Abstract
There are a number of reports indicating correlation between outbreaks of campylobacteriosis and the consumption of raw vegetables. This study is a meta-analysis on the prevalence of Campylobacter in fresh vegetables and fruits without any location limitation, which was performed through a documented review of the available resources. Relevant literature was reviewed by trained reviewers, who examined the results for the inclusion of articles in the meta-analysis. The prevalence of Campylobacter in raw vegetables, the sample source, the Campylobacter species, and the method of detection were extracted. The prevalence of Campylobacter in vegetables, fruits, and fresh produce were estimated to be 0.53%. Analysis of the various sample groups initially showed that the bean and sprouts group was the vegetable with the highest prevalence of Campylobacter (11.08%). The rate of contamination was higher when both the molecular and conventional methods were employed. The highest prevalence of Campylobacter was found in Asia (33.4%). Despite the low prevalence, consumption of raw vegetables is inherently risky because no treatment is used to inactivate the pathogens. Therefore, proper sanitation methods are recommended to treat the raw products.
Keywords: Campylobacter, Raw vegetable, Foodborne infections
Background
In recent years, it is emphasized that consuming the organic food is associated with a healthier lifestyle. Thus, new food consumption trends indicate that people are interested in freshly produced organic foods. Among them, the consumption of fresh cut or minimally-processed fruit and vegetables have undergone a sharp increase. Such trends have been reflected in an increase in the popularity of salad bars in many countries [1–3]. In terms of retail, vegetables can be sold intact or minimally processed to provide a ready-to-eat product and can be contaminated at any point in the chain, starting from the farm to the plate. As they are not subjected to any treatment to eliminate pathogens, a diverse range of human enteric pathogens can contaminate them. There are a number of reports showed the correlation between foodborne illness outbreaks and the consumption of raw vegetables, annually [4, 5]. Several bacterial pathogens have been implicated in foodborne illnesses associated with the consumption of raw vegetables, such as Salmonella spp., thermo-tolerant Campylobacter, Listeria monocytogenes, and certain enteric viruses [6]. These may contaminate vegetables during any stage of production. The yearly average frequency of foodborne outbreaks linked with fresh produce contamination between 2002 and 2012 was reported by Wadamori et al. [7] with the prevalence of 57% (USA), 8% (Japan), and 6% (New Zealand). Infection by Campylobacter spp., specifically Campylobacter jejuni and Campylobacter coli, are the major cause of the mild bacterial diarrhea disease in the world [8]. Campylobacter spp. is estimated as the third most common bacterial cause of foodborne illness, but relatively few outbreaks have been detected [5]. Studies in high-income countries have estimated the annual incidence between 4.4 and 9.3 per 1000 population. While, the disease is usually self-limiting within 3–7 days, an acute infection can have serious long-term consequences, including severe neurological dysfunctions, such as Guillain–Barré syndrome (GBS) and Miller Fisher syndrome (MFS), and functional bowel diseases, such as irritable bowel syndrome (IBS) [9]. In 2013, the overall national incidence of campylobacteriosis infections per 100,000 population was estimated to be 6.621, which led to 1010 hospitalizations and 12 death [10]. In 2011, the Euro surveillance editorial team reported that out of a total of 5048 outbreaks of foodborne diseases, Campylobacter was responsible for 220,209 cases which occurred in the European Union (EU) [11]. It has been estimated that 75% [12] and 82% [13] of Campylobacter disease in Australia was associated with food. Most fruits and many vegetables are typically consumed raw and may also be as an important vehicle for Campylobacter spp. It is essential to assess Campylobacter as a relevant microbial risk for raw vegetables, fruits and minimally processed packaged salads, because can be pail of the indigenous microflora of fresh produce. A number of reports refer to fresh produce harboring potential foodborne pathogens. Lettuce and spinach are described in the international literature as the main vegetable sources of human infection by Campylobacter spp. [1, 16, 25, 26]. An increased interest in the campylobacteriosis risk assessment of raw vegetables is driven by several outbreaks of infections caused by consumption of fresh produce, such as leafy vegetables and salads [14], lettuce [15], and sprout and cabbage [16]. Studies have revealed that travelling to Asia, Africa, Latin America, the Caribbean, and Southern Europe significantly increased the risk of acquiring campylobacteriosis as compared to travelling within Western Europe [17–19]. Between 2004 and 2012, total of seven and three outbreaks of campylobacteriosis associated with the consumption of fresh vegetables have occurred in the United States and Europe, respectively [20]. Studies such as Evans et al. [21]; Mellou et al. [22] and Danis et al. [3] reported that fresh vegetables and fruits could be considered as risk factors for Campylobacter infection.
Role of fresh vegetable as a risk factor in campylobacteriosis, was previously addressed. Previous studies reported different prevalence of infection in assorted fresh vegetables. Present systematic review and meta-analysis study was aimed to focus on the more precise prevalence of infection. Therefore our study will be useful to find out the role of each vegetable to cause the infection.
Methods
Search strategy
A comprehensive scientific search on the presence of Campylobacter spp. in freshly produced food was carried out in three valid electronic global databases: PubMed, Scopus, and Science Direct using the same keywords. The search was performed through systematic research from the year 1990 till 2017. Keywords used to filter through the databases were: Campylobacter, vegetable, lettuce, spinach, leafy vegetable, sprout, fruits, salad, rocket, onion, carrot, cilantro, tomato, cucumber, broccoli, cabbage, cantaloupe, parsley, arugula, pepper, blueberry, strawberry, apple, peach, and melon. Articles containing any of these keywords in their abstracts or titles were included. A total of 135 articles were finally selected.
Study selection
After screening these relevant abstracts, 80 articles were selected. Articles that did not use the English language in the main text, review articles, and book chapters, as well as publications, related to the surveillance of case control study, risk factors, outbreaks of campylobacteriosis, genotyping, food handlers with their hygienic practices, and artificially contaminated samples were excluded from the study. Thereafter, full text screening of all the eligible primary studies was carried out from the databases. In case that full text of the articles were not available, they were finally excluded. To improve the reliability, our included articles was screened by two independent researchers.
Data extraction
Population of the study included vegetables, fruits, and freshly produced food investigated in each relevant primary study. Food that has been considered as fresh produces in this study are vegetables [fresh cut, organic, leafy, root crops, and ready-to-eat (RTE)], beans and sprouts, salad (mixed, gravy), and fruits (fresh cut, mixed, or fruit crops). Various samples were collected from restaurants, retail shops, farm, supermarkets, and ready-to-eat street-vended foods. Studies that apply any treatment, such as heat, pressure, irradiation, and bactericidal on fresh produce, and those found to report effects of cross-contamination were disregarded from the assay. Different kinds of salads and vegetables were categorized into a few subgroups.
Statistical analysis
All the data was analyzed using the Stata® 13.0 software (StataCorp LP., College Station, Texas, USA). Confidence interval of the prevalence rate of Campylobacter spp. in every study was calculated on the basis of binomial proportion formula. Statistical heterogeneity was assessed with the help of the I2 and Chi square test. For heterogeneity recognition, p < 0.05 and I square > 50%. Random-effects model was used to calculate the prevalence estimate after the heterogeneity test.
Results and discussion
Systematic review
Search results and selection of studies
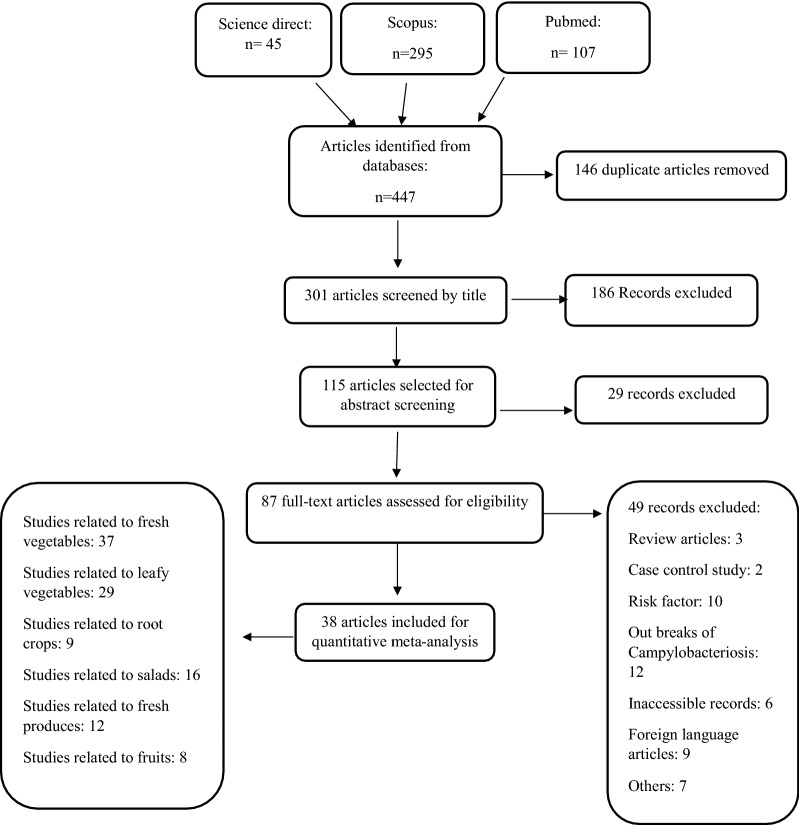
Following research using electronic global databases, a list of titles and abstracts from all the articles provided by the researcher was evaluated independently based on the selected keywords and elimination of similar articles in order to determine and select related topics. From a total of 447 records, at least 301 studies selected as related articles. These articles were assessed by their titles; 115 articles were included. After screening of relevant abstracts, full text of 87 articles were obtained and assessed for eligibility. Out of these, 49 studies were excluded based on inclusion and exclusion criteria mentioned in the methodology. Considering all the requirements, at least 38 studies were finally included in the quantitative meta-analysis. Some studies related to basic scientific, quality, quantity, and methodologies were selected for additional assessment (Fig. 1). All the selected articles were classified based on total samples, prevalence, commodity, isolation method, and region, and were collected for the preparation of a check list by the researcher. Sample collections were grouped into seven categories: vegetables, RTE vegetables, leafy vegetables, root crops, salad, beans and sprouts, and fruit and evaluated using two dimensions of scientific principles and methodology accuracy.
Fig. 1.
Flowchart stages of the entry studies into a systematic review and meta-analysis
Characteristics of studies and data extraction
The summary plan of this study has been presented in Table 1. Although in most studies the prevalence of Campylobacter was low, the highest prevalence of Campylobacter spp. was reported by Khalid et al. [16]. Out of the seven food categories, freshly produced food showed the highest prevalence, while the lowest rate of contamination was associated with the consumption of salads. Among Campylobacter species, C. jejuni has reflected the highest prevalence in targeted population, while only one study confirmed the isolation in lettuce. The major detection methods were included the selective culture, molecular, and a combination of culture/molecular techniques. The presence of pathogen was confirmed by of the selective culture method (n = 29). Thirty studies were performed to isolate different species of Campylobacter regardless of any limitation. This analysis revealed seven researches in Asia, three in Africa, nineteen in Europe, one in Oceania, two in South America, and six in North America.
Table 1.
Information of included studies in the meta-analysis of prevalence of Campylobacter spp. in vegetables, fruits and fresh produces
| References | Na | nb | P (%) | 95% Cl | Cam.Sp | Sample | Method | Country | V (g)c |
|---|---|---|---|---|---|---|---|---|---|
| [1] | 5 | 0 | 0 | 0–49.06 | spp. | Arugula | Culture | Spain | 25 |
| 18 | 0 | 0 | 0–20.95 | spp. | Carrot | Culture | Spain | 25 | |
| 21 | 0 | 0 | 0–18.63 | spp. | Corn salad | Culture | Spain | 25 | |
| 21 | 0 | 0 | 0–18.63 | spp. | Endive | Culture | Spain | 25 | |
| 29 | 0 | 0 | 0–14.1 | spp. | Lettuce | Culture | Spain | 25 | |
| 10 | 0 | 0 | 0–32.17 | spp. | Spinach | Culture | Spain | 25 | |
| 15 | 0 | 0 | 0–21.28 | spp. | Sprouts | Culture | Spain | 25 | |
| 132 | 0 | 0 | 0–3.37 | spp. | Mixed salads | Culture | Spain | 25 | |
| 21 | 0 | 0 | 0–18.63 | spp. | Fresh–cut fruit | Culture | Spain | 25 | |
| 28 | 0 | 0 | 0–14.63 | spp. | Whole vegetables | Culture | Spain | 25 | |
| [23] | 40 | 0 | 0 | 0–9.75 | spp. | Fresh vegetable | Culture | Austria | 25 |
| 36 | 0 | 0 | 0–11.75 | spp. | Mixed salad | Culture | Austria | 25 | |
| [24] | 128 | 0 | 0 | 0–3.58 | spp. | Lettuce | Culture | Canada | 25 |
| 59 | 0 | 0 | 0–7.37 | spp. | Spinach | Culture | Canada | 25 | |
| 129 | 0 | 0 | 0–3.56 | spp. | Green onions | Culture | Canada | 25 | |
| 206 | 0 | 0 | 0–2.26 | spp. | Carrots | Culture | Canada | 25 | |
| 120 | 0 | 0 | 0–3.8 | spp. | Tomatoes | Culture | Canada | 25 | |
| 31 | 0 | 0 | 0–13.38 | spp. | Strawberry | Culture | Canada | 25 | |
| [25] | 40 | 2 | 5 | 0–11.75 | jejuni | Lettuce | Molecular | Brazil | 25 |
| 40 | 1 | 2.5 | 0–7.33 | coli | Lettuce | Molecular | Brazil | 25 | |
| 40 | 0 | 0 | 0–10.62 | spp. | Spinach | Molecular | Brazil | 25 | |
| [26] | 80 | 0 | 0 | 0–4.6 | spp. | Strawberry | Culture/molecular | Belgium | 25 |
| 241 | 8 | 3.3 | 1.7 –6.4 | spp. | Leafy greens | Culture/molecular | Belgium | 25 | |
| [8] | 40 | 4 | 10 | 0.7–19.3 | spp. | Yard long bean | Culture/molecular | Malaysia | 10 |
| 39 | 18 | 46.1 | 31–61 | spp. | Winged bean | Culture/molecular | Malaysia | 10 | |
| 41 | 23 | 56.09 | 41–71 | spp. | Mung bean sprout | Culture/molecular | Malaysia | 10 | |
| 36 | 20 | 55.5 | 40–70 | spp. | Vietnamese coriander | Culture/molecular | Malaysia | 10 | |
| 39 | 21 | 53.8 | 38–70 | spp. | Japanese parsley | Culture/molecular | Malaysia | 10 | |
| 37 | 22 | 59.4 | 43–74 | spp. | Indian pennywort | Culture/molecular | Malaysia | 10 | |
| 38 | 13 | 34.2 | 19–49 | spp. | Wild cosmos | Culture/molecular | Malaysia | 10 | |
| [27] | 49 | 4 | 8.16 | 0–15.7 | spp. | Vegetable from farm | Culture/molecular | Malaysia | 10 |
| [28] | 27 | 0 | 0 | 0–14.3 | jejuni | Vegetable | Culture | Vietnam | 250 |
| [29] | 5170 | 0 | 0 | 0–0.09 | spp. | Leafy vegetables | Culture | Canada | 25 |
| 3696 | 0 | 0 | 0–0.13 | spp. | Leafy herbs | Culture | Canada | 25 | |
| [30] | 400 | 2 | 0.5 | 0.0–1.2 | jejuni | Grated vegetables | Culture/molecular | France | 20 |
| [31] | 50 | 1 | 2 | 0.0–5.88 | spp. | Parsley | Culture/molecular | Mexico | 25 |
| [32] | 88 | 8 | 9 | 3.02–14.97 | spp. | Lettuce | Culture | Belgium | 25 |
| [15] | 48 | 4 | 8.3 | 0.5–16.1 | spp. | Greenhouse lettuce | Culture | Belgium | 25 |
| 40 | 4 | 10 | 0.7–19.3 | spp. | Open field farm lettuce | Culture | Belgium | 25 | |
| [33] | 22 | 9 | 40.9 | 19.52–60.47 | jejuni | Vegetable/fruit salads | Culture | Pakistan | 10 |
| [34] | 80 | 0 | 0 | 0–5.5 | spp. | Strawberry | Culture/molecular | Norway | 10 |
| [16] | 61 | 22 | 36.06 | 24–48 | jejuni | Winged bean | Culture/molecular | Malaysia | 10 |
| 60 | 40 | 66.6 | 54–78 | jejuni | Long yard bean | Culture/molecular | Malaysia | 10 | |
| 20 | 11 | 55 | 34–76 | jejuni | Indian pennywort | Culture/molecular | Malaysia | 10 | |
| 47 | 20 | 42.5 | 28.4–56.6 | jejuni | Japanese parsley | Culture/molecular | Malaysia | 10 | |
| 10 | 7 | 70 | 42–98 | jejuni | Vietnamese coriander | Culture/molecular | Malaysia | 10 | |
| 23 | 12 | 52.2 | 31.6–72.4 | jejuni | Cucumber | Culture/molecular | Malaysia | 10 | |
| 30 | 21 | 70 | 54–86 | jejuni | Cabbage | Culture/molecular | Malaysia | 10 | |
| 10 | 8 | 80 | 56–104 | jejuni | Mung bean sprout | Culture/molecular | Malaysia | 10 | |
| 70 | 50 | 71.4 | 70–81.9 | jejuni | Wild cosmos | Culture/molecular | Malaysia | 10 | |
| [35] | 9 | 1 | 11.11 | 0–31.44 | jejuni | Spinach | Culture | India | 25 |
| 9 | 1 | 11.11 | 0–31.44 | jejuni | Fenugreek | Culture | India | 25 | |
| 9 | 0 | 0 | 0–34.86 | spp. | Cauliflower | Culture | India | 25 | |
| 9 | 0 | 0 | 0–34.86 | spp. | Cabbage | Culture | India | 25 | |
| 10 | 0 | 0 | 0–32.48 | spp. | Coriander | Culture | India | 25 | |
| 4 | 0 | 0 | 0–55 | spp. | Raddish | Culture | India | 25 | |
| 6 | 0 | 0 | 0–44.79 | spp. | Carrot | Culture | India | 25 | |
| [36] | 151 | 0 | 0 | 0–2.9 | spp. | Lettuce | Culture | UK | 25 |
| [37] | 1372 | 12 | 0.9 | 0.4–1.4 | spp. | Fresh leafy vegetable | Culture/molecular | Italy | 25 |
| 1160 | 6 | 0.5 | 0.1–0.9 | spp. | Ready to Eat vegetable | Culture/molecular | Italy | 25 | |
| [38] | 86 | 0 | 0 | 0–5.23 | spp. | Organic vegetable | Culture | North Ireland | 25 |
| [39] | 42 | 0 | 0 | 0–10.16 | spp. | RTE vegetables | Culture | Canada | 100 |
| [40] | 1260 | 0 | 0 | 0–0.36 | spp. | Fruit and vegetables | Culture | UK | 25 |
| 224 | 0 | 0 | 0–2.07 | spp. | Mixed salads | Culture | UK | 25 | |
| 226 | 0 | 0 | 0–2.05 | spp. | Coleslaw (Salad) | Culture | UK | 25 | |
| [41] | 12 | 0 | 0 | 0–28.7 | spp. | Salad | Culture | South Africa | 25 |
| [42] | 22 | 0 | 0 | 0–17.78 | jejuni | Salad/gravy prepared | Culture | South Africa | 20 |
| 22 | 0 | 0 | 0–17.78 | jejuni | Salad/gravy during holding | Culture | South Africa | 20 | |
| 22 | 0 | 0 | 0–17.78 | jejuni | Salad/gravy raw materials | Culture | South Africa | 20 | |
| [43] | 65 | 0 | 0 | 0–6.85 | spp. | RTU vegetables | Culture | Canada | 25 |
| 296 | 0 | 0 | 0–1.47 | spp. | RTU vegetable | Culture | Canada | 25 | |
| [44] | 183 | 2 | 1.09 | 0–2.4 | spp. | Spinach | Culture | Canada | 50 |
| 348 | 2 | 0.57 | 0–1.24 | spp. | Lettuce | Culture | Canada | 50 | |
| 174 | 2 | 1.15 | 0.0–2.65 | spp. | Radish | Culture | Canada | 200 | |
| 160 | 1 | 0.62 | 0–1.8 | spp. | Green onion | Culture | Canada | 50 | |
| 177 | 1 | 0.56 | 0–1.54 | spp. | Parsley | Culture | Canada | 50 | |
| 153 | 1 | 0.65 | 0.0–1.82 | spp. | Potatoes | Culture | Canada | 200 | |
| 150 | 0 | 0 | 0.0–3.09 | spp. | Celery | Culture | Canada | 50 | |
| 130 | 0 | 0 | 0.0–3.55 | spp. | Cabbage | Culture | Canada | 200 | |
| 149 | 0 | 0 | 0–3.09 | spp. | Carrot | Culture | Canada | 200 | |
| 123 | 0 | 0 | 0.0–3.61 | spp. | Cucumber | Culture | Canada | 200 | |
| 482 | 14 | 2.9 | 1.5–4.5 | spp. | Fresh vegetables | Culture | Canada | 50/200 | |
| [45] | 90 | 20 | 22.2 | 13.5–30.5 | spp. | MAP mixed salad | Culture | UK | 10 |
| [46] | 2870 | 0 | 0 | 0–0.165 | spp. | RTE salads | Culture | UK | 25 |
| [47] | 3852 | 0 | 0 | 0–0.122 | spp. | RTE salad vegetables | Culture | UK | 25 |
| [48] | 3200 | 0 | 0 | 0–0.148 | spp. | RTE organic vegetables | Culture | UK | 25 |
| [49] | 94 | 0 | 0 | 0–4.93 | spp. | Chicken salad | Culture/molecular | UK | 25 |
| 35 | 0 | 0 | 0–12 | spp. | Ham salad | Culture/molecular | UK | 25 | |
| 12 | 0 | 0 | 0–28.7 | spp. | Salmon salad | Culture/molecular | UK | 25 | |
| [50] | 28 | 0 | 0 | 0–14.6 | jejuni | Vegetable | Culture | Malawi | 10 |
| [51] | 40 | 0 | 0 | 0–10.6 | spp. | Vegetable | Culture | United States | 25 |
| [52] | 11 | 1 | 9.1 | 0–25.9 | jejuni | Cucumber | Culture | Malaysia | 25 |
| 9 | 0 | 0 | 0–34.8 | jejuni | Lettuce | Culture | Malaysia | 25 | |
| [53] | 55 | 0 | 0 | 0–7.85 | jejuni | Asparagus | Culture | New Zealand | 50 |
| 55 | 0 | 0 | 0–7.85 | jejuni | Mung bean sprouts | Culture | New Zealand | 50 | |
| 55 | 0 | 0 | 0–7.85 | jejuni | Watercress | Culture | New Zealand | 50 | |
| 55 | 0 | 0 | 0–7.85 | jejuni | Spinach | Culture | New Zealand | 50 | |
| 55 | 0 | 0 | 0–7.85 | jejuni | Silver beet | Culture | New Zealand | 50 | |
| [14] | 1157 | 2 | 0.17 | 0.02–0.62 | spp. | Fruit crops | Culture | Netherland | 25 |
| 196 | 0 | 0 | 0–1.86 | spp. | Root crops | Culture | Netherland | 25 | |
| 127 | 0 | 0 | 0–2.86 | spp. | Cabbage | Culture | Netherland | 25 | |
| 8 | 0 | 0 | 0–36.94 | spp. | Mushrooms | Culture | Netherland | 25 | |
| 42 | 0 | 0 | 0–8.41 | spp. | Onions, garlic | Culture | Netherland | 25 | |
| 50 | 1 | 2 | 0.05–10.65 | spp. | Stem and sprout crops | Culture | Netherland | 25 | |
| 2549 | 5 | 0.2 | 0.06–0.46 | spp. | Mixed salads/vegetables | Culture | Netherland | 25 | |
| 159 | 1 | 0.6 | 0.02–3.45 | spp. | Vegetable-fruit mix | Culture | Netherland | 25 | |
| 11 | 0 | 0 | 0–28.49 | spp. | Fruit | Culture | Netherland | 25 | |
| 779 | 2 | 0.3 | 0.03–0.92 | spp. | Mixed fruit | Culture | Netherland | 25 | |
| 562 | 2 | 0.36 | 0.04–1.28 | spp. | Leafy vegetables | Culture | Netherland | 25 | |
| [54] | 217 | 2 | 0.9 | 0.0–2.2 | jejuni | Mushrooms | Culture | Ireland | 10 |
| 62 | 0 | 0 | 0–7.11 | spp. | Vegetables/salad | Culture | Ireland | 10 | |
| [55] | 1810 | 3 | 0.22 | 0.06–0.48 | spp. | Raw vegetable | Culture | Netherland | 25 |
| 764 | 0 | 0 | 0–0.5 | spp. | Vegetable | Culture | Netherland | 25 | |
| 1151 | 0 | 0 | 0–0.4 | spp. | Vegetable | Culture | Netherland | 25 |
a Number of samples, b Number of positive samples, c Sample volume
Meta-analysis results
Overall prevalence
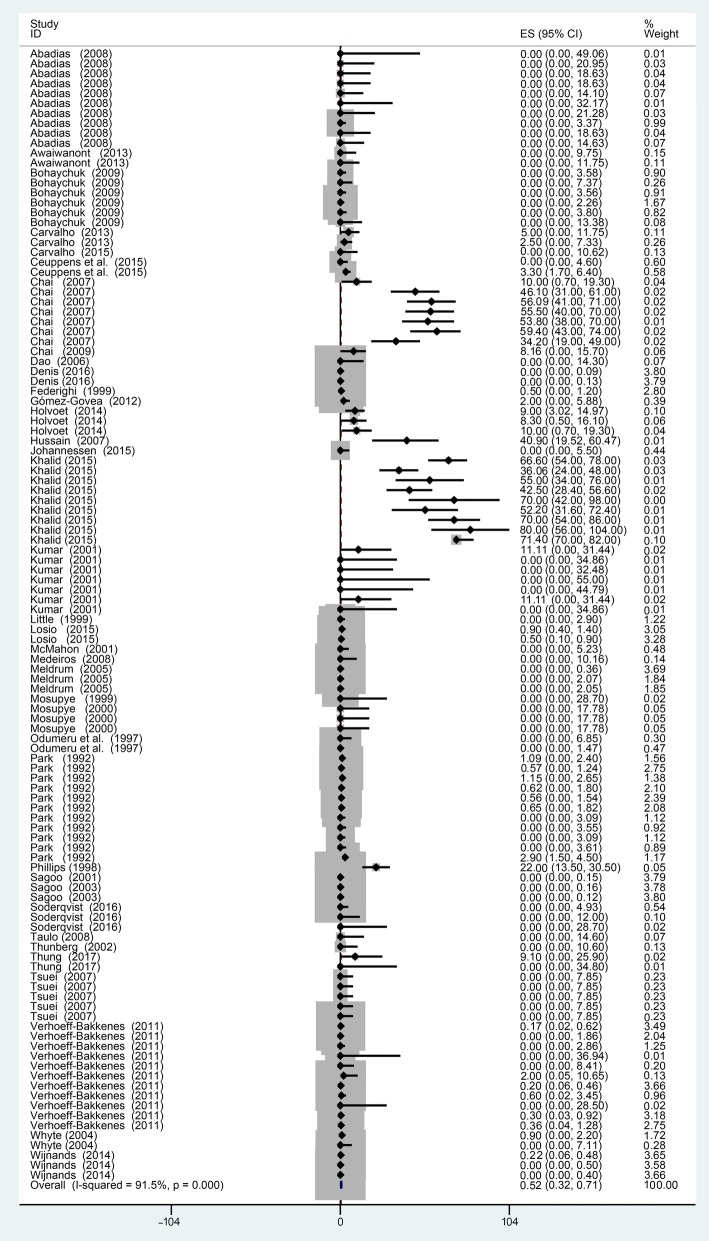
The total prevalence of Campylobacter in vegetables, fruits, and fresh products was estimated at 0.53% (Fig. 2). The results showed a low occurrence of Campylobacter based on the reports of Losio and Verhoeff-Bakkenes, where the prevalence was less than one percent in vegetables and fruits [30, 37]. Lower rates of isolation were probably due to problems in the growth and recovery of microorganisms. Based on many scientific research reports, foods of animal origin, such as raw milk [56], turkey, chicken, beef, pork [57] and manure [58] were considered as the major sources of Campylobacter spp. Hence, it is likely that the occurrence of Campylobacter spp. in the targeted resource of this study was due to cross-contamination during growth, irrigation, harvest, transportation, and further processing and handling. Danis and Pintar both supported this hypothesis [3, 59].
Fig. 2.
Forest plot for meta-analysis of Campylobacter spp. prevalence
Type of samples
All of the target samples included for this review have been listed in Table 2. Fresh produce, in particular fruit, does not receive any lethal treatment that kills all pathogens prior to consumption. Results related to the prevalence of pathogen in the different types of produce subgroups have been presented in Table 3. The results of the meta-analysis demonstrated that, among the different group of samples, the beans and sprouts (11.08%) revealed the highest prevalence, followed by the vegetable, detected in 1.73% of samples from supermarkets, retails, and farm lands. The minimum prevalence of Campylobacter was belong to the salad and fruit, which estimated at around 0.02% and 0.20%, respectively. As shown in Table 2, the highest prevalence of Campylobacter was found in the Indian pennywort and wild cosmos. Fields on which livestock or wild animals have grazed are more likely to be contaminated with enteric pathogens. Factors, such as bacterial presence in livestock, companion animals, wild animals, insects, and the natural environment, including soil and surface waters, lack of good agricultural practices (GAP), and cross-contamination with manure, could be related to the presence of pathogens in these vegetables [60]. Also, high prevalence was found in beans and sprouts. Lots of outbreak reports throughout the world have been linked to the consumption of raw and lightly cooked sprouts [61, 62]. Sprout production involves a unique seed germination process that can support the growth of pathogens because its germination is ideal for bacterial proliferation [63]. Additional factors, such as nutritive value, root nature of sprout, cross-contamination by manure, and irritation might have influenced the microbial contamination of these products. When manure is spread on agricultural fields, it possibly goes into the surface water. Hence, along with weak good manufacturing practice (GMP) and GAP, the presence of environmental bacteria may occur in food. Low prevalence in salad vegetables (0.02%) and fruits (0.20%) may be due to the accurate and sufficient attention paid towards hygiene of salad commodities and also sensitivity to acidic conditions (pH < 5.0) for fruits. Human or animal sources, as well as handling in the stores, may also be associated with increasing the microorganisms at the surface of fresh produce. The low temperature and lack of nutrients at the surface of fruits cause a reduction in Enterobacteriaceae during storage. It can also be due to the breaking of the cold chain during shelf-life or handling by the shoppers. Therefore, it is not surprising to find Campylobacter on the surface of fresh produce [64].
Table 2.
Meta-analysis of prevalence of Campylobacter in all of foods
| Sourcea | Total inputsb | Total sample sizec | Overall prevalence (%) | 95% confidence interval | I2 (%) | P for χ2 |
|---|---|---|---|---|---|---|
| Pennywort | 2 | 57 | 57.84 | 45.37–70.31 | 0.00 | 0.74 |
| Wild cosmos | 2 | 108 | 53.46 | 17.02–89.89 | 95.10 | 0.00 |
| Coriander | 3 | 56 | 41.00 | 0.00–83.65 | 93.60 | 0.00 |
| Bean | 4 | 200 | 39.47 | 13.81–65.13 | 94.70 | 0.00 |
| Sprouts | 5 | 171 | 23.68 | 6.68–40.68 | 95.60 | 0.00 |
| Parsley | 4 | 313 | 18.58 | 8.54–28.62 | 96.10 | 0.00 |
| Cucumber | 3 | 157 | 18.30 | 0.00–42.00 | 92.50 | 0.00 |
| Fenugreek | 1 | 9 | 11.11 | 0.00–26.83 | – | – |
| Cabbage | 4 | 296 | 10.42 | 2.38–18.45 | 95.90 | 0.00 |
| Lettuce | 10 | 921 | 1.53 | 0.12–2.94 | 54.00 | 0.02 |
| Radish | 2 | 178 | 1.14 | 0.00–2.47 | 0.00 | 0.93 |
| Spinach | 6 | 356 | 0.91 | 0.00–1.98 | 0.00 | 0.81 |
| Mushroom | 2 | 225 | 0.89 | 0.00–1.99 | 0.00 | 0.92 |
| Potato | 1 | 153 | 0.65 | 0.00–1.56 | – | – |
| Fresh cut vegetables | 2 | 421 | 0.50 | 0.00–1.10 | – | – |
| Green Onion | 2 | 289 | 0.49 | 0.00–1.29 | 0.00 | 0.54 |
| Fruits | 4 | 1968 | 0.21 | 0.00–0.45 | 0.00 | 0.97 |
| RTE vegetables | 5 | 4763 | 0.13 | 0.00–0.40 | 31.00 | 0.21 |
| Vegetables | 15 | 8535 | 0.12 | 0.00–0.28 | 38.40 | 0.06 |
| Leafy vegetables | 5 | 11,041 | 0.10 | 0.00–0.25 | 81.10 | 0.00 |
| Salad | 16 | 7692 | 0.02 | 0.00–0.26 | 63.50 | 0.00 |
| Onion | 1 | 42 | 0.00 | 0.00–4.20 | – | – |
| Crops | 1 | 196 | 0.00 | 0.00–0.93 | – | – |
| Beet | 1 | 55 | 0.00 | 0.00–3.92 | – | – |
| Water cress | 1 | 55 | 0.00 | 0.00–3.92 | – | – |
| Asparagus | 1 | 55 | 0.00 | 0.00–3.92 | – | – |
| Celery | 1 | 150 | 0.00 | 0.00–1.54 | – | – |
| Cauliflower | 1 | 9 | 0.00 | 0.00–17.43 | – | – |
| Strawberry | 3 | 191 | 0.00 | 0.00–1.70 | 0.00 | 1.00 |
| Tomatoes | 1 | 120 | 0.00 | 0.00–1.90 | – | – |
| Endive | 1 | 21 | 0.00 | 0.00–9.31 | 0.00 | 0.00 |
| Arugula | 2 | 60 | 0.00 | 0.00–24.53 | 0.00 | 0.00 |
| Carrot | 4 | 379 | 0.00 | 0.00–0.90 | 0.00 | 1.00 |
a Different type of fresh vegetables and fruits
b Number of distinctive prevalence values is reported
c Number of vegetable and fruit samples used to determine each estimate
Table 3.
Prevalence of Campylobacter in subgroups of freshly produced foods
| Sourcea | Total inputsb | Total sample sizec | Overall prevalence (%) | 95% confidence interval | I2 (%) | P for χ2 |
|---|---|---|---|---|---|---|
| Vegetables | ||||||
| Organic vegetable, asparagus, parsley, coriander, tomatoes, green onion, cucumber, endive, mushroom, arugula, cosmos, fenugreek, cauliflower, Celery | 39 | 10,094 | 1.73 | 1.04–2.41 | 95.10 | 0.00 |
| RTE vegetables | ||||||
| Fresh cut vegetables, RTU and RTE vegetables | 3 | 1602 | 0.49 | 0.16–0.83 | 0.00 | 0.98 |
| Leafy vegetables | ||||||
| Spinach, lettuce, cabbage, pennywort, water cress | 29 | 12,726 | 0.49 | 0.17– 0.82 | 87.00 | 0.00 |
| Root crops | ||||||
| Radish, potato, carrot, beet | 9 | 961 | 0.34 | 0.00–0.82 | 0.00 | 0.93 |
| Salad | ||||||
| MAP mixed salad, RTE salads, chicken salad, ham salad, salmon salad | 16 | 7692 | 0.02 | 0.00–0.26 | 63.50 | 0.00 |
| Bean and sprouts | ||||||
| Winged bean, long yard bean, sprouts, mung bean sprout | 12 | 3932 | 11.08 | 7.82–14.33 | 96.20 | 0.00 |
| Fruit | ||||||
| Fruits, strawberry, fruit salads | 8 | 2168 | 0.20 | 0.00–0.45 | 0 | 1.00 |
aSample collections were grouped into seven categories: vegetables, RTE vegetables, leafy vegetables, root crops, salad, beans and sprouts, and fruit
bNumber of distinctive prevalence values is reported
cNumber of vegetable and fruit samples used to determine each estimate
Campylobacter species
Results of the statistical analysis also showed that the highest prevalence of Campylobacter was observed for C. jejuni, with a percentage of 18.20%, whereas other Campylobacter spp. had the minimum prevalence, with a percentage of 0.23% (Table 4). Actually, among different species, C. jejuni showed the highest prevalence [54, 65]. It is worth mentioning that the aim of majority of the papers assessed in this study was to consider no specific species of Campylobacter. The highest prevalence of Campylobacter was identified by molecular approaches. C. jejuni mainly resided in the intestinal tract of warm-blooded animals and birds, and, therefore, the excreta may act as a source of contamination. Isolation of C. jejuni from vegetables was possibly due to the fecal contamination of these commodities and water at any step of the production chain. However, contact with the utensils used to process raw chicken was also important as they were the main reservoirs of C. jejuni [66]. In developed countries, C. jejuni was the most frequent cause of acute diarrheal infections. An improvement in the survival of C. jejuni in soil and rhizosphere is possibly a substantial factor in the environmental cycle of bacteria [67].
Table 4.
Prevalence values and sample sizes for Campylobacter species provided in Table 1
| Speciesa | Total inputsb | Total sample sizec | Overall prevalence (%) | 95% confidence interval | I2 (%) | P for χ2 |
|---|---|---|---|---|---|---|
| Campylobacter spp. | 86 | 37,682 | 0.23 | 0.11–0.35 | 77.8 | 0.000 |
| Campylobacter jejuni | 27 | 1444 | 18.20 | 13.63–22.77 | 97.2 | 0.000 |
| Campylobacter coli | 1 | 40 | 2.50 | 0.0–6.16 | _ | _ |
aDifferent species of Campylobacter
bNumber of distinctive prevalence values is reported
cNumber of vegetable and fruit samples used to determine each estimate
Methods of detection
Various isolation methods have been applied according to the literature. The results of the meta-analysis have shown on more than one method for better identification of the bacterium, and thus the estimated prevalence in this method was 21.52% (Table 5). Higher prevalence rates were reported using most probable number PCR (MPN-PCR) by Khalid et al. [16] and Chai et al. [8]. Additionally, there have been articles documenting the positive efficacy of this method for the isolation of food-borne pathogens in various food types. Norinaga et al. [68] compared two methods, MPN-PCR and MPN- thiosulfate citrate bile sucrose agar (MPN- TCBS agar), for the detection and enumeration of Vibrio parahaemolyticus in sea foods. The results showed that MPN-PCR was more convenient and reliable compared to MPN-TCBS, which was also supported by Luan et al. [69].
Table 5.
Prevalence values and sample sizes for detection method of Campylobacter
| Methoda | Total inputsb | Total sample sizec | Overall prevalence (%) | 95% confidence interval | I2 (%) | P for χ2 |
|---|---|---|---|---|---|---|
| Culture | 85 | 34,922 | 0.06 | 0.01–0.12 | 23.7 | 0.03 |
| Molecular | 3 | 120 | 2.38 | 0.0–5.07 | 0.0 | 0.46 |
| Culture/molecular | 26 | 4124 | 21.52 | 18.60–24.44 | 97.9 | 0.000 |
aDifferent method of detection
bNumber of distinctive prevalence values is reported
cNumber of vegetable and fruit samples used to determine each estimate
Strength and weaknesses of this study
In few studies, the heterogeneity as high as 75%. This finding indicated a high proportion of heterogeneity to assess weighted mean between studies. Factors influencing variations that were not clarified in our study may have associated with this heterogeneity. This phenomenon is common for this kind of study due to limited number of published data. One of the limitations was due to English inclusion criteria, therefore other non-English reports were not included in our study. Data for most Oceania, Africa and South American countries were inadequate for analysis. As such, we were not able to estimate the prevalence of campylobacter in fresh vegetables among those countries.
The current systematic review and meta-analysis was the first study estimating the prevalence of Campylobacter in different kinds of fresh vegetables and fruits in various geographical areas. In addition the specific role of each species of bacteria was studied. The more applicable method of detection was also investigated.
Conclusion
As final conclusion it seems that in spite of general low prevalence of the Campylobacter contamination in vegetable and fruits and the high level of consumption of these products raises it total risk of infection. Food chain is increasing the risk of contamination by different routes, for instances, primary production (the most effective one), postharvest contamination during transportation, food processing steps, packaging, distribution and cross contamination in the retail market are among the health hazards. Therefore, employing proper sanitation techniques is highly recommended during all the steps of food preparation.
Authors’ contributions
EB: study design; review relevant articles, analysis and interpretation of data; drafting and finalizing the manuscript; study supervision. HM, MM and SH: review relevant articles, analysis and interpretation of data; drafting the manuscript. MZ: analysis and interpretation of data; drafting the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by Shiraz University of Medical Sciences (Grant No. 1396-01-106-15153).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abadias M, Usall J, Anguera M, Solsona C, Vinas I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int J Food Microbiol. 2008;123(1–2):121–129. doi: 10.1016/j.ijfoodmicro.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Jongen W. Improving the safety of fresh fruit and vegetables. Boca Raton: CRC Press; 2005. [Google Scholar]
- 3.Danis K, Di Renzi M, O’Neill W, Smyth B, McKeown P, Foley B, Tohani V, Devine M. Risk factors for sporadic Campylobacter infection: an all-Ireland case-control study. Euro Surveill. 2009;14(7):19123. [PubMed] [Google Scholar]
- 4.Danyluk M, Goodrich-Schneider R, Schneider K, Harris L, Worobo R. Outbreaks of foodborne disease associated with fruit and vegetable juices, 1922–2010. EDIS publication, FSHN12-04; 2012. http://ucfoodsafety.ucdavis.edu/files/223883.pdf.
- 5.Painter JA, Hoekstra RM, Ayers T, Tauxe RV, Braden CR, Angulo FJ, Griffin PM. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg Infect Dis. 2013;19(3):407. doi: 10.3201/eid1903.111866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taban BM, Halkman AK. Do leafy green vegetables and their ready-to-eat [RTE] salads carry a risk of foodborne pathogens? Anaerobe. 2011;17(6):286–287. doi: 10.1016/j.anaerobe.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Wadamori Y, Gooneratne R, Hussain MA. Outbreaks and factors influencing microbiological contamination of fresh produce. J Sci Food Agri. 2017;97(5):1396–1403. doi: 10.1002/jsfa.8125. [DOI] [PubMed] [Google Scholar]
- 8.Chai LC, Robin T, Ragavan UM, Gunsalam JW, Bakar FA, Ghazali FM, Radu S, Kumar MP. Thermophilic Campylobacter spp. in salad vegetables in Malaysia. Int J Food Microbiol. 2007;117(1):106–111. doi: 10.1016/j.ijfoodmicro.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . The global view of campylobacteriosis: report of an expert consultation, Utrecht. Berlin: World Health Organization; 2013. p. 2012. [Google Scholar]
- 10.Crim SM, Iwamoto M, Huang JY, Griffin PM, Gilliss D, Cronquist AB, Cartter M, Tobin-D’Angelo M, Blythe D, Smith K. Incidence trends of infection with pathogens transmitted commonly through food. Foodborne Dis Act Surve Net US sites. 2014;10:2006–2013. [PMC free article] [PubMed] [Google Scholar]
- 11.Team EE. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011 has been published. EFSA J. 2013;18(5):20449. doi: 10.2903/j.efsa.2013.3129. [DOI] [PubMed] [Google Scholar]
- 12.Hall G, Kirk MD, Becker N, Gregory JE, Unicomb L, Millard G, Stafford R, Lalor K. OzFoodNet Working Group. Estimating foodborne gastroenteritis, Australia. Emerg Infect Dis. 2005;11(8):1257. doi: 10.3201/eid1108.041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unicomb LE, Fullerton KE, Kirk MD, Stafford RJ. Outbreaks of campylobacteriosis in Australia, 2001 to 2006. Foodborne Pathog Dis. 2009;6(10):1241–1250. doi: 10.1089/fpd.2009.0300. [DOI] [PubMed] [Google Scholar]
- 14.Verhoeff-Bakkenes L. Jansen HAPM, in ‘t Veld PH, Beumer RR, Zwietering MH, van Leusden FM. Consumption of raw vegetables and fruits: a risk factor for Campylobacter infections. Int J Food Microbiol. 2011;144(3):406–412. doi: 10.1016/j.ijfoodmicro.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Holvoet K, Sampers I, Seynnaeve M, Jacxsens L, Uyttendaele M. Agricultural and management practices and bacterial contamination in greenhouse versus open field lettuce production. Int J Environ Res Public Health. 2014;12(1):32–63. doi: 10.3390/ijerph120100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalid MI, Tang JY, Baharuddin NH, Rahman NS, Rahimi NF, Radu S. Prevalence, antibiogram, and cdt genes of toxigenic Campylobacter jejuni in salad style vegetables (ulam) at farms and retail outlets in Terengganu. J Food Prot. 2015;78(1):65–71. doi: 10.4315/0362-028X.JFP-14-109. [DOI] [PubMed] [Google Scholar]
- 17.Ekdahl K, Andersson Y. Regional risks and seasonality in travel-associated campylobacteriosis. BMC Infect Dis. 2004;4(1):54. doi: 10.1186/1471-2334-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakanen A, Jousimies-Somer H, Siitonen A, Huovinen P, Kotilainen P. Fluoroquinolone resistance in Campylobacter jejuni isolates in travelers returning to Finland: association of ciprofloxacin resistance to travel destination. Emerg Infect Dis. 2003;9(2):267. doi: 10.3201/eid0902.020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mughini-Gras L, Smid JH, Wagenaar JA, De Boer A, Havelaar AH, Friesema IH, French NP, Graziani C, Busani L, Van Pelt W. Campylobacteriosis in returning travellers and potential secondary transmission of exotic strains. Epidemiol Infect. 2014;142(6):1277–1288. doi: 10.1017/S0950268813002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callejón RM, Rodríguez-Naranjo MI, Ubeda C, Hornedo-Ortega R, Garcia-Parrilla MC, Troncoso AM. Reported foodborne outbreaks due to fresh produce in the United States and European Union: trends and causes. Foodborne Pathog Dis. 2015;12(1):32–38. doi: 10.1089/fpd.2014.1821. [DOI] [PubMed] [Google Scholar]
- 21.Evans MR, Ribeiro CD, Salmon RL. Hazards of healthy living: bottled water and salad vegetables as risk factors for Campylobacter infection. Emerg Infect Dis. 2003;9(10):1219. doi: 10.3201/eid0910.020823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellou K, Sourtzi P, Tsakris A, Saroglou G, Velonakis E. Risk factors for sporadic Campylobacter jejuni infections in children in a Greek region. Epidemiol Infect. 2010;138(12):1719–1725. doi: 10.1017/S0950268810001196. [DOI] [PubMed] [Google Scholar]
- 23.Awaiwanont N, Smulders FJ, Paulsen P. Microbiological quality of typical foods served in Thai restaurants in Vienna, Austria. J Food Safe Food Qual. 2013;64(3):78–83. doi: 10.2376/0003-925X-64-78. [DOI] [Google Scholar]
- 24.Bohaychuk V, Bradbury R, Dimock R, Fehr M, Gensler G, King R, Rieve R, Barrios PR. A microbiological survey of selected Alberta-grown fresh produce from farmers’ markets in Alberta, Canada. J Food Prot. 2009;72(2):415–420. doi: 10.4315/0362-028X-72.2.415. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho AF, Silva DM, Azevedo SS, Piatti RM, Genovez ME, Scarcelli E. Detection of CDT toxin genes in Campylobacter spp. strains isolated from broiler carcasses and vegetables in São Paulo, Brazil. Braz J Microbiol. 2013;44(3):693–699. doi: 10.1590/S1517-83822013000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceuppens S, Johannessen GS, Allende A, Tondo EC, El-Tahan F, Sampers I, Jacxsens L, Uyttendaele M. Risk factors for Salmonella, shiga toxin-producing Escherichia coli and Campylobacter occurrence in primary production of leafy greens and strawberries. Int J Environm Res Pub Health. 2015;12(2):9809–9831. doi: 10.3390/ijerph120809809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chai L, Ghazali F, Bakar F, Lee H, Suhaimi L, Talib S, Nakaguchi Y, Nishibuchi M, Radu S. Occurrence of thermophilic Campylobacter spp. contamination on vegetable farms in Malaysia. J Microbiol Biotechnol. 2009;19(11):1415–1420. doi: 10.4014/jmb.0901.0002. [DOI] [PubMed] [Google Scholar]
- 28.Dao HTA, Yen PT. Study of Salmonella, Campylobacter, and Escherichia coli contamination in raw food available in factories, schools, and hospital canteens in Hanoi, Vietnam. Ann N Y Acad Sci. 2006;1081(1):262–265. doi: 10.1196/annals.1373.033. [DOI] [PubMed] [Google Scholar]
- 29.Denis N, Zhang H, Leroux A, Trudel R, Bietlot H. Prevalence and trends of bacterial contamination in fresh fruits and vegetables sold at retail in Canada. Food Control. 2016;67:225–234. doi: 10.1016/j.foodcont.2016.02.047. [DOI] [Google Scholar]
- 30.Federighi M, Magras C, Pilet M, Woodward D, Johnson W, Jugiau F, Jouve J. Incidence of thermotolerant Campylobacter in foods assessed by NF ISO 10272 standard: results of a 2-year study. Food Microbiol. 1999;16(2):195–204. doi: 10.1006/fmic.1998.0223. [DOI] [Google Scholar]
- 31.Gómez-Govea M, Solís-Soto L, Heredia N, García S, Moreno G, Tovar O, Isunza G. Analysis of microbial contamination levels of fruits and vegetables at retail in Monterrey, Mexico. J Food Agric Environ. 2012;10(1):152–156. [Google Scholar]
- 32.Holvoet K, Sampers I, Seynnaeve M, Uyttendaele M. Relationships among hygiene indicators and enteric pathogens in irrigation water, soil and lettuce and the impact of climatic conditions on contamination in the lettuce primary production. Int J Food Microbiol. 2014;171:21–31. doi: 10.1016/j.ijfoodmicro.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Hussain I, Shahid Mahmood M, Akhtar M, Khan A. Prevalence of Campylobacter species in meat, milk and other food commodities in Pakistan. Food Microbiol. 2007;24(3):219–222. doi: 10.1016/j.fm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Johannessen GS, Eckner KF, Heiberg N, Monshaugen M, Begum M, Økland M, Høgåsen HR. Occurrence of Escherichia coli, Campylobcter, Salmonella and shiga-toxin producing E. coli in norwegian primary strawberry production. Int J Environ Res Pub Health. 2015;12(6):6919–6932. doi: 10.3390/ijerph120606919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A, Agarwal RK, Bhilegaonkar KN, Shome BR, Bachhil VN. Occurrence of Campylobacter jejuni in vegetables. Int J Food Microbiol. 2001;67(1–2):153–155. doi: 10.1016/S0168-1605(01)00433-0. [DOI] [PubMed] [Google Scholar]
- 36.Little C, Roberts D, Youngs E, De Louvois J. Microbiological quality of retail imported unprepared whole lettuces: a PHLS food working group study. J Food Prot. 1999;62(4):325–328. doi: 10.4315/0362-028X-62.4.325. [DOI] [PubMed] [Google Scholar]
- 37.Losio MN, Pavoni E, Bilei S, Bertasi B, Bove D, Capuano F, Farneti S, Blasi G, Comin D, Cardamone C, Decastelli L, Delibato E, De Santis P, Di Pasquale S, Gattuso A, Goffredo E, Fadda A, Pisanu M, De Medici D. Microbiological survey of raw and ready-to-eat leafy green vegetables marketed in Italy. Int J Food Microbiol. 2015;210:88–91. doi: 10.1016/j.ijfoodmicro.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 38.McMahon MAS, Wilson IG. The occurrence of enteric pathogens and Aeromonas species in organic vegetables. Int J Food Microbiol. 2001;70(1–2):155–162. doi: 10.1016/S0168-1605(01)00535-9. [DOI] [PubMed] [Google Scholar]
- 39.Medeiros DT, Sattar SA, Farber JM, Carrillo CD. Occurrence of Campylobacter spp. in raw and ready-to-eat foods and in a Canadian food service operation. J Food Prot. 2008;71(10):2087–2093. doi: 10.4315/0362-028X-71.10.2087. [DOI] [PubMed] [Google Scholar]
- 40.Meldrum R, Ribeiro C, Smith R, Walker A, Simmons M, Worthington D, Edwards C. Microbiological quality of ready-to-eat foods: results from a long-term surveillance program (1995 through 2003) J Food Prot. 2005;68(8):1654–1658. doi: 10.4315/0362-028X-68.8.1654. [DOI] [PubMed] [Google Scholar]
- 41.Mosupye FM, Von Holy A. Microbiological quality and safety of ready-to-eat street- vended foods in Johannesburg, South Africa. J Food Prot. 1999;62(11):1278–1284. doi: 10.4315/0362-028X-62.11.1278. [DOI] [PubMed] [Google Scholar]
- 42.Mosupye FM, von Holy A. Microbiological hazard identification and exposure assessment of street food vending in Johannesburg, South Africa. Int J Food Microbiol. 2000;61(2–3):137–145. doi: 10.1016/S0168-1605(00)00264-6. [DOI] [PubMed] [Google Scholar]
- 43.Odumeru JA, Mitchell SJ, Alves DM, Lynch JA, Yee AJ, Wang SL, Styliadis S, Farber JM. Assessment of the microbiological quality of ready-to-use vegetables for health-care food services. J Food Prot. 1997;60(8):954–960. doi: 10.4315/0362-028X-60.8.954. [DOI] [PubMed] [Google Scholar]
- 44.Park CE, Sanders GW. Occurrence of thermotolerant campylobacters in fresh vegetables sold at farmers’ outdoor markets and supermarkets. Can J Microbiol. 1992;38(4):313–316. doi: 10.1139/m92-052. [DOI] [PubMed] [Google Scholar]
- 45.Phillips CA. The isolation of Campylobacter spp. from modified atmosphere packaged foods. Int J Environ Health Res. 1998;8(3):215–221. doi: 10.1080/09603129873499. [DOI] [Google Scholar]
- 46.Sagoo SK, Little CL, Mitchell RT. Microbiological quality of open ready-to-eat salad vegetables: effectiveness of food hygiene training of management. J Food Prot. 2003;66(9):1581–1586. doi: 10.4315/0362-028X-66.9.1581. [DOI] [PubMed] [Google Scholar]
- 47.Sagoo S, Little C, Ward L, Gillespie I, Mitchell R. Microbiological study of ready-to-eat salad vegetables from retail establishments uncovers a national outbreak of salmonellosis. J Food Prot. 2003;66(3):403–409. doi: 10.4315/0362-028X-66.3.403. [DOI] [PubMed] [Google Scholar]
- 48.Sagoo SK, Little CL, Mitchell RT. The microbiological examination of ready-to-eat organic vegetables from retail establishments in the United Kingdom. Lett Appl Microbiol. 2001;33(6):434–439. doi: 10.1046/j.1472-765X.2001.01026.x. [DOI] [PubMed] [Google Scholar]
- 49.Söderqvist K, Lambertz ST, Vågsholm I, Boqvist S. Foodborne bacterial pathogens in retail prepacked ready-to-eat mixed ingredient salads. J Food Prot. 2016;79(6):978–985. doi: 10.4315/0362-028X.JFP-15-515. [DOI] [PubMed] [Google Scholar]
- 50.Taulo S, Wetlesen A, Abrahamsen R, Kululanga G, Mkakosya R, Grimason A. Microbiological hazard identification and exposure assessment of food prepared and served in rural households of Lungwena. Malawi. Int J Food Microbiol. 2008;125(2):111–116. doi: 10.1016/j.ijfoodmicro.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 51.Thunberg RL, Tran TT, Bennett RW, Matthews RN, Belay N. Microbial evaluation of selected fresh produce obtained at retail markets. J Food Prot. 2002;65(4):677–682. doi: 10.4315/0362-028X-65.4.677. [DOI] [PubMed] [Google Scholar]
- 52.Thung TY, Siti Norshafawatie BM, Premarathne JM, Chang WS, Loo YY, Kuan CH, New CY, Ubong A, Ramzi OS, Mahyudin NA, Dayang FB. Isolation of food-borne pathogen bacteriophages from retail food and environmental sewage. Int Food Res J. 2017;24(1):450–454. [Google Scholar]
- 53.Tsuei AC, Carey-Smith GV, Hudson JA, Billington C, Heinemann JA. Prevalence and numbers of coliphages and Campylobacter jejuni bacteriophages in New Zealand foods. Int J Food Microbiol. 2007;116(1):121–125. doi: 10.1016/j.ijfoodmicro.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 54.Whyte P, McGill K, Cowley D, Madden RH, Moran L, Scates P, Carroll C, O’Leary A, Fanning S, Collins JD, McNamara E, Moore JE, Cormican M. Occurrence of Campylobacter in retail foods in Ireland. Int J Food Microbiol. 2004;95(2):111–118. doi: 10.1016/j.ijfoodmicro.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Wijnands LM, Delfgou-Van Asch EHM, Beerepoot-Mensink ME, Van Der Meij-Florijn A, Fitz-James I, Van Leusden FM, Pielaat A. Prevalence and concentration of bacterial pathogens in raw produce and minimally processed packaged salads produced in and for the Netherlands. J Food Prot. 2014;77(3):388–394. doi: 10.4315/0362-028X.JFP-13-135. [DOI] [PubMed] [Google Scholar]
- 56.Christidis T, Pintar K, Butler A, Nesbitt A, Thomas M, Marshall B, Pollari F. Campylobacter spp. prevalence and levels in raw milk: a systematic review and meta-analysis. J Food Prot. 2016;79(10):1775–1783. doi: 10.4315/0362-028X.JFP-15-480. [DOI] [PubMed] [Google Scholar]
- 57.Korsak D, Mackiw E, Rozynek E, Żylowska M. Prevalence of Campylobacter spp. in retail chicken, turkey, pork, and beef meat in Poland between 2009 and 2013. J Food Prot. 2015;78(5):1024–1028. doi: 10.4315/0362-028X.JFP-14-353. [DOI] [PubMed] [Google Scholar]
- 58.Frey SK, Topp E, Khan IU, Ball BR, Edwards M, Gottschall N, Sunohara M, Lapen DR. Quantitative Campylobacter spp., antibiotic resistance genes, and veterinary antibiotics in surface and ground water following manure application: influence of tile drainage control. Sci Total Environ. 2015;532:138–153. doi: 10.1016/j.scitotenv.2015.03.114. [DOI] [PubMed] [Google Scholar]
- 59.Pintar KDM, Thomas KM, Christidis T, Otten A, Nesbitt A, Marshall B, Pollari F, Hurst M, Ravel A. A comparative exposure assessment of Campylobacter in Ontario, Canada. Risk Anal. 2017;37(4):677–715. doi: 10.1111/risa.12653. [DOI] [PubMed] [Google Scholar]
- 60.Doyle ME. Multidrug-resistant pathogens in the food supply. Foodborne Pathog Dis. 2015;12(14):261–279. doi: 10.1089/fpd.2014.1865. [DOI] [PubMed] [Google Scholar]
- 61.Chapman B. 2009. Sprout associated outbreaks in North America, 1990–2009. Viewed 05/14/2009 from. Ben Chapman Bites, Food Safety Network. http://foodsafety.k-state.edu/en/article-details.php.
- 62.Centers for Disease Control and Prevention. 2013. OutbreakNet, foodborne outbreak online database.
- 63.Ding H, Fu TJ, Smith MA. Microbial contamination in sprouts: how effective is seed disinfection treatment? J Food Sci. 2013 doi: 10.1111/1750-3841.12064. [DOI] [PubMed] [Google Scholar]
- 64.Abadias M, Cañamás T, Asensio A, Anguera M, Viñas I. Microbial quality of commercial ‘Golden Delicious’ apples throughout production and shelf-life in Lleida (Catalonia, Spain) Int J Food Microbiol. 2006;108(3):404–409. doi: 10.1016/j.ijfoodmicro.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Jørgensen F, Bailey R, Williams S, Henderson P, Wareing D, Bolton F, Frost J, Ward L, Humphrey T. Prevalence and numbers of Salmonella and Campylobacter spp. on raw, whole chickens in relation to sampling methods. Int J Food Microbiol. 2002;76(1):151–164. doi: 10.1016/S0168-1605(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 66.Butzler J-P, Oosterom J. Campylobacter: pathogenicity and significance in foods. Int J Food Microbiol. 1991;12(1):1–8. doi: 10.1016/0168-1605(91)90043-O. [DOI] [PubMed] [Google Scholar]
- 67.Brandl MT, Haxo AF, Bates AH, Mandrell RE. Comparison of survival of Campylobacter jejuni in the phyllosphere with that in the rhizosphere of spinach and radish plants. Appl Environ Microbiol. 2004;70(2):1182–1189. doi: 10.1128/AEM.70.2.1182-1189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norinaga M, Tomohiro N, Yono A, Fumihiko K, Takashi M, Akiyama M. Evaluation of MPN method combined with PCR procedure for detection and enumeration of Vibrio parahaemolyticus in seafood. Food Hyg Safe Sci. 2003;44(6):289–293. doi: 10.3358/shokueishi.44.289. [DOI] [PubMed] [Google Scholar]
- 69.Luan X, Chen J, Liu Y, Li Y, Jia J, Liu R, Zhang XH. Rapid quantitative detection of Vibrio parahaemolyticus in seafood by MPN-PCR. Curr Microbiol. 2008;57(3):218–221. doi: 10.1007/s00284-008-9177-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.