Abstract
Tumefactive multiple sclerosis (MS) is characterized by the presence of a single MS-plaque in the brain. It mimics tumors due to large size, mass effect, and enhancement patterns. Refractory intracranial hypertension due to tumefactive MS requiring decompressive craniectomy (DC) was reported in five cases. However, none of these cases were documented new lesions during the follow-up. We report a case of a 28-year-old female admitted with acute right hemiparesis, headache, and nausea. A brain magnetic resonance imaging (MRI) revealed a left parietal lobe lesion. Within 4 days, she became comatose. Computed tomography (CT) scan revealed the left uncal herniation. DC and resection of the lesion were carried out. Histopathology revealed active demyelinating disease. After 11 years of the first attack, she went to the emergency department due to headache and left hemiparesis. Head CT scan revealed a hypodense area in the right frontal lobe. Three months later, the patient was asymptomatic, and new MRI did not show new lesions.
Keywords: Decompressive craniectomy, pseudotumoral multiple sclerosis, tumefactive multiple sclerosis
Introduction
Tumefactive multiple sclerosis (MS), also known as pseudotumoral MS, it is characterized by the presence of a single MS plaque in the brain. The lesion mimics intracerebral neoplasms due to its large size, mass effect, and enhancement patterns. Refractory intracranial hypertension due to tumefactive MS requiring decompressive craniectomy (DC) was reported in five cases.[1,2,3,4,5] However, none of these cases were documented new lesions during the follow-up. From now on, we report a case of a young woman who had tumefactive MS in the left parietal lobe, which required DC. Eleven years after, she presented a new lesion in the right frontal lobe.
Case Report
A 28-year-old female was admitted to our hospital due to right hemiparesis, headache, and nausea within 1 day of onset. She was previously healthy, and her family history was unremarkable. In the neurological examination, the right Babinski sign and bilateral papilledema were present. A brain magnetic resonance imaging (MRI) at that time revealed a lesion in the left parietal lobe with 4.1 cm × 3 cm × 4 cm; hypointense on T1-weighted and hyperintense on T2-weighted; there was restricted diffusion and a subtle “C”-ring enhancement occurred after injection of contrast; and magnetic resonance spectroscopy (MRS) showed a peak of choline and lactate with decrease of N-acetyl-aspartate. The diagnosis of a tumor was suggested. Corticosteroids, mannitol, and antiepileptics were started.
On the 4th admission day, she became unresponsive with fixed and dilated left pupil. Head computed tomography (CT) scan revealed the left uncal herniation. Emergency DC and an extensive resection of the lesion were carried out. Cerebrospinal fluid (CSF) analysis showed a moderate increase of protein concentration and the absence of oligoclonal bands with immunoglobulin G index of 0.50. Histopathologic examination revealed features of active demyelinating disease with an abundance of foamy macrophages, reactive gliosis, hypercellularity with myelin loss, and relative axonal preservation. One month later, she developed focal epilepsy. Her bone flap was replaced within 3 months of the DC. At 6 months, the corticosteroid was discontinued, and she had a mild loss of strength in the right lower limb and lesional epilepsy. Two years from the disease-onset, none new radiological, or clinical manifestations were detected.
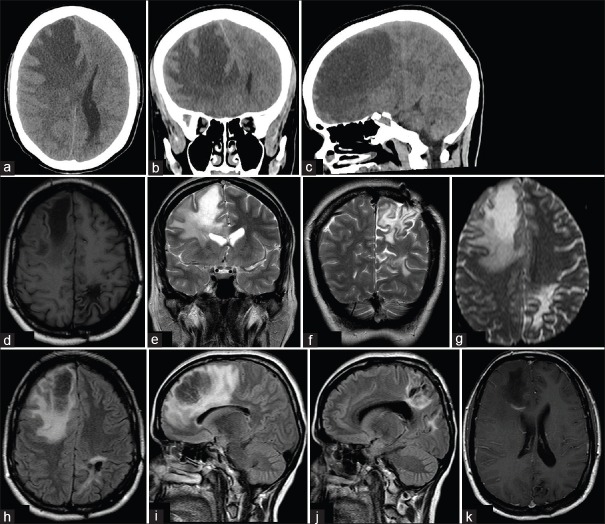
After 11 years of the first attack, she went to the emergency department due to a headache and left hemiparesis within 6 h of onset. In the neurological examination, the right and left lower limb weakness, Babinski sign in the left lower limb, and bilateral papilledema were seen. Head CT scan revealed a hypodense area in the right frontal lobe [Figure 1]. Corticosteroids and mannitol were started. In 2 weeks, a brain MRI was requested [Figure 1]. Three months later, the patient was asymptomatic, and a new MRI did not show new lesions. A second biopsy and CSF analysis showed the same alterations as before. In a routine evaluation, 6 months after, she was clinically and radiologically stable.
Figure 1.
Neuroimage is showing tumefactive demyelinating area. Axial (a), coronal (b), and sagittal (c) view of noncontrast head computed tomography scan showing a hypodense area in the right frontal lobe, with mass effect and subfalcine herniation. Axial T1-weighted (d), Coronal T2-weighted (e and f), Axial diffusion-weighted (g), Axial (h), and sagittal (i and j) Fluid-attenuated inversion recovery. Axial contrast showing “C”-shaped ring enhancement (k). The magnetic resonance imaging images show the left parietal lobe where there is a residual lesion from the first attack (d, f, g-j); and the right frontal lobe, with the second new lesion (d, e, g-i, k)
Discussion
The tumefactive MS is part of the spectrum of primary demyelinating diseases. Studies throughout the 1990s have shown that the tumefactive MS may occur in 1–2/1000 cases of MS.[6] Nevertheless, the recent cohort of Lucchinetti et al. showed that this prevalence is possibly even higher, and more epidemiological studies are needed.[7]
State of unconsciousness presenting as an initial clinical manifestation is found in 2% of patients with tumefactive MS.[7] One of the possible mechanisms involved in the pathophysiology of the comatose state is the extensive cerebral edema leading to intracranial hypertension, which commonly resolves with the administration of hyperosmolar agents. In rare cases, however, the intracranial hypertension is refractory to initial therapy and can increase progressively, resulting in uncal herniation, a life-threatening situation. In these cases, the DC is a treatment choice.
There are only a few case reports of tumefactive MS complicated by brain herniation, and that required emergency DC. We identified five cases after a thorough review of the English literature and we compared them with the present case [Table 1].[1,2,3,4,5]
Table 1.
Reported cases of tumefactive (pseudotumoral) multiple sclerosis requiring emergency decompressive craniectomy
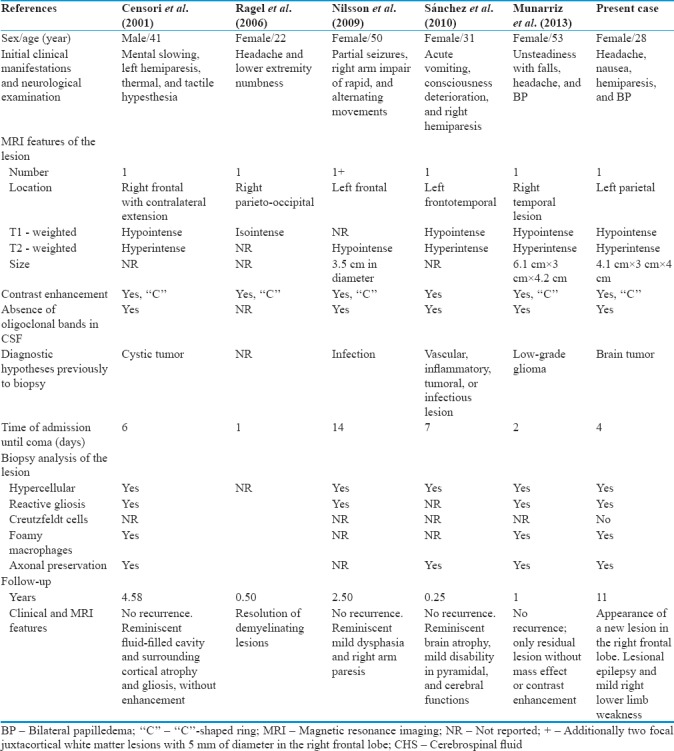
In all of the reports, including this, corticosteroids and at least one osmotic diuretic were used in the initial management.[1,2,3,4,5] In the study from Censori et al., azathioprine (AZA) was added to the treatment. Then, prednisone was used with AZA during 1 year, and in the subsequent 5 months of follow-up, AZA was used alone in a dosage of 150 mg/day.[1] Another case report also suggested the use of AZA when corticosteroid alone fails in the management of tumefactive MS.[8]
It is still not well established if only the clinical manifestations associated with MRS, without a brain biopsy, could be enough to make a reliable diagnosis of tumefactive MS, excluding safely brain tumors among which glioma is the most common differential diagnosis.[7] MRS was used in the investigation of two cases and in the present case.[1,5] Furthermore, apparently, the only MRS parameter sensible to detect differences between glioma and tumefactive MS is the N-acetylaspartate/creatine ratio in the central region of the affected area, where, in gliomas, the ratio is lower than in tumefactive MS.[9] Unfortunately, this ratio was not evaluated in any of the cases reported.[1,2,3,4,5]
Comparing the data of Table 1 with the cohort data from Lucchinetti et al., there seems to be a female predominance of tumefactive MS; the median age of onset is 37.5 years; and the most common initial presentation is polysymptomatic including at least one motor symptom. As for MRI features, the most frequent lesion location was frontal or parietal, in association with ring enhancement. Yet, more studies are needed to evaluate the contribution of clinical predictors for outcomes in tumefactive MS.[1,2,3,4,5,7]
To the best of authors’ knowledge, this is the first study to report the appearance of a second pseudotumoral lesion in a patient with previously refractory intracranial hypertension due to tumefactive MS that required DC. Probably, this clinical-radiological course was observed due to the long-term follow-up of almost 11 years. In the other studies, only one isolated demyelinating episode was reported, but their follow-up, the majority of the cases of <2.5 years was probably too small to detect a recurrence since according to Lucchinetti et al., the median time to the second attack is 4.8 years.[1,2,3,4,5,7]
If we evaluate the attacks reported in this case, regarding clinical-radiological differences, we can notice that the first lesion was more external than the second. This fact may be related to the evolution of the first attack, which was refractory to drug therapy since more external injuries in the brain tend to be associated with a greater increase in the intracranial pressure and brain herniation.[10] Moreover, a possible explanation for recurrence after long-term remission is that the patient started smoking tobacco with 35 years, approximately 10–20 cigarettes/day, and she refused to stop even with appropriate counseling. Data about smoking were not reported in the other studies that described cases of tumefactive MS that required DC,[1,2,3,4,5] but smoking has already been reported to be a risk factor for development and progression of MS.[11]
The DC is a possible choice of therapy when intracranial hypertension is refractory to pharmacological therapy. The best current evidence in favor of DC arises from randomized control trials in the management of refractory intracranial hypertension secondary to malignant middle cerebral artery infarction.[12] In the cases reported and also in the present case, the DC was done within less 24 h and none of the patients had any complication related to the surgery.[1,2,3,4,5] Thus, probably, an early intervention plays a fundamental role in the prognosis.
Conclusion
Tumefactive MS requiring emergency craniectomy has been rarely reported in the literature. This is the first case to document the occurrence of a second lesion in this context. In this way, it is possible that individuals with tumefactive MS need long-term follow-up. Moreover, it appears that DC can be safely performed with minimal risks in these critically ill patients, and an early neurosurgical intervention in selected cases may result in a greater benefit.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Censori B, Agostinis C, Partziguian T, Gazzaniga G, Biroli F, Mamoli A. Large demyelinating brain lesion mimicking a herniating tumor. Neurol Sci. 2001;22:325–9. doi: 10.1007/s10072-001-8176-5. [DOI] [PubMed] [Google Scholar]
- 2.Ragel BT, Fassett DR, Baringer JR, Browd SR, Dailey AT. Decompressive hemicraniectomy for tumefactive demyelination with transtentorial herniation: Observation. Surg Neurol. 2006;65:582–3. doi: 10.1016/j.surneu.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson P, Larsson EM, Kahlon B, Nordström CH, Norrving B. Tumefactive demyelinating disease treated with decompressive craniectomy. Eur J Neurol. 2009;16:639–42. doi: 10.1111/j.1468-1331.2009.02547.x. [DOI] [PubMed] [Google Scholar]
- 4.González Sánchez JJ, Nora JE, de Notaris M, Arboix JR, García CG, Rodríguez EF, et al. A case of malignant monophasic multiple sclerosis (Marburg's disease type) successfully treated with decompressive hemicraniectomy. J Neurol Neurosurg Psychiatry. 2010;81:1056–7. doi: 10.1136/jnnp.2007.142133. [DOI] [PubMed] [Google Scholar]
- 5.Munarriz PM, Castaño-Leon AM, Martinez-Perez R, Hernandez-Lain A, Ramos A, Lagares A, et al. Tumefactive multiple sclerosis requiring emergency craniotomy: Case report and literature review. Neurocirugia (Astur) 2013;24:220–4. doi: 10.1016/j.neucir.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Poser S, Lüer W, Bruhn H, Frahm J, Brück Y, Felgenhauer K, et al. Acute demyelinating disease. Classification and non-invasive diagnosis. Acta Neurol Scand. 1992;86:579–85. doi: 10.1111/j.1600-0404.1992.tb05490.x. [DOI] [PubMed] [Google Scholar]
- 7.Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131:1759–75. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda KM, Lee DH, Fraser JA, Mirsattari S, Morrow SA. Plasma exchange in a patient with tumefactive, corticosteroid-resistant multiple sclerosis. Int J MS Care. 2015;17:231–5. doi: 10.7224/1537-2073.2014-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saindane AM, Cha S, Law M, Xue X, Knopp EA, Zagzag D. Proton MR spectroscopy of tumefactive demyelinating lesions. AJNR Am J Neuroradiol. 2002;23:1378–86. [PMC free article] [PubMed] [Google Scholar]
- 10.Fatigba HO, Savi de Tove MK, Tchaou BA, Mensah E, Allode AS, Padonou J, et al. Surgical management of head trauma: Problems, results, and perspectives at the departmental teaching hospital of Borgou, Benin. World Neurosurg. 2013;80:246–50. doi: 10.1016/j.wneu.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Riise T, Nortvedt MW, Ascherio A. Smoking is a risk factor for multiple sclerosis. Neurology. 2003;61:1122–4. doi: 10.1212/01.wnl.0000081305.66687.d2. [DOI] [PubMed] [Google Scholar]
- 12.Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): A randomized, controlled trial. Stroke. 2007;38:2518–25. doi: 10.1161/STROKEAHA.107.485649. [DOI] [PubMed] [Google Scholar]