Abstract
Background and Objectives:
Thyroid disease largely affects young females, but the incidence is also increasing among males. In an effort to avoid the scarring of the neck that is synonymous with conventional thyroidectomy, endoscopic techniques have been developed over the years. The transoral endoscopic approach is the latest of these innovations that promises a scarless surgical outcome. This review evaluates whether this technique is safe and feasible in live patients and outlines the outcomes in published literature so far.
Database:
PubMed, Medline, BioMed Central, Cochrane Library, OVID and Web of Science were systematically searched by using a Medical Subject Heading (MeSH)–optimized search strategy. The selection of papers followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines after setting strict inclusion and exclusion criteria. Sixteen studies were included in the final analysis.
Discussion:
This systematic review presents cases of 785 patients. Surgeons in 15 of the studies used a completely vestibular approach, whereas those in the remaining 2 used the floor of the mouth for primary access. Conversion to open surgery took place in 1.3%. In total, 4.3% of patients experienced transient laryngeal nerve palsy, whereas 0.1% had permanent recurrent incidences of the condition. Transient hypocalcemia occurred in 7.4% of cases, with no recorded permanent cases. Carbon dioxide embolism occurred in 0.6% of cases, and another 0.6% had a deep-seated neck infection. The complication rates within the review were deemed acceptable and the overall technique feasible. A prospective randomized controlled trial was proposed to compare this technique with conventional thyroidectomy.
Keywords: Natural orifice endoscopic surgery, Oral endoscopy, Scarless, Thyroidectomy, Transoral
INTRODUCTION
Over the past centuries, procedures to surgically remove all or part of the thyroid gland from the neck have gone from infamy to fame. What Samuel D. Gross in the 19th century considered to be “horrid butchery,” through the brave work of surgeons like Emil Theodor Kocher, has become one of the most common and safest of surgeries.1–3 The gold-standard approach for thyroidectomy has been open or conventional surgery. Recently, there has been increased interest in applying the principles of minimally invasive surgery to thyroid surgery. This development was initially promoted by Miccoli and his colleagues4 in 1999 and has continued to expand and improve throughout recent years. The aims of minimally invasive surgery include better cosmesis and earlier recovery without compromising the excellent results achieved with open surgery.5 The approaches taken in thyroid surgery include mainly a transaxillary approach with later additions of areolar, anterior chest wall, and mixed approaches.6–10 The extent of dissection and difficulty of these procedures despite robotic help has limited the uptake of these techniques.11,12
The transoral endoscopic technique, an adaptation of the concept of natural orifice transluminal endoscopic surgery (NOTES) to the neck, is a technique that promises to improve the aesthetic aspect by offering a scarless operation while retaining the advantages of minimally invasive surgery.13,14 The pioneers of this technique were the group led by Witzel and his colleagues,15 who presented their first paper on the subject in 2008. In their study on cadavers and live pigs, they managed to present a proof of concept that formed the basis for the extensive work that is being carried out by multiple groups around the world.
Transoral endoscopic thyroidectomy is performed with the patient under general anesthesia. The patient is placed supine with the neck extended. The mouth is cleansed with 0.05% chlorhexidine in water, and the patient is usually given a broad-spectrum antibiotic (eg, amoxicillin/clavulanic acid). The primary surgeon stands at the patient's head facing the monitor and the first assistant stands to the patient's left. Access through the mouth is gained with the use of a 10-mm port and two 5-mm ports, the position of which varies according to different techniques. The subplatysmal space is developed with the use of hydrodissection. Carbon dioxide insufflation is usually used to keep the working space open. The working space is developed keeping the larynx as the superior border, the suprasternal notch as the inferior border, and the anteromedial borders of the sternocleidomastoid muscles bilaterally as lateral borders. Strap muscles are divided at the linea alba cervicalis. The dissection continues in a craniocaudal fashion with the use of energy sealing devices. The thyroid isthmus is first identified, elevated, and divided. The veins and arteries supplying the thyroid gland are sealed as close as possible to the thyroid gland. The superior laryngeal nerve, recurrent laryngeal nerve, and parathyroid glands should be identified and protected. Berry's ligament is then dissected. The thyroid specimen is retrieved in an Endobag (Medtronic, Minneapolis, Minnesota, USA). The space is irrigated with saline, and drains are inserted if needed. The mucosal wounds are closed with an absorbable 5-0 suture.16
The procedure was shown to be feasible and safe in several cadaveric17,18 and animal model studies19,20 and subsequently was translated to implementation in human patients. In view of these developments, this systematic review was conducted to identify the current experience of transoral endoscopic thyroidectomy and to assess the safety and feasibility of this technique according to the outcomes set.
MATERIALS AND METHODS
For this systematic review, 6 databases were searched up to January 31, 2018: PubMed, Medline, BioMed Central, Cochrane Library, OVID, and Web of Science. Keywords used for the search strategy included “human,” “transoral,” “floor of mouth,” “vestibule,” “endoscopic,” “scarless,” “video-assisted,” “natural orifice,” and “thyroidectomy.” The comprehensive search strategy was adapted to the different search engines. In addition to the aforementioned searches, back-chaining of references and manual searches of key journals were conducted. Key experts in the field were also contacted. The study protocol was registered on PROSPERO (CRD42017075758).
The primary outcome was to elucidate whether, from the available literature, the technique of transoral video-assisted endoscopic thyroidectomy is safe and feasible. Several secondary outcomes were identified for this systematic review. These included the following: population demographics, type of access used, use of carbon dioxide insufflation, blood loss, length of surgery, use of intraoperative neuromonitoring (IONM), use of drains, rate of conversion to open, rate of complications associated with the procedure (including recurrent laryngeal nerve damage, parathyroid damage, and mental nerve damage), time to oral intake, and time to discharge. The Population, Intervention, Comparison, and Outcomes (PICO) framework was used to develop the research question. Inclusion criteria encompassed studies involving patients with thyroid disease who underwent transoral endoscopic thyroidectomy, with or without the use of gas insufflation and in whom the primary and secondary outcomes were investigated. Observational studies, randomized controlled studies, case–control studies, and case series were included. Only literature published in English was considered for this systematic review. No restrictions on date of publication or country of origin were applied. Studies investigating neck pathology other than thyroid or in which robotic or open procedures or endoscopic surgery that did not use the transoral approach were performed were excluded. Qualitative studies, case reports, abstracts, editorials, conference proceedings, and systematic reviews were also excluded.
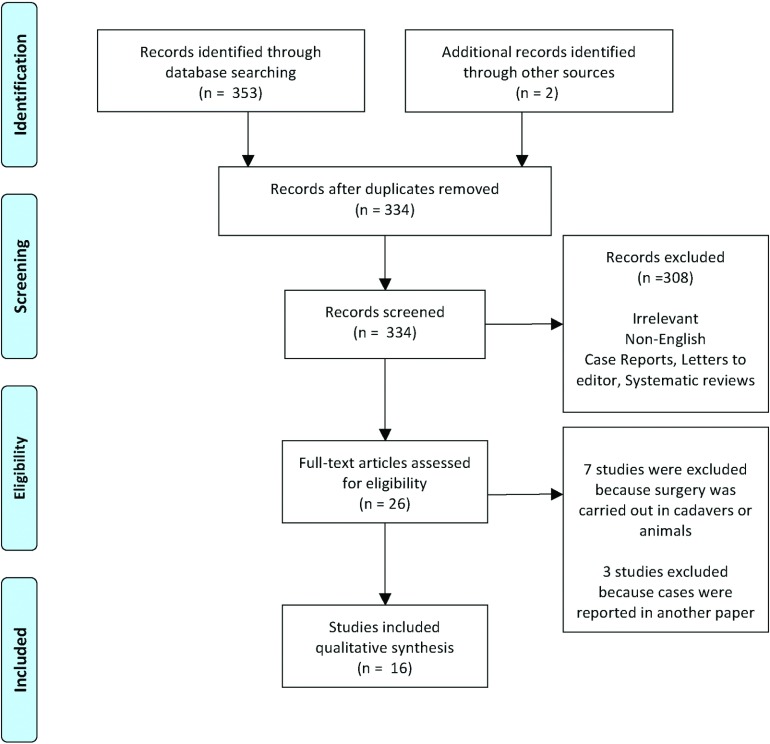
After excluding all duplicates, 2 separate researchers independently reviewed the titles and abstracts of all downloaded citations to decide whether to include or exclude the studies. When it was not possible to determine whether a citation was relevant, it was included at this stage. In cases of disagreement between the reviewers about a citation, the citation was also included. Full copies of the potentially included papers were obtained and reviewed by the independent reviewers. In cases of disagreement a third researcher was involved to discuss and settle any divergences. The number of studies included and excluded at each stage of the review was recorded with a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart diagram that illustrated the number of studies screened, the number of studies assessed for eligibility, and the number of studies included in the review (Figure 1). Data was extracted from all the studies that fulfilled the inclusion criteria. A data extraction form was developed for the purpose of standardization. Data quality assessment was carried out by 2 independent reviewers using the Quality Appraisal Tool for Case Series studies developed by the National Institutes of Health.21
Figure 1.
The PRISMA flow diagram for the selection of articles for review. The articles present at each stage of the review are represented and reasons for exclusion are given.
RESULTS
Sixteen studies are included in this review. The earliest published series was in 2011 by Wilhelm et al22 from Germany. Patients in that study were integrated into a later paper by the same team, and therefore this original paper was excluded. Since then, several other case series have been published, mostly from Thailand, Republic of Korea, and China. The studies reviewed sum to a total of 785 patients of which 713 (91%) were female and 68 (8%) were male. Zeng et al23 did not specify the gender of the 4 participants in their study. The weighted mean age of the patients in the included studies was 35 years. Fourteen of the studies included were considered to be of good quality.24–37 The other 2 lacked detail and focus and were therefore considered to be of fair quality.23,38
The inclusion and exclusion criteria used in the different studies were heterogeneous; however, general themes were present. Fourteen studies declared their inclusion criteria. Patients with benign disease (proven on imaging and cytology) or papillary microcarcinoma, in whom the nodules' maximum diameter was <5 cm, the total thyroid volume was ≤45 mL, and in whom there was no lymph node metastasis, were included. Fourteen studies declared the exclusion criteria. Patients in whom there was history of neck surgery or radiotherapy, recurrent disease, presence of intraoral infection or characteristics that did not fit the inclusion criteria, were excluded. Altogether there were 396 hemithyroidectomies, 249 total thyroidectomies, 10 isthmusectomies, and 31 subtotal thyroidectomies. Zeng and his colleagues23 did not indicate what type of thyroidectomy their 4 participants underwent (Table 1).
Table 1.
Demographic Characteristics
| Study | Number (Gender) | Inclusion Criteria | Exclusion Criteria | Mean Age (years) |
|---|---|---|---|---|
| Nakajo et al.24 | 8 (7 F, 1 M) | Follicular neoplasm, symptomatic large nodular goiter, Graves' disease and papillary microcarcinoma. | Evidence of lymph node metastasis. | 54.9 |
| Wang et al.25 | 12 (10 F, 2 M) | Benign tumor on B-scan US and CT confirmed on cytology, patient consent, mass ≤6 cm, healthy patient. | Mass >6 cm, advanced cardiac or pulmonary disease, patient not a candidate for minimally invasive techniques, patient indifferent regarding unscarred skin. | 24 |
| Yang et al.26 | 41 (33 F, 8 M) | 18–50 years old, maximum tumor diameter <5 cm, hyperthyroidism not exceeding degree II, suspicious cancer without metastasis in cervical lymph nodes, endoscopic surgery required. | Infected lesions (e.g. oral ulcer), history of neck surgery, substernal goiter. | 31.9 |
| Udelsman et al.27 | 5 F | Toxic thyroid adenoma, multinodular goiter, indeterminate thyroid nodule and papillary thyroid microcarcinoma. | NA | 42.8 |
| Zeng et al.23 | 4 (gender not reported) | NA | Malignancy, hyperthyroidism, previous neck surgery/radiotherapy, ≥5 cm nodule. | NA |
| Yang et al.28 | 6 (5 F, 1 M) | Tumor with maximum diameter <5 cm, good mobility under palpation, clear boundary, I- or II-degree thyroid enlargement. | Hyperthyroidism or parathyroid disease, history of neck surgery, adhesions to surrounding benign tissue. | 34.3 |
| Jitpratoom et al.29 | 45 (40 F, 5 M) | Controlled Graves' disease with suspicious nodule or toxic multinodular goiter, failure of medical treatment, or local compressive symptoms. | Unfit for surgery, previous neck surgery/radiotherapy, could not tolerate general anesthesia, thyroid diameter ≥10 cm. | F, 32.47 ± 4.74; M, 35.80 ± 11.70 |
| Wilhelm et al.30 | 96 (92 F, 4 M) | 12 adenomas, 1 cystic lesion, 66 uninodular and 14 multinodular changes, 11 Hashimoto thyroiditis and 2 micropapillary carcinoma. | NA | F, 36 ± 10; M, 48 ± 4 |
| Dionigi et al.31 | 15 (12 F, 3 M) | US showing estimated thyroid gland diameter ≤10 cm, thyroid volume ≤45 mL and max nodule size ≤5 cm; benign nodules, follicular neoplasms, papillary microcarcinoma without metastasis. | Patients unfit for surgery or cannot tolerate general anesthesia, previous radiation or surgery in neck, recurrent goiter, thyroid volume >45 mL, dominant nodule size >5 cm, evidence of LN metastasis or tracheal/esophageal invasion, RLN palsy, hyperthyroidism, oral abscess. | 29.4 |
| Russell et al.38 | 6 F | Hypertrophic scarring, motivated to avoid cervical scar. | Thyroiditis, external beam radiotherapy, nodule >6 cm. | 37.2 |
| Sivakumar et al.32 | 11 F | Nodular thyroid disease, nodule size <4 cm, thyroid volume ≤30 mL. | History of neck surgery, neck radiotherapy, nodules or thyroid volumes large than inclusion criteria. | 24.8 |
| Park et al.33 | 18 (13 F, 4 M) | Consent for the new approach, thyroid cancer <2.5 cm or a benign tumor <8 cm. | Extrathyroid extension or lymph node metastasis on preoperative US, surgical treatment of head and neck. | F, 39.6; M, 48.3 |
| Chai et al.35 | 10 F | Papillary microcarcinoma. | Suspected capsular invasion and lymph node metastasis. | 43.3 ± 11.5 |
| Bakker et al.36 | 5 F | Solitary thyroid nodule, Bethesda II, III, or IV, US thyroid volume of ≤45 mL, thyroid diameter ≤10 cm, noncancerous nodules up to 5 cm. | Previous neck irradiation. | 36 |
| Anuwong et al.34 | 422 (389 F, 33 M) | NA | Previous neck/chin surgery, substernal goiter, clinical evident lateral neck lymph node, distant/ local metastasis. | 35.3 |
| Fu J. et al.37 | 81 (79 F, 2 M) | Benign tumor diameter <5 cm evaluated by US, malignant tumor, patients with cosmetic requirements. | Maximum tumor diameter >5 cm, cancer with metastasis to cervical LN, Graves' disease, history of surgery or radiation to neck, severe coagulation disorders. | 34.2 ± 9.4 |
US, ultrasonography; LN, lymph node; RLN, recurrent laryngeal nerve; GA, general anesthesia.
The access to the neck through the mouth was mostly through a completely vestibular approach (14 out of 16 studies). This approach involves a central 10-mm port that is used to accommodate the endoscope and two 5-mm ports inserted at the junction between the incisors and canine bilaterally; these ports are used as routes of access for the working instruments.24–29,31–36,38 Zeng et al23 modified this technique by using a central 12-mm port. The only exceptions to this approach are presented by Wilhelm et al and Fu et al.30,37 Both of these groups used the floor of mouth as the access point for the 10-mm port that received the endoscope. The working 5-mm ports were inserted at the previously described position in the vestibule (Figure 2).
Figure 2.
Position of ports. Red lines: position of 5-mm ports in both techniques. Blue line: position of the 10-mm port in the totally vestibular approach. Yellow circle: 10-mm port position of the floor-of-the-mouth approach.
In most studies, the working space was kept open with the use of carbon dioxide insufflation. Fourteen studies reported the use of carbon dioxide. The maximum insufflation pressures used in most instances was 6 mm Hg.25–29,31,32,34,36 Some researchers allowed a range of pressures ranging from as low as 4 mm Hg37 and up to 8 mm Hg.23,37,38 Wilhelm et al30 reported the use of carbon dioxide insufflation but did not report the pressures accepted. Nakajo et al24 attempted to eliminate carbon dioxide insufflation in the neck by using Kirschner wires of 1.2-mm thickness. These were inserted through the skin of the neck and used to elevate the skin and platysma, therefore maintaining the working space needed.
IONM was used in only 4 of the studies.30,31,35,38 The weighted average length of operation reported in the 16 studies included for isthmusectomy was 53.3 minutes, for hemithyroidectomy was 85.5 minutes, for subtotal thyroidectomy was 115.4 minutes, and for total thyroidectomy was 136.6 minutes, giving an overall weighted average operating time of 94.9 minutes. In total, 10 (1.3%) conversions to open surgery were reported: 3 by Wilhelm et al,30 3 by Anuwong et al,34 1 by Jitpratoom et al,29 1 by Bakker et al,36 and 2 by Fu et al.37 The weighted average loss of blood reported in 12 studies was 34.3 mL. Three groups used drains routinely after both total and hemithyroidectomy,24,29,32 whereas another 3 groups used drains almost exclusively for total thyroidectomy only.31,33,34 Fu et al37 never used drains in the first 49 cases and subsequently used them in all the remaining 32 cases. The rest of the study groups used no drains in their procedures.23,25–28,30,35,36,38 (Table 2).
Table 2.
Operative Characteristics
| Study | Type of Surgery (n) | Access | Average Length of Surgery (min) | Conversion to Open (n) | IONM | CO2 Insufflation and Pressure (mm Hg) | Blood Loss (mL) | Drain |
|---|---|---|---|---|---|---|---|---|
| Nakajo et al.24 | Hemithyroidectomy, 5; subtotal thyroidectomy, 3. | Completely vestibular (midline 10 mm and two 5 mm ports). | Hemithyroidectomy, 208; subtotal thyroidectomy, 361. | None | No | No, skin retraction with Kirschner 1.2 mm wires. | Hemithyroidectomy, 108; subtotal thyroidectomy, 80. | Yes |
| Wang et al.25 | Hemithyroidectomy, 8 total thyroidectomy, 4. | Completely vestibular (midline 10 mm and two 5 mm ports). | 60.4 ± 7.4 | None | No | Yes, 6 | 10.8 ± 7.3 | No |
| Yang et al.26 | Hemithyroidectomy, 19; subtotal/near total, 18; total thyroidectomy, 4. | Completely vestibular (midline 10 mm and two 5 mm ports). | 72.1 ± 19.5 | None | No | Yes, 6 | 11.1 ± 7.1 | No |
| Udelsman et al.27 | Hemithyroidectomy, 3; total thyroidectomy, 2. | Completely vestibular (midline 10 mm and two 5 mm ports). | Hemithyroidectomy, 214.3; total thyroidectomy, 290.5. | None | No | Yes, 6 | <20 | No |
| Zeng et al.23 | NA | Completely vestibular (midline 12 mm and two 5 mm ports). | 189 ± 39.14 | None | No | Yes, 8 | 7.50 ± 2.89 | No |
| Yang et al.28 | Hemithyroidectomy, 6 | Completely vestibular (midline 10 mm and two 5 mm ports). | 122 | None | No | Yes, 6 | 30 | No |
| Jitpratoom et al.29 | Total thyroidectomy, 45 | Completely vestibular (midline 10 mm and two 5 mm ports). | 134.11 ± 31.48 | 1 | NO | Yes, 6 | 62.81 ± 37.37 | Yes |
| Wilhelm et al.30 | Isthmusectomy, 10; hemithyroidectomy, 66; subtotal thyroidectomy. 10; and total thyroidectomy, 7. | Bivestibular and sublingual. | Isthmusectomy, 38; hemithyroidectomy, 49; subtotal thyroidectomy, 88; and total thyroidectomy, 126. | 3 | No | Yes but no pressure noted. | Isthmusectomy 15, hemi-thyroidectomy 20, subtotal thyroidectomy 30 and total thyroidectomy 67. | No |
| Dionigi et al.31 | Hemithyroidectomy, 10; total thyroidectomy, 5. | Completely vestibular (midline 10 mm and two 5 mm ports). | Hemithyroidectomy, 86.8; total thyroidectomy, 115.2. | None | Yes | Yes, 6 | Hemithyroidectomy, 36.5; total thyroidectomy, 62. | Yes, for total thyroidectomy only |
| Russell et al.38 | Hemithyroidectomy, 6. | Completely vestibular (midline 10 mm and two 5 mm ports). | 245.5 | None | Yes | Yes, 5–7 | NA | No |
| Sivakumar et al.32 | Total thyroidectomy, 11. | Completely vestibular (midline 10 mm and two 5 mm ports). | 126.3 | None | No | Yes, 6 | 1.8 | Yes |
| Park et al.33 | Hemithyroidectomy, 16; total thyroidectomy, 2 | Completely vestibular (midline 10 mm and two 5 mm ports). | Hemithyroidectomy, 168.8; total thyroidectomy, 185. | None | No | Yes, 5–6 | NA | Yes, for total thyroidectomy and 1 case of hemithyroidectomy. |
| Chai et al.35 | Hemithyroidectomy, 7; isthmusectomy, 3. | Completely vestibular (midline 10 mm and two 5 mm ports). | Isthmusectomy, 90.0 ± 9.2; hemithyroidectomy, 121.1 ± 30.7. | None | Yes | Yes, 6 | NA | No |
| Bakker et al.36 | Hemithyroidectomy, 5. | Completely vestibular (midline 10 mm and two 5 mm ports). | 122 | 1 | No | Yes, 6 | NA | No |
| Anuwong et al.34 | Hemithyroidectomy, 245; total thyroidectomy, 177. | Completely vestibular (midline 10 mm and two 5 mm ports). | Hemithyroidectomy, 78.6; total thyroidectomy, 135.1. | 3 | No | NA | Hemithyroidectomy, 26.8; total thyroidectomy, 52.6. | Yes, for total thyroidectomy only. |
| Fu J. et al.37 | Hemithyroidectomy (subtotal), 65; isthmusectomy, 6; subtotal thyroidectomy, 5; total thyroidectomy, 5. | 10 mm port in floor of mouth anterior to frenulum, 5 mm vestibular port. | Hemithyroidectomy (subtotal), 90.1± 39.8; isthmusectomy, 60.3 ± 8.2; subtotal thyroidectomy, 100.0 ± 35.4; and total thyroidectomy, 115.0 ± 14.1. | 2 | No | Yes, 4–8 | Hemi-thyroidectomy (subtotal), 26.3 ± 20.6; isthmusectomy, 15.2 ± 7.8; subtotal thyroidectomy, 28.6 ± 16.4; total thyroidectomy, 55.0 ± 31.6. | Yes (last 32 cases). |
IONM, intraoperative neuromonitoring.
Several complications were reported in the studies. Overall, 34 (4.3%) cases of temporary recurrent laryngeal nerve palsy were described.26,29,34,35,38 One (0.1%) case was reported by Wilhelm et al,30 and 1 case by Nakajo et al24; however, they did not clarify whether the condition was temporary or permanent.24 Fifty-eight (7.4%) cases of transient postoperative hypocalcemia were reported.29,31,33,34 In all cases, there was full recovery of the function of the parathyroid glands with no resultant permanent hypocalcemia. Fu et al37 did not report any cases of postoperative hypocalcemia but stated that 2 patients had perioral numbness. Eighteen (2.29%) cases of temporary mental nerve palsy occurred overall.24,30,34 Nakajo et al24 did not report any specific cases of mental nerve damage; however, they mentioned that all their participants had altered sensation in the chin area that persisted for a period exceeding 6 months. Twenty-two (2.8%) occurrences of seroma are reported throughout the studies.33,34 There were 6 (0.8%) cases of subcutaneous emphysema that were all self-limiting31,36 and an additional case (0.1%) of mediastinal emphysema that did not carry long-term consequences.30 Yang et al26 reported 1 case of anterior flap perforation and another of neck skin burn, and Bakker et al36 also reported 1 case of flap perforation (0.3%). Five cases (0.6%) of carbon dioxide–induced gas embolism were reported; 3 by Wilhelm et al30 and 2 by Fu et al.37 In 4 cases (0.5%), the patients developed extensive ecchymosis after surgery.26,39 One case (0.1%) of operative site hematoma required emergency decompression through an open neck incision.34 Five cases (0.6%) of deep-seated neck infections were reported by Wilhelm et al.30 In addition, 6 cases (0.8%) of wound infections were reported.28,30,37 Fu et al37 had 4 cases of inflammatory masses in the neck, and they reported 2 patients with pain on opening the mouth, 2 with excessive salivation, and 2 with neck discomfort.37 The occurrence of lower lip swelling is common after transoral endoscopic thyroidectomy; however, this condition is usually self-limiting.24,31
From the 9 studies that recorded time to oral intake, most patients were allowed oral intake either on the day of surgery26,31,33–35,37 or on day 1 after surgery.24,29,32 Time for discharge, in the 12 studies that reported it, had a weighted mean of 4.3 d but this varied widely between the different studies, ranging from 1 day38 to more than 8 days28 (Table 3).
Table 3.
Postoperative Outcomes
| Study | RLN Injury | Hypocalcaemia | Mental Nerve Injury | Other Complications (n) | Time to Oral Intake (days) | Average Time to Discharge (days) |
|---|---|---|---|---|---|---|
| Nakajo et al.24 | 1 (not specified) | NA | No | Lower lip swelling and lower lip altered sensation lasting 6 months in all patients. | 1 | 4.25 |
| Wang et al.25 | No | No | No | Ecchymosis, 2. | NA | 4.9 |
| Yang et al.26 | 1 transient | No | No | Ecchymosis, 2; skin pierced, 1; anterior skin burn. 1. | 0 | 5 ± 1.4 |
| Udelsman et al.27 | No | No | No | No | NA | 1.1 |
| Zeng et al.23 | No | No | No | No | NA | 5 |
| Yang et al.28 | No | No | No | Wound infection, 1. | NA | 8.2 |
| Jitpratoom et al.29 | 4 transient | 10 transient | No | No | 1 | NA |
| Wilhelm et al.30 | 1 permanent | NA | 15 transient (resolved in three weeks). | Intraoral infection, 1; neck site infection, 5; CO2 embolism, 3; mediastinal emphysema, 1. | NA | NA |
| Dionigi et al.31 | No | 1 transient | No | Mild emphysema. | 0 | 2.1 |
| Russell et al.38 | 1 temporary | NA | No | No | NA | 1 |
| Sivakumar et al.32 | No | No | No | No | 1 | NA |
| Park et al.33 | No | 1 transient | No | 2 seroma | 0 | 4.1 |
| Chai et al.35 | 2 transient | NA | No | No | 4 hours | 3 |
| Bakker et al.36 | No | NA | No | 5 subcutaneous emphysema, 1 flap perforation. | NA | 1.2 |
| Anuwong et al.34 | 25 transient | 46 transient | 3 transients | 20 seroma, 1 hematoma needed cervical incision for decompression. | 0 | NA |
| Fu J. et al.37 | No | No | No | 2 CO2 embolism, 2 perioral numbness, 2 pains on opening mouth, 2 patients excessive salivation, 2 patients had neck discomfort, 4 wound infection, 4 inflammatory mass. | 6 hours | 4.77 ± 2.61 |
NA, not available.
DISCUSSION
This systematic review extensively explores the published experience of transoral endoscopic thyroidectomy. The technique has been gaining ground, with this systematic review presenting the experience of various groups in 785 patients. As is usual with thyroid disease, most the patients were young females. The indications for surgery varied widely among the studies, and therefore there is no standard set of indications that can be proposed based on this systematic review. However, the contraindications of previous neck surgery or neck irradiation, intraoral infection, and recurrent disease seemed to be common throughout the studies. Other contraindications for this procedure, not encountered in this systematic review, include smoking and morbid obesity.40
Two slightly different access techniques were reported in the studies. Wilhelm et al30 and Fu et al37 (a group that reportedly was trained by Wilhelm) used a floor-of-the-mouth access in which the first camera port was inserted just anterior to the frenulum, whereas the working ports were inserted through the vestibule. The rest of the groups used a technique in which both the camera port and working ports were inserted through the vestibule. When comparing the complications reported from the 2 techniques, it was evident that using the floor-of-the-mouth access led to more carbon dioxide embolism, mediastinal emphysema, and both neck and surgical site infection. Otherwise, there was no major difference between the 2 reported techniques. Combining the transoral technique with other minimally invasive techniques has also been proposed.41 Although the latter procedures may be easier, they have not been included in this systematic review, because they go against the scarless principle of the transoral technique.
Most groups used carbon dioxide insufflation to keep the working space open during the procedures. The pressure used varied, with the most commonly used pressure at a maximum of 6 mm Hg, but Zeng et al,23 Russell et al,38 and Fu et al37 allowed higher pressures. Nakajo et al24 did not use carbon dioxide and opted to keep the working space open by pulling the skin of the neck with Kirschner wires. Although the results of this group were comparable to those of the rest of the groups that used insufflation, the technique has been criticized as going against the scarless concept of the transoral technique, because several small stab incisions were needed to anchor the wires in the skin. Wang et al42 described a hybrid method in which the surgical working space is kept open by combining a cosmetically acceptable suspensory mechanism with carbon dioxide insufflation. Data regarding the outcomes of this technique are limited; further research is needed to assess its feasibility.
It is recognized that the transoral endoscopic thyroidectomy technique is challenging and the dissection can prove to be difficult. The rate of conversion to conventional dissection in this review was 1.3%, which is acceptable, given the length of experience with the technique and the arduous task involved. In terms of outcomes, the rates of recurrent laryngeal nerve palsy, postoperative hypocalcemia, infection, and bleeding after transoral thyroidectomy are comparable to those after conventional thyroidectomy.29,43–45 Recurrent laryngeal nerve monitoring through a long probe has been used31,38 and found to be safe and feasible,46 thus enhancing the safety of the procedure. However, the technique presents several new complications that, although not common, have to be given due consideration. Mental nerve injury was reported in 2.29% of cases; however, a level of chin paresthesia was common in many patients. This condition is usually self-limiting, but patients should be cautioned regarding the possibility. Carbon dioxide embolism, flap perforation, and surgical emphysema are other examples of uncommon but potential complications that this technique presents. These findings are comparable to those published by Shan and Liu.47
Patients who underwent the procedure recovered early and were given fluids, either on the same day or the day after surgery, without any appreciable complications, despite having surgical wounds in the mouth. Notwithstanding the fast recovery, the technique was not performed as day surgery. Patients spent an average of 4.3 d in the hospital, although some groups discharged their patients on postoperative day 1. There is no clear explanation, given the studies, of the reason for this prolonged length of stay. Studies including patients who had longer hospital stays do not consistently report more complications than other groups. It could be that given that the technique is novel, some groups opted for longer postoperative observation to exclude complications including bleeding, airway impairment, and neck space infections. As experience and confidence in the procedure increase, the length of stay should decrease.
Excluding literature that was not in English meant excluding several studies in which extensive patient populations were studied. One such example is the case series published in Chinese by Wang et al39 which included 150 cases. The reporting only of studies in English is one of the limitations of this study. The robotic experience was purposefully excluded from this review to keep the study topic clear. Notwithstanding the exclusion, robotic assistance in the transoral technique is an interesting concept that is being evaluated and developed. Another limitation of this review is the level of evidence carried by the included papers. The technique has now gained enough ground to be compared to conventional thyroidectomy in a prospective randomized trial that would carry a high level of evidence. Jitpratoom et al29 and Anuwong et al34 have performed this exercise; however, both studies were retrospective. In both the latter studies, the transoral approach was found to be as safe as the conventional cervical approach.
CONCLUSION
Transoral endoscopic thyroidectomy is a safe and feasible technique with acceptable complication rates and good outcomes. The completely vestibular approach seems to offer a safer alternative to floor-of-the-mouth access. More research, particularly using newly developed tools to further improve this technique, are needed, thus making it more available to patients worldwide.
Contributor Information
Christian Camenzuli, Department of Anatomy, Faculty of Medicine and Surgery, Biomedical Sciences, University of Malta, Msida, Malta..
Pierre Schembri Wismayer, Department of Anatomy, Faculty of Medicine and Surgery, Biomedical Sciences, University of Malta, Msida, Malta..
Jean Calleja Agius, Department of Anatomy, Faculty of Medicine and Surgery, Biomedical Sciences, University of Malta, Msida, Malta..
References:
- 1. Tapscott WJ. A brief history of thyroid surgery. Curr Surg. 2001;58:464–466. [DOI] [PubMed] [Google Scholar]
- 2. Hannan SA. The magnificent seven: a history of modern thyroid surgery. Int J Surg. 2006;4:187–191. [DOI] [PubMed] [Google Scholar]
- 3. Fortuny JV, Guigard S, Karenovics W, Triponez F. Surgery of the thyroid: recent developments and perspective. Swiss Med Wkly. 2015;145:w14144. [DOI] [PubMed] [Google Scholar]
- 4. Miccoli P, Berti P, Conte M, Bendinelli C, Marcocci C. Minimally invasive surgery for thyroid small nodules: preliminary report. J Endocrinol Invest. 1999;22:849–851. [DOI] [PubMed] [Google Scholar]
- 5. Mohamed SE, Noureldine SI, Kandil E. Alternate incision-site thyroidectomy. Curr Opin Oncol. 2014;26:22–30. [DOI] [PubMed] [Google Scholar]
- 6. Hakim Darail NA, Lee SH, Kang S, Jeong JJ, Nam K, Chung WY. Gasless transaxillary endoscopic thyroidectomy: a decade on. Surg Laparosc Endosc Percutan Tech. 2014;24:211. [DOI] [PubMed] [Google Scholar]
- 7. Gao W, Liu L, Ye G, Lu W, Teng L. Bilateral areolar approach endoscopic thyroidectomy for low-risk papillary thyroid carcinoma: a review of 137 cases [published correction in Surg Laparosc Endosc Percutan Tech. 2015;25:373]. Surg Laparosc Endosc Percutan Tech. 2015;25:19–22. [DOI] [PubMed] [Google Scholar]
- 8. Woo J, Kim SK, Park I, Choe JH, Kim J, Kim JS. A novel robotic surgical technique for thyroid surgery: bilateral axillary approach (BAA). Surg Endosc. 2017;31:667–672. [DOI] [PubMed] [Google Scholar]
- 9. Chowbey PK, Soni V, Khullar R, Sharma A, Baijal M. Endoscopic neck surgery. J Minim Access Surg. 2007;3:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang EHE, Kim HY, Koh YW, Chung WY. Overview of robotic thyroidectomy. Gland Surg. 2017;6:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berber E, Bernet V, Fahey TJ, et al. American Thyroid Association Statement on Remote-Access Thyroid Surgery. Thyroid. 2016;26:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inabnet WB, Fernandez-Ranvier G, Suh H. Transoral endoscopic thyroidectomy-an emerging remote access technique for thyroid excision. JAMA Surg. 2018;153:376–377. [DOI] [PubMed] [Google Scholar]
- 13. Witzel K, Hellinger A, Kaminski C, Benhidjeb T. Endoscopic thyroidectomy: the transoral approach. Gland Surg. 2016;5:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pai VM, Muthukumar P, Prathap A, Leo J, Rekha A. Transoral endoscopic thyroidectomy: a case report. Int J Surg Case Rep. 2015;12:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Witzel K, von Rahden BHA, Kaminski C, Stein HJ. Transoral access for endoscopic thyroid resection. Surg Endosc. 2008;22:1871–1875. [DOI] [PubMed] [Google Scholar]
- 16. Anuwong A, Kim HY, Dionigi G. Transoral endoscopic thyroidectomy using vestibular approach: updates and evidences. Gland Surg. 2017;6:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park JO, Kim CS, Song JN, et al. Transoral endoscopic thyroidectomy via the tri-vestibular routes: results of a preclinical cadaver feasibility study. Eur Arch Otorhinolaryngol. 2014;271:3269–3275. [DOI] [PubMed] [Google Scholar]
- 18. Cai C, Huang Y, Zhang T, et al. Anatomical study of surgical approaches for minimally invasive transoral thyroidectomy: eMIT and TOPP. Minim Invasive Ther Allied Technol. 2015;24:340–344. [DOI] [PubMed] [Google Scholar]
- 19. Lee HY, Hwang SB, Ahn K, Lee JB, Bae JW, Kim HY. The safety of transoral periosteal thyroidectomy: results of swine models. J Laparoendosc Adv Surg Tech A. 2014;24:312–317. [DOI] [PubMed] [Google Scholar]
- 20. Lee HY, You JY, Woo SU, et al. Transoral periosteal thyroidectomy: cadaver to human. Surg Endosc. 2015;29:898–904. [DOI] [PubMed] [Google Scholar]
- 21. Study Quality Assessment Tools. Washington DC: U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools Accessed February 24, 2018. [Google Scholar]
- 22. Wilhelm T, Metzig A. Endoscopic minimally invasive thyroidectomy (eMIT): a prospective proof-of-concept study in humans. World J Surg. 2011;35:543–551. [DOI] [PubMed] [Google Scholar]
- 23. Zeng Y, Li Z, Xuan W, He J. Trans-oral glasses-free three-dimensional endoscopic thyroidectomy-preliminary single center experiences. Gland Surg. 2016;5:628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakajo A, Arima H, Hirata M, et al. Trans-oral video-assisted neck surgery (TOVANS): a new transoral technique of endoscopic thyroidectomy with gasless premandible approach. Surg Endosc. 2013;27:1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang C, Zhai H, Liu W, et al. Thyroidectomy: a novel endoscopic oral vestibular approach. Surgery. 2014;155:33–38. [DOI] [PubMed] [Google Scholar]
- 26. Yang J, Wang C, Li J, et al. Complete endoscopic thyroidectomy via oral vestibular approach versus areola approach for treatment of thyroid diseases. J Laparoendosc Adv Surg Tech A. 2015;25:470–476. [DOI] [PubMed] [Google Scholar]
- 27. Udelsman R, Anuwong A, Oprea AD, et al. Trans-oral vestibular endocrine surgery: a new technique in the United States. Ann Surg. 2016;264:e16. [DOI] [PubMed] [Google Scholar]
- 28. Yang K, Ding B, Lin C, Li W, Li X. The novel transvestibule approach for endoscopic thyroidectomy: a case series. Surg Laparosc Endosc Percutan Tech. 2016;26:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jitpratoom P, Ketwong K, Sasanakietkul T, Anuwong A. Transoral endoscopic thyroidectomy vestibular approach (TOETVA) for Graves' disease: a comparison of surgical results with open thyroidectomy. Gland Surg. 2016;5:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilhelm T, Wu G, Teymoortash A, Güldner C, Günzel T, Hoch S. Transoral endoscopic thyroidectomy: current state of the art: a systematic literature review and results of a bi-center study. Trans Cancer Res. 2016;5(suppl 7):S1521–S1530. [Google Scholar]
- 31. Dionigi G, Bacuzzi A, Lavazza M, et al. Transoral endoscopic thyroidectomy: preliminary experience in Italy. Updates Surg. 2017;69:225–234. [DOI] [PubMed] [Google Scholar]
- 32. Sivakumar T, Amizhthu RA. Transoral endoscopic total thyroidectomy vestibular approach: a case series and literature review. J Minim Access Surg. 2018;14:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park J, Sun D. Transoral endoscopic thyroidectomy: our initial experience using a new endoscopic technique. Surg Endosc. 2017;31:5436–5441. [DOI] [PubMed] [Google Scholar]
- 34. Anuwong A, Ketwong K, Jitpratoom P, Sasanakietkul T, Duh Q. Safety and outcomes of the transoral endoscopic thyroidectomy vestibular approach. JAMA Surg. 2018;153:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chai YJ, Chung JK, Anuwong A, et al. Transoral endoscopic thyroidectomy for papillary thyroid microcarcinoma: initial experience of a single surgeon. Ann Surg Treat Res. 2017;93:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bakkar S, Al Hyari M, Naghawi M, Corsini C, Miccoli P. Transoral thyroidectomy: a viable surgical option with unprecedented complications-a case series. J Endocrinol Invest. 2018;41:809–813. [DOI] [PubMed] [Google Scholar]
- 37. Fu J, Luo Y, Chen Q, et al. Transoral endoscopic thyroidectomy: review of 81 cases in a single institute. J Laparoendosc Adv Surg Tech A. 2018;28:286–291. [DOI] [PubMed] [Google Scholar]
- 38. Russell JO, Clark J, Noureldine SI, et al. Transoral thyroidectomy and parathyroidectomy: a North American series of robotic and endoscopic transoral approaches to the central neck. Oral Oncol. 2017;71:75–80. [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Xie QP, Yu X, et al. [Preliminary experience with transoral endoscopic thyroidectomy via vestibular approach: a report of 150 cases in a single center] (in Chinese). Zhonghua Wai Ke Za Zhi. 2017;55:587–591. [DOI] [PubMed] [Google Scholar]
- 40. Razavi CR, Russell JO. Indications and contraindications to transoral thyroidectomy. Ann Thyroid. 10.21037/aot.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Witzel K, Benhidjeb T, Kaminski C, Messenbaeck FG, Weitzendorfer M. Hybrid techniques and patients' safety in implementing transoral sublingual thyroidectomy. Endocrine. 2018;60:50–55. [DOI] [PubMed] [Google Scholar]
- 42. Wang Y, Zhang Z, Zhao Q, et al. Transoral endoscopic thyroid surgery via the tri-vestibular approach with a hybrid space-maintaining method: a preliminary report. Head Neck. DOI. 10.1002/hed.25157. [DOI] [PubMed] [Google Scholar]
- 43. Cannizzaro MA, Borzì L, Lo Bianco S, Okatyeva V, Cavallaro A, Buffone A. Comparison between Focus Harmonic scalpel and other hemostatic techniques in open thyroidectomy: a systematic review and meta-analysis. Head Neck. 2016;38:1571–1578. [DOI] [PubMed] [Google Scholar]
- 44. Chadwick DR. Hypocalcaemia and permanent hypoparathyroidism after total/bilateral thyroidectomy in the BAETS Registry. Gland Surg. 2017;6(Suppl 1):S69–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aytac B, Karamercan A. Recurrent laryngeal nerve injury and preservation in thyroidectomy. Saudi Med J. 2005;26:1746–1749. [PubMed] [Google Scholar]
- 46. Dionigi G, Wu C, Tufano RP, et al. Monitored transoral endoscopic thyroidectomy via long monopolar stimulation probe. J Vis Surg. 2018;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shan L, Liu J. A systemic review of transoral thyroidectomy. Surg Laparosc Endosc Percutan Tech. 2018;28:135–138. [DOI] [PubMed] [Google Scholar]