Abstract
Stress affects cellular aging and inflammatory and chromosomal processes, including telomere length, thereby potentially compromising health and facilitating disease onset and progression. Stress-related diseases and strategies to manage stress usually require integrative or behavioral therapeutic approaches that also operate on cellular levels. Mind-body medicine (MBM) uses the interaction between the mind, body, behavior, and the environment to correct physical and psychological malfunctions, thus ameliorating disease states and improving health. The relaxation response (RR) is a physiological opponent of stress and the stress response (SR) (i.e., fight-or-flight response), also invoking molecular anti-stress processes. Techniques that elicit the RR are at the core of practically all MBM interventions. We surmise that these techniques can also affect chromosomal and telomere processes, molecular aging, and the modulation of inflammatory states on cellular levels.
MeSH Keywords: Mind-Body Therapies; Nitric Oxide; Psychophysiology; Relaxation; Stress, Physiological; Telomerase
Background
Stress has gained remarkable significance in modern times. Various causes may account for this. It has been reported that the acceleration of human activities, challenges, productivities, and behaviors, accompanied by increasing levels of noise, pollution, and “daily stressors”, such as employment, are related to over-population or the actual financial pressures on micro- and macro-levels [1–3]. Its existence addresses the presence of homeostatic perturbations in all living organisms (e.g., physiological) and transcends into the cognitive realm, thus gaining in significance with humans [4]. Therefore, we see a surge of stress-related complaints and diseases, many of them leading to medical interventions and, quite often, to expensive treatments and disabilities [3].
Chronic Stress, Cell Aging, and Chromosomal Markers
The effects that chronic stress – and a high dose of supposedly threatening, stressful situations – can have on the entire body, as they literally make us “look old”, can be seen on a molecular genetic level, among others, in our chromosomes. One marker and potential factor that seems to provide evidence of individual vulnerability (and “molecular resilience” consisting of cellular resistance and regeneration capacity) is the length of chromosomal telomeres and activity of the cellular telomerase [5].
Human chromosomes have at their ends a structural complex called telomeres, which protects the chromosome strands from degradation by defects in the replication and repair processes [6–8]. Hence, these telomeres, consisting of repetitive deoxyribonucleic acid (DNA) sequences, are physiologically subject to shortening by the discontinuous replication at each cell division. The enzyme telomerase contributes significantly to maintenance of the protective effect of the telomeres by their extension. The level of telomerase enzyme activity is limited in many cells and it is believed that the gradual loss of telomeric DNA leads to accelerated cell aging and, ultimately, causes cell death [6]. At the same time, excessive enzyme activity can increase the risk of certain neoplastic diseases [7], as cellular life cycles, including those of cancer cells, may be prolonged.
The ability of the telomeres to protect the chromosomes critically depends on the binding of sufficient quantities of functional shelterin, a 6-unit protein complex with specific and crucial roles in telomere maintenance and function. Insufficient telomere length, leading to insufficient concentration of shelterin at chromosome ends, or otherwise crippled shelterin function, causes telomere deprotection [9]. While contributing to aging-related pathologies, loss of telomere protection can act as a barrier to tumorigenesis, as dysfunctional telomeres activate DNA-damage-like checkpoint responses that halt cell proliferation or trigger cell death. In addition, dysfunctional telomeres affect cancer development and progression by being a source of genomic instability [9].
As stated above, it has been hypothesized that stress affects health by modulating the rate of cellular aging. In fact, evidence has been provided that psychological stress (PS) – both perceived stress and chronicity of stress – is significantly associated with higher oxidative stress, lower telomerase activity, and shorter telomere length, which are known modulators of cell senescence and cellular life span [5]. PS can contribute to numerous diseases and, as noted before, may do so in part through damage to the protective non-coding segments on the ends of chromosomes (i.e., the telomeres). Accordingly, in a systematic review, increased PS was associated with a small decrease in telomere length, adjusting for age, and this relationship was similar between sexes and within studies using validated measures of PS, and marginally stronger among samples recruited for stress exposure [10].
Clearly, at the cellular level, stress can promote earlier onset of age-related diseases [11]. Hence, with aging, increasing telomere shortening is observed in humans, even though interindividual differences in length also exist regardless of age [6,7,12]. In addition to the individual genetic predisposition, telomere length is directly influenced by exo- and endogenous noxae, oxidative stress, chronic stress, and personal health behaviors [13]. For example, oxidative DNA damages are usually repaired by base excision repair and nucleotide excision repair, thereby ensuring telomeric and genomic stability [13]. However, this process can be challenged by cellular stress and proinflammation. Accordingly, depression, as an example, which also relates to proinflammatory processes within the nervous system (e.g., in the brain) [14,15], can presumably lead to a shortening of telomeres [16].
Using yeast as a model organism, it has been shown that stressors and noxae can have very different outcomes: alcohol and acetic acid elongate telomeres, whereas caffeine and high temperatures shorten telomeres [17]. In addition, acute exposure to severe hypoxia in rats has been demonstrated to cause damage to heart and lung tissues due to the production of reactive oxygen species (i.e., oxidative stress), but at the same time, and perhaps counterintuitively, to promote telomere length and adaptive responses by upregulating mRNA and protein levels of telomerase reverse transcriptase (TERT) [18]. By combining genome-wide expression measurements with a systematic genetic screen, the Rap1/Rif1 pathway has been identified as a central mediator of the telomeric response to environmental signals [17]. These results demonstrate that telomere length can be meaningfully manipulated, and that a carefully regulated homeostasis may become markedly deregulated in opposing directions in response to different environmental cues.
Taken together, the above observations suggest that part of the negative impact of chronic stress on our health (e.g., at cellular levels) is caused by an acceleration of cell aging and earlier onset of age-associated diseases, since increased levels of oxidative/cellular stress, decreased telomerase activity, and thus telomere shortening, could all be associated with stress, including perceived stress and chronic stress.
Mind-Body Practice and Health Behaviors
Substantial evidence exists demonstrating that behavioral cues, such as exercise, and other mind-body medical procedures can have a protective effect on telomere length [13,19–21]. The research group led by Nobel laureate Elizabeth Blackburn, who has been awarded the prize for her research on telomeres, has examined, among other things, how healthy behaviors in healthy people (e.g., healthy eating, exercise, and sleep quality, as well as mindfulness meditation) have a direct impact on telomere length. The length of the telomeres also appears to be variable over a short period of time. For example, over an observation period of 1 year, studies revealed that drastic or traumatic life events as well as challenging life situations can lead to a direct shortening of telomeres. Remarkably, however, this phenomenon was negligible in individuals with pronounced health-promoting behaviors and markedly reduced in those who maintained moderate health behaviors [8].
Ornish et al. also provided evidence that during a 3-month lifestyle intervention involving diet, exercise, and stress management, patients with low-rate prostate cancer had increased telomerase activity and telomere lengthening compared to a control group. This was interpreted as a positive effect of the mind-body practices applied. The relative lengthening of telomeres was also described in the follow-up after 5 years [22]. In patients with coronary heart disease (CHD), it was shown that patients with telomere prolongation over the 5-year follow-up period tended to have decreased mortality independent of other cardiovascular risk factors [23].
Results from telomere biology suggest that psychophysiological stress processing may have a direct effect on cell aging and, moreover, on the individual disease risk. The “good news”, according to the Blackburn group: With the practice of various mind-body medical techniques, a reduction of cognitive-mental (or emotional) stress or “stress arousal” can be achieved, together with more positive mood states [12]. Accordingly, the modulation of catecholamine and cortisol hormone levels has been demonstrated in mind-body practice, and both are associated with a salutary effect on telomeres [12]. However, the specific pathways incorporated, as well as cause-effect associations and effect estimates, in this regard, are largely unknown.
Relaxation Physiology, Stress, and Proinflammation
At the center of many mind-body techniques and interventions are relaxation techniques and relaxation response (RR) procedures. The RR has been described by Herbert Benson as a set of integrated physiological mechanisms and adjustments that are elicited when a subject engages in a repetitive mental or physical activity and passively ignores distracting thoughts [24]. These behaviors are associated with instantly occurring physiological changes that include decreased oxygen consumption or carbon dioxide elimination (i.e., reduced metabolism) and lowered heart rate, arterial blood pressure, and respiratory rate. Benson identified it as the physiological counterpart of the stress or fight-or-flight response [24].
At the molecular genetic level, the RR involves changes and modulations that are opposed to stress-induced gene expression (genetic “stress pattern”). With modern techniques, Benson’s original postulate (i.e., the assumption of a relaxation-stress-antagonism [11]) can now be confirmed experimentally. In addition, the field of social genetics shows that our everyday living conditions influence which genetic information (i.e., which of the different genes) are “read” (transcribed) and appear and act through the synthesis of proteins in the body. Social living conditions, such as low socioeconomic status, social isolation, and social insecurity or threat, are associated with a variety of altered genetic scripts (e.g., in white blood cells and diseased tissues) [25]. Chronic stress and social disadvantage induce a characteristic gene expression pattern, which is an increased development of inflammatory processes (proinflammation), and a reduction of the antiviral and specific immune defense. This specific pattern is also known today as “conserved transcriptional response to adversity” (CTRA) and is considered a molecular signature of chronic stress [26], which is ultimately a part of the biological stress response (SR) already described. While the acute inflammatory response is a time-limited adaptation of the body to increased activity of the immune system to fight injury or infection, the chronic inflammatory response is a maladaptive condition [15,27,28]. This is associated with an increased risk of certain cancers and neurodegenerative and cardiovascular diseases, as well as asthma, arthritis, and mental disorders [29,30]. This suggests that RR procedures would have an ameliorating potential in this context.
Relaxation/Mind-Body Techniques: Inflammation Modulation and Nitric Oxide
The RR has been shown to contribute to the symptomatic improvement of a variety of stress-associated diseases [26,31,32]. However, the underlying molecular genetic mechanisms are mostly less well known. It is assumed that the RR can to some extent counteract stress-induced gene expression and thus lead to a partial improvement of the negative stress effects on the human body via various biochemical signaling pathways [33,34]. Moreover, we speculate that the RR is a synchronized metabolic down-regulation, thereby facilitating longevity and health via protection of genetic integrity, also allowing for metabolic “cleansing” procedures.
In particular, a modulating effect of the RR on inflammatory processes at the cellular level is frequently discussed and investigated. In most of the cases described, the use of different RR-eliciting techniques exerts an effect on specific gene expression, or epigenetic alterations, which are involved in the development of inflammatory and immunological processes as well as the regulation of cell survival [35,36]. The activity of these genes is regulated, among various factors, including the nuclear (cellular) transcription factor NF-κB (nuclear factor kappa B), which also is modulated by local nitric oxide (NO) production and plays an important role in the development and maintenance of chronic inflammatory processes [15,37–39].
Studies have often reported that mind-body interventions (MBI) can cause down-regulation of the proinflammatory transcription factor NF-κB [2,20,38]. Compared to, for example, control groups with no MBI, this effect was particularly evident in experienced practitioners (meditators), newcomers, healthy or ill patients, and in the use of different meditation methods, as well as in yoga and tai chi [19,20,33,40]. NO-mediated cellular signaling is involved in this immune mechanism [2,39]. The physiology of the RR presumably leads to an increased release of constitutive NO, which in turn influences the activity of NF-κB (c.f., [34]). NF-κB activity is blocked by association with the specific inhibitor IκB kinase (IKKB) complex [37,41]. The presence of NO additionally stabilizes this complex, which is likely to reduce NF-κB-mediated activation of proinflammatory genes. As a result, a diminished synthesis of proinflammatory mediators is expected, which would be generated in several steps (theoretically) by the RR and would be beneficial to health [26,42].
Several studies have been able to directly correlate meditation or relaxation practice (MBI) with biological consequences of demonstrably altered gene expression. For example, altered levels of some inflammatory mediators, such as IL-6, IL-12, and IL-10, have been reported [43]. In addition to the modulation of NF-κB activity, there is a range of other mechanisms in which an influence on cell metabolism and apoptosis regulation (controlled cell death) is suggested by the molecular effect of the RR. Again, differences between people with regular, long-term practice and novices are observed. For experienced practitioners, in particular, increased expression of genes associated with mitochondrial energy metabolism signaling pathways, as well as increased insulin secretion, has been demonstrated [20,21,33,44]. In addition, physiological adaptations concerning the signaling pathways of cellular oxidation processes were shown. An increased reduction of oxidative stress is observed in MBI, which could represent an important process of cell repair and may thus slow cell aging [21,33].
Another molecular phenomenon influenced by the RR is an increase in brain-derived neurotropic factor (BDNF) in the blood plasma, mediated, for example, by yoga as meditation or mind-body practice (MBI). This growth factor is responsible for the genesis, survival, and plasticity of neurons, showing the adaptability in the central and peripheral nervous systems. The protein shows its greatest activity in brain areas that are of central importance for learning and memory functions, and higher states of consciousness [2,43]. Although this research is in its early stages, there is substantial and mounting evidence that supports the concept of strong mind-body-cell-gene-epigenetic connections.
The Role of Mitochondrial and Microbiome Regulation
The underlying molecular mechanisms of relaxation/mind-body regulation, with the ability to down-regulate energy metabolism and (cell) aging, reside within the origin of cellular life. We speculate that it originated in prokaryotes and evolved in the symbiotic mitochondria. The establishment of this process would involve the utilization of nitrogen in primordial life forms, such as nucleic bases and NO, serving, in part, as the original cellular chemical messengers with roles in energy metabolism [45]. This is noted in the interaction of nitrates and nitrites and the effects of NO on mitochondrial metabolism. These molecules can thus down-regulate aerobic metabolism. In this regard, endogenous morphine releases mitochondrial constitutive NO via the mu3 opiate receptor subtype on mitochondrial inner membranes, which diminishes oxygen utilization [46,47].
We surmise this molecular action at this base level of modulation provides cells with the ability to periodically rejuvenate their intrinsic life-sustaining activities simply by decreasing energy transduction into adenosine triphosphate (ATP), both in anaerobic and aerobic metabolism. This down-regulation allows time to rid the cell of harmful byproducts, such as free radicals. In part, this process derives its significance from the fact that ATP is not stored. As multicellular organisms evolved with ever increasing sophistication (e.g., cognition), this molecular mitochondrial “down-regulation” also became more complex and eventually evolved into synchronized periods of time when an entire animal would appear to be in a state of “rest”, such as “sleep”. Mitochondrial restoration, or rejuvenation, would then be a “healthy” mechanism to counteract mitochondrial decline, since a decline in mitochondrial function contributes to various disease states and aging, including cellular senescence and chronic inflammation [48]. We therefore surmise that cognitive induced meditation (i.e., the RR) is similar in nature (Figure 1). Furthermore, once cognition took hold as a major coping strategy for survival, the restorative properties of rest, or relaxation, were fully realized.
Figure 1.
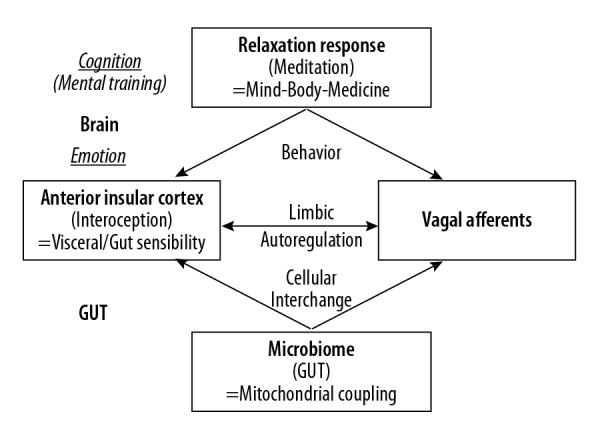
Brain-Gut Coupling and Insular-Vagal Integration. It has become apparent that the molecular and biochemical integrity of interactive families, genera, and species of human gut microflora is critically linked to maintaining complex metabolic and behavioral processes mediated by peripheral organ systems and central nervous system neuronal groupings [49]. In addition, the vagus nerve may be the primary route through which gut signaling and pathological triggers such as alpha-synuclein travel from the intestines to the brain. Here, limbic autoregulation would integrate afferent peripheral inputs with central interoception, insular cortex activity, and emotion/emotional contexts. Accordingly, with mental training and MBI, i.e., cognitively induced health behaviors, the brain (via its motivation and reward circuitries, imbedded in the limbic system) may downregulate upstream (hyper-) activating processes [2,32,42,49–51].
With regard to microbiome regulation as part of these processes, it has become apparent that the molecular and biochemical integrity of interactive families, genera, and species of human gut microflora is critically linked to maintaining complex mitochondrial, metabolic, and behavioral processes mediated by peripheral organ systems and central nervous system neuronal groupings [49]. In shaping human gut microbiota, environment dominates host genetics [52]. In fact, the gut microbiome does not seem to be significantly associated with genetic ancestry, since similarities in the compositions of the microbiomes occur in unrelated individuals who share a household, and 20% of the inter-person microbiome variability is related to other behavioral factors and anthropometric measurements [52]. Clearly, microbiome composition data have improved the prediction accuracy for obesity and other lifestyle or behavioral measures.
The gut microbiota is subject to micro-fluctuations, meaning that it is finely tuned, as it also displays diurnal rhythmicity and functional oscillations over the course of a day, which potentially affects metabolic host homeostasis [53]. Thus, the microbiome is highly sensitive to various cues, both external and internal, including circadian activity changes. This diurnal microbial behavior also drives the global programming of the host circadian transcriptional, epigenetic, and metabolite oscillations [53]. Disruption of homeostatic microbiome rhythmicity therefore affects diurnal fluctuations of host physiology and disease susceptibility [53].
A particular disruptor with extensive (patho-) physiological impact on human gut microbiota is drug application [54]. This relates not only to antibiotics, but also to a broad array of commonly used drug classes; it has been estimated that a quarter of the drugs with human targets, including members of all therapeutic classes, inhibited gut microbiota [54]. Particular classes of psychotropic drugs, such as the chemically diverse antipsychotics, were overrepresented in this group [54]. Taken together, metabolic and mitochondrial cues, as well as behavioral and other external cues, have a high significance for microbiota function and gut integrity, and vice versa. In principle, however, the microbiome, as well as mitochondrial processes related to it, is prone to influence by host behaviors and, possibly, cognitive behavioral training. Accordingly, with mental training and MBI such as cognitively induced health behaviors, the brain, via its motivation and reward circuitries imbedded in the limbic system, can steer or down-regulate upstream (hyper-) activating processes that were originally induced by the gut microbiome (Figure 1).
Limitations
The effects of MBI on telomere elongation, or possibly shortening, in particular cases, and its significance and specificity in various disease states, remain to be determined. In particular, the role of MBI in cancer therapy and prevention is still a matter of ongoing debate and thus under further scientific scrutiny. Here, as well as in the general understanding of the underlying cellular physiology, additional studies are under way and their results are eagerly awaited. So far, the findings described must be seen as promising but limited (i.e., preliminary), but constitute a new model for understanding the chromosomal processes in mind-body medicine, and defining a role for chronic stress, cell aging, and telomere length.
Conclusions
Stress is a growing problem in our modern societies, with profound impact on economic and medical outcomes. High levels of stress or chronic, prolonged states affect cellular aging, as well as inflammatory and chromosomal processes, including telomere lengths, and this has a close connection to a variety of chronic health conditions. It can be surmised that stress-related, behavioral, or chronic diseases usually require a holistic or integrative therapeutic approach. Mind-body medicine (MBM) uses the interaction between the mind, body, behavior, and the environment to affect physical and psychological health and functions. The concept of a general down-regulatory potential in physiology and autoregulation is critical to MBM. The RR is a physiological opponent of stress and the SR (i.e., the fight-or-flight response). Hence, techniques that elicit the RR are at the core of practically all MBM interventions. Accordingly, we further surmise that these techniques and elements can also affect chromosomal and telomere processes, molecular aging, and the modulation of inflammatory processes. In addition, mitochondrial and other intracellular procedures, including NO and endogenous opioid signaling, as well as the general nitrogenic or the actual microbiome status in the extracellular space (i.e., gut) may also be involved in stress and its counteraction through MBM/MBI. Further studies will reveal more details on these possible correlations.
Acknowledgements
The authors would like to thank Mrs. Gudrun Berlinghoff for her assistance in the formatting of the content of this work.
Footnotes
Source of support: Self financing
Conflicts of interest
None.
References
- 1.Esch T. Health in stress: Change in the stress concept and its significance for prevention, health and life style. Gesundheitswesen. 2002;64(2):73–81. doi: 10.1055/s-2002-20275. [DOI] [PubMed] [Google Scholar]
- 2.Esch T, Stefano GB. The neurobiology of stress management. Neuro Endocrinol Lett. 2010;31(1):19–39. [PubMed] [Google Scholar]
- 3.Werdecker L, Esch T. Stress and health. In: Haring R, editor. Health Sciences. Heidelberg: Springer; 2018. [in press] [Google Scholar]
- 4.Stefano GB, Cadet P, Zhu W, et al. The blueprint for stress can be found in invertebrates. Neuro Endocrinol Lett. 2002;23:85–93. [PubMed] [Google Scholar]
- 5.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Nati Acad Sci USA. 2004;101(49):17312–15. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–98. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 8.Puterman E, Lin J, Krauss J, et al. Determinants of telomere attrition over 1 year in healthy older women. Stress and health behaviors matter. Mol Psychiatry. 2015;20(4):529–35. doi: 10.1038/mp.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs JJL. Loss of telomere protection: Consequences and opportunities. Front Oncol. 2013;3:88. doi: 10.3389/fonc.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathur MB, Epel E, Kind S, et al. Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav Immun. 2016;54:158–69. doi: 10.1016/j.bbi.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefano GB, Benson H, Fricchione GL, Esch T. The stress response: Always good and when it is bad. Med Sci; New York: 2005. [Google Scholar]
- 12.Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann NY Acad Sci. 2009;1172:34–53. doi: 10.1111/j.1749-6632.2009.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trajano LADSN, Trajano ETL, Silva MADS, et al. Genomic stability and telomere regulation in skeletal muscle tissue. Biomed Pharmacother. 2018;98:907–15. doi: 10.1016/j.biopha.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuroendocrinol Lett. 2002;23(3):199–208. [PubMed] [Google Scholar]
- 15.Esch T, Stefano GB. Proinflammation: A common denominator or initiator of different pathophysiological disease processes. Med Sci Monit. 2002;8(5):HY1–9. [PubMed] [Google Scholar]
- 16.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13(10):693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romano GH, Harari Y, Yehuda T, et al. Environmental stresses disrupt telomere length homeostasis. PLoS Genet. 2013;9(9) doi: 10.1371/journal.pgen.1003721. e1003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Zhao Z, Zhu Z, et al. Telomere elongation protects heart and lung tissue cells from fatal damage in rats exposed to severe hypoxia. J Physiol Anthropol. 2018;37(1):5. doi: 10.1186/s40101-018-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black DS, Cole St W, Irwin MR, et al. Yogic meditation reverses NF-kappaB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2013;38(3):348–55. doi: 10.1016/j.psyneuen.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhasin MK, Dusek JA, Chang B-H, et al. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0062817. e62817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denninger J, Bhasin MK, Corrigan AA, et al. Functional genomics in the study of mind-body therapies. Ochsner J. 2014;(14):681–95. [PMC free article] [PubMed] [Google Scholar]
- 22.Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer. 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112–20. doi: 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- 23.Goglin SE, Farzaneh-Far R, Epel ES, et al. Change in leukocyte telomere length predicts mortality in patients with stable coronary heart disease from the heart and soul study. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0160748. e0160748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benson H. The relaxation response. William Morrow; New York: 1975. [Google Scholar]
- 25.Cole SW. Human social genomics. PLoS Genet. 2014;10(8) doi: 10.1371/journal.pgen.1004601. e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buric I, Farias M, Jong J, et al. What is the molecular signature of mind-body interventions? A systematic review of gene expression changes induced by meditation and related practices. Front Immunol. 2017;8:670. doi: 10.3389/fimmu.2017.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benz D, Cadet P, Mantione K, et al. Tonal nitric oxide and health: A free radical and a scavenger of free radicals. Med Sci Monit. 2002;8(1):RA1–4. [PubMed] [Google Scholar]
- 28.Stefano GB, Scharrer B. Endogenous morphine and related opiates, a new class of chemical messengers. Adv Neuroimmunol. 1994;4:57–68. doi: 10.1016/s0960-5428(05)80001-4. [DOI] [PubMed] [Google Scholar]
- 29.Esch T, Mantione KJ, Stefano GB. Stress, proinflammation, autoregulation, and cardiovascular diseases. Brain Immune. 2011. Available from: http://www.brainimmune.com/stress-proinflammation-autoregulation-and-cardiovascular-diseases/
- 30.Slavich GM. Understanding inflammation, its regulation, and relevance for health. A top scientific and public priority. Brain Behav and Immun. 2015;45:13–14. doi: 10.1016/j.bbi.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kearney DJ, Brown-Chang J. Complementary and alternative medicine for IBS in adults. Mind-body interventions. Nat Clin Pract Gastroenterol Hepatol. 2008;5(11):624–36. doi: 10.1038/ncpgasthep1257. [DOI] [PubMed] [Google Scholar]
- 32.Esch T, Stefano GB, Fricchione GL. The therapeutic use of the relaxation response in stress-related diseases. Med Sci Monit. 2003;9(2):RA23–34. [PubMed] [Google Scholar]
- 33.Dusek JA, Otu HH, Wohlhueter AL, et al. Genomic counter-stress changes induced by the relaxation response. PLoS One. 2008;3(7) doi: 10.1371/journal.pone.0002576. e2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esch T, von Bernus L. In: Dobos G, Paul A, editors. [Mind-Body-Medizin]. Munich: Elsevier; 2018. [in press] [Google Scholar]
- 35.Bhasin M, Fan X, Libermann T, et al. Epigenetic alterations associated with short-term relaxation response training in healthy subjects. Presentation and Abstract, World Congress of Integrative Medicine; 2017 May 3–5; Berlin. [Google Scholar]
- 36.Fyfe-Johnson A, Taylor D, Zierold C, Dusek J. Effects of relaxation response intervention on endogenous progenitor cells in a hypertensive population. BMC Complement Altern Med. 2012;12(Suppl 1):O13. [Google Scholar]
- 37.Welters ID, Fimiani C, Bilfinger TV, Stefano GB. NF-κB, nitric oxide and opiate signaling. Medical Hypotheses. 1999;54:263–68. doi: 10.1054/mehy.1999.0032. [DOI] [PubMed] [Google Scholar]
- 38.Kuo B, Bhasin M, Jacquart J, et al. Genomic and clinical effects associated with a relaxation response mind-body intervention in patients with irritable bowel syndrome and inflammatory bowel disease. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123861. e0123861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefano GB, Esch T, Bilfinger TV, Kream RM. Proinflammation and preconditioning protection are part of a common nitric oxide mediated process. Med Sci Monit. 2010;16(6):125–30. [PubMed] [Google Scholar]
- 40.Creswell JD, Irwin MR, Burklund LJ, et al. Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults. A small randomized controlled trial. Brain Behav Immun. 2012;26(7):1095–101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefano GB, Goumon Y, Bilfinger TV, et al. Basal nitric oxide limits immune, nervous and cardiovascular excitation: Human endothelia express a mu opiate receptor. Prog Neurobiol. 2000;60:513–30. doi: 10.1016/s0301-0082(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 42.Stefano GB, Esch T. Integrative medical therapy: Examination of meditation‘s therapeutic and global medicinal outcomes via nitric oxide. Int J Mol Med. 2005;16:621–30. [PubMed] [Google Scholar]
- 43.Cahn BR, Goodman MS, Peterson CT, et al. Meditation and mind-body health. Increased BDNF, cortisol awakening response, and altered inflammatory marker expression after a 3-month yoga and meditation retreat. Front Hum Neurosci. 2017;11:315. doi: 10.3389/fnhum.2017.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manchanda SC, Madan K. Yoga and meditation in cardiovascular disease. Clin Res Cardiol. 2014;103(9):675–80. doi: 10.1007/s00392-014-0663-9. [DOI] [PubMed] [Google Scholar]
- 45.Stefano GB, Kream RM. Alkaloids, nitric oxide, and nitrite reductases: Evolutionary coupling as key regulators of cellular bioenergetics with special relevance to the human microbiome. Med Sci Monit. 2018;24:3153–58. doi: 10.12659/MSM.909409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefano GB, Mantione KJ, Capellan L, et al. Morphine stimulates nitric oxide release in human mitochondria. J Bioenerget Biomembr. 2015;47(5):409–17. doi: 10.1007/s10863-015-9626-8. [DOI] [PubMed] [Google Scholar]
- 47.Stefano GB, Mantione KJ, Casares FM, Kream RM. Anaerobically functioning mitochondria: Evolutionary perspective on modulation of energy metabolism in Mytilus edulis. Invertebr Surv J. 2015;12:22–28. [Google Scholar]
- 48.Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell. 2016;61(5):654–66. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stefano GB, Pilonis N, Ptacek R, et al. Gut, microbiome, and brain regulatory axis: Relevance to neurodegenerative and psychiatric disorders. Cell Mol Neurobiol. 2018;38(6):1197–206. doi: 10.1007/s10571-018-0589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esch T, Stefano GB. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuroendocrinol Lett. 2004;25(4):235–51. [PubMed] [Google Scholar]
- 51.Esch T. The Neurobiology of meditation and mindfulness. In: Schmidt S, Walach H, editors. Meditation – neuroscientific approaches and philosophical implications. Vol. 2. Springer International Publishing; New York: 2014. pp. 153–73. (Springer Series: Studies in Neuroscience, Consciousness and Spirituality). [Google Scholar]
- 52.Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–15. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 53.Thaiss CA, Levy M, Korem T, et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167(6):1495–510. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–28. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]


