Abstract
Background:
Attention-deficit hyperactivity disorder (ADHD) is overrepresented among individuals seeking treatment for substance use disorders. We previously reported that treatment with extended release mixed amphetamine salts (MAS-XR) increased abstinence, compared to placebo, among patients with co-occurring ADHD and cocaine dependence. This secondary analysis investigates the temporal relationship between ADHD improvement and cocaine abstinence in the first six weeks of the trial.
Methods:
The study was a three-arm, randomized, double-blinded, placebo-controlled, 14-week trial comparing MAS-XR (60 mg or 80 mg daily) versus placebo among 126 participants with ADHD and cocaine dependence. An autoregressive cross-lagged structural equation model was fit and evaluated weekly ADHD improvement (de-fined as ≥30% reduction in the Adult ADHD Investigator Symptom Rating Scale) and urine-confirmed abstinence over the first six weeks.
Results:
The proportion of patients with each of the possible overall patterns of response was: ADHD improves before cocaine abstinence: 24%; Cocaine abstinence occurs before ADHD improvement: 12%; ADHD improvement and abstinence occur during the same week: 6%; ADHD improves but abstinence never achieved: 34%; Abstinence achieved but ADHD never improves: 6%; Neither ADHD improvement nor abstinence: 18%. A significant cross-lagged association was found; subjects with ADHD improvement at week 2 had significantly higher odds of cocaine abstinence at week 3 (p = .014).
Conclusion:
When treating co-occurring ADHD and cocaine dependence with stimulant medication, abstinence is most likely preceded by improvement in ADHD, which tends to occur early with medication treatment. Other observed temporal patterns suggest the potential complexity of the relationship between ADHD and cocaine dependence.
Keywords: Cocaine dependence, ADHD, Treatment, Adderall
1. Introduction
Attention deficit hyperactivity disorder, characterized by problems with executive deficits and impulsive behavior, is one of the most common childhood disorders and has prevalence rates up to 18% in children and adolescents (CDC, 2005). While some individuals have remission of their symptoms, most continue to have impairing symptoms into adulthood (Biederman et al., 2010; Sibley et al., 2016). Perhaps not surprisingly, ADHD is overrepresented among individuals with substance use disorders; a meta-analysis found an overall rate of 22% (van Emmerik-van Oortmerssen et al., 2012).
Initially it was thought that individuals with cocaine use disorders would have elevated rates of ADHD compared to other substance use disorders because they would choose cocaine (or other stimulants) to self-medicate their underlying ADHD symptoms (even if the patient did not recognize it as such). Early work found elevated rates of ADHD among cocaine-dependent individuals (Levin et al., 1998a; Perez de Los Cobos et al., 2011), but the findings have been mixed regarding whether cocaine-dependent patients have higher rates of ADHD than other groups of substance dependent individuals (van de Glind et al., 2014; van Emmerik-van Oortmerssen et al., 2014). As argued by Khantzian (1997) and Mariani et al. (2014), individuals with psychiatric disorders may use alcohol or drugs to lessen intolerable affect or other psychiatric symptoms, albeit often unsuccessfully. Further, individuals may initially use alcohol, nicotine or marijuana to mitigate these psychiatric symptoms, but a number of other factors, such as drug availability, positive subjective effects, the pharmacologic properties of the drug to induce tolerance or withdrawal, and the individual’s perception of harm may influence which drug or drugs an individual with ADHD initiates, consistently uses, and potentially develops a SUD from.
Because cocaine use disorder has remained an intractable disorder for which there is no FDA-approved pharmacologic treatment, one approach has been to focus on treating a common psychiatric comorbidity such as ADHD in cocaine-dependent adults. Some early open pilot studies found that ADHD improved when administering stimulants to active cocaine users (Levin et al., 1998b; Somoza et al., 2004), but other studies have been negative or mixed (Levin et al., 2006; Levin et al., 2007; Carpentier et al., 2005; Schubiner et al., 2002). None of these studies found that cocaine abstinence was more likely to occur among those receiving stimulant medication.
There are two prevailing putative mechanisms of how stimulants might reduce both ADHD and cocaine use among ADHD individuals with cocaine use disorders. One hypothesis is that stimulants improve executive functioning (Rubio Morell and Hernandez Exposito, 2017) resulting in better prefrontal control leading to less impulsive choices (Arnsten, 2009). Moreover, stimulants given to ADHD individuals have been shown to improve ability to delay reward (Shiels et al., 2009). Thus, stimulants may improve ADHD in individuals with substance use disorders and help them to achieve abstinence, particularly if cognitive behavioral strategies or other psychotherapeutic approaches are also utilized to resist cocaine use. Alternatively, stimulants might directly reduce cocaine use as any agonist might in treating a substance use disorder. Several double-blind studies have shown that amphetamines may reduce cocaine use among cocaine-dependent individuals not specifically diagnosed with ADHD (Grabowski et al., 2001, 2004a; Mooney et al., 2009). Some of the most effective FDA-approved interventions for other substance use disorders have used an agonist approach (i.e., buprenorphine and methadone for opioid use disorders (Ayanga et al., 2016) and varenicline and nicotine replacement therapies for nicotine use disorders (Elrashidi and Ebbert, 2014)).
Hypothesizing that earlier studies targeting ADHD and cocaine use disorders may have produced negative results due to ineffective dosing and poor adherence, a two-site study coordinated by our research group conducted a double-blind, placebo-controlled trial targeting cocaine-dependent adults using robust dosing of extended-release mixed amphetamine salts. Specifically, we evaluated the effectiveness of 60 mg/day or 80 mg/day of extended release mixed amphetamine salts (MASXR) for adults with ADHD and cocaine dependence. We found that, for both doses, more than half of the sample (60 mg: 75%, 80 mg; 58%) exhibited reductions in ADHD symptoms by at least 30% at the end of study compared to baseline, whereas 40% achieved this response in the placebo arm. Similarly, the odds of cocaine abstinence were more than four times greater for the pooled 60-mg and 80-mg dose groups compared to the placebo (Levin et al., 2015). What was not assessed was the temporal relationship of these improvements.
Repeatedly, double blind treatment trials in adult ADHD populations have found substantial improvement in ADHD symptoms early on in treatment, often within the first few of weeks (Mattingly et al., 2013; Rosler et al., 2009). Thus, we expected that this would be likely to occur for our trial (Levin et al., 2015). Among individuals who become abstinent in cocaine treatment clinical trials, it is less clear how soon abstinence generally occurs. We hypothesized that clinically significant ADHD improvement would occur first or simultaneously with cocaine abstinence, when both occur, early on in treatment.
To date, one previous study of methylphenidate treatment for cigarette smokers with ADHD showed that improvement in ADHD was associated with abstinence (Nunes et al., 2013), although the sequence of improvement was not examined. Here we explore this relationship by reporting a secondary analysis of a recently published trial in cocaine-dependent adults with ADHD who received either extended-release amphetamine salts or placebo (Levin et al., 2015). Using a path analysis that applied an autoregressive cross-lagged model, we explored within the first six weeks of the trial whether improvement in ADHD symptoms precede cocaine abstinence or occur after cocaine abstinence.
2. Methods
2.1. Participants and criteria
The methods utilized for the antecedent clinical trial and primary analyses have been described elsewhere (Levin et al., 2015). Briefly, patients seeking treatment for cocaine dependence were enrolled at the Columbia University/New York State Psychiatric Institute (NYSPI) Substance Treatment and Research Service (STARS) or at the Ambulatory Research Center (ARC) at the University of Minnesota (UMN), Department of Psychiatry. Inclusion criteria for the study required participants to be aged 18–60, to meet criteria for adult ADHD and current cocaine dependence as diagnosed by the Conners Adult ADHD Diagnostic Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (Epstein et al., 2001) and the Structured Clinical Interview for DSM-IV Disorders (SCID) (First et al., 1995), respectively, and to be medically and psychiatrically stable. Exclusion criteria included a history of mania, schizophrenia, or any psychosis beyond transient symptoms related to drug use, evidence of abnormal cardiac function, or any unstable medical or psychiatric conditions.
2.2. Procedures
2.2.1. Study design
The original clinical trial was approved by the Institutional Review Board at both the UMN and NYSPI. All participants provided written informed consent. Participants were enrolled in the trial from December 2007 through March 2013, and the study was completed in June 2013. The study was a three-arm, randomized, double-blinded, placebo-controlled, parallel group, 14-week trial comparing placebo, MAS-XR 60 mg daily, and MAS-XR 80 mg daily. It included a placebo lead-in during week 1 for all participants followed by randomization, an 11-week active trial, and a 2 week taper down.
Randomization was in fixed blocks of 4 stratified by baseline cocaine use (measured via quantitative urine testing) during the lead-in week and was supervised by statisticians independent from the study groups at both sites. Participants unable to tolerate the assigned MASXR dose underwent dose reduction based on clinical evaluation and a predetermined schedule. All participants were tapered off the study medication in the final week of the trial. All participants received a standardized weekly Cognitive-Behavioral Therapy/Relapse Prevention Treatment, that focused on addressing cocaine use, conducted by experienced therapists.
2.2.2. Study measures
Self-reported drug use data were obtained via the timeline followback method, which has been validated for cannabis and cocaine use (Robinson et al., 2014). This information included reports of all substance use by day for 28 days prior to evaluation and then continuing weekly throughout the study. Patients were scheduled to attend the clinic 3 times a week. Urine samples were obtained at each visit and tested for cocaine.
Participants’ ADHD was assessed biweekly using the Adult ADHD Investigator Symptom Rating Scale (AISRS) (Spencer et al., 2010).
2.3. Statistical analyses
A cocaine-abstinent week was defined as one in which (1) at least 2 urine drug screens were collected, and all collected urine samples (either 2 or 3) were cocaine negative, and (2) all self-reported cocaine use for the week was negative. A cocaine-positive week was defined as at least 1 positive result on the urine screen or positive self-report. Weeks with insufficient data to determine cocaine abstinence were designated as cocaine-positive weeks.
ADHD improvement was defined as at least a 30% reduction in AISRS score compared with week 0 and was measured every 2 weeks. This definition has been routinely used as a clinical measure of improvement (Mattingly et al., 2013). Weeks with insufficient data to determine ADHD improvement were considered missing for the analyses. To summarize the temporal ordering of the main outcomes throughout the entire trial, the sample of all participants was divided into six groups: (1) achieved ADHD improvement before cocaine abstinence, (2) achieved cocaine abstinence before ADHD improvement, (3) achieved ADHD improvement and cocaine abstinence at the same time, (4) achieved ADHD improvement only, (5) achieved cocaine abstinence only, and (6) neither ADHD improvement nor cocaine abstinence achieved. A two-sample proportion test was used to compare the proportions of participants falling in groups (1) and (2).
The temporal and cross-temporal relationship between the treatment and placebo groups on bi-weekly ADHD improvement and weekly cocaine use abstinence, during week 2 to week 6 of the trial, was examined using an autoregressive cross-lagged structural equation modeling design (Laursen et al., 2012) (see Fig. 1A). Although the study included an 11-week active trial phase, the cross-lagged analysis was performed on data up to week 6 to avoid type I error that may occur as additional paths are tested, i.e., during weeks 7–12, and to focus on early effects of MAS-ER on ADHD and cocaine use. The logit link was used to model the dichotomous measures of ADHD improvement (at least 30% reduction from baseline vs. not) and cocaine use (abstinent vs. not). The effect of treatment group (60 mg or 80 mg daily MAS-XR vs. placebo) was included as a predictor of ADHD improvement and cocaine use at week 2. Because no significant differences between the 60 mg and 80 mg dosing were found in the corresponding primary paper, subjects who received MAS-XR medication (60 mg or 80 mg daily) were combined into one MAS-XR group and compared to the placebo arm. Using separate groups of subjects randomized to the 60 mg and 80 mg doses would lead to a more complex path model which would require a higher number of subjects to be robustly assessed.
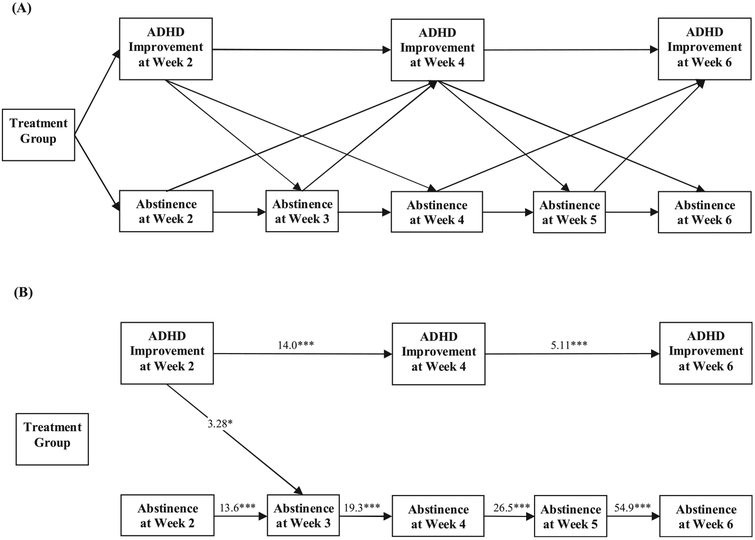
Fig. 1.
(A). Diagram of the autoregressive cross-lagged model of MAS-XR treatment vs. placebo on ADHD improvement and cocaine abstinence within the first 6 weeks of the trial (n = 126). Autoregressive effects are represented as horizontal arrows; cross-lagged effects are illustrated by diagonal arrows between abstinence and ADHD improvement. (B). Paths achieving statistical significance based on the autoregressive cross-lagged model. Corresponding model estimated odds ratios (OR) and 95% confidence intervals (CI) are presented in Table 1.
Structural equation modeling allows multiple factors (i.e., ADHD improvement and cocaine abstinence) to be entered into the model at the same time, providing estimates and tests of significance for associations between bi-weekly ADHD improvement and weekly cocaine abstinence over time. To account for the stability of each measure over time, e.g., ADHD improvement at week 2 and ADHD improvement at week 4, autoregressive path weights between two consecutive time points were estimated (as represented by the horizontal arrows between each measure; see Fig. 1A). All analyses were performed on the intent-to-treat sample of all randomized participants and were performed in Mplus version 7.11 (Muthen and Muthen, 2007). Mplus uses full information maximum likelihood (FIML) to account for missing data. Since ADHD measures were collected bi-weekly, i.e., at week 0, 2, 4, 6, etc., the cross-lagged analysis started at week 2 for analyses. All statistical tests were 2-sided with a significance level of 5%.
3. Results
3.1. Sample
The sample of 126 participants from the primary study (Levin et al., 2015) was predominantly male, unmarried, and unemployed. Approximately half were white, and half were African American or Hispanic. Baseline ADHD scores reflected moderate ADHD symptoms, and the average cocaine use at baseline was 11.65 (standard deviation = 7.35) days/month.
3.2. ADHD symptoms vs. cocaine abstinence
Of the 126 subjects randomized to either MAS-XR or placebo, 24% (n = 30) improved in ADHD first before achieving cocaine abstinence. Inversely, 12% (n = 15) of subjects achieved cocaine abstinence first before improving in ADHD. The proportion of subjects who improved first in ADHD was significantly different than the proportion who achieved cocaine abstinence (24% vs. 12%; p = .013). Only 6% (n = 8) of subjects improved in ADHD and achieved cocaine abstinence during the same week. A larger proportion of subjects (34%, n = 43) improved in ADHD only but never achieved abstinence compared to subjects who only achieved abstinence but never improved in ADHD (6%, n = 7). The proportion of subjects who never achieved abstinence or improved in ADHD throughout the trial was 18% (n = 23).
3.3. Time of first improvement
For the 30 subjects who improved in ADHD before achieving cocaine abstinence, the median week of their first improved in ADHD was week 2, which was followed by first cocaine abstinence at median week 4. Inversely, for the 15 subjects who achieved cocaine abstinence first, the median week of their first cocaine abstinence was week 2, and ADHD improvement followed at median week 6. For the 8 subjects who improved in ADHD and achieved abstinence simultaneously, the median week when this occurred was week 2.
The autoregressive cross-lagged model of MAS-XR treatment vs. placebo on ADHD improvement and cocaine abstinence was performed on data from week 2 to week 6, as the aim of the paper is to understand the early effects of MAS-XR. The majority of first improvement in ADHD or cocaine abstinence was observed during the first 6 weeks. Fig. 1A shows all the model paths. Autoregressive effects are represented as horizontal arrows between the same variable at one time point to the next; cross-lagged effects are illustrated by diagonal arrows between abstinence and ADHD improvement. Fig. 1B shows the same model with only the paths that reached statistical significance. Corresponding model estimated odds ratios (OR) and 95% confidence intervals (CI) are presented in Table 1. The model fit indices were: Akaike Information Criteria (AIC) = 921.58 and Bayesian Information Criteria (BIC) = 989.65.
Table 1.
Odds Ratios and 95% confidence intervals of all paths in the autoregressive cross-lagged model of MAS-XR treatment vs. placebo on ADHD improvement and cocaine abstinence within the first 6 weeks of the trial (n = 126).
| Model Paths | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Treatment Group to Week 2 Outcome | ||
| Treatment Group → ADHD Improvement at Week 2 | 1.59 | 0.69, 3.64 |
| Treatment Group → Cocaine Abstinence at Week 2 | 2.04 | 0.54, 7.74 |
| Cross-lagged Paths | ||
| ADHD → Cocaine Use | ||
| ADHD Improvement at Week 2 → Cocaine Abstinence at Week 3 | 3.28* | 1.27, 8.45 |
| ADHD Improvement at Week 2 → Cocaine Abstinence at Week 4 | 1.63 | 0.60, 4.40 |
| ADHD Improvement at Week 4 → Cocaine Abstinence at Week 5 | 1.83 | 0.62, 5.34 |
| ADHD Improvement at Week 4 → Cocaine Abstinence at Week 6 | 1.36 | 0.40, 4.66 |
| Cocaine Use → ADHD | ||
| Cocaine Abstinence at Week 2 → ADHD Improvement at Week 4 | 2.28 | 0.48, 10.9 |
| Cocaine Abstinence at Week 3 → ADHD Improvement at Week 4 | 1.11 | 0.32, 3.82 |
| Cocaine Abstinence at Week 4 → ADHD Improvement at Week 6 | 1.46 | 0.40, 5.34 |
| Cocaine Abstinence at Week 5 → ADHD Improvement at Week 6 | 1.11 | 0.30, 4.07 |
| Autoregressive Paths | ||
| ADHD Improvement at Week 2 → ADHD Improvement at Week 4 | 14.0*** | 4.29, 45.6 |
| ADHD Improvement at Week 4 → ADHD Improvement at Week 6 | 5.11*** | 2.13, 12.3 |
| Cocaine Abstinence at Week 2 → Cocaine Abstinence at Week 3 | 13.6*** | 3.64, 51.1 |
| Cocaine Abstinence at Week 3 → Cocaine Abstinence at Week 4 | 19.3*** | 6.86, 54.5 |
| Cocaine Abstinence at Week 4 → Cocaine Abstinence at Week 5 | 26.5*** | 9.38, 74.9 |
| Cocaine Abstinence at Week 5 → Cocaine Abstinence at Week 6 | 54.9*** | 16.5, 182.8 |
Note: The significant model path are denoted with
p < .05, and
p < .001. For instance, those who achieved ADHD improvement by at least 30% at week 2 had 3.28 higher odds of achieving cocaine abstinence at week 3.
As can be seen in Fig. 1B and Table 1, at week 2 there was no significant effect of treatment group on ADHD improvement (OR = 1.59, 95% CI = 0.69, 3.64; p = .275) or cocaine use (OR = 2.04, 95% CI = 0.54, 7.74; p = .296). Results demonstrated a significant cross-lagged association: ADHD improvement at week 2 was significantly associated with cocaine abstinence at week 3 (OR = 3.28, 95% CI = 1.27, 8.45; p = .014). Autoregressive associations between consecutive bi-weekly measurements of ADHD improvement and cocaine abstinence were all significant.
The cross-lagged model was also fit for each treatment group separately. Within the active medication treatment group (n = 83; AIC = 481.77, BIC = 529.16), the significant association between ADHD improvement at week 2 and subsequent cocaine abstinence at week 3 remained (OR = 3.13, 95% CI = 1.04, 5.25; p = .041). However, in the placebo group (n = 43; AIC = 182.35, BIC = 215.11), this association was not found significant (OR = 2.55, 95% CI = 0.31,24.1; p = .383).
4. Discussion
We examined the timing of response of ADHD symptoms and cocaine use over the first six weeks of the trial in a placebo-controlled trial of stimulant medication (MAS-XR) for patients with both ADHD and Cocaine Use Disorder. Among patients who had both a significant reduction in ADHD symptoms and achieved abstinence, a greater proportion (24% of total sample) experienced an improvement in ADHD symptoms prior to initiation of abstinence compared to individuals who achieved abstinence before ADHD symptom improvement (12%). A smaller proportion (6%) had both ADHD symptom improvement and cocaine abstinence occur simultaneously. ADHD improvement tended to occur quickly (median at week 2) followed by cocaine abstinence first occurring at a median of week 4.
The cross-lagged structural equation model found one significant connection across time points between improvement in ADHD and abstinence: the association between ADHD improvement at week 2 and cocaine abstinence at week 3. That means improvement in ADHD symptoms at week 2 was associated with higher likelihood of cocaine abstinence a week later (at week 3). This association was significant when analyzed on all randomized subjects and also remained significant in the sample of only subjects in the active medication treatment group. The predominance of the significant autoregressive paths suggests that the most predictive effect of either condition (ADHD improvement or cocaine use) was the corresponding past week’s (or 2 weeks’) behavior. The single significant cross-lagged association over and above the effect of the previous week suggests that effective treatment of ADHD may be the precursor to reducing cocaine use, though no claim of causality can be supported since this association was no longer observed at later weeks. The significant autoregressive paths suggest that each week’s outcome strongly depends on the corresponding observations in the past weeks. Future studies on larger samples may show more cross-lagged associations throughout the trial instead of just one.
The path model findings have several possible explanations. First, the effective treatment of ADHD might result in reduction of ADHD symptoms that are associated with reduced impulsive behavior thereby decreasing cocaine use. Second, improved cognitive control emerges after stimulant administration for ADHD. Third, if one ascribes to the self-medication hypothesis, then improvement in ADHD symptoms reduces the need to use cocaine (or other drugs) to mitigate impairing symptoms. Our findings show that subjects who improved in ADHD at week 2 were more likely to attain cocaine abstinence a week later; however, this does not mean ADHD symptom improvement can be treated as the underlying mechanism for promoting cocaine use for all subjects at any time during the study. For instance, across the study weeks, about one third of participants achieved a reduction in ADHD symptoms without attaining abstinence.
Additionally, stimulant medication may exert a direct agonist effect. Studies conducted by Grabowski et al. (2001, 2004b), Mooney et al. (2009), as well as Nuijten et al. (2016) found that amphetamines were more likely than placebo to promote abstinence among cocaine dependent individuals not specifically diagnosed with ADHD. However, abstinence did not occur immediately; instead, it required several weeks, as stimulant medication was titrated to a robust dose. Given that there is a delay in cessation of cocaine use, albeit a small one, it may be that chronic cocaine use leads to dopamine dysregulation that may take longer to be compensated for by a long-acting amphetamine formulation that has dopamine enhancing properties. And, over the course of that, ADHD symptoms may improve earlier than cocaine use and may serve as a marker, in a sense, of whether the underlying deficit has improved enough to eventually result in abstinence from cocaine.
Finally, and most likely, the two mechanisms in tandem altered the ultimate course of cocaine use in this population. What can be concluded from these findings is that if titration is conducted rapidly, as was done in this clinical trial, improvement of ADHD and possibly cocaine abstinence may be associated with each other within the first 2–3 weeks of treatment. When each arm was analyzed separately, the significant relationship between week 2 improvement in ADHD and week 3 cocaine abstinence was found only in the treatment arm but not in the placebo arm. This suggests that the mechanism of change may be different for those receiving medication compared to those not receiving medication.
One inference from these findings is the importance of addressing ADHD comorbidity in individuals with active substance use disorders. While extended-release mixed amphetamine salts may not effectively treat all individuals (in the primary trial, over 50% in both active treatment arms had a clinical improvement), addressing ADHD with medication options or combined pharmacotherapy and psychotherapy may be necessary for most individuals with both ADHD and a cocaine use disorder. Notably, few (6%) got abstinent without improvement in ADHD symptoms.
Limitations of this study include the choice of primary outcomes (e.g., 30% reduction in ADHD symptoms and abstinent weeks) to ascertain the temporal relationships of improvement of ADHD and cocaine use. The findings obtained were from a sample that was not sufficiently large to ensure enough power for such analyses and had minor or no additional psychiatric comorbidities; thus, the findings may not generalize to other more psychiatrically unstable populations. Further, our first assessment of ADHD improvement occurred at week 2, so the 12% of the sample that had cocaine abstinence improve before the ADHD improvement might be an overestimate if both disorders improved within the first week, but this was not captured by our methodology.
5. Conclusions
This paper is, to our knowledge, the first to evaluate the cross-temporal behavior of improvement in ADHD and cocaine use in participants receiving treatment for their ADHD and cocaine use disorder. This study suggests that when individuals show at least a 30% improvement in both ADHD and achieve cocaine abstinence, ADHD most commonly improves first. However, there are other pathways, such as cocaine abstinence first, ADHD improvement without cocaine abstinence, cocaine abstinence without ADHD improvement, and neither abstinence nor improvement in ADHD. Future studies might explore these pathways in more naturalistic settings and other substance use disordered ADHD adults.
Acknowledgments
We acknowledge the contributions of the staffs of the Substance Treatment and Research Service (STARS) at the New York State Psychiatric Institute and the Ambulatory Research Center, Department of Psychiatry, University of Minnesota for their clinical support.
Role of funding source
Funding for this research was provided by the National Institute on Drug Abuse (NIDA) grants R01DA 023652, K24 DA029647, and K24 DA022412. NIDA had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Conflict of interest
Dr. Levin served as a consultant to GW Pharmaceuticals, Eli Lily, and served on an advisory board to Shire in 2006–2007. Dr. Levin also serves as a consultant to Major League Baseball regarding the diagnosis and treatment of ADHD. Dr. Grabowski served on an advisory board to Shire in 2005–2007. Dr. Nunes served on an advisory board for Eli Lily and Company in January 2012. Dr. Nunes receives medication from Alkermes for ongoing studies that are sponsored by the National Institute on Drug Abuse. Drs. Mariani, Pavlicova and Ms. Mahony, Ms. Choi, and Mr. Brooks report no competing interests and no financial relationships with commercial interests.
References
- Arnsten AF, 2009. Toward a new understanding of attention-deficit hyperactivity disorder pathology: an important role for prefrontal cortex dysfunction. CNS Drugs 23 (Suppl. 1), 33–41. [DOI] [PubMed] [Google Scholar]
- Ayanga D, Shorter D, Kosten TR, 2016. Update on pharmacotherapy for treatment of opioid use disorder. Expert Opin. Pharmacother 17, 2307–2318. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Evans M, Small J, Faraone SV, 2010. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res 177, 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2005. Mental health in the United States. Prevalence of diagnosis and medication treatment for attention-deficit hyperactivity disorder—United States, 2003. MMWR Morb. Mortal. Wkly. Rep 54, 842–847. [PubMed] [Google Scholar]
- Carpentier PJ, de Jong CA, Dijkstra BA, Verbrugge CA, Krabbe PF, 2005. A controlled trial of methylphenidate in adults with attention deficit/hyperactivity disorder and substance use disorders. Addiction 100, 1868–1874. [DOI] [PubMed] [Google Scholar]
- Elrashidi MY, Ebbert JO, 2014. Emerging drugs for the treatment of tobacco dependence: 2014 update. Expert Opin. Emerg. Drugs 19, 243–260. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Johnson DE, Conners CK, 2001. Conners’ Adult ADHD Diagnostic Interview for DSM-IV (CAADID) Multi-Health Systems, North Tonawanda, NY. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1995. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P), Version 2.0 Biometrics Research New York State Psychiatric Institute, New York. [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG, 2001. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J. Clin. Psychopharmacol 21, 522–526. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J, 2004a. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacol 29, 969–981. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS, 2004b. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict. Behav 29, 1439–1464. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ, 1997. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv. Rev. Psychiatry 4, 231–244. [DOI] [PubMed] [Google Scholar]
- Laursen BP, Little TD, Card NA, 2012. Handbook of Development Research Methods Guilford Press, New York, NY. [Google Scholar]
- Levin FR, Evans SM, Kleber HD, 1998a. Prevalence of adult attention-deficit hyperactivity disorder among cocaine abusers seeking treatment. Drug Alcohol Depend 52, 15–25. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, McDowell DM, Kleber HD, 1998b. Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J. Clin. Psychiatry 59, 300–305. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evan SM, Brooks DJ, Kalbag AS, Garawi F, Nunes EV, 2006. Treatment of methadone-maintained patients with adult ADHD: Double blind comparison of methylphenidate, bupropion and placebo. Drug Alcohol Depend 81, 137–148. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Garawi F, 2007. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend 87, 20–29. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Specker S, Mooney M, Mahony A, Brooks DJ, Babb D, Bai Y, Eberly LE, Nunes EV, Grabowski J, 2015. Extended-release mixed amphetamine salts vs placebo for comorbid adult attention-deficit/hyperactivity disorder and cocaine use disorder: a randomized clinical trial. JAMA Psychiatry 72, 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani JJ, Khantzian EJ, Levin FR, 2014. The self-medication hypothesis and psychostimulant treatment of cocaine dependence: an update. Am. J. Addict 23, 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly GW, Weisler RH, Young J, Adeyi B, Dirks B, Babcock T, Lasser R, Scheckner B, Goodman DW, 2013. Clinical response and symptomatic remission in short- and long-term trials of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2013, 13–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J, 2009. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 101, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO, 2007. Mplus User’s Guide, 6th edition. Muthen and Muthen, Los Angeles, CA. [Google Scholar]
- Nuijten M, Blanken P, van de Wetering B, Nuijen B, van den Brink W, Hendriks VM, 2016. Sustained-release dexamfetamine in the treatment of chronic cocaine-dependent patients on heroin-assisted treatment: a randomised, double-blind, placebo-controlled trial. Lancet 387, 2226–2234. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Covey LS, Brigham G, Hu MC, Levin FR, Somoza EC, Winhusen TM, 2013. Treating nicotine dependence by targeting attention-deficit/hyperactivity disorder (ADHD) with OROS methylphenidate: the role of baseline ADHD severity and treatment response. J. Clin. Psychiatry 74, 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de Los Cobos J, Sinol N, Puerta C, Cantillano V, Lopez Zurita C, Trujols J, 2011. Features and prevalence of patients with probable adult attention deficit hyperactivity disorder who request treatment for cocaine use disorders. Psychiatry Res 185, 205–210. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI, 2014. Reliability of the timeline followback for cocaine, cannabis, and cigarette use. Psychol. Addict. Behav 28, 154–162. [DOI] [PubMed] [Google Scholar]
- Rosler M, Fischer R, Ammer R, Ose C, Retz W, 2009. A randomized, placebo-controlled, 24-week, study of low-dose extended-release methylphenidate in adults with attention-deficit/hyperactivity disorder. Eur. Arch. Psychiatry Clin. Neurosci 259, 120–129. [DOI] [PubMed] [Google Scholar]
- Rubio Morell B, Hernandez Exposito S, 2017. Differential long-term medication impact on executive function and delay aversion in ADHD. Appl. Neuropsychol. Child 15, 1–18. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, Edwards A, Donlin J, Pihlgren E, 2002. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp. Clin. Psychopharmacol 10, 286–294. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Reynolds B, Mazullo RJ, Rhodes JD, Pelham WE, Waxmonsky JG, Gangloff BP, 2009. Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Exp. Clin. Psychopharmacol 17, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Mitchell JT, Becker SP, 2016. Method of adult diagnosis influences estimated persistence of childhood ADHD: A systematic review of longitudinal studies. Lancet 12, 1157–1165. [DOI] [PubMed] [Google Scholar]
- Somoza EC, Winhusen TM, Bridge TP, Rotrosen JP, Vanderburg DG, Harrer JM, Mezinskis JP, Montgomery MA, Ciraulo DA, Wulsin LR, Barrett JA, 2004. An open-label pilot study of methylphenidate in the treatment of cocaine dependent patients with adult attention deficit/hyperactivity disorder. J. Addict. Dis 23, 77–92. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Adler LA, Meihua Q, Saylor KE, Brown TE, Holdnack JA, Schuh KJ, Trzepacz PT, Kelsey DK, 2010. Validation of the adult ADHD investigator symptom rating scale (AISRS). J. Atten. Disord 14, 57–68. [DOI] [PubMed] [Google Scholar]
- van Emmerik-van Oortmerssen K, van de Glind G, van den Brink W, Smit F, Crunelle CL, Swets M, Schoevers RA, 2012. Prevalence of attention-deficit hyperactivity disorder in substance use disorder patients: a meta-analysis and meta-regression analysis. Drug Alcohol Depend 122, 11–19. [DOI] [PubMed] [Google Scholar]
- van Emmerik-van Oortmerssen K, van de Glind G, Koeter MW, Allsop S, Auriacombe M, Barta C, Bu ET, Burren Y, Carpentier PJ, Carruthers S, Casas M, Demetrovics Z, Dom G, Faraone SV, Fatseas M, Franck J, Johnson B, Kapitany-Foveny M, Kaye S, Konstenius M, Levin FR, Moggi F, Moller M, Ramos-Quiroga JA, Schillinger A, Skutle A, Verspreet S, IASP Research Group, van den Brink W, Schoevers RA, 2014. Psychiatric comorbidity in treatment-seeking substance use disorder patients with and without attention deficit hyper-activity disorder: results of the IASP study. Addiction 109, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Glind G, Konstenius M, Koeter MW, van Emmerik-van Oortmerssen K, Carpentier PJ, Kaye S, Degenhardt L, Skutle A, Franck J, Bu ET, Moggi F, Dom G, Verspreet S, Demetrovics Z, Kapitany-Foveny M, Fatseas M, Auriacombe M, Schillinger A, Moller M, Johnson B, Faraone SV, Ramos-Quiroga JA, Casas M, Allsop S, Carruthers S, Schoevers RA, Wallhed S, Barta C, Alleman P, Levin FR, van den Brink W, IASP Research Group, 2014. Variability in the prevalence of adult ADHD in treatment seeking substance use disorder patients: results from an international multi-center study exploring DSM-IV and DSM-5 criteria. Drug Alcohol Depend 134, 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]