Abstract
Background
Due to limitations of conventional medicine for atopic eczema (AE), complementary and alternative medicine (CAM) is widely used as an alternative, maintaining, or simultaneous treatment for AE. We aimed to evaluate the beneficial and harmful effects of CAM for children with AE under 14 years old.
Methods
We searched for randomized trials on CAM in 12 Chinese and English databases from their inception to May 2018. We included children (< 14 years) diagnosed with AE, who received CAM therapy alone or combined with conventional medicine. We extracted data, and used the Cochrane “Risk of bias” tool to assess methodological quality. Effect was presented as relative risk (RR) or mean difference (MD) with 95% confidence interval (CI) using RevMan 5.3.
Results
Twenty-four randomized controlled trials involving 2233 children with AE were included. Methodological quality was of unclear or high risk of bias in general. The trials tested 5 different types of CAM therapies, including probiotics, diet, biofilm, borage oil, and swimming. Compared to placebo, probiotics showed improved effect for the SCORAD index (MD 9.01, 95% CI 7.12–10.90; n = 5). For symptoms and signs such as itching, skin lesions, CAM combined with usual care was more effective for symptom relief ≥95% (RR 1.47, 95% CI 1.30–1.68; n = 8), and for ≥50% symptoms improvement (RR 1.34, 1.25–1.45; n = 9) compared to usual care. There was no statistic significant difference between CAM and usual care on ≥95% improvement or ≥ 50% improvement of symptoms. However, swimming, diet and biofilm showed improvement of clinical symptoms compared with usual care. At follow-up of 8 weeks to 3 years, CAM alone or combined with usual care showed lower relapse rate (RR 0.38, 0.28–0.51, n = 2; RR 0.31, 0.24–0.40, n = 7; respectively) compared to usual care. Twelve out of 24 trials reported no occurrence of severe adverse events.
Conclusions
Low evidence demonstrates that some CAM modalities may improve symptoms of childhood AE and reduce relapse rate. Safety remains unclear due to insufficient reporting. Further well-designed randomized trials are needed to confirm the potential beneficial effect and to establish safety use.
Electronic supplementary material
The online version of this article (10.1186/s12906-018-2306-6) contains supplementary material, which is available to authorized users.
Keywords: Complementary and alternative medicine, CAM, Atopic eczema, Children, Randomized controlled trials, Systematic review, Meta-analysis, Clinical evidence
Background
Eczema, as defined by the World Allergy Organization, encompasses both atopic and non-atopic conditions, and is commonly referred to as atopic eczema (AE) or atopic dermatitis (AD) [1]. AE is a chronically relapsing inflammatory skin disease, often found in children under the age of 14 years. It impairs people’s quality of life [2] and the prevalence of AE is estimated to be 15–20% in children worldwide [3]. As one of the most common inflammatory skin diseases, AE has a prevalence exceeding 10% of children in some populations [4]. There is an increasing number of studies focusing on AE, such as clinical trials and systematic reviews [5].
AE can be caused by multiple and complex risk factors such as irritants, contact allergens, food, inhaled allergens, stress or infection [6]. The pathogenesis of eczema is a complex interplay of numerous elements including immune, genetic, infection and neuroendocrine factors and their interaction with the environment [2]. Moreover, the diagnosis of AE relies on the assessment of clinical features because there is no definitive/conclusive test to diagnose the condition. The clinical characteristics are itching, skin inflammation, a skin barrier abnormality, and susceptibility to skin infection [7]. Although not always recognized by health-care professionals as being a serious medical condition, AE can have a significant negative impact on quality of life for children and their parents and care takers [8]. Children with AE may suffer from lack of sleep, irritability, daytime tiredness, emotional stress, lowered self-esteem and psychological disturbance [9]. Moreover, many cases of AE clear or improve during childhood, whereas others persist into adulthood [8]. Thus, there is a substantial need for cure and symptom relief as early as possible. However, despite the common claims for curative interventions, there is currently no known cure for AE in allopathic medicine [9].
Therefore, there is an increasing number of trials studying complementary and alternative medicine (CAM) to treat children with AE. There is a growing interest in CAM as a primary, maintenance, or simultaneous treatment for AE [10]. These studies suggest that CAM may improve health related quality of life of children. In fact, many people rely on these treatments as their primary approach to relieve their illness or at least to improve the duration and quality of symptomatic relief [10]. The most frequently used CAM modalities are herbal medicine, vitamins, Ayurveda, naturopathy, homeopathy, traditional healing [6], and probiotics [11]. However, current literature, published protocols and systematic reviews have not involved or included all kinds of CAM modalities. Moreover, we were not able to find any systematic review focusing on CAM with AE in children (< 14 years). Therefore, we conducted a comprehensive literature search involving CAM for AE in children (< 14 years) to add to current available evidence in order to inform clinical practice further.
Methods
The protocol of the review was registered in PROSPERO (CRD42017071267) on 7th of August 2017 (Available from: http://www.crd.york.ac.uk/PROSPERO/). The content of the review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [12].
Eligibility criteria
Type of studies
Randomized controlled trials (RCTs) were included in the systematic review.
Type of participants
Children (< 14 years) diagnosed with AE by defined criteria or validated instruments or tools based on either the UK Working Party, Hanifin and Rajka (Hanifin 1980) or explicitly stated provider based diagnostic criteria [13] were included. Trials without clear diagnostic criteria but with detailed description of clinical features to be diagnosed as AE were also eligible for inclusion in a subgroup analysis. The limited age of < 14 years was set because of the maximum age as younger adolescents defined by WHO. No gender or ethnicity limitations were set.
Type of intervention
CAM modalities used alone or in combination with conventional therapies for children (< 14 years) were included. CAM terms have different concepts: If a non-mainstream practice is used together with conventional medicine, it’s considered as “complementary”. If a non-mainstream practice is used in place of conventional medicine, it is considered “alternative” [14]. Since a separate review on Traditional Chinese Medicine (TCM) for AE will be prepared due to clinical heterogeneity, we included the following CAM modalities: dietary advices/restriction, dietary supplements, probiotics, prebiotics, psychological interventions, oral evening primrose oil or borage oil, specific allergen immunotherapy, aromatherapy, bath therapy, bioresonance, chromotherapy, homeopathy, hypnotherapy and relaxation techniques in addition to some other CAM modalities that are known to be used for treating AE [10].
However, CAM is different from the new drug to estimate effectiveness, but to focus on its efficacy. So, it is sometimes difficult to to split CAM modalities up into parts to investigate effectiveness and safety of CAM modalities separately, except the placebo-controlled randomized trials [15]. Therefore, from the component level, CAM in the intervention group can be classified as above specific modalities such as probiotics, bath therapy, and so on. And from the system level, CAM can be considered as an integrated “whole system” of intervention.
Type of outcomes
Primary outcomes included clinical disease severity measured by one or more of the following instruments: (1) global improvement in objective AE outcomes as measured by scoring atopic dermatitis index (SCORAD); eczema area and severity index score (EASI); Nottingham eczema severity score (NESS) reported by a clinician; global improvement in subjective AE outcomes as measured by patient oriented eczema measure (POEM); itching visual analogue score (VAS); dermatology life quality index (DLQI) reported by participants or their parents. (2) Frequency of treatment discontinuation due to adverse effects. Secondary outcomes included (1) relapse rate; (2) proportion of participants with ≥50% symptoms and signs improvement in a given outcome as assessed by a clinician; (3) type, frequency, and severity of adverse events.
Search strategy
We conducted systematic literature searches in 12 electronic databases, including 4 Chinese databases (China National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Scientific Journal Database (VIP), and SinoMed), and 8 English databases: PubMed, EMBASE via OVID, AMED (Allied and Complementary Medicine Database) via OVID, CINAHL (Cumulative Index to Nursing and Allied Health Literature) via EBSCO, PsychoInfo, CAM-QUEST, the GREAT database (the Global Resource for Eczema Trials: www.Greatdatabase.org.uk), and the Cochrane Library from their inception date until May 2018. The filters were English and Chinese language (Additional file 1). We also searched in the grey literature such as conference proceedings and dissertations in CNKI and Wanfang for unpublished trials and trial protocols. References of all included studies were hand searched for additional eligible studies.
Study selection and data extraction
Two authors (CL Lu and SB Liang) independently examined the full text to identify the eligible trials. Four authors in pair (CL Lu, XH Liu, X Wang, and X Bai) extracted data independently from the included studies according to a predesigned data sheet. Any disagreement was resolved by discussion with a third author (JP Liu). Following items were extracted: publication year, study type, funding, inclusion/exclusion criteria, diagnostic criteria, study methodology, demographic characteristics of the participants, details of intervention and controls, outcome measures methods, adverse events, and results.
Quality assessment
Two authors (CL Lu and XH Liu) used the risk of bias tool [16] to assess the methodological quality of the included trials. Seven items including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias such as pharmaceutical funding, were used to be judged as “low risk”, “high risk”, or “unclear risk”. Any disagreements were resolved by discussion with a third author (JP Liu).
Data analysis
We used RevMan 5.3 software for data analysis. For continuous data, we used mean difference (MD) with 95% confidence intervals (CI), and for dichotomous data we used relative risk (RR) with 95% CI. We performed meta-analyses for trials if the study design, participants, interventions, control, and outcome measures were similar. Bulk data were synthesized quantitatively by descriptive counting. Other data not suitable for pooling analysis were synthesized qualitatively.
We used I-square (I2) to test the statistical heterogeneity as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered I2 statistic value greater than 50% as a suggestion that there might be substantial heterogeneity [16]. We used random effects model for data pooling with significant heterogeneity (I2 ≥ 50%), otherwise a fixed effect model was applied. If the data were available, we did subgroup analyses for subcategories of CAM modalities.
A sensitivity analysis was conducted to explore the influence of the type of randomized trials (parallel or cross-over randomized) and the quality of trials (high or low) if the data were available. A funnel plot was generated to explore possible publication bias if more than ten trials were included in a meta-analysis.
Results
Description of studies
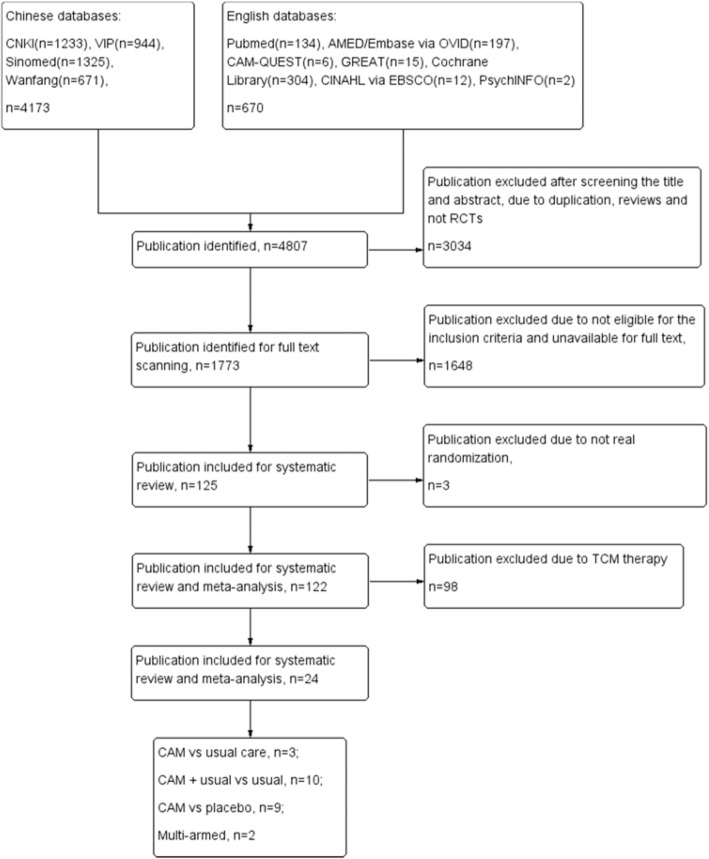
Our searches identified 4807 citations. After reviewing the titles and abstracts, 3034 citations were excluded due to duplication, reviews, and non RCTs. After scanning the full texts to identify the participants who were over 14 years, we excluded 1648 publications. Among 125 publications that were eligible, three publications were excluded [17–19] due to inappropriate allocation of participants. We excluded 98 trials for the intervention of TCM in separate systematic review. Finally, there were 24 trials [20–43] with a total of 2233 children (< 14 years) included in this review (Fig. 1). Eleven trials [33–43] were published in English, and 13 trials [20–32] were in Chinese. We did not identify any unpublished study. Twenty-two trials [20–41] had two arms with parallel groups, one trial [42] had three arms, and one trial [43] had five arms.
Fig. 1.
Flow diagram of study selection and different sub-groups interventions included in this review
Study characteristics
The details of the 24 trials are presented in Table 1. The sample size of these studies ranged from 15 to 298 participants. The age ranged from 2 months to 13 years. We defined the conventional therapy with more than two modalities (e.g. topical and systemic anti-allergic, and immunomodulatory therapy) as “usual care” in 24 trials [9]. Every trial had more than two modalities of conventional therapy except placebo. Therefore, from the component level, CAM modalities of 22 trials [21, 23–43] in the intervention group could be classified as probiotics, diet, biofilm, and borage oil (undershirts coated with oil) (more in Table 2). Moreover, two trials [20, 22] had their main modality referring to swimming and biofilm while accompanying other modalities. So, we considered for three different comparisons in the two-arm trials from system level: CAM versus usual care [20–22], CAM plus usual care versus usual care [23–32], and CAM (probiotics) versus placebo [33–41]. For the two trials with three or more arms [42, 43], probiotics was compared with other formula of probiotics, placebo, usual care, or observation with no intervention.
Table 1.
Characteristics of included randomized clinical trials on CAM therapies for childhood atopic eczema
| Study ID | Sample size | Age | Sex M/F | Comparisons | Outcome | Follow up |
|---|---|---|---|---|---|---|
| CAM vs usual care, 3 studies | ||||||
| Liu CH 2009 [20] | T:150 C:148 | T:1-6 m, 80 cases; 6-12 m, 50 cases; 1-2 y, 20 cases C:1-6 m, 83 cases; 6-12 m, 44 cases; 1-2 y, 21 cases |
T:88/62 C:86/44 |
Swimming therapy + Baibu (Stemona japonica) lotion (bath) + Tuina vs Cyproheptadine (oral) + Boric lotion (external application with cold lotion)/Hydrocortisone butyrate cream (external use)/Zinc oxide cream (external use) (7-15d) | Improvement of symptoms and signs and signs; IgG; Relapse (T:26/150, 3 m; 37/150, 6 m C:80/148, 3 m; 96/148, 6 m) |
3 m, 6 m |
| Liu WQ 2016 [21] | T:60 C:60 | T:(4.5 ± 3.8) y C:(5.3 ± 4.5) y | T:37/23 C:33/27 |
Fasting and rotation diet vs Pevisone paste (external use) (3 m) | Improvement of symptoms and signs; IgG; Relapse (T:4/60 C:11/60) |
3 m |
| Wu YQ 2014 [22] | T:74 C:74 | T:(6.35 ± 1.36) m C:(5.98 ± 1.23) m | T:41/33 C:31/43 |
Velvetfeeling lotion (external application) + Moisturizing cream (external use) + Saline water (dipping) vs Boric lotion (external application) + Vitamin E (external use) + Saline water (dipping) (7d) | Improvement of symptoms and signs; CGI-EI |
NR |
| CAM + usual care vs usual care, 10 studies | ||||||
| Chen DX 2015 [23] | T:20 C:20 | T:(3.82 ± 0.7) m C:(2.35 ± 1.3) m | NR | Bifid triple viable capsules (oral) + Hydrocortisone butyrate cream (external use) vs Hydrocortisone butyrate cream (external use) (180d) | Improvement of symptoms and signs | 6 m |
| Chen YL 2015 [24] | T:58 C:58 | T:(11 ± 5) m C:(12 ± 5) m |
T:31/27 C:30/28 |
Probiotics (oral) + Chlorphenamine maleate tablets (oral) + Vitamin B6 + Fluocinonide cream (external use) + Calcium supplement (oral) vs Chlorphenamine maleate tablets (oral) + Vitamin B6 + Fluocinonide Cream (external use) + Calcium supplement (oral) (28d) | Improvement of symptoms and signs; CGI-EI; Interleukin; Interferon; Relapse (T:9/58 C:29/58) |
NR |
| Guo YH 2015 [25] | T:90 C:90 | T:2 m-3 y C:2 m-3 y |
total: 98/82 | Tetralogy of viable bifidobacterium tablets (oral) + Usual care (Calamine lotion/ Zinc oxide cream/ Loratadine syrup/ Mometasone furoate cream) vs Usual care (Calamine lotion/Zinc oxide cream/Loratadine syrup/Mometasone furoate cream) (30d) | Improvement of symptoms and signs; IL-4, IL-10, IFN-γ, IgE, Th1/Th2; Relapse (T:24/90 C:62/90) |
3 m |
| Jiang YX 2013 [26] | T:65 C:60 | T:2 y C:2 y |
total: 72/53 | Velvetfeeling lotion (external application) + Usual care (eg. Chlorphenamine maleate tablets) vs Usual care (eg. Chlorphenamine maleate tablets) + Boric lotion (external application) (28d) | Improvement of symptoms and signs | NR |
| Li DY 2012 [27] | T:32 C:30 | T:(7.15 ± 2.06) m C:(6.89 ± 2.54) m | T:17/15 C:16/14 |
Bifid triple viable capsules (oral) + Zinc oxide cream (external use) + Boric lotion (external application) vs Zinc oxide cream (external use) + Boric lotion (external application) (14d drugs for external use/28d Oral bifid-triple viable capsule) | Improvement of symptoms and signs; Relapse (T:6/32 C:20/30) |
NR |
| Mao HX 2013 [28] | T:50 C:50 | T:2 m-5 y C:2 m-5 y |
T:24/26 C:28/22 |
Probiotics (oral) + Antihistamines (oral) + Calcium supplement (oral) + Skincare cream (external use) vs Antihistamines (oral) + Calcium supplement (oral) + Skincare cream (external use) | Improvement of symptoms and signs; Relapse (T:2/50 C:12/50) |
NR |
| Wei MX 2010 [29] | T:38 C:36 | T:(6.78 ± 2.62) m C:(7.14 ± 2.10) m | T:22/16 C:19/17 |
Viable Bacillus coagulans tablets (oral) + Boric lotion (wash-out) + Zinc oxide cream (external use) vs Boric lotion (wash-out) + Zinc oxide cream (external use) (28d) | Improvement of symptoms and signs; Relapse (T:7/38 C:24/36) |
6 m |
| Ye CQ 2017 [30] | T:48 C:48 | T:(6.9 ± 2.4) m C:(6.8 ± 2.6) m |
T:27/21 C:28/20 |
Condensation living bacterium bacillus (oral) + Boric lotion (wash-out) + Zinc oxide cream (external use) vs Boric lotion (wash-out) + Zinc oxide cream (external use) (28d) | Improvement of symptoms and signs; Relapse (T:6/48 C:28/48) |
6 m |
| Zhang MH 2013 [31] | T:35 C:35 | T:(5 ± 3) m C:(6 ± 3) m |
T:27/8 C:25/10 |
Bifico lriple viable (oral) + Boric lotion (wash-out) + Zinc oxide cream (external use) + Cetirizine hydrochloride drops (oral) vs Boric lotion (wash-out) + Zinc oxide cream (external use) + Cetirizine hydrochloride drops (oral) (30d) | IFN-γ; Interleukin; T-Cell; Relapse (T:6/35 C:16/58) |
6 m |
| Zhang XN 2013 [32] | T:36 C:34 | total:(7.06 ± 3.48) y | total:32/38 | Velvetfeeling lotion (external application) + Butyl flufenamate ointment (external use) + Antihistamine (oral) vs Saline water (external application) + Butyl flufenamate ointment (external use) + Antihistamine (oral) (14d) | Improvement of symptoms and signs; CGI-EI |
NR |
| CAM vs placebo, 9 studies | ||||||
| D. Sistek 2006 [33] | T:30 C:29 | T:3.8 y C:4.4 y |
T:15/14 C:17/13 |
Probiotics (oral) vs Placebo (oral) (12w) | Improvement of symptoms and signs | 4w |
| Hyeon-Jong Yang 2014 [34] | T:50 C:50 | T:(58.7 ± 29.9) m C:(47.4 ± 28.1) m | T:29/21 C:24/26 |
Probiotics (oral) vs Placebo (oral) (6w) | Improvement of symptoms and signs; Fecal cell counts; IL-4; TNF-α |
NR |
| Reza 2011 [35] | T:19 C:21 | T:(28.68 ± 40.86) m C:(22.76 ± 34.03) m | T:11/8 C:14/7 |
Synbiotic (oral) vs Placebo (oral) (8w) | Improvement of symptoms and signs Mononuclear cells |
NR |
| S Weston 2017 [36] | T:28 C:28 | T:(11.5 ± 4.2) m C:(10.3 ± 3.2) m | T:14/14 C:16/12 |
VRI-003 PCC freeze dried powder probiotics (oral) vs Placebo (oral) (8w) | Improvement of symptoms and signs; total IgE levels; radioallergosorbent test |
8w |
| Sergei V. Gerasimov 2010 [37] | T:48 C:48 | T:(25.6 ± 7.7) m C:(24.1 ± 6.3) m | T:28/15 after withdrew C:28/19 after withdrew |
Probiotics (oral) vs Placebo (oral) (8w) | Improvement of symptoms and signs; Quality of life; Total IgE; Eosinophil count |
NR |
| Shoko 2007 [38] | T:16 C:16 | T:4.44 y C:5.56 y |
T:9/7 C:12/4 |
Borage oil (undershirts coated with oil) vs Placebo (non-coated undershirts) (2w) | Improvement of symptoms and signs; Changes of transepidermal water loss |
NR |
| Wu YJ 2017 [39] | T:33 C:33 | T:(1.5 ± 1.1) m C:(7.14 ± 2.10) m |
T:25/8 C:19/14 |
Probiotics (Lactobacillus rhamnosus) (oral) vs Placebo (oral) (8w) | Improvement of symptoms and signs; Quality of Life (Infant Dermatitis Quality of Life Questionnaires and Dermatitis Family Impact Questionnaires); |
NR |
| Yavuz 2012 [40] | T:20 C:20 | total:1-13 y | total: 23/17 | Probiotic bacteria (oral) vs Placebo (oral) (8w) | Improvement of symptoms and signs; cytokine analyse/IgE/Eosinophil cationic protein |
10w |
| Youngshin 2012 [41] | T:58 C:60 | T:(4.6 ± 3.3) y C:(5.1 ± 3.3) y | T:34/24 C:35/25 |
Probiotics (L. plantarum CJLP133) (oral) vs Placebo (oral) (12w) | Improvement of symptoms and signs; total IgE levels/specific IgE |
2w |
| Multi-armed trials, 2 studies | ||||||
| Pasi E. Kankaanpa 2002 [42] | T1:5 T2:5 C1:5 |
T1:(4.5 ± 2) m T2:(5.7 ± 2.2) m C1:(5.6 ± 2.1) m | T1:2/3 T2:3/2 C1:2/3 | Probiotics (Lactobacillus GG) (oral) vs Probiotics (Bifidobacterium Bb12) (oral) vs placebo (oral) (①4.4 ± 1.7 m ②7.3 ± 0.7 m ③5.7 ± 2.0 m) | Fatty acid analysis | ≥36 m follow until panticipants at the age of 3 years |
| C. Gore 2011 [43] | T1:45 T2:45 C1:47 C2:22 C3:49 |
T1:19 [16-23] w T2:20.5 [17-23] w C1:20 [16-23] w C2:15 [13-19.5] w C3:19 [15-21.5] w | T1:28/17 T2:24/21 C1:28/19 C2:16/6 C3:25/24 | Probiotics (Lactobacillus paracasei) (oral) vs Probiotics (Bifidobacterium) (oral) vs Placebo (oral) vs Exclusively breastfed vs Standard formula-fed (12w) | Improvement of symptoms and signs; Quality of life; Stool; GI-Permeability; Specific serum IgE; Urinary EPX |
NR |
T treatment group, C control group, y year, m month, d day, w week, NR not report
Table 2.
Description and trials numbers of different CAM therapies
| Therapy | Administration | Dosage | Frequency | Detail | Formula | Trials numbers | %(/22) |
|---|---|---|---|---|---|---|---|
| Probiotics | oral | depend on formula | 2–3/d | Oral probiotics preparation | The probiotic formulation was a mixture of Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABLA-12 with fructo-oligosaccharide in a rice maltodextrin powder. | 18 | 72% |
| Diet | oral | None | 3 m | To avoid to severe and moderate intolerant food, and take mild intolerant food per 6 days | None | 2 | 8% |
| Biofilm | external application | appropriate amount | 2–3/d | The main ingredient is chitosan with low polymer (OPC). External application help form a protective film on the skin. | Velvetfeeling | 3 | 12% |
| Borage oil | undershirts coated withoil | 498 mg of GLA per 100 g of cotton) |
2 w | Borage oil was chemically bonded to the cotton fibers of the undershirts that made of pure organic cotton. The borage oil was gradually released from the cotton fibers and absorbed into the skin. The undershirts were designed such that the sutures did not touch and stimulate the skin directly. | Borage oil | 1 | 4% |
| Swimming | Passively by nurse; Autonomous swimming |
None | 1/d | Trained nurse helps baby to stretch the limb as passively swimming or baby swim autonomously. | None | 1 | 4% |
m month, d day, w week
Risk of bias of included trials
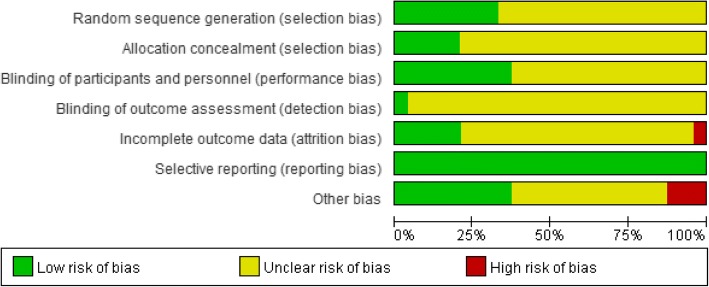
The trials only reporting that the study was “randomized” were defined as “unclear” risk of bias, while the trials describing a specific method of randomized sequences generation, allocation concealment, and blinding as “low” risk of bias. Other bias accessed the funding scheme. Trials supported by non-commercial funding were defined as “low” risk of bias, trials funded by pharmaceutical companies were classified as “high” risk of bias, no information as “unclear” risk of bias. Eight trials [22, 24, 29, 31, 33, 34, 37, 41] reported the random allocation by random number table or computer generated-list. Only five trials [33, 34, 37, 38, 40, 41] reported the allocation concealment by using computer-generated random numbers or randomization software, which can conceal the allocation automatically. Ten trials [33, 34, 36–43] reported double blinding. Eight trials [33–37, 39, 41, 43] reported the drop out in both intervention group and control group, and only four trials [33, 39, 41, 43] used intention-to-treat (ITT) to analyze for all outcome [33, 39, 41] and primary outcome [43], and the other four trials [34–37] analyzed data by per-protocol (PP) and reported the data of available participants. Besides, two trials [39, 41] analyzed by both ITT and PP. We considered one trial [34] as high risk of incomplete data for loss to follow up without ITT analysis because of 13 withdrawals in intervention group and 16 withdrawals in control group among the 100 participants in the trial. Four trials [33, 39, 41, 43] were considered as low risk of bias and the others as unclear. Eleven trials [21, 25, 29, 33, 35–37, 40–43] mentioned non-commercial funding. Three trials [33, 39, 43] reported that probiotic manufacturers produced the drugs used. We considered these three trials [33, 39, 43] as high risk of bias for conflict of interest (Fig. 2). Sample size calculation was reported in five trials [33, 35, 37, 41, 43] according to disease prevalence [33], symptoms and signs reduction in treatment group by 30% [34, 41], 34% [37] and in placebo group by 15% [34], 17% [37], 10% [41], and symptoms and signs scale of SCORAD for a standard deviation increments of 7.65 [43]. The other trials did not report any detail of sample size calculation. We considered the other bias of power calculation as unclear.
Fig. 2.
Risk of bias graph
Effects of interventions
The 24 trials tested five CAM interventions: combination of probiotics (72%, n = 18) [23–25, 27–43], biofilm (12%, n = 3) [22, 26, 32], diet (8%, n = 2) [21, 43], swimming (4%, n = 1) [20], and undershirts coated with borage oil (4%, n = 1) [38] (Table 2).
Twenty-two two-arms trials [20–41] involved CAM modalities, including probiotics, swimming, diet, borage oil, and biofilm. Three different comparisons were summarized: CAM versus placebo, CAM versus usual care, and CAM plus usual care versus usual care alone. In addition to this, two multi-arm trials [42, 43] used different probiotic formulae in different groups to compare with placebo, observation with no intervention or diet. Table 3 showed the detailed results of the effect estimation.
Table 3.
Summary of findings of CAM for childhood atopic eczema in randomized controlled trials
| Study ID | Sample size | Main intervention | Estimate effect [95% CI] | Outcome | P |
|---|---|---|---|---|---|
| CAM vs usual care | |||||
| Liu CH 2009 [20] | T:150 C:148 | Swimming therapy | RR 1.01 [0.92, 1.11] RR 1.01 [0.96, 1.07] RR 0.38 [0.28, 0.52] |
Clinical effectiveness rate 50% Improvement of symptoms and signs Relapse rate |
P = 0.8318 P = 0.5671 P < 0.00001 |
| Liu WQ 2016 [21] | T:60 C:60 | Fasting and Rotation diet | RR 1.57 [1.04, 2.38] RR 1.32 [1.09, 1.60] RR 0.36 [0.12, 1.08] |
Clinical effectiveness 50% Improvement of symptoms and signs; Relapse rate |
P = 0.0323 P = 0.0049 P = 0.0681 |
| Wu YQ 2014 [22] | T:74 C:74 | Velvetfeeling lotion (external application) | RR 1.92 [1.36, 2.72] RR 1.33 [1.10, 1.61] |
Clinical effectiveness rate 50% Improvement of symptoms and signs |
P = 0.0002 P = 0.0031 |
| CAM + usual care vs usual care | |||||
| Chen DX 2015 [23] | T:20 C:20 | Bifid Triple Viable capsules (oral) | RR 1.63 [0.87, 3.04] RR 1.73 [1.15, 2.60] |
Clinical effectiveness rate 50% Improvement of symptoms and signs |
P = 0.1283 P = 0.0088 |
| Chen YL 2015 [24] | T:58 C:58 | Probiotics (oral) | RR 1.21 [0.87, 1.68] RR 1.19 [0.99, 1.42] RR 0.31 [0.16, 0.60] |
Clinical effectiveness rate 50% Improvement of symptoms and signs Relapse rate |
P = 0.2659 P = 0.0624 P = 0.0004 |
| Guo YH 2015 [25] | T:90 C:90 | Tetralogy of viable bifidobacterium tablets (oral) | RR 1.37 [1.01, 1.85] RR 1.27 [1.04, 1.55] RR 0.39 [0.27, 0.56] |
Clinical effectiveness rate 50% Improvement of symptoms and signs Relapse rate |
P = 0.0400 P = 0.0172 P < 0.00001 |
| Jiang YX 2013 [26] | T:65 C:60 | Velvetfeeling lotion (external application) | RR 1.85 [0.90, 3.79] RR 1.91 [1.46, 2.50] |
Clinical effectiveness rate 50% Improvement of symptoms and signs |
P = 0.0947 P < 0.00001 |
| Li DY 2012 [27] | T:32 C:30 | Bifid Triple Viable capsules (oral) | RR 1.36 [1.03, 1.79] RR 1.32 [1.06, 1.65] RR 0.28 [0.13, 0.60] |
Clinical effectiveness rate 50% Improvement of symptoms and signs; Relapse rate |
P = 0.0295 P = 0.0151 P = 0.0011 |
| Mao HX 2013 [28] | T:50 C:50 | Probiotics (oral) | RR 0.17 [0.04, 0.71] | Relapse rate | P = 0.0151 |
| Wei MX 2010 [29] | T:38 C:36 | Viable Bacillus Coagulans tablets (oral) | RR 1.33 [1.05, 1.68] RR 1.30 [1.07, 1.58] RR 0.28 [0.14, 0.56] |
Clinical effectiveness rate 50% Improvement of symptoms and signs; Relapse rate |
P = 0.0189 P = 0.0089 P = 0.0004 |
| Ye CQ 2017 [30] | T:48 C:48 | Condensation living bacterium bacillus (oral) | RR 1.57 [1.21, 2.02] RR 1.31 [1.10, 1.55] RR 0.21 [0.10, 0.47] |
Clinical effectiveness rate 50% Improvement of symptoms and signs; Relapse rate |
P = 0.0005 P = 0.0019 P = 0.0001 |
| Zhang MH 2013 [31] | T:35 C:35 | Bifico Lriple Viable (oral) | RR 1.15 [0.91, 1.46] RR 0.38 [0.17, 0.85] |
50% Improvement of symptoms and signs; Relapse rate |
P = 0.2371 P = 0.0180 |
| Zhang XN 2013 [32] | T:36 C:34 | Velvetfeeling lotion (external application) | RR 6.61 [1.62, 26.96] RR 1.25 [1.00, 1.56] |
Clinical effectiveness rate 50% Improvement of symptoms and signs |
P = 0.0084 P = 0.0542 |
| CAM vs placebo | |||||
| Reza 2011 [35] | T:19 C:21 | Synbiotic (oral) | MD 19.10 [7.60, 30.60] | Clinical effectiveness scores | P = 0.0017 |
| Sergei V. Gerasimov 2010 [37] | T:48 C:48 | Probiotics (oral) | MD 6.40 [2.71, 10.09] | Clinical effectiveness scores | P = 0.0009 |
| Wu YJ 2017 [39] | T:33 C:33 | Probiotics (Lactobacillus rhamnosus) (oral) | MD 10.85 [3.82, 17.88] | Clinical effectiveness scores | P = 0.0035 |
| Yavuz 2012 [40] | T:20 C:20 | Probiotic (oral) | MD 10.20 [7.45, 12.95] | Clinical effectiveness scores | P < 0.00001 |
| Youngshin 2012 [41] | T:58 C:60 | Probiotics (L. plantarum CJLP133) (oral) | MD 7.30 [2.63, 11.97] | Clinical effectiveness scores | P = 0.0029 |
CAM complementary and alternative medicine, RR risk ratio, MD mean difference, CI confidence interval
Global improvement (symptoms and signs improvement ≥95%, such as itching, skin lesions, swelling, and papula)
Global improvement was better for CAM (probiotics) compared with placebo in five trials [35, 37, 39–41] including 323 participants (MD 9.01, 7.12–10.90; I2 = 37%). Three trials [20–22] with 566 participants showed no difference between CAM alone (swimming, diet, or biofilm) and usual care (RR 1.43, 0.82–2.48; I2 = 91%). Eight trials [23–27, 29, 30, 32] involving 763 participants showed better effect from CAM plus usual care (RR 1.47, 1.30–1.68; I2 = 11%) compared with usual care.
Apart from statistical heterogeneity, the interventions in three trials [20–22] were totally different, including swimming [20], diet [21], biofilm [22], and one trial [20] investigated swimming in combination with Chinese herbal medicine lotion and tuina (Chinese massage for children). We conducted a qualitative description on these three trials. One trial [20] tested swimming, Chinese herbal medicine lotion and tuina compared to usual care. The intervention group showed more symptom reduction than the control group, however, not at a significant level. One trial [21] compared fasting and rotation diet with Pevisone paste, and reported beneficial effects of diet on symptom improvement. Another trial [22] showed statistically significant effects of Velvetfeeling Lotion (biofilm) on symptom improvement when compared with usual care.
Relapse rate
CAM showed lower relapse rate compared to usual care (RR 0.38, 0.28–0.51; n = 2, 418 participants) [20, 21]. CAM plus usual care showed lower relapse rate compared to usual care (RR 0.31, 0.24–0.40; n = 7, 698 participants) [24, 25, 27–31]. Nine trials [33–41] with 622 participants compared probiotics with placebo, but did not report the relapse, and rest of two trials [22, 23] with 188 participants did not report the relapse either.
≥50% improvement of symptoms and signs (such as itching, skin lesions, swelling, and papula)
Three trials [20–22] with 566 participants showed no difference between CAM alone (RR 1.20, 0.90–1.60; I2 = 92%) and usual care. Nine trials [23–27, 29–32] with 833 participants showed improvement from CAM (RR 1.34, 1.25–1.45; I2 = 35%) in addition to usual care compared with usual care alone. Nine trials [33–41] with 622 participants compared probiotics with placebo reported as continuous data resulting in unavailable outcome for ≥50% improvement of symptoms.
Apart from statistical heterogeneity, three trials had clinical heterogeneity for different CAM modalities [20–22]. One trial [20] showed that swimming had more children with improvement of symptoms and signs of ≥50% than the control group, but not at a significant level. One trial [21] showed significant effects of diet. Another trial [22] reported the positive effect of biofilm compared with usual care.
Adverse events
Only 12 trials (50%) [20, 22, 24, 25, 29, 30, 32, 35–37, 39, 43] reported the outcome of adverse events. Among these, four trials [27, 29, 35, 36] reported no occurrence of adverse events in either groups, and one trial [39] reported no relation between adverse events and the tested product without any details about adverse events, while seven trials [20, 22, 24, 30, 32, 37, 43] reported that children (< 14 years) with adverse events gradually adapted to treatments without extra treatment or that the adverse events was not related to the medications under investigation (Table 4). No severe adverse event such as death or hospitalization were reported. The reported adverse events included crying, irritability, and worsening of skin lesions (reddening) (Table 4).
Table 4.
Adverse events of CAM for childhood atopic eczema in randomized controlled trials
| Study ID | Total sample num | Sample num in intervention group | Sample num in control group | Adverse events cases | Intervention group | Control group | Treatment for adverse event |
|---|---|---|---|---|---|---|---|
| C. Gore 2011 [43] | 208 | 137 | 71 | T: 42/137 C: NR |
green loose stools; increased vomiting; feed-refusal; colic |
NR | 24/137 (17.5%) participants stopped the study formula. |
| Chen YL 2015 [24] | 116 | 58 | 58 | T: 1/58 C: 2/58 |
1 for dizzy. | 1 for dizzy; 1 for drowsy. |
NR |
| Liu CH 2009 [20] | 298 | 150 | 148 | T: 0/150 C: 57/148 |
None | 21 for facial flushing; 18 for dry skin; 8 for partial facial skin thinning; 10 for facial skin with mild pigmentation; Unclear for other hormonal dermatitis symptoms. |
NR |
| Sergei V. Gerasimov 2010 [37] | 96 | 48 | 48 | T: 26/48 C: 24/48 |
11 for upper respiratory tract infections; 4 for lower respiratory tract infections; 7 for herpetic stomatitis; 3 for diarrhea; 6 for constipation; 5 for abdominal colic; 2 for burn and croup with severe adverse events. |
10 for upper respiratory tract infections; 5 for lower respiratory tract infections; 5 for herpetic stomatitis; 2 for diarrhea; 6 for constipation; 4 for abdominal colic; 3for head injury, food poisoning with severe adverse events. |
None was related to the medications under investigation. |
| Wu YQ 2014 [22] | 148 | 74 | 74 | T: 2/74 C: 5/74 |
2 for crying and mildly red skin lesions on the 2nd day. | 5 for crying, irritability, and red skin lesions. | Without any treatment, to ease soon and symptoms disappearance. |
| Ye CQ 2017 [30] |
96 | 48 | 48 | T:3/48 C:14/48 |
1 for diarrhea; 2 for constipation. |
6 for diarrhea; 8 for constipation. |
NR |
| Zhang XN 2013 [32] | 70 | 36 | 34 | T: 1/36 C: 2/34 |
1 for mild skin irritation after using Velvetfeeling Lotion | 2 forskin lesions reddening after using Butyl Flufenamate Ointment. | Without any treatment, to ease soon and symptoms disappearance. |
T treatment group, C control group, NR not report
Additional analysis
Since the fact that each comparison did not include more than 10 trials, we were not able to conduct meaningful funnel plot analysis in order to identify the publication bias. Due to the same quality and the type of randomized trials, we could not conduct sensitivity analysis in this aspect. Besides, significant heterogeneity in two outcomes with two comparisons was more than 50% (I2 ≥ 50%), so we conducted a subgroup meta-analysis or a meaningful sensitivity analysis.
The global improvement (≥95% improvement) and ≥ 50% improvement of symptoms and signs in probiotics compared with usual care in three trials with 566 participants [20–22] showed I2 as 91 and 92%. The interventions were very heterogeneous in the trials including swimming [20], diet [21], and biofilm [22]. One trial [20] investigated not only swimming but also Chinese herbal medicine lotion and tuina. We conducted a sensitivity analysis, which showed improvement from CAM both for global improvement ≥95% improvement) (RR 1.77, 1.36–2.31; n = 2) and ≥ 50% improvement of symptoms and signs (RR 1.33, 1.16–1.52; n = 2).
Discussion
Summary of findings
This review identified 24 RCTs involving 2233 children (< 14 years) with AE. The findings suggest that some of the CAM modalities used alone or in combination with usual care may relieve the symptoms and signs of AE with ≥95% and ≥ 50% improvement, such as itchiness, skin lesions, swelling, and papula, in addition to reduce relapse of eczema. Moreover, some CAM modalities (such as probiotics) showed significant effect compared with placebo. The evaluated modalities appear to be safe and tolerated for lower relapse rate in CAM modality group. In spite of unclear pathogenesis of AE, CAM modalities may reduce symptoms and signs, and relapse of AE compared to conventional therapies.
The majority of trials had unclear risk of bias in many domains such as allocation concealment, blinding, missing data, and sample size calculation. Due to the unclear risk of bias of included trials, we could not come to firm conclusions from the evidence of the included trials in this review.
Comparison with previous studies
By searching the Cochrane Library with “AE”, “AD”, and “CAM”, there are 19 Cochrane reviews and 11 other reviews published, and after scanning, 9 reviews [13, 44–51] with CAM related to children (< 14 years) for treating or preventing. These reviews or protocols included both children and adults, and even pregnant women to prevent, cure and explore the pathogenesis of AE. We found only one protocol [44] similar to our review, but was withdrawn as the author gave up the title “Complementary and alternative medicine treatments for AE”. In addition, the previous studies did not exclude the allergic diseases (such as asthma, and intestinal diseases) to focus on AE. Our review included children (< 14 years) suffering only from AE. Moreover, most reviews investigated the specific treatment like probiotics, based on pathological mechanisms but ignoring the complex and unclear pathogenesis of AE. The findings of our review are based on the symptom relief of AE and we included more comprehensive trials involving CAM for children (< 14 years).
Strengths and limitations
Although great effort was made to retrieve all trials, we still cannot confirm that we were able to cover all the evidence due to non identified unpublished data. Besides, selecting and extracting data may also lead to some bias. We included only children under the age of 14 as this is the maximum age as younger adolescents defined by WHO, which may exclude some studies due to unavailable data for their participants over the age of 14 years. In addition, due to the various treatment for AE with an integrated “whole system” of care approach, we considered the control group with usual care as the system effect. The intervention group with CAM for a specific modality as the component efficacy, which cannot be used to document or disprove the effectiveness of a “whole system” treatment approach [15]. Additionally, in terms of the statistical heterogeneity and the variability in the CAM modalities, we were not able to conduct a subgroup meta-analysis, meaningful sensitivity analysis and funnel plot analysis. These factors limit the conclusiveness and robustness of this systematic review.
Implications for research
In fact of the limitations of this review, future trials should be designed as multi-center, double-blind placebo controlled trials with sufficient power, and reported according to the CONSORT (Consolidated Standards for Reporting Trials) Statement [52]. In addition, trials should record the relapse with sufficient length of follow-up. Besides, it is important to provide the definite safe treatments to the patients. Thus, adverse events in each group should be reported respectively so that we could retrospect the reason of different modalities and be easy to estimate the safety of CAM modalities.
Conclusion
Based on evidence from this systematic review we found some promising effect of CAM modalities on reducing symptoms and signs, and relapse of AE. However, it is still premature to recommend the therapy in clinical practice due to the limited number of trials and general low methodological quality of the included trials. Further rigorously double-blind, placebo-controlled trials are warranted to confirm efficacy of the CAM modalities for AE.
Additional file
Searching strategy for electronic databases. (DOCX 20 kb)
Acknowledgements
Much appreciation goes to Xun Li and Li-qiong Wang for their advice about data analysis.
Funding
This work was supported by the Capacity Building in Evidence-based Chinese Medicine and Internationalization Project by Beijing University of Chinese Medicine (No. 1000061020008).
Availability of data and materials
All data analyzed during this study are supported by the published articles in databases, including 12 opening electronic databases (details in search strategy section), and all data generated are included in this published article.
Abbreviations
- < 14 years
Under 14 years old
- AD
Atopic dermatitis
- AE
Atopic eczema
- CAM
Complementary and alternative medicine
- CHM
Chinese herbal medicine
- CI
Confidence interval
- CNKI
China National Knowledge Infrastructure
- DLQI
Dermatology life quality index
- EASI
Eczema area and severity index score
- I2
I-square
- ITT
Intention-to-treat
- MD
Mean difference
- NESS
Nottingham eczema severity score
- POEM
Patient oriented eczema measure
- PP
Per-protocol
- RCTs
Randomized clinical trials
- RR
Relative risk
- SCORAD
Scoring atopic dermatitis index
- SMD
Standardized mean difference
- VAS
Itching visual analogue score
- VIP
Chinese Scientific Journal Database
Authors’ contributions
JPL and VF conceived the review. CLL drafted the protocol and searched literature to identify eligible trials, extracted and analyzed data, and drafted the manuscript. XHL extracted and analyzed data. SBL did searches to identify eligible trials and revised on the tables in the drafted manuscript. XW and XB extracted the data. TS, AEK, AJN, FM, TA, VF, and JPL revised and commented on the drafted protocol and manuscript. All authors approved the final manuscript.
Ethics approval and consent to participate
All data during this study drew from publicly available data and this study did not recruit human participants, so ethical approval was not required. Not applicable.
Consent for publication
The manuscript does not contain data from an individual person. Not applicable.
Competing interests
The author JPL is the associate editor of the journal, but all authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chun-li Lu, Email: annyzhenni@163.com.
Xue-han Liu, Email: xuehan_liu@foxmail.com.
Trine Stub, Email: trine.stub@uit.no.
Agnete E. Kristoffersen, Email: agnete.kristoffersen@uit.no
Shi-bing Liang, Email: zyi20126185@163.com.
Xiao Wang, Email: wangxiao9311@126.com.
Xue Bai, Email: baixue667788@163.com.
Arne Johan Norheim, Email: arne.johan.norheim@uit.no.
Frauke Musial, Email: frauke.musial@uit.no.
Terje Araek, Email: terje.alrak@uit.no.
Vinjar Fonnebo, Email: vinjar.fonnebo@uit.no.
Jian-ping Liu, Phone: +86-13718004410, Email: Liujp@bucm.edu.cn, Email: Jianping.Liu@uit.no.
References
- 1.Helen N, Alan M, Williams HC. Mapping randomized controlled trials of treatments for eczema-The GREAT database (The Global Resource of Eczema Trials: a collection of key data on randomized controlled trials of treatments for eczema from 2000 to 2010) BMC Dermatol. 2011;11(1):10. doi: 10.1186/1471-5945-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manjra AI, Du PP, Weiss R, et al. Childhood atopic eczema consensus document. S Afr Med J. 2005;2:435. [PubMed] [Google Scholar]
- 3.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66:8–16. doi: 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- 4.Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 national survey of children’s health. J Investig Dermatol. 2011;131(1):67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genuneit J, Seibold AM, Apfelbacher CJ, et al. Overview of systematic reviews in allergy epidemiology. Allergy. 2017;72:849–856. doi: 10.1111/all.13123. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25(5):246–254. doi: 10.1097/DER.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 7.McAleer MA, Flohr C, Irvine AD. Management of difficult and severe eczema in childhood. BMJ. 2012;345:e4770. doi: 10.1136/bmj.e4770. [DOI] [PubMed] [Google Scholar]
- 8.NICE . Atopic eczema in under 12s: diagnosis and management. 2016. [PubMed] [Google Scholar]
- 9.Leung TN, Hon KL. Eczema therapeutics in children: what do the clinical trials say? Hong Kong Med J. 2015;21(3):251. doi: 10.12809/hkmj144474. [DOI] [PubMed] [Google Scholar]
- 10.Jadotte YT, Santer M, Vakirlis E, et al. Complementary and alternative medicine treatments for atopic eczema (Protocol). Cochrane Database Syst Rev. 2014; 10.1002/14651858.CD010938.pub2.
- 11.Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289–295. doi: 10.1016/j.nut.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu S, Yang AWH, Xue CCL, et al. Chinese herbal medicine for atopic eczema. Cochrane Database Syst Rev. 2013; 10.1002/14651858.CD008642.pub2. [DOI] [PMC free article] [PubMed]
- 14.National Center for Complementary and Alternative Medicine (NCCAM). Complementary, alternative, or integrative health: what’s in a name? http://nccam.nih.gov/health/whatiscam. Accessed 7 June 2017.
- 15.Fønnebø V, Grimsgaard S, Walach H, et al. Researching complementary and alternative treatments-the gatekeepers are not at home. BMC Med Res Methodol. 2007;7(1):7. doi: 10.1186/1471-2288-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0.2011. https://training.cochrane.org/handbook. Accessed 7 June 2017.
- 17.Wang LH, Zhou LB. Clinical observation on 648 cases of infantile allergic eczema treated by “heat-clarifying and dampness-removing mixture”. J Shanghai Univ Tradit Chin Med. 2002;16(1):26–27. [Google Scholar]
- 18.Zhang YZ. Clinical observation on 1840 cases of infantile allergic eczema treated by qibaixiaoruan ointment. Chin Community Doct. 2013;15(1):211. [Google Scholar]
- 19.Cheng XM. Observation on the curative effect of Yinyanjing on infantile eczema. Pract Prev Med. 2005;12(3):680. [Google Scholar]
- 20.Liu CH, Fu R, Wan H, et al. The clinical study of swimming therapy, Tuina and Baibu Heji lotion to treat infantile eczema. Hubei J Tradit Chin Med. 2009;31(12):21–23. [Google Scholar]
- 21.Liu WQ, Luo DJ, Teng LZ, et al. Clinical effect observation of diet taboos combined with alternative therapy in childhood eczema with food intolerance. Med Innov China. 2016;13(10):133–136. [Google Scholar]
- 22.Wu YQ, Zhao WQ, Pan JS. Assessment of therapeutic effect of medical skin healing biological film combined with moisturizer on infantile acute eczema. China J Lepr Skin Dis. 2014;30(1):38–39. [Google Scholar]
- 23.Chen DX. Clinical effect of oral probiotics and butyric acid hydrocortisone cream in the treatment of infantile eczema. Chin Foreign Med Res. 2015;10:21–23. [Google Scholar]
- 24.Chen YL. The clinical efficacy and safety of probiotics to treat infantile eczema. China J Pharm Econ. 2015;11:37–38. [Google Scholar]
- 25.Guo YH, Mou YD, Wang HS, et al. Clinical effect of microecologics as an adjuvant therapy on infants’ eczema. J Dalian Med Univ. 2015;6:571–573,588. [Google Scholar]
- 26.Jiang YX. Velvetfeeling lotion to treat 125 cases of infantile eczema. Health Horiz. 2013;6:583–584. [Google Scholar]
- 27.Li DY. The efficacy observation of bifid-triple viable capsule to treat infantile eczema. Public Med Forum Mag. 2012;29:3860–3861. [Google Scholar]
- 28.Mao HX, Hu XH. Clinical observation of supplement the intestinal probiotics as an adjuvant therapy to treat 50 cases of infant eczema. J Aerospace Med. 2013;24(3):337–338. [Google Scholar]
- 29.Wei MX, Yan R, Luo HB, et al. The clinical observation of bacillus coagulans tablets to treat 36 cases of infantile eczema. Chin J Pract Pediatr. 2010;25(12):943–945. [Google Scholar]
- 30.Ye CQ. Observation of curative effect on 48 cases of infant eczema treated by coagulative bacillus granulosa bioactive tablets. China Prac Med. 2017;12(18):120–121. [Google Scholar]
- 31.Zhang MH. The study of adhibition and the influence of intestinal flora, immunologic function and cytokines of probiotics to treat infantile eczema. Chin J Clin Ration Drug Use. 2013;6(6):13–14. [Google Scholar]
- 32.Zhang XN. Observation of efficacy and safety of the velvetfeeling combined with butyl flufenamate ointment in the treatment of child eczema. China Pract Med. 2013;8(25):26–27. [Google Scholar]
- 33.Sistek D, Kelly R, Wickens K, et al. Is the effect of probiotics on atopic dermatitis confined to food sensitized children? J Brit Soc Allergy Clin Immunol. 2006;36(5):629–633. doi: 10.1111/j.1365-2222.2006.02485.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang HJ, Min TK, Lee HW, et al. Efficacy of probiotic therapy on atopic dermatitis in children: a randomized, double-blind, placebo-controlled trial. Allergy, Asthma Immunol Res. 2014;6(3):208–215. doi: 10.4168/aair.2014.6.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reza F, Hamid A, Farahzad J, et al. Effect of a new synbiotic mixture on atopic dermatitis in children: a randomized-controlled trial. Iran J Pediatr. 2011;21(2):225–230. [PMC free article] [PubMed] [Google Scholar]
- 36.Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90(9):892–897. doi: 10.1136/adc.2004.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI. Probiotic supplement reduces atopic dermatitis in preschool children: a randomized, double-blind, placebo-controlled, clinical trial. Am J Clin Dermatol. 2010;11(5):351–361. doi: 10.2165/11531420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Kanehara S, Ohtani T, Uede K, et al. Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: a double-blind, placebo-controlled clinical trial. J Dermatol. 2007;34(12):811–815. doi: 10.1111/j.1346-8138.2007.00391.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu YJ, Wu WF, Hung C-W, et al. Evaluation of efficacy and safety of lactobacillus rhamnosus in children aged 4-48 months with atopic dermatitis: an 8-week, double-blind, randomized, placebo-controlled study. J Microbiol Immunol Infect. 2017;50:684–692. doi: 10.1016/j.jmii.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Yeşilova Y, Ömer C, Akdeniz N, et al. Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol. 2012;24(2):189–193. doi: 10.5021/ad.2012.24.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han Y, Kim B, Ban J, et al. A randomized trial of lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr Allergy Immunol. 2012;23(7):667–673. doi: 10.1111/pai.12010. [DOI] [PubMed] [Google Scholar]
- 42.Kankaanpää PE, Yang B, Kallio HP, et al. Influence of probiotic supplemented infant formula on composition of plasma lipids in atopic infants. J Nutr Biochem. 2002;13(6):364–369. doi: 10.1016/S0955-2863(02)00185-7. [DOI] [PubMed] [Google Scholar]
- 43.Gore C, Custovic A, Tannock GW, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow-up until age 3 years. Clin Exp Allergy. 2012;42(1):112–122. doi: 10.1111/j.1365-2222.2011.03885.x. [DOI] [PubMed] [Google Scholar]
- 44.Jadotte YT, Santer M, Vakirlis E, et al. Complementary and alternative medicine treatments for atopic eczema (Protocol). Cochrane Database Syst Rev. 2017; 10.1002/14651858.CD010938.pub2.
- 45.Küster D, Spuls PI, Flohr C, et al. Effects of systemic immunosuppressive therapies for moderate-to-severe eczema in children and adults (Protocol). Cochrane Database Syst Rev. 2015; 10.1002/14651858.CD011939.
- 46.Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012; 10.1002/14651858.CD000133.pub3. [DOI] [PMC free article] [PubMed]
- 47.Osborn DA, Sinn JKH. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007; 10.1002/14651858.CD006475.pub2. [DOI] [PubMed]
- 48.Kramer MS. Maternal antigen avoidance during lactation for preventing atopic eczema in infants. Cochrane Database Syst Rev. 1996; 10.1002/14651858.CD000131. [DOI] [PubMed]
- 49.Bath-Hextall FJ, Delamere FM, Williams HC. Dietary exclusions for established atopic eczema. Cochrane Database Syst Rev. 2008; 10.1002/14651858.CD005203.pub2. [DOI] [PMC free article] [PubMed]
- 50.Bamford JTM, Ray S, Musekiwa A, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013; 10.1002/14651858.CD004416.pub2. [DOI] [PMC free article] [PubMed]
- 51.Ersser SJ, Cowdell F, Latter S, et al. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev. 2014; 10.1002/14651858.CD004054.pub3. [DOI] [PMC free article] [PubMed]
- 52.CONSORT Statement 2001-Checklist: items to include when reporting arandomized trial. 2001. http://www.consort-statement.org. Accessed 7 June 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Searching strategy for electronic databases. (DOCX 20 kb)
Data Availability Statement
All data analyzed during this study are supported by the published articles in databases, including 12 opening electronic databases (details in search strategy section), and all data generated are included in this published article.