Abstract
AZD3965, a pyrole pyrimidine derivative is a potent and orally bioavailable inhibitor of monocarboxylate transporter 1 (MCT1), currently in a Phase I clinical trial in UK for lymphomas and solid tumors. There is currently no published assay for AZD3965. The objectives of this study were to develop and validate a LC/MS/MS assay for quantifying AZD3965 in mouse plasma and tumor tissue. Protein precipitation with 0.1% formic acid in acetonitrile was used for sample preparation. Chromatographic separation was achieved on a C18 column followed by tandem mass spectrometry detection in multiple reaction monitoring mode with utilizing Atmospheric Pressure Chemical Ionization. AR-C155858 was used as the internal standard. The inter-day and intra-day precision and accuracy of quality control samples evaluated in plasma and tumor tissue were less than ±7% of the nominal concentrations. The extraction recovery, matrix effect and stability values were all within acceptable levels. Sample dilution integrity, accessed by diluting plasma spiked with AZD3965 10-fold with blank plasma, was 101%. The lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ) were 0.15 ng/mL and 12 μg/ml, respectively, in plasma. The assay in tumor tissue was also validated with good precision and accuracy. The LLOQ was 0.15 ng/mL in tumor tissue. This assay was successfully applied to pharmacokinetic and murine 4T1 breast tumor xenograft studies of AZD3965 in mice.
Keywords: AZD3965, Monocarboxylate transporters, LC/MS/MS, Pharmacokinetics, Plasma, Tumor tissue
Graphical Abstract

1. INTRODUCTION
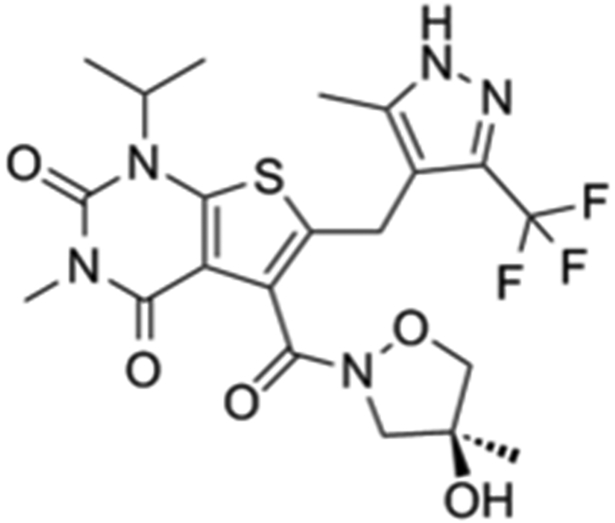
AZD3965, a pyrole pyrimidine derivative and an analogue of AR-C155858, represents a first-in-class monocarboxylate transporter 1 (MCT1) inhibitor that is currently being investigated in a Phase I clinical trial in the UK for solid tumors and lymphoma treatment (NCT01791595) (Scheme-1A). Targeting lactate transporters (MCTs) as potential therapeutic targets in cancer has been proposed in recent years, due to the increasing reports of MCT1 and/or MCT4 up-regulation in various cancers [1-3]. MCTs inhibition or silencing RNA have shown to limited the survival of cancer cells and decreased tumor growth in various preclinical tumor models [4-7].
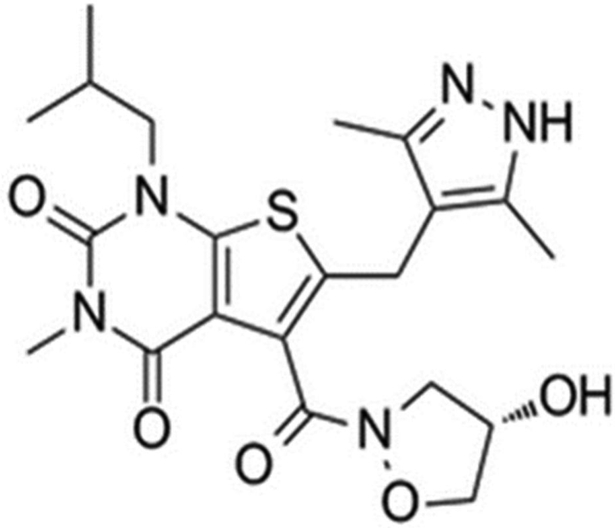
Scheme-1A-1B.
In this study, we developed and validated a LC/MS/MS method for quantifying AZD3965 in mouse plasma and breast tumor tissue using AR-C155858 (Scheme-1B) as the internal standard. The method is able to quantitate AZD3965 in mouse plasma and tumor tissue selectively and accurately over a wide range of concentrations. Our results demonstrate the applicability of our assay in pharmacokinetic and murine 4T1 breast tumor xenograft studies in mice following the oral and intraperitoneal administration of 100 mg/kg AZD3965, respectively.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
AZD3965 (>98% purity) and AR-C155858 (≥ 98% purity) were purchased from MedKoo Biosciences (Chapel Hill, NC) and Chemscene (Monmouth Junction, NJ), respectively. All other reagents used were HPLC grade and were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Cell Culture
Mouse mammary tumor, 4T1 cells were kindly provided by Dr. Elizabeth A. Repasky (Roswell Park Cancer Institute, Buffalo NY). Cells were maintained at 37°C in a humidified atmosphere with 5% CO2/95% air. 4T1 cells were cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 units penicillin and 100 μg/mL of streptomycin. Culture medium was changed every 2-3 days, and cells were passaged with 0.25% Trypsin/EDTA.
2.3. LC/MS/MS and Chromatographic Conditions
The LC/MS/MS assay was performed on Shimadzu Prominence HPLC with binary pump and autosampler (Shimadzu Scientific, Marlborough, MA) connected to a Sciex API 3000 triple quadruple tandem mass spectrometer with utilizing Atmospheric Pressure Chemical Ionization (APCI) (Sciex, Foster City, CA). Chromatographic separation was achieved by injecting 15 or 4 μL of the sample on to an Xterra MS C18 column (250 × 2.1 mm i.d., 5-μm particle size; Waters, Milford, MA) for the low or high calibration curve, respectively. Mobile phase A consisted of acetonitrile/water (5/95, v/v) with 0.1% acetic acid and mobile phase B was acetonitrile/water (95/5, v/v) with 0.1% acetic acid. The flow rate was 250 μL/min with a gradient elution profile and a total run time of 15 min. The mass spectrometer was operated in multiple reaction monitoring (MRM) mode utilizing APCI for specific detection of AZD3965 and the I.S. by measuring the characteristic ion transition. The mass spectrometer parameters were optimized: declustering potential of 35 V, entrance potential of 10 V, and collision cell exit potential of 25 V. The nebulizer current was 3 uA with the source temperature at 450°C. The selected mobile phase, column, chromatographic conditions and sample preparation are modified from our preliminary validated assay for AR-C155858 (an analogue of AZD3965) in rat plasma [8]. The data was analyzed using Analyst version 1.4.2 (Sciex, Foster City, CA).
2.4. Standard and Quality Control Solutions
AZD3965 and the I.S., AR-C155858 were dissolved in DMSO at a concentration of 1 mg/mL. Separate stock solutions for calibration standards and quality control (QC) samples were then diluted in acetonitrile/water (40/60, v/v). These stock solutions were further diluted in acetonitrile/water (20/80, v/v) to obtain working solutions. All the stock and working solutions were stored at −20°C. The concentrations of the calibration standard samples were 0.15, 0.5, 5, 20, 50, 100, 250, and 500 ng/mL for the low calibration curve and 100, 200, 500, 1000, 2500, 5000, 8000, and 12000 ng/mL for the high calibration curve. Low-, medium- and high-QC samples were prepared to yield concentration of 10, 150, and 400 ng/mL for the low calibration curve and 1000, 6000, and 10000 ng/mL for the high calibration curve. The I.S. concentrations were 15 and 200 ng/mL for the low and high calibration standard and QC samples, respectively.
2.5. Sample Preparation
For tumor samples, tumors were thawed on ice and homogenized in methanol/water (5/95 v/v) (5 mL/g tumor). Samples were prepared by adding 5 μL of I.S. solution containing AR-C155858 to 35 μL of the plasma or tumor homogenate sample. To ensure the concentrations quantified were below the upper limit of quantification (ULOQ), part of the plasma samples were diluted 10-fold with blank plasma prior to the sample preparation. Standards and QCs were prepared by adding 5 μL of I.S. solution and 5 μL of stock solution containing AZD3965 to 30 μL of blank plasma or blank tumor homogenate. Plasma and tumor homogenate proteins were precipitated by the addition of 600 μL 0.1% formic acid in acetonitrile. Samples were vortexed, followed by centrifugation at 10,000 × g for 20 min at 4°C. Then 540 μL of the supernatant was collected and evaporated under a stream of nitrogen gas, followed by reconstitution in 200 and 1,000 μL of acetonitrile/water (40/60, v/v) for low and high calibration curve, respectively.
2.6. Assay Validation
2.6.1. Linearity and Lower Limit of Quantification
Regression analysis of peak area ratios of AZD3965/AR-C155858 to AZD3965 concentrations was used to assess linearity of the curve. The linearity of the calibration curves was determined utilizing 1/Χ weighting factor (Χ=concentration). The guideline used to determine the lower limit of quantification (LLOQ) and detection (LOD) was based on a signal-to-noise ratio (S/N) of at least 10:1 and 3:1, respectively.
2.6.2. Precision and Accuracy
The intraday precision and accuracy were determined by analyzing QC samples in triplicate on each day. Whereas for the interday precision and accuracy, QC samples were analyzed over three different days. A calibration curve was run on each analysis day along with the QCs. The precision was determined by the coefficient of variation, and accuracy was measured by comparing the calculated concentration with the known concentration. The acceptable precision and accuracy were required to be within ±15%.
2.6.3. Recovery and Matrix Effect
The recoveries of the AZD3965 and I.S. were determined by comparing the peak areas of extracted standard samples with the peak areas of post-extraction plasma spiked at the corresponding QC concentrations (low, middle and high). The matrix effect was evaluated by comparing the peak areas of post-extraction plasma and tumor homogenate spiked with QC (low and high) to the neat QC solutions. For post-extraction spiked plasma and tumor homogenate samples, 40 uL of blank plasma or tumor homogenate were precipitated with 0.1% formic acid in acetonitrile and centrifuged as described in Section 2.5. The resulting supernatant (540 μL) was dried and reconstituted in 190 and 990 μL of acetonitrile/water (40/60, v/v) for low and high calibration curve, respectively plus 5 uL of I.S solution and 5 uL of QC stock solution. The neat QC solutions were prepared similarly as the post-extraction spiked plasma and tumor homogenate samples but in the absence of matrix. Both recovery and matrix effect were examined in triplicate.
2.6.4. Effect of Dilution
The effect of dilution was evaluated for the analysis of AZD3965 in plasma at concentrations higher than the ULOQ by analyzing duplicates of plasma spiked with AZD3965 at 10-fold dilution of the three QC concentrations (10,000, 60,000 and 100,000 ng/mL) and diluting with blank plasma to the corresponding QC concentrations for the high calibration curve.
2.6.5. Stability
The post preparative stability was determined by rerunning QC samples that were kept in the autosampler at 15 °C for at least 16 h. The stability of AZD3965 in mouse plasma and tumor tissue were assessed after three freeze-thaw cycles (−80°C to room temperature) by analyzing QCs samples in triplicate. For long-term stability evaluations, QC samples in mouse plasma (low, medium and high concentrations of the low calibration curve), obtained from the same aliquots that were used to assess interday variability, were evaluated in duplicate after storage for 15 months in −80°C.
2.7. Application in a Pharmacokinetic and Breast Tumor Xenograft Studies
Female, BALB/c mice weighting 18 to 20 g were purchased from Envigo (Indianapolis, IN). Animals were housed in a filtered laminar airflow room in standard vinyl cages with air filter tops. Water and food were autoclaved and provided ad libitum. Animals were maintained under the standard 12-h light/dark cycle at 22-24°C. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University at Buffalo.
For the pharmacokinetic study mice were administered an oral dose of 100 mg/kg AZD3965 through oral gavage. AZD3965 stock solution (10 mg/mL) was prepared in 20% w/v cyclodextrin in normal saline. Three mice per time-point were sacrificed after 15 and 30 min, and 1, 2, 3, 6, 9, 12, and 24 h through aortic exsanguination. Blood samples were collected and centrifuged within 1 h for plasma extraction. All the samples were stored at −80°C until analysis.
For the tumor study, mice were lightly anesthetized and injected with 100 uL of cell suspension subcutaneously into the 4th inguinal mammary fat pad at a cell density of 2.5× 105 cells/mL in 1× PBS. Prior to inoculation, 4T1 cells were treated with trypsin, washed and resuspended in 1× PBS. When the tumor volume reached 100 mm3, three animals were given AZD3965 intraperitoneally (i.p.) twice daily for 17 days. Twelve hours after the last AZD3965 dose, the primary tumor was excised and snapped frozen in liquid nitrogen. Terminal blood samples were collected in a lithium-heparinized tube. Blood samples were centrifuged and plasma was extracted. All the samples were stored at −80°C until analysis.
2.8. Data and Statistical Analysis
Noncompartmental analysis was performed using Phoenix WinNonlin version 7.0 (Pharsight, Mountain View, CA) to determine AZD3965 pharmacokinetic parameters. Area under the plasma concentration-time curve (AUC) was determined using the trapezoidal method; with AUC values extrapolated to time infinity. The terminal half-life (t½) was calculated as 0.693/k, where k was the slope of the terminal regression line. Vz/F (apparent volume of distribution/bioavailability (F)) represents the apparent volume of distribution estimated from the terminal phase. The maximal concentration (Cmax) was also determined by visual inspection. Oral clearance (clearance/F) was determined by the oral dose/AUC. All the data are presented as mean ± SD. Data analysis was performed using GraphPad Prism (GraphPad Software Inc., San Diego CA). Student’s t-test was used to assess the statistical significance with p value < 0.05.
3. RESULTS
The assay presented here has been validated according to the US Food and Drug Administration (FDA) guidelines: Bioanalytical Method Validation (2001) and Analytical Procedures and Methods Validation for Drugs and Biologics (2015).
3.1. LC/MS/MS and Chromatograms
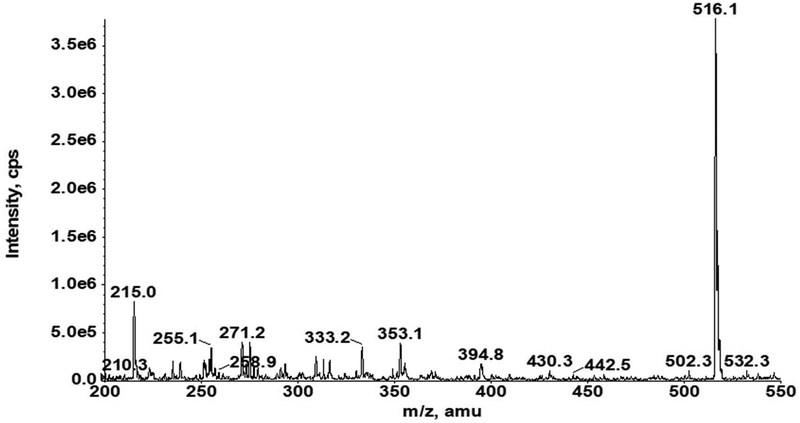
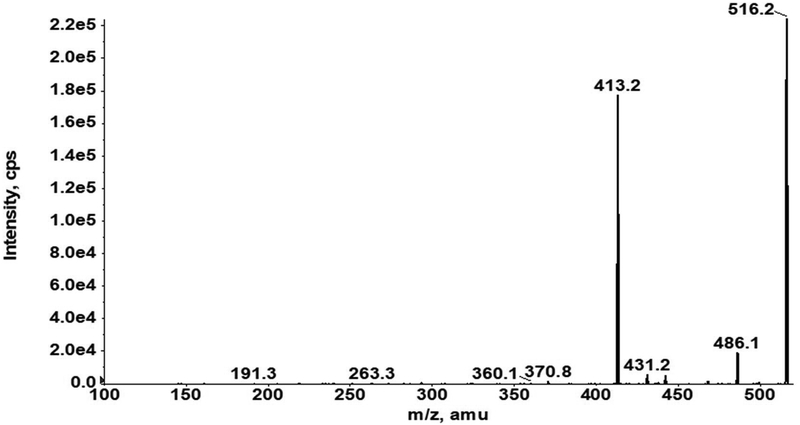
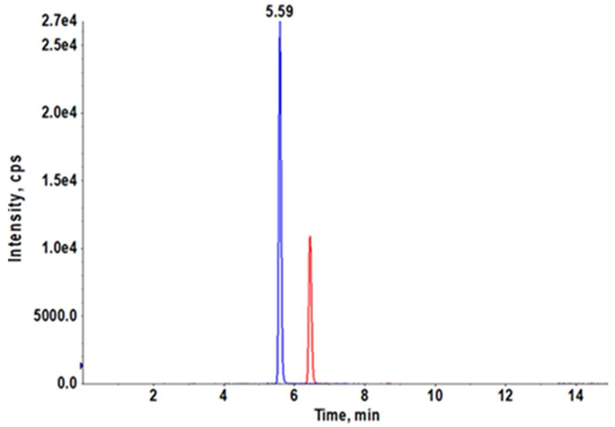
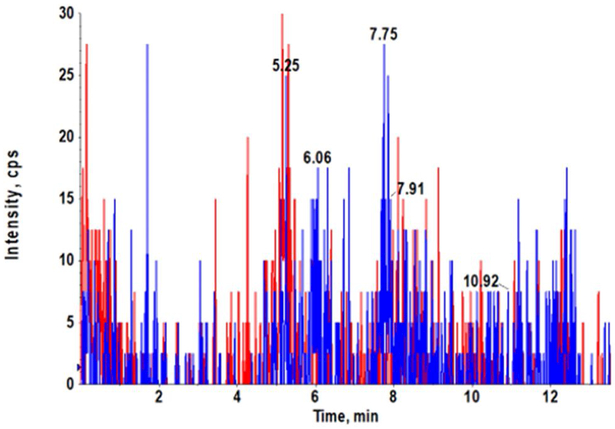
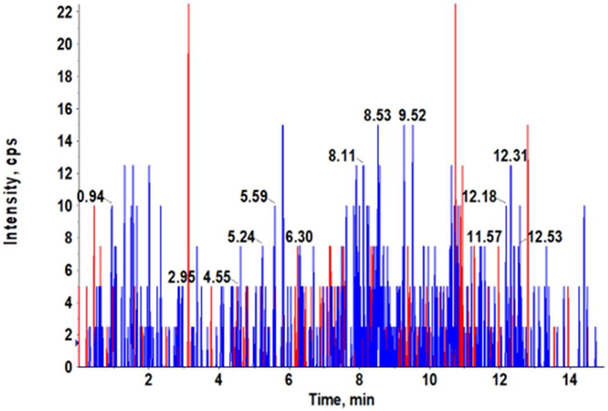
Our AZD3965 method was optimized to obtain good peak shapes, chromatographic separation and reproducibility. The Q1 (first quadruple) full scan and product ion scan mass spectra of AZD3965 are presented in Fig. 1A and 1B, respectively. Q1/Q3 m/z ratio for the precursor/product ion of AZD3965 and AR-C15585 (I.S.) was 516.4/413.2 and 462.3/373.2, respectively. The representative chromatograms of mouse plasma and tumor tissue homogenate spiked with AZD3965 and I.S. are shown in Fig. 2A and2B, respectively. The retention time of AZD3965 and AR-C155858 (I.S.) were 5.77 and 4.91 min, respectively. Additional system suitability parameters such as tailing factor and theoretical plates were also evaluated and the results are summarized in Table 1. No peaks were observed in the blank mouse plasma and tumor tissue samples at the retention time of AZD3965 and I.S. (Fig. 2C and 2D), indicating no matrix interference is presence.
Fig. 1.
Q1 full scan (A) and product ion scan (B) mass spectra of AZD3965. The mass spectra of AZD3965 was obtained from direct injection of 5 μg/mL of AZD3965 in acetonitrile/water (40/60, v/v) into the mass spectrometer.
Fig. 2.
Representative chromatograms of LC/MS/MS analyses of mouse plasma (A) and tumor tissue (B) spiked with AZD3965 (10 ng/mL) and internal standard (15 ng/mL). Chromatograms of double blank mouse plasma (C) and tumor tissue (D). AZD3965 and AR-C155858 (I.S.) peaks are denoted in red and blue, respectively. The retention times of AZD3965 and AR-C1558585 were 5.77 and 4.91 min, respectively.
Table 1.
System suitability parameters of AZD3965 and AR-C155858 (I.S.)
| Standard Curve |
AZD3965 | AR-C155858 | ||
|---|---|---|---|---|
| TF | N | TF | N | |
| Low | 1.09 | 2.66×104 | 1.09 | 2.14×104 |
| High | 1.12 | 2.5×104 | 1.15 | 3.08×104 |
TF, tailing factor measured at 5% of peak height; N, number of theoretical plates.
3.2. Validation of the Assay
3.2.1. Linearity and Lower Limit of Quantification
The calibration curves were linear over the AZD3965 concentration range of 0.15–500 ng/mL in both plasma and tumor tissue. The high concentration calibration curves were also linear over the AZD3965 concentration range of 100–12000 ng/mL in plasma. The correlation coefficients (r2 values) were all greater than 0.999. Using our assay, the LLOQ and ULOQ for AZD3965 was found to be 0.15 and 12000 ng/mL, respectively in plasma. The LLOQ in tumor tissue was found to be 0.15 ng/mL.
3.2.2. Precision and Accuracy
Intra-day and inter-day precision and accuracy were evaluated by analyzing QC samples (low, medium and high concentration) in both plasma and tumor tissue matrix in triplicate. The results of the assay performance are summarized in Table 2 and 3. The intra-day and inter-day precision and accuracy of the assay were less than ±7% of the nominal concentrations.
Table 2.
AZD3965 recovery, matrix effect, intra-day and inter-day accuracy and precision in mouse plasma. The measured concentrations represent the mean of triplicate measurements. The analyses were performed over 3 days.
| Intra-day | Inter-day | Recovery | Matrix Effect |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard Curve |
Nominal concentration (ng/mL) |
Mean (ng/mL) |
SD | Precision (CV%) |
Accuracy (%) |
Mean (ng/mL) |
SD | Precision (CV%) |
Accuracy (%) |
% | % | SD |
| Low | 10 | 10.3 | 0.06 | 0.56 | 103 | 10.1 | 0.225 | 2.23 | 101 | 101 | 103 | 4.18 |
| 150 | 153 | 4.93 | 3.22 | 102 | 148 | 4.36 | 2.94 | 98.9 | 101 | -- | -- | |
| 400 | 415 | 8.33 | 2.01 | 104 | 402 | 17.7 | 4.39 | 101 | 102 | 108 | 1.93 | |
| High | 1,000 | 1.04×103 | 15.3 | 1.47 | 104 | 1.01×103 | 30.1 | 2.98 | 101 | 103 | 101 | 1.84 |
| 6,000 | 6.00×103 | 124 | 2.07 | 100 | 5.96×103 | 144 | 2.42 | 99.4 | 101 | -- | -- | |
| 10,000 | 9.76×103 | 262 | 2.68 | 97.6 | 9.57×103 | 161 | 1.68 | 95.7 | 105 | 97.8 | 1.41 | |
Table 3.
AZD3965 matrix effect, intra-day and inter-day accuracy and precision in the murine 4T1 breast tumor tissue. The measured concentrations represent the mean of triplicate measurements. The analyses were performed over 3 days.
| Intra-day | Inter-day | Matrix Effect |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal concentration (ng/mL) |
Mean (ng/mL) |
SD | Precision (CV%) |
Accuracy (%) |
Mean (ng/mL) |
SD | Precision (CV%) |
Accuracy (%) |
% | SD | |
| 10 | 10.2 | 0.12 | 1.13 | 102 | 9.93 | 0.932 | 9.39 | 99.3 | 101 | 3.59 | |
| 150 | 140 | 1.53 | 1.09 | 93.6 | 140 | 1.58 | 1.13 | 93.2 | -- | -- | |
| 400 | 406 | 17.5 | 4.03 | 102 | 384 | 21.9 | 5.71 | 95.9 | 98.8 | 5.27 | |
3.2.3. Effect of Dilution
A total of six replicate of plasma spiked with AZD3965 (duplicate of each of high calibration QC concentrations) were diluted 10-fold with blank plasma were evaluated for sample dilution integrity. The average accuracy and CV% from the plasma dilution were 101% and 5.37%, respectively.
3.2.4. Recovery and Matrix Effect
The recovery of AZD3965 in plasma is shown in Table 2 and the overall mean extraction was 102%. The matrix effects of AZD3965 in both matrices are summarized in Table 2 and 3 and the results indicate no matrix effect in mouse plasma and mouse tumor tissue.
3.2.5. Stability
Our stability studies demonstrated AZD3965 was stable under different storage conditions. The concentrations of AZD3965 from the stability analysis in mouse plasma (long term, three freeze-thaw cycles and post-preparation) were all less than ±15% of the nominal values. The stock solutions were demonstrated to be stable in −20 °C for a least 12 months with standard deviations less than 10% of the mean.
3.3. Application in a PK and Breast Tumor Xenograft Studies
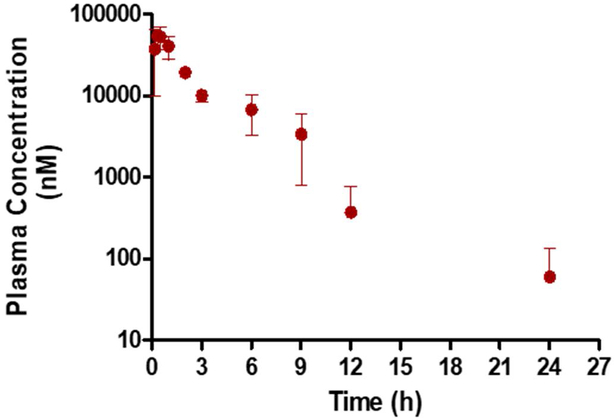
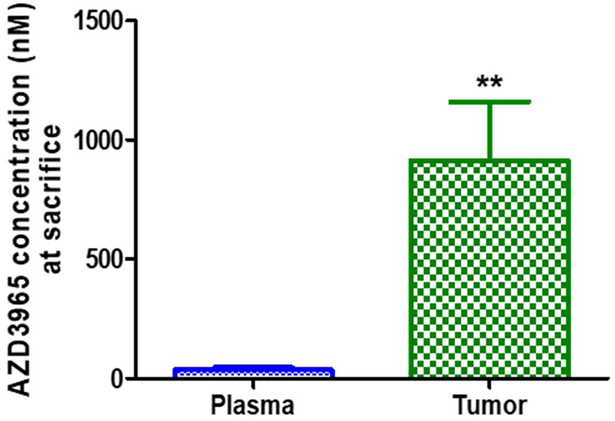
The applicability of the validated assay was demonstrated by analyzing plasma samples obtained in a preliminary pharmacokinetic study in mice. The pharmacokinetic parameters of AZD3965 after oral administration demonstrated a half-life of 4.45 h, maximal plasma concentration of 54.7 nmol/mL, time of maximal concentration of 0.333 h, oral clearance (clearance/bioavailability (CL/F)) of 1.41 L/h/kg, and apparent volume of distribution (V/F) of 9.07 L/kg. The AZD3965 concentration time profile in plasma following an oral dose of 100 mg/kg is presented in Fig. 3A. The applicability of the validated assay was also applied to assess total tumor AZD3965 concentrations in a preliminary murine 4T1 breast tumor xenograft study. Plasma and total tumor AZD3965 concentrations measured 12 h after the last AZD3965 dose (100 mg/kg twice daily for 17 days) are presented in Fig. 3B.
Fig. 3.
Plasma concentration of AZD3965 after oral administration of 100 mg/kg of AZD3965 in female Balb/c mice (A). Plasma and total tumor AZD3965 concentration measured 12 h after the last AZD3965 dose (100 mg/kg, i.p) in the murine 4T1 breast tumor xenograft (B). Assuming tumor density of 1 g/cm3, tumor AZD3965 concentrations were normalized per gram of tumor tissue and expressed as nM. ** P< 0.01, compared to the plasma AZD3965 concentration (nonparametric Student’s t-test). All the data are presented as mean ± SD, n=3.
4. DISCUSSION
Studies have demonstrated potency and efficacy of AZD3965 in tumor xenograft models such as those of human small cell lung cancer and various types of lymphoma and breast cancer [9–14]. AZD3965, a first-in-class monocarboxylate transporter 1 (MCT1) inhibitor is being evaluated in a Phase I clinical trial in the UK for solid tumors and lymphoma treatment (NCT01791595). However, the PK of AZD3965 and the concentration-effect relationships of AZD3965 have not been previously investigated. Although free plasma AZD3965 concentrations in a Raji lymphoma tumor xenograft model has been reported by Curtis et al. [15], there is no report of an analytical method developed and validated for quantification of AZD3965 concentration in biological fluids. Therefore, in our current study, we developed and validated an LC/MS/MS assay for use in preclinical animal studies. Previously, our laboratory has reported a preliminary validated LC/MS/MS assay for AR-C155858 (an analogue of AZD3965) in rat plasma [8]. With the incorporation of AR-C155858 as an internal standard, our current LC/MS/MS assay was successfully validated in mouse plasma and 4T1 breast tumor tissue over a wide range of AZD3965 concentrations with high selectivity and good precision and accuracy.
From our preliminary PK study, the mean maximum plasma concentration occurred around 0.33 h, indicating the oral absorption of AZD3965 is relatively fast. This short tmax observed in our study was in good agreement with previously reported results [15]. The mice used in our PK study were not fasted, and we observed a small reentry peak in plasma of AZD3965 between 6 and 9 hours, which might suggest the enterohepatic recycling of AZD3965. The apparent volume of distribution was 9.07 L/kg indicating extensive tissue distribution and binding is likely. Further AZD3965 PK studies are needed to confirm our findings. In our murine 4T1 breast tumor xenograft study, the total tumor AZD3965 concentration was found to be significantly higher (25.5-fold higher) than that in the plasma, suggesting extensive tumor uptake and accumulation of AZD3965.
5. CONCLUSION
The LC/MS/MS assay described in our current study shows high selectivity, precision and accuracy for the determination of AZD3965 in mouse plasma and tumor tissue over a wide range of concentrations. The assay was validated according to the FDA guidelines on analytical procedure and method validation. Our assay was successfully applied for the analysis of plasma samples in preliminary pharmacokinetic and breast tumor xenograft studies of oral AZD3965 in mice.
Highlights.
A validated LC/MS/MS assay is developed for quantifying a wide range of AZD3965 concentrations in biological fluids with high selectivity.
The assay is successfully used to analyze AZD3965 concentrations in pharmacokinetic and breast cancer tumor xenograft studies in mice.
AZD3965 shows extensive tumor accumulation in a murine 4T1 breast cancer tumor model.
ACKNOWLEDGMENTS
We thank Kristin Follman for her assistance in assay development.
FUNDING
This work was funded by the National Institute of Health National Institute on Drug Abuse [grant DA023223]. X.G. was funded in part by an Allen Barnett Fellowship.
ABBREVIATIONS:
- LC/MS/MS
liquid chromatography tandem mass spectrometry
- APCI
atmospheric pressure chemical ionization
- I.S.
internal standard
- QC
quality control
- MCTs
monocarboxylate transporters
- LLOQ
lower limit of quantification
- QC
quality control
- ULOQ
upper limit of quantification
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors report no conflict of interest.
REFERENCES
- 1.Pinheiro C, et al. , Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J Biomed Biotechnol, 2010. 2010: p. 427694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinheiro C, et al. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol, 2011. 26(10): p. 1279–86. [DOI] [PubMed] [Google Scholar]
- 3.Pinheiro C, et al. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology, 2010. 56(7): p. 860–7. [DOI] [PubMed] [Google Scholar]
- 4.Sonveaux P, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest, 2008. 118(12): p. 3930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colen CB, et al. Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study. Neoplasia, 2011. 13(7): p. 620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong SC, et al. Monocarboxylate Transporters MCT1 and MCT4 Regulate Migration and Invasion of Pancreatic Ductal Adenocarcinoma Cells. Pancreas, 2016. 45(7): p. 1036–47. [DOI] [PubMed] [Google Scholar]
- 7.Doherty JR, et al. Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis. Cancer Res, 2014. 74(3): p. 908–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijay N, Morse BL, and Morris ME, A Novel Monocarboxylate Transporter Inhibitor as a Potential Treatment Strategy for gamma-Hydroxybutyric Acid Overdose. Pharm Res, 2015. 32(6): p. 1894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bola BM, et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol Cancer Ther, 2014. 13(12): p. 2805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izumi H, et al. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci, 2011. 102(5): p. 1007–13. [DOI] [PubMed] [Google Scholar]
- 11.Polanski R, et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin Cancer Res, 2014. 20(4): p. 926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, et al. Synthesis and structure-activity relationships of pteridine dione and trione monocarboxylate transporter 1 inhibitors. J Med Chem, 2014. 57(17): p. 7317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble RA, et al. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica, 2017. 102(7): p. 1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong CS, et al. MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4. Cell Rep, 2016. 14(7): p. 1590–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis NJ, et al. Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt’s lymphoma anti-tumor activity. Oncotarget, 2017. 8(41): p. 69219–69236. [DOI] [PMC free article] [PubMed] [Google Scholar]