveries |
|---|---|---|---|
| ng/L | ng/L | % | |
| Propranolol | 1000 | 844 ± 157 | 84 |
| Propranolol | 10000 | 11078 ± 204 | 111 |
| Metoprolol | 2000 | 2056 | 103 |
| Metoprolol | 20000 | 17294 ± 181 | 87 |
3.2. Cellular damage markers
3.2.1. Lysosomal destabilization
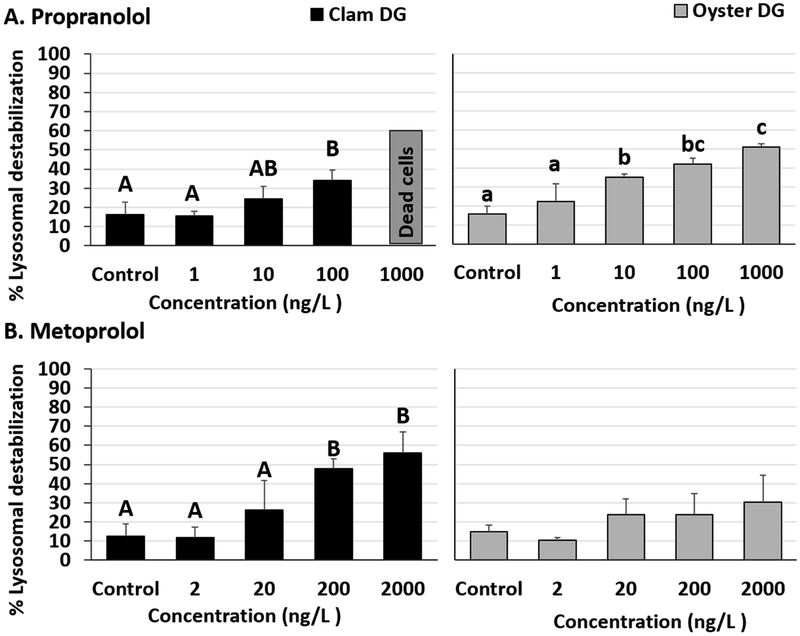
Lysosomal membrane destabilization was observed in oyster and clam DG tissues exposed to β-blockers. The lowest observed effect concentration (LOEC), which represents the lowest exposure concentration at which significantly elevated lysosomal damage was noted in comparison to controls, for this study was 10 ng/L for propranolol in oyster tissues (Fig 1A, Tables S3 and S4). DG tissues from clams and oysters exposed to 100 ng/L propranolol were found to have significantly higher lysosomal damage than controls and 1 ng/L propranolol exposure groups. (Fig 1A, Tables S3 and S4). Additionally, oyster and clam DG tissue damage levels were found to be significantly different from each other for 10 ng/L propranolol exposures (two-tailed p value= 0.047). Clam DG exposed to the highest test propranolol concentration (1000 ng/L) did not have enough viable cells in our preparations to provide reliable values. At the same propranolol concentration, oysters were found to have significant differences compared to the control, 1 and 10 ng/L test concentrations with destabilization rates reaching 50.7% (Fig 1A, Table S4). Although oyster DG tissues exposed to 1000 ng/L propranolol were not significantly different from 100 ng/L (Post-hoc pairwise comparison, Student-Newman-Keuls method, P = 0.053, Table S4), the high destabilization rate is biologically relevant and represents extensive damage to the lysosomes. Significantly elevated lysosomal destabilization rates were also noted in metoprolol exposed clam DG tissues at exposure concentrations ≥ 200 ng/L (Fig 1B, Tables S3 and S4). Metoprolol exposed oyster DG tissues did not have significant increases in lysosomal damage in our 24-hour in vitro study (Fig 1B, Table S3). The highest percent destabilization, 56%, was measured in clam tissues exposed to 2000 ng/L metoprolol (Fig 1B).
Fig 1.
Percentage lysosomal destabilization in clam and oyster digestive gland (DG) tissues exposed to (A) Propranolol and (B) Metoprolol for 24 hours. Different letters indicate significant differences obtained from Student-Newman-Keuls post hoc pairwise multiple comparison tests (p ≤ 0.05). No letters indicate no significant differences between treatments.
3.2.2. Lipid peroxidation
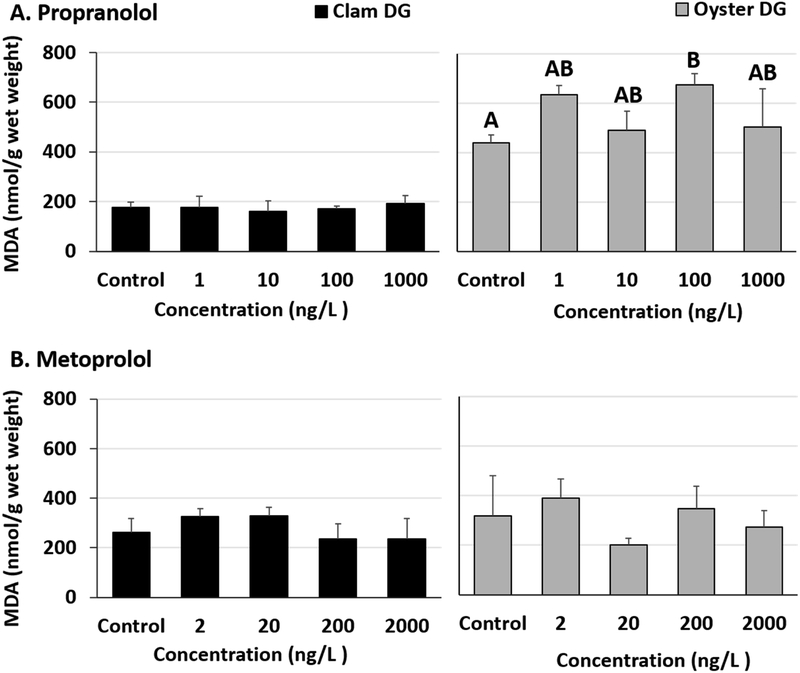
Malondialdehyde levels were elevated in oyster DG tissues exposed to propranolol. Significant increases compared to controls were observed in oysters exposed to 100 ng/L propranolol (Fig 2A, Tables S5 and S6). Although not significant, oyster DG tissues exposed to 1 ng/L propranolol also showed elevated damage levels (p = 0.069, Table S6). No increases were observed in propranolol-exposed clam malondialdehyde levels in DG tissues (Fig 2A, Table S5). Malondialdehyde levels were not affected in metoprolol exposed oyster (Fig 2B) or clam tissues (Fig 2B) (Table S5).
Fig 2.
Malondialdehyde (MDA) levels in clam and oyster digestive gland (DG) tissues exposed to (A) Propranolol and (B) Metoprolol for 24 hours. Different letters indicate significant differences obtained from Student-Newman-Keuls post hoc pairwise multiple comparison tests (p ≤ 0.05). No letters indicate no significant differences between treatments.
3.3. Total antioxidant capacity
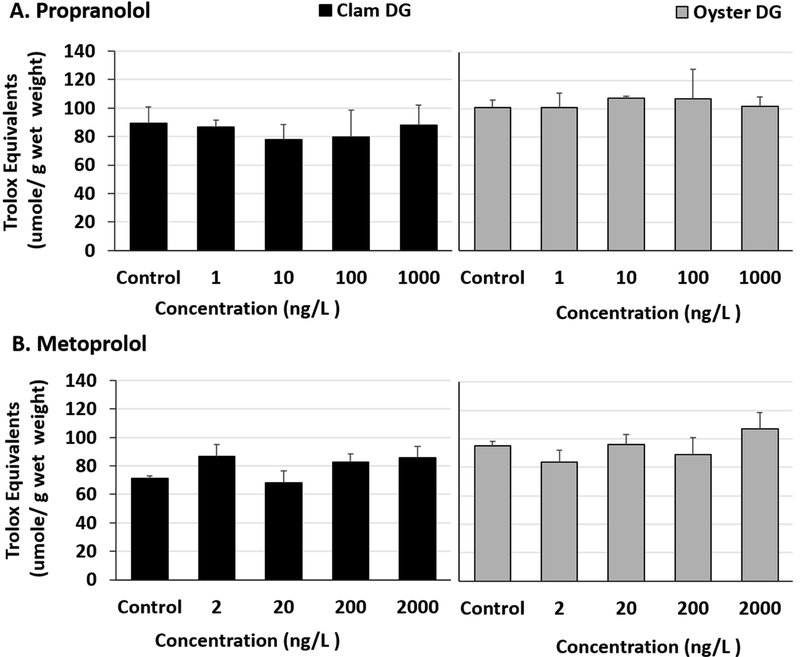
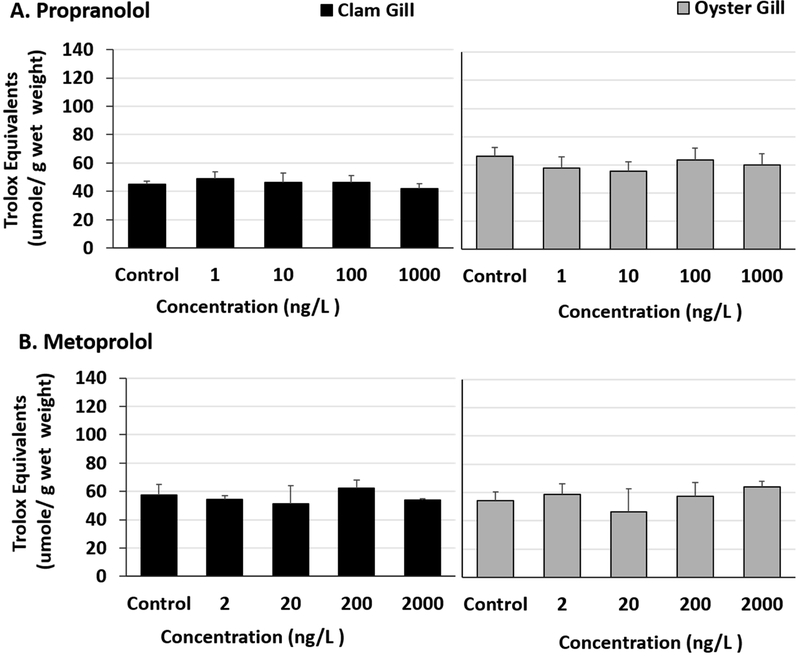
Total antioxidant capacity was unaffected in the DG tissues of propranolol exposed bivalves (Fig 3A, Table S7) Although pairwise multiple comparisons following ANOVA were not significant in metoprolol exposed clam DG tissues (Fig 3B, Table S7 and S8), trolox equivalents were found to be slightly elevated in 2 ng/L, 200 ng/L and 2000 ng/L exposed clam DG tissues than controls (two-tailed t-test p values 0.038, 0.027, and 0.04 respectively). No differences in total antioxidant capacities of the DGs of metoprolol exposed oysters were observed (Fig 3B, Table S7). Overall, the antioxidant capacities of gill tissues were lower than that of DGs. No significant changes were noted in exposed bivalve gill tissues for either β-blocker (Fig 4A and B, Table S9).
Fig 3.
Total antioxidant capacity represented as trolox equivalents in clam and oyster digestive gland (DG) tissues exposed to (A) Propranolol and (B) Metoprolol for 24 hours.
Fig 4.
Total antioxidant capacity represented as trolox equivalents in clam and oyster gill tissues exposed to (A) Propranolol and (B) Metoprolol for 24 hours.
3.4. Glutathione-s-transferase (GST) activity
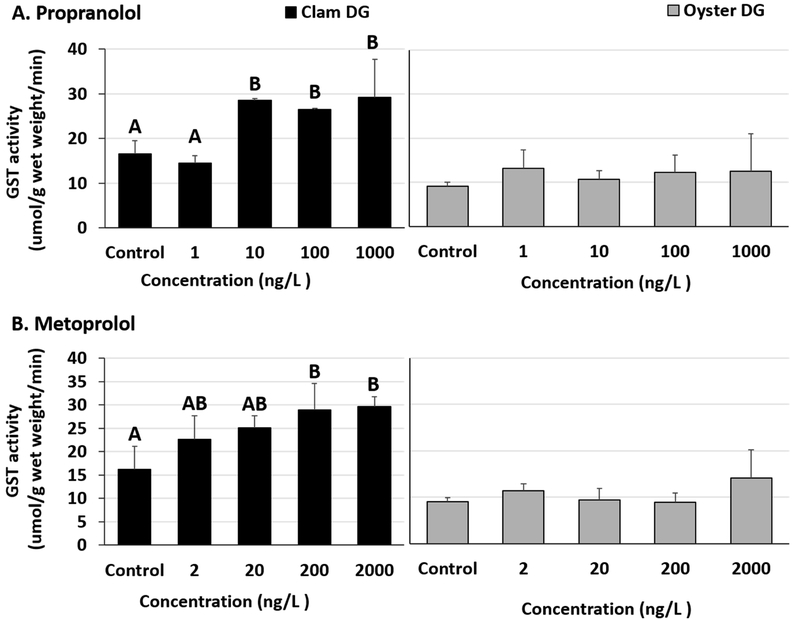
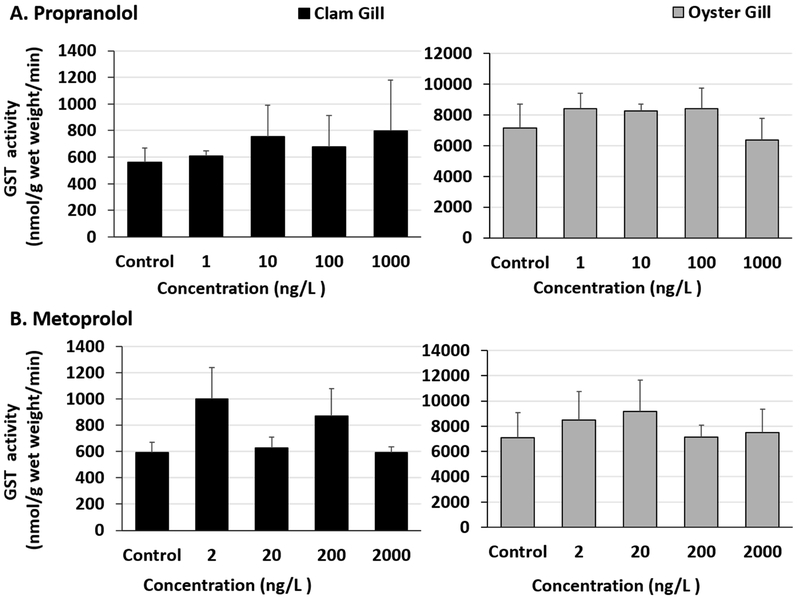
Significant increases in GST activities were observed in clam DG tissues exposed to propranolol and metoprolol (Fig 5A and B, Table S10). The LOEC for propranolol and metoprolol in our study was 10 ng/L and 200 ng/L, respectively, in clam DG tissues (Fig 5A and B, Table S11). The enzyme activities stayed elevated at the test concentrations above LOEC. Increases in clam DG enzyme activities at 10, 100 and 1000 ng/L propranolol were also significantly higher than in clams exposed to the lowest (1 ng/L) propranolol test concentration (Table S11). Digestive gland tissues of oysters exposed to β-blockers had no changes in GST activities (Fig 5A and B, Table S10). No differences were observed in gill tissues of oysters exposed to either propranolol or metoprolol (Fig 6A and B, Table S12). Additionally, clam gills exposed to propranolol or metoprolol didn’t show any significant GST activity changes (Fig 6A and B, Tables S12 and 13).
Fig 5.
Glutathione-s-transferase activity in clam and oyster digestive gland (DG) tissues exposed to (A) Propranolol and (B) Metoprolol for 24 hours. Different letters indicate significant differences obtained from Student-Newman-Keuls post hoc pairwise multiple comparison tests (p ≤ 0.05). No letters indicate no significant differences between treatments.
Fig 6.
Glutathione-s-transferase activity in clam and oyster gills tissues exposed to (A) Propranolol and (B) Metoprolol for 24 hours.
4. Discussion
Elevated consumption of prescription antihypertensive β-blocking agents, such as metoprolol and propranolol, by humans and their incomplete removal from WWTPs have raised concerns about pharmaceutical burdens in aquatic ecosystems and their environmental effects. In the Narragansett Bay area (RI, USA), current reported concentrations of metoprolol and propranolol are in the range 1-313 ng/L and below 15 ng/L, respectively (Cantwell, Katz et al. 2016). Elevated concentrations have been found around the world in freshwater systems and in coastal areas receiving higher riverine inputs with measured concentrations reaching up to 8 - 10 μg/L for metoprolol and 0.59 μg/L for propranolol (Hughes, Kay et al. 2012, Gaw, Thomas et al. 2014, Godoy, Kummrow et al. 2015, Cantwell, Katz et al. 2017, Varano, Fabbri et al. 2017). Coastal organisms, such as filter-feeding bivalve mollusks, can bioconcentrate pharmaceuticals (Ericson, Thorsén et al. 2010, Franzellitti, Buratti et al. 2014, Gaw, Thomas et al. 2014) and hence may serve as good model organisms for β-blocker toxicity studies.
Beta-receptor binding signaling molecules, such as NE, contribute to a range of stress related responses in vertebrates. Octopamine (OA), which is structurally similar to NE and is also a tyrosine derivative, plays a vital role in immune function as well as stress responses in invertebrates (Gloriam, K Bjarnadóttir et al. 2005, Adamo 2008). Vertebrates predominantly use NE, while many invertebrates, primarily insects, use OA in stress signaling pathways. Mollusks are unique in using NE as well as OA (Adamo 2008, Massarsky, Trudeau et al. 2011). Mollusks have been proposed to possess adrenergic receptors of the α- and β- type (Lacoste, Malham et al. 2001, Fabbri and Capuzzo 2010) as well as octopaminergic receptors (C Gerhardt, A Bakker et al. 1997, Blais, Bounif et al. 2010, Pryce, Samuel et al. 2015). Sequence homology between molluscan octopaminergic receptors and mammalian adrenergic receptors (C Gerhardt, A Bakker et al. 1997) may make bivalves further vulnerable to β-blocker exposures.
Bivalve β-blocker exposure studies are limited in number, but both freshwater (Contardo-Jara, Pflugmacher et al. 2010) and marine mussels (Fabbri, Franzellitti et al. 2009, Franzellitti, Buratti et al. 2011, Franzellitti, Buratti et al. 2015) have been shown to mount cellular responses to such exposures. Our short-term in vitro study found extensive lysosomal damage in bivalve digestive gland tissues exposed to β-blockers (Fig. 1). Lysosomal damage is a sensitive marker of toxicity and destabilization rates above 30% are biologically relevant (Ringwood, Conners et al. 1998, Ringwood, Conners et al. 1999, ViarengoLowe et al. 2007). Lysosomal destabilization is accompanied by the release of lysosomal contents (degradation enzymes and very low pH conditions) into the cytoplasm, and leads to cell death following hydrolase and protease induced degradation of cellular macromolecules (Aits and Jӓӓttelӓ 2013). Release of lysosomal contents could be due to dysfunction of the membrane Mg2+ATPase-dependent proton pump which maintains the internal acidic environment in lysosomes. Direct interaction of contaminants with the proton pump could lead to dysfunction and membrane damage. Additionally, numerous stimuli, including oxidative stress, lysosomotropic compounds, and stress protein p53 can induce lysosomal membrane permeabilization (Terman, Kurz et al. 2006, Aits and Jӓӓttelӓ 2013). Lysosomal rupture has also been suggested as an upstream event in many forms of apoptosis (Terman, Kurz et al. 2006). Overall, lysosomal membrane stability is reflective of overall organismal health, and elevated damage to lysosomal membranes represents cell injury and tissue dysfunction in bivalves (Allen and Moore 2004, Moore, Allen et al. 2006). Bivalve DG plays a vital role in nutritional and assimilatory processes, hence elevated damage and dysfunction in these tissues could result in physiological adverse effects. In our study, damage in oysters was observed at an environmentally relevant propranolol exposure concentration of 10 ng/L. Lysosomal labilization effects of propranolol have been reported in Mytilus at concentrations as low as 0.3 ng/L (Franzellitti, Buratti et al. 2011). Cell preparations from clams exposed to the highest test propranolol concentration (1000 ng/L) in our study did not yield enough readable viable cells to detect intact lysosomes, suggesting protein damage and cell death could be an outcome of pharmaceutical exposures in aquatic invertebrates. A metoprolol exposure study with zebra mussels, Dreissena polymorpha, found elevated expression of heat shock protein (hsp)70 (Contardo-Jara, Pflugmacher et al. 2010) which is a marker of protein damage and altered homeostasis. The study reports elevated hsp70 expression in gill and DG tissues at metoprolol concentrations as low as 0.534 μg/L after a day of exposure and 5.34 μg/L after four days of exposure, respectively. Upregulation of metallothioneins and antioxidant enzymes in β-blocker exposures has also been reported (Contardo-Jara, Pflugmacher et al. 2010, Franzellitti, Buratti et al. 2011) which suggests involvement of cellular oxidative stress pathways (Ringwood, Conners et al. 1998, Viarengo, Burlando et al. 2000, Livingstone 2001). For our study, lipid peroxidation, an oxidative damage marker, was only noted to increase in 100 ng/L propranolol exposed oysters. Furthermore, as noted in Figs 3 and 4, no significant changes were found in the total antioxidant capacity of the exposed bivalve digestive gland or gill tissues. Future investigations are required to evaluate interactions between oxidative stress signaling and β-adrenergic signaling pathways in marine oysters and clams. Lack of changes in antioxidant capacities, along with limited changes in lipid peroxidation levels in the exposed bivalves as observed in our study, represent short term in vitro responses. It must be noted that changes in the expression of antioxidant enzymes such as superoxide dismutase and catalase in bivalves exposed to β-blockers have not been reported until four or more days of exposure (Contardo-Jara, Pflugmacher et al. 2010, Franzellitti, Buratti et al. 2011). This may explain some of the non-dose response patterns and lack of effects observed. Chronic β-blocker exposures and assessments of oxidative lipid damage are currently underway in our laboratory.
For our short-term studies presented here, clams were found to be more responsive than oysters to test biomarkers. Along with elevated lysosomal damage, exposed clams also showed increases in GST activities. Glutathione-s-transferases are a superfamily of enzymes which catalyze the conjugation of reduced glutathione to a variety of electrophiles and contribute to cell survival by detoxification of xenobiotics. In its catalytic role, GST participates in biotransformation phase II of drug metabolism which follows cytochrome P450 mediated phase I of the detoxification process. Cytochrome P450 enzyme system, particularly CYP2D6, is involved in β-blocker metabolism in humans (Anzenbacher and Anzenbacherova 2001, Mehvar and Brocks 2001). In mollusks, GSTs have a tissue-specific distribution (Balabaskaran, Chew et al. 1986) and its high activity in digestive gland contributes to increased detoxification capacity. Increases in GST activity in clams may represent a detoxification response following β-blocker uptake. While we did not measure tissue drug concentrations in these studies, bioaccumulation of β-blockers has been reported in mussels (Contardo-Jara, Pflugmacher et al. 2010, Ericson, Thorsén et al. 2010, Gaw, Thomas et al. 2014). Similar to lysosomal damage results in our study, the effects of propranolol on GST activity were observed at lower exposure concentrations than metoprolol. Such differences in toxicities could be reflective of the non-selective binding properties of propranolol and its high accumulation rates in bivalves (Contardo-Jara, Pflugmacher et al. 2010, Ericson, Thorsén et al. 2010). It has also been suggested that the effects of propranolol exposures could be associated with multiple receptor classes as it can block β-adrenergic as well as 5-HT serotonin receptors (Huggett, Brooks et al. 2002). In agreement with our findings, other researchers have found green algae Desmodesmus and planktonic crustacean Daphnia to be more sensitive to propranolol compared to metoprolol (Cleuvers 2005). The molecular basis of differences in toxicities of β-blockers to bivalves remains to be explored and is a vital step towards assessing environmental risks of pharmaceutical exposures and the development of AOPs.
In addition to their catalytic role in detoxification, GSTs are also involved in non-enzymatic processes including ligand binding, signal transduction and protein-protein interactions. An example of GST controlled modulation of protein function is c-Jun-N-terminal kinase (JNK) pathway. JNKs are stress kinases, regulated by GST, and their role in cellular responses to oxidative, heat, and osmotic stress has been documented (Davis 2000, Tew and Townsend 2012). Xenobiotic or ROS mediated stress is believed to assist in activation of JNK signaling, leading to cellular stress response events such as apoptosis or proliferation. The cyclic adenosine monophosphate (cAMP) pathway, reported to be affected in propranolol exposed mussels (Franzellitti, Buratti et al. 2011), also suppresses apoptosis via inhibition of JNK activation (Zhang, Wang et al. 2008). Beta-blocker induced changes in cAMP levels could affect JNK signaling as well as apoptotic pathways, and eventually lead to cell damage or death. Changes in GST activity has been established as a xenobiotic stress biomarker (Contardo-Jara, Pflugmacher et al. 2010, Franzellitti, Buratti et al. 2011), which our findings support, and may serve as a key event in AOP development for β-blocking agents. Due to its antioxidant role, induction of GST could have limited oxidative stress and malondialdehyde levels in our short-term in vitro exposures. Antiperoxidative properties of GSTs and its participation in the metabolism of 4-hydroxynonenal (a product of lipid peroxidation) as well as the reduction of DNA hydroperoxides have also been documented (Bao, Jemth et al. 1997). Non-catalytic effects of GST upregulation in bivalves during chemical exposures is a promising area of biomarker research that could clarify associated key events and their relationships with signaling pathways. As more chronic toxicity studies emerge on the involvement of oxidative stress pathways in β-blocker effects, the relationship between lipid peroxides and GSTs and their potential as biomarkers of exposure should become clearer.
Short-term in vitro studies serve as rapid screening tools to evaluate sensitive biomarkers and are logistically simple to conduct. They offer new information on a suite of toxicity endpoints in a relatively short exposure time and play an effective role in informing further investigations (Parolini, Binelli et al. 2009) and more expensive and time-consuming in vivo studies. Isolated tissue studies evaluate sensitivity of different tissues which lays groundwork for physiological implications and susceptibility to adverse outcomes. Emerging contaminants, such as β-blockers, especially benefit from such initial in vitro assessments because experimental information on their effects is often scarce. In vitro assessments, as presented in the current study, allow us to focus our toxicological approach and evaluate the sensitivity of species, tissue types, biomarkers and signaling pathways.
We report bivalve DG tissues to be more responsive to β-blocker exposures than gills and hence a better target tissue for future studies aimed at the identification of key events that can contribute to AOP development. In agreement with our tissue-specific results, the DG tissues of Mytilus were found to be more sensitive to propranolol than gills (Franzellitti, Buratti et al. 2011). Freshwater mussel studies with metoprolol have reported effects in gills and DG, but oxidative stress was only reported in the DG tissues (Contardo-Jara, Pflugmacher et al. 2010). The differences in tissue specificity could be due to differences in detoxification and antioxidant capacities. Additionally, differences in the distribution of uptake carriers and receptors across species and tissue types could contribute to vulnerabilities to pharmaceutical exposures.
5. Conclusions
Our short term in vitro β-blocker study was designed to evaluate tissue-specific stress responses and detoxification potential of two commercially and ecologically valuable species of bivalves. Based on our results, digestive gland tissues are more responsive than gill tissues to low-concentration β-blocker exposures in bivalves. Furthermore, lysosomal destabilization and changes in GST activities are sensitive biomarkers of such exposures. We did not observe oxidative stress in our short-term studies but our results suggest potential direct effects of contaminant exposure on lysosomal integrity possibly via proton pump dysfunction, and induction of GST as a detoxification response against β-blockers. This study qualifies as a tier 1 comparative screening approach to test the environmental effects of commonly prescribed adrenergic antagonists for human cardiovascular diseases. Isolated tissue studies help us identify potential areas of investigation for large scale, in vivo exposure studies. Further investigations are required to characterize tissue-specific receptor distribution including the 5-HT serotonin receptor class, and β- blocker effects on cell signaling. Our ongoing work is focused on identification of reliable molecular biomarkers for oysters and clams using long-term investigations which mimic environmental exposures. Together, these studies are aimed at contributing to the development of AOPs for β- blocking agents.
Supplementary Material
Highlights.
β-blocker exposures can induce lysosomal damage in marine bivalves
Changes in activities of glutathione-s-transferase were noted in exposed bivalves
Digestive gland tissues are more sensitive to β-blocker exposures than gills
Species-specific differences were noted in responses to β-blocker exposures
Acknowledgements:
The authors would like to acknowledge Lesley Mills, Bryan Clark and Amy Ringwood for their contributions towards the review of this manuscript. We would also like to thank Perry Raso for the oysters and Karin Tammi for assistance with the algal cultures. This research was performed while B Khan held an NRC Research Associateship award at the U.S. EPA, ORD/NHEERL Atlantic Ecology Division.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: We declare no conflicts of interest.
Disclaimer:
The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency (EPA). Mention of trade names and products does not imply an endorsement or recommendation for use by the U.S. Government or the U.S. EPA. The present study is number ORD-026481of the Atlantic Ecology Division of the U.S. EPA, Office of Research and Development, National Health and Environmental Effects Research Laboratory.
References
- Adamo S (2008). "Norepinephrine and Octopamine: Linking Stress and Immune Function Across Phyla." Invertebrate Survival Journal 5: 12–19. [Google Scholar]
- Aits S and Jӓӓttelӓ M (2013). "Lysosomal Cell Death At A Glance." Journal of Cell Science 126: 1905–1912. [DOI] [PubMed] [Google Scholar]
- Allen JI and Moore MN (2004). "Environmental Prognostics: Is the Current Use of Biomarkers Appropriate for Environmental Risk Evaluation?" Marine Environmental Research 58(2-5): 227–232. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL and Schmieder PK (2010). "Adverse Outcome Pathways: A Conceptual Framework to Support Ecotoxicology Research and Risk Assessment." Environmental Toxicology and Chemistry 29(3): 730–741. [DOI] [PubMed] [Google Scholar]
- Anzenbacher P and Anzenbacherova E (2001). "Cytochromes P450 and Metabolism of Xenobiotics." Cellular and Molecular Life Sciences CMLS 58(5-6): 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, Hill SJ and Summers RJ (2011). "Evolution of β-Blockers: From Anti-Anginal Drugs to Ligand-Directed Signalling." Trends in Pharmacological Sciences 32(4): 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabaskaran S, Chew S and Segaran M (1986). "Studies on Glutathione S-Transferase in Molluscs." Comparative Biochemistry and Physiology 85(1): 183–192.2876815 [Google Scholar]
- Bao Y, Jemth P, Mannervik B and Williamson G (1997). "Reduction of Thymine Hydroperoxide by Phospholipid Hydroperoxide Glutathione Peroxidase and Glutathione Transferases." FEBS Letters 410(2-3): 210–212. [DOI] [PubMed] [Google Scholar]
- Blais V, Bounif N and Dubé F (2010). "Characterization of a Novel Octopamine Receptor Expressed in the Surf Clam Spisula solidissima." General and Comparative Endocrinology 167(2): 215–227. [DOI] [PubMed] [Google Scholar]
- Böhm M and Maack C (2000). "Treatment of Heart Failure with Beta-Blockers. Mechanisms and Results." Basic Research in Cardiology 95(1): I15–I24. [DOI] [PubMed] [Google Scholar]
- Bristow MR (1997). "Mechanism of Action of Beta-Blocking Agents in Heart Failure." American Journal of Cardiology 80(11A): 26L–40L. [DOI] [PubMed] [Google Scholar]
- C Gerhardt C, A Bakker R, J Piek G, J Planta R, Vreugdenhil E, Leysen J and van Heerikhuizen H (1997). Molecular Cloning and Pharmacological Characterization of a Molluscan Octopamine Receptor. 51(2): 293–300. [DOI] [PubMed] [Google Scholar]
- Cantwell MG, Katz DR, Sullivan JC, Ho K and Burgess RM (2017). "Temporal and Spatial Behavior of Pharmaceuticals in Narragansett Bay, Rhode Island, United States." Environmental Toxicology and Chemistry. 36(7): 1846–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell MG, Katz DR, Sullivan JC, Ho K, Burgess RM and Cashman M (2016). "Selected Pharmaceuticals Entering an Estuary: Concentrations, Temporal Trends, Partitioning, and Fluxes." Environmental Toxicology and Chemistry 35(11): 2665–2673. [DOI] [PubMed] [Google Scholar]
- Cantwell MG, Katz DR, Sullivan JC, Shapley D, Lipscomb J, Epstein J, Juhl AR, Knudson C and O'Mullan GD (2017). "Spatial Patterns of Pharmaceuticals and Wastewater Tracers in the Hudson River Estuary." Water Research 137: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleuvers M (2005). "Initial Risk Assessment for Three β-Blockers Found in the Aquatic Environment." Chemosphere 59(2): 199–205. [DOI] [PubMed] [Google Scholar]
- Cole SW and Sood AK (2012). "Molecular Pathways: Beta-Adrenergic Signaling in Cancer." Clinical Cancer Research 18(5): 1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contardo-Jara V, Pflugmacher S, Nützmann G, Kloas W and Wiegand C (2010). "The β-Receptor Blocker Metoprolol Alters Detoxification Processes in the Non-Target Organism Dreissena polymorpha." Environmental pollution 158(6): 2059–2066. [DOI] [PubMed] [Google Scholar]
- Coon S and Bonar D (1987). "Pharmacological Evidence That Alpha1.-Adrenoceptors Mediate Metamorphosis of the Pacific Oyster, Crassostrea gigas." Neuroscience 23(3): 1169–1174. [DOI] [PubMed] [Google Scholar]
- Davis RJ (2000). Signal Transduction by the JNK Group of MAP Kinases. Cell 103(2): 239–252. [DOI] [PubMed] [Google Scholar]
- Ericson H, Thorsén G and Kumblad L (2010). "Physiological Effects of Diclofenac, Ibuprofen, and Propranolol on Baltic Sea Blue Mussels." Aquatic Toxicology 99(2): 223–231. [DOI] [PubMed] [Google Scholar]
- Fabbri E (2015). "Pharmaceuticals in the Environment: Expected and Unexpected Effects on Aquatic Fauna." Annals of the New York Academy of Sciences 1340(1): 20–28. [DOI] [PubMed] [Google Scholar]
- Fabbri E and Capuzzo A (2010). "Cyclic AMP Signaling in Bivalve Molluscs: an Overview." Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 313A(4): 179–200. [DOI] [PubMed] [Google Scholar]
- Fabbri E and Franzellitti S (2016). "Human Pharmaceuticals in the Marine Environment: Focus on Exposure and Biological Effects in Animal Species." Environmental Toxicology and Chemistry 35(4): 799–812. [DOI] [PubMed] [Google Scholar]
- Fabbri E, Franzellitti S and Capuzzo A (2009). "Effects of the Beta-Blocker Propranolol on Health Status and Gene Regulation in the Mussel, Mytilus galloprovincialis." Comparative Biochemistry and Physiology 154(1): S13–S22. [Google Scholar]
- Fent K, Weston AA and Caminada D (2006). "Ecotoxicology of Human Pharmaceuticals." Aquatic Toxicology 76(2): 122–159. [DOI] [PubMed] [Google Scholar]
- Franzellitti S, Buratti S, Capolupo M, Du B, Haddad SP, Chambliss CK, Brooks BW and Fabbri E (2014). "An Exploratory Investigation of Various Modes of Action and Potential Adverse Outcomes of Fluoxetine in Marine Mussels." Aquatic Toxicology 151: 14–26. [DOI] [PubMed] [Google Scholar]
- Franzellitti S, Buratti S, Du B, Haddad SP, Chambliss CK, Brooks BW and Fabbri E (2015). "A Multibiomarker Approach to Explore Interactive Effects of Propranolol and Fluoxetine in Marine Mussels." Environmental Pollution 205: 60–69. [DOI] [PubMed] [Google Scholar]
- Franzellitti S, Buratti S, Valbonesi P, Capuzzo A and Fabbri E (2011). "The β-Blocker Propranolol Affects cAMP-Dependent Signaling and Induces the Stress Response in Mediterranean Mussels, Mytilus galloprovincialis." Aquatic Toxicology 101(2): 299–308. [DOI] [PubMed] [Google Scholar]
- Gaw S, Thomas KV and Hutchinson TH (2014). "Sources, Impacts and Trends of Pharmaceuticals in the Marine and Coastal Environment." Phil. Trans. R. Soc B 369(1656): 20130572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloriam D, K Bjarnadóttir T, B Schiöth H and Fredriksson R (2005). "High Species Variation Within the Repertoire of Trace Amine Receptors." 1040(1): 323–327. [DOI] [PubMed] [Google Scholar]
- Godoy AA, Kummrow F and Pamplin PAZ (2015). "Occurrence, Ecotoxicological Effects and Risk Assessment of Antihypertensive Pharmaceutical Residues in the Aquatic Environment-A Review." Chemosphere 138: 281–291. [DOI] [PubMed] [Google Scholar]
- Gómez MJ, Petrović M, Fernández-Alba AR and Barceló D (2006). "Determination of Pharmaceuticals of Various Therapeutic Classes by Solid-Phase Extraction and Liquid Chromatography–Tandem Mass Spectrometry Analysis in Hospital Effluent Wastewaters." Journal of Chromatography A 1114(2): 224–233. [DOI] [PubMed] [Google Scholar]
- Huggett D, Brooks B, Peterson B, Foran C and Schlenk D (2002). "Toxicity of Select Beta Adrenergic Receptor-Blocking Pharmaceuticals (B-Blockers) on Aquatic Organisms." Archives of Environmental Contamination and Toxicology 43(2): 229–235. [DOI] [PubMed] [Google Scholar]
- Huggett D, Khan I, Foran C and Schlenk D (2003). "Determination of Beta-Adrenergic Receptor Blocking Pharmaceuticals in United States Wastewater Effluent." Environmental Pollution 121(2): 199–205. [DOI] [PubMed] [Google Scholar]
- Hughes SR, Kay P and Brown LE (2012). "Global Synthesis and Critical Evaluation of Pharmaceutical Data Sets Collected From River Systems." Environmental Science & Technology 47(2): 661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K, Havrilla CM, Brady TC, Abramo KH and Levin ED (1998). "Oxidative Stress in Toxicology: Established Mammalian and Emerging Piscine Model Systems." Environmental Health Perspectives 106(7): 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste A, Malham S, Cueff A and A Poulet S (2001). Noradrenaline Modulates Oyster Hemocyte Phagocytosis Via a β-Adrenergic Receptor–cAMP Signaling Pathway 122: 252–259. [DOI] [PubMed] [Google Scholar]
- Livingstone D (2001). "Contaminant-Stimulated Reactive Oxygen Species Production and Oxidative Damage in Aquatic Organisms." Marine Pollution Bulletin 42(8): 656–666. [DOI] [PubMed] [Google Scholar]
- Mann DE and Gautieri RF (1984). "Influence of α- and β-Adrenergic Antagonists on Dopamine-Induced Responses in the Isolated Heart of Mercenaria mercenaria." Journal of Pharmaceutical Sciences 73(10): 1491–1493. [DOI] [PubMed] [Google Scholar]
- Massarsky A, Trudeau VL and Moon TW (2011). "β-Blockers as Endocrine Disruptors: the Potential Effects of Human β-Blockers on Aquatic Organisms." Journal of Experimental Zoology 315(5): 251–265. [DOI] [PubMed] [Google Scholar]
- Mehvar R and Brocks DR (2001). "Stereospecific Pharmacokinetics and Pharmacodynamics of Beta-Adrenergic Blockers in Humans." Journal of Pharmacy & Pharmaceutical Science 4(2): 185–200. [PubMed] [Google Scholar]
- Moore MN, Allen JI and McVeigh A (2006). "Environmental Prognostics: an Integrated Model Supporting Lysosomal Stress Responses as Predictive Biomarkers of Animal Health Status" Marine Environmental Research 61(3): 278–304. [DOI] [PubMed] [Google Scholar]
- Moore MN, Lowe D and Köhler A (2004). Biological Effects of Contaminants: Measurement of Lysosomal Membrane Stability, International Council for the Exploration of the Sea. [Google Scholar]
- Moore MN, Viarengo AG, Somerfield PJ and Sforzini S (2012). Linking Lysosomal Biomarkers and Ecotoxicological Effects at Higher Biological Levels Ecological Biomarkers: Indicators of Ecotoxicological Effects, CRC Press; Boca Raton, FL: 107–130. [Google Scholar]
- Parolini M, Binelli A, Cogni D, Riva C and Provini A (2009). "An in vitro biomarker approach for the evaluation of the ecotoxicity of non-steroidal anti-inflammatory drugs (NSAIDs)." Toxicology in Vitro 23(5): 935–942. [DOI] [PubMed] [Google Scholar]
- Pryce K, Samuel D, Lagares E, Myrthil M, Bess F, Harris A, Welsh C, Carroll MA and Catapane EJ (2015). "Presence of Octopamine and an Octopamine Receptor in Crassostrea virginica." In Vivo 37(1): 16–24. [PMC free article] [PubMed] [Google Scholar]
- Ramil M, El Aref T, Fink G, Scheurer M and Ternes TA (2010). "Fate of Beta Blockers in Aquatic-Sediment Systems: Sorption and Biotransformation." Environmental Science & Technology 44(3): 962–970. [DOI] [PubMed] [Google Scholar]
- Ringwood A, Conners D and DiNovo A (1998). "The Effects of Copper Exposures on Cellular Responses in Oysters." Marine Environmental Research 46(1-5): 591–595. [Google Scholar]
- Ringwood A, Hoguet J, Keppler C, Gielazyn M, Ward B and Rourk A (2003). "Cellular biomarkers (lysosomal destabilization, glutathione & lipid peroxidation) in three common estuarine species: a methods handbook." [Google Scholar]
- Ringwood AH, Conners DE, Keppler CJ and Dinovo AA (1999). "Biomarker Studies with Juvenile Oysters (Crassostrea virginica) Deployed In-Situ." Biomarkers 4(6): 400–414. [DOI] [PubMed] [Google Scholar]
- Smith C and Teitler M (1999). "Beta-Blocker Selectivity at Cloned Human Beta1-and Beta2-Adrenergic Receptors." Cardiovascular Drugs and Therapy 13(2): 123–126. [DOI] [PubMed] [Google Scholar]
- Terman A, Kurz T, Gustafsson B and Brunk U (2006). "Lysosomal Labilization." IUBMB Life 58(9): 531–539. [DOI] [PubMed] [Google Scholar]
- Tew KD and Townsend DM (2012). "Glutathione-s-Transferases as Determinants of Cell Survival and Death." Antioxidants & Redox Signaling 17(12): 1728–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebskorn R, Casper H, Scheil V and Schwaiger J (2007). "Ultrastructural Effects of Pharmaceuticals (Carbamazepine, Clofibric Acid, Metoprolol, Diclofenac) in Rainbow Trout (Oncorhynchus mykiss) and Common Carp (Cyprinus carpio)" Analytical and Bioanalytical Chemistry 387(4): 1405–1416. [DOI] [PubMed] [Google Scholar]
- Varano V, Fabbri E and Pasteris A (2017). "Assessing the Environmental Hazard of Individual and Combined Pharmaceuticals: Acute and Chronic Toxicity of Fluoxetine and Propranolol in the Crustacean Daphnia magna." Ecotoxicology 26(6): 711–728. [DOI] [PubMed] [Google Scholar]
- Viarengo A, Burlando B, Ceratto N and Panfoli I (2000). "Antioxidant Role of Metallothioneins: A Comparative Overview." Cellular and Molecular Biology (Noisy-le-Grand, France) 46(2): 407–417. [PubMed] [Google Scholar]
- Viarengo A, Lowe D, Bolognesi C, Fabbri E and Koehler A (2007). "The Use of Biomarkers in Biomonitoring: a 2-Tier Approach Assessing the Level of Pollutant-Induced Stress Syndrome in Sentinel Organisms." Comparative Biochemistry and Physiology Part C 146(3): 281–300. [DOI] [PubMed] [Google Scholar]
- Wang G, Liu B, Tang B, Zhang T and Xiang J (2006). "Pharmacological and Immunocytochemical Investigation of the Role of Catecholamines on Larval Metamorphosis by β-Adrenergic-Like Receptor in the Bivalve Meretrix meretrix." Aquaculture 258(1-4): 611–618. [Google Scholar]
- Yang J-L, Li W-S, Liang X, Li Y-F, Chen Y-R, Bao W-Y and Li J-L (2014). "Effects of Adrenoceptor Compounds on Larval Metamorphosis of the Mussel Mytilus coruscus." Aquaculture 426-427: 282–287. [Google Scholar]
- Zhang J, Wang Q, Zhu N, Yu M, Shen B, Xiang J and Lin A (2008). "Cyclic AMP Inhibits JNK Activation by CREB-Mediated Induction of c-FLIP L and MKP-1, Thereby Antagonizing UV-Induced Apoptosis." Cell Death and Differentiation 15(10): 1654–1662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.