Abstract
Giant pituitary adenomas (GPAs) are defined as pituitary lesions larger than 40 mm of diameter. Surgical resection remains the gold standard to decompress the optic apparatus, reduce lesion load, and preserve hormonal function. The endoscopic endonasal approach (EEA) has been increasingly used for the treatment of pituitary adenomas and skull base tumors due to the wide angle of view and exposure. Through the description of an exemplificative case of EEA resection of a nonsecreting GPA in the setting of a multimodal treatment, the authors discuss the advantages and disadvantages of this management strategy and provide a detailed review of the literature.
Keywords: Endoscopic endonasal approach, giant pituitary adenoma, radiosurgery, transcranial route
Introduction
Giant pituitary adenomas (GPAs) are defined as pituitary adenomas larger than 40 mm of diameter; depending on the patient neurological status, hormonal profile, and lesion boundaries, the management options available include pharmacological treatment in functional GPA and surgical resection with or without radiotherapy/radiosurgery for nonfunctional ones.[1,2]
Surgical removal of GPA is challenging due to their size and the proximity of neurovascular structures, which are commonly invaded by these lesions.[3] Microsurgical and endoscopic approaches as standalone options or combinations of the two are available and chosen on a case by case basis. The endoscopic endonasal approach (EEA) generally allows visualization of neurovascular structures, lesion boundaries, and its suprasellar extension;[4,5] it certainly offers a series of advantages in the multimodal management of patients harboring GPA.
This article describes an exemplificative case of EEA resection of a nonfunctional GPA in the setting of a multimodal treatment, highlights the advantages and disadvantages of this management strategy, and provides a detailed review of the literature.
Case Report
A 62-year-old woman presented with an 18-month history of progressive headache, generalized weakness, and bilateral exophthalmus more relevant on the right. Neurological examination demonstrated diplopia on the right lateral gaze and bilateral decrease of visual acuity, especially in the right eye, without visual field deficit. Endocrine workup revealed hypocortisolism (cortisol: 49.3 μg/l, normal: 70–250 μg/l; ACTH: 8.3 ng/l, normal: 9–60 ng/l) and hypothyroidism (TSH: 0.84 mUI/l, normal: 0.270–4.20 mUI/l; T4: 5.1 ng/l, normal: 9.5–18 ng/l). Computer tomography (CT) and magnetic resonance imaging (MRI) scans showed a large (62 mm) extradural lesion invading sphenoid, right maxillary sinus, both cavernous sinuses (CSs), anterior and posterior ethmoid bone, orbits, and anterior and middle skull base with bone invasion extending to the clivus [Figure 1]. The patient was initially managed with a 15-day course of steroids (prednisolone 80 mg twice a day): bilateral exophthalmus remarkably improved, and a MRI (1 week after steroid interruption) showed a significant volume reduction (44 mm) [Figure 2]. At our skull base meeting, it was decided to proceed with Stage I EEA for tissue biopsy that suggested the diagnosis of a pituitary adenoma and therefore induced to offer a Stage II EEA surgical resection to the patient. Surprisingly, the MRI realized for navigation protocols the day before surgery (6 weeks after discontinuing steroids) showed a dramatic volumetric increase of the lesion. Surgery was undertaken using image-guided surgery with MRI and CT imaging fusion. The first step consisted in making a nasoseptal flap.[6] The lesion consistency was quite soft in its upper and superficial-anterior part, whereas the lower and deeper posterior portion involving the clivus, the internal carotid artery (ICA), and the CS was firmer, oozy, and fairly adherent. The tumor invaded and eroded the petrous bone; the ICAs were uncovered and identified with the help of a micro-Doppler probe. At the end, the resection was subtotal and the nasoseptal flap was lined over tuberculum sellae, sellar floor, clival recess, and ICAs and sealed with fibrin glue. The postoperative course was uneventful, and the patient was discharged after 3 days. The histopathology of the lesion confirmed a GPA with a Ki-67 of 1%. Immunohistochemistry analysis showed that cells had an epithelial phenotype with positivity of anti-pan-keratin staining and neuroendocrine differentiation with positive anti-chromogranin A, synaptophysin, CD56, and NSE antibodies. PS100, vimentin, GFAP, and TTF1 were absent. Furthermore, there was a positivity of 5%–10% of the cells to anti-ACTH antibodies (polyclonal) and negativity for all other hormones.
Figure 1.
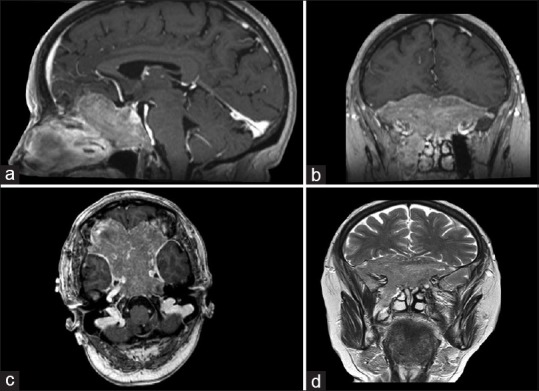
Preoperative sagittal (a), coronal (b), and axial (c) T1-magnetic resonance imaging showing slightly hyperintense and preoperative coronal (d) T2-magnetic resonance imaging showing isointense: anterior and middle skull base lesion with suprasellar extension, invading sphenoid sinus, clivus, ethmoid bone, anterior cranial fossa, and cavernous sinus extending in the right masticator space and encasing internal carotid artery
Figure 2.
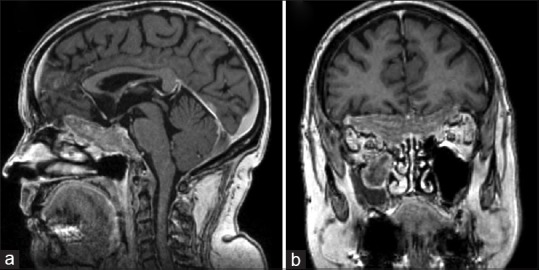
T1-magnetic resonance imaging ([a], sagittal, [b], coronal) showing the consistent lesion volume reduction after steroid treatment
Early postoperative MRI confirmed residues in the right orbit, the right frontobasal region as well as the lower clivus, and both CSs [Figure 3]. At 6-week follow-up, the clinical examination showed the resolution of diplopia and exophthalmus, with improvement of the visual acuity; hydrocortisone and thyroid replacement therapy were weaned off after 6 months due to normal hormonal assessment. At 6 months, MRI showed once again a limited progression of the residue. The case was discussed at our neuro-oncology meeting and given the lesion's aggressive behavior despite a Ki-67 of 1%, and it was decided to start a radiotherapy treatment. No further progression of the residue was seen at the 12-, 24-, 36-, and 48-month follow-up, and the patient remained asymptomatic with a normal pituitary function.
Figure 3.
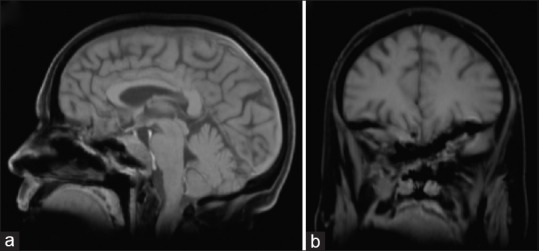
Postoperative T1-magnetic resonance imaging ([a], sagittal, [b], coronal) after an early injection of gadolinium showing large lesion debulking with residual mass in the clival, right subfrontal area, and the right orbit
Discussion
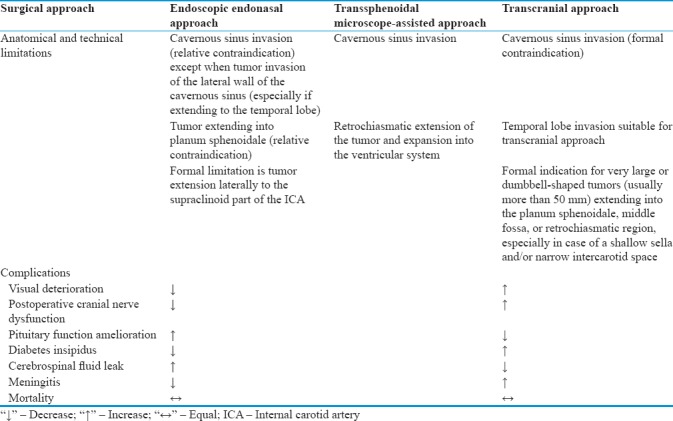
In the present article, we describe a case of GPA invading sphenoid, right maxillary sinus, CSs, anterior and posterior ethmoid bone, orbits, and anterior and middle skull base, in which EEA followed by the administration of adjuvant radiotherapy allowed a long-term (48-month) clinical remission with lesion control and normal pituitary function. Management of GPA is challenging because of their size, their consistency, their vascularization, and tendency to encase ICA and optic nerves as well as to invade the CSs.[7,8,9,10] The most common surgical routes used to address these lesions have been the transsphenoidal microscope-assisted approach (TSMA) (a.k.a. Hardy procedure)[11] and transcranial approaches (TCAs).[6,12,13] Since the introduction of EEA, the management of GPA has significantly improved;[14] multiple advantages such as reduction of surgical time and intraoperative risks will probably make this the standard treatment for Stages I and II (biopsy and excision) of most GPA in the near future. On the other hand, surgeons possessing only training in conventional microsurgery (TSMA or TCA) may find the learning curve very steep.[14,15,16] The most recent literature[17,18,19,20] highlights that EEA could be considered a valid alternative to more traditional TSMA and TCA, especially in the context of a multimodal management. The different surgical approaches with their anatomical, technical limitations and complications are summarized in Table 1. In general, the main goals for GPA management are reversal of the visual field deficits, improvement of the endocrine deficits, and neurological recovery. Preoperative visual field deficit can improve in up to 80% of patients;[6,21] nonetheless, postoperative visual worsening can also occur in up to 22% of cases, being more frequent following TCA (especially for lesions extending to the CS) than transsphenoidal surgery.[6,22] Improvement of pituitary function after surgery for GPA has not been explored in detail; although the reported improvement rate for hormonal function in patients with macroadenomas ranges between 35% and 50%, this might not be consistent in GPA given that the long-standing hypopituitarism is more unlikely to recover.[23,24] Transient or permanent hypopituitarism is a relatively common complication of EEA although its rate is similar to that of TSMA; nevertheless, due to the greater respect of pituitary stalk, decreased hormonal function following transsphenoidal approaches (either EEA or TSMA) remains lower than of TCA.[7,6,21] Postoperative diabetes insipidus (DI) is also more common after TCA than transsphenoidal surgery for GPA;[6,21] in TSMA series, the reported incidence of permanent postoperative DI ranges between 8.2%[6] and 10.4%.[8] The most common complication encountered in multistaged EEA is postoperative rhinorrhea (16.7%); great attention should be paid during reconstruction using multilevel grafts (fascia lata, fat, bone, glue, etc.). Nevertheless, in the last years, the cerebrospinal fluid (CSF) leak rate has dramatically improved thanks to the implementation of pedicled nasoseptal flap.[25,26,27] In the existing literature, a gross total removal of GPA is described in only 50% of cases;[6,12] because subtotal resection may be associated with early postoperative hemorrhages, acute hydrocephalus, and persistent optic nerve compression, a multimodal approach is warranted in most cases which can lead to a control of the disease as happened in this case report. In fact, the concomitant use of pharmacological therapy and radiotherapy or radiosurgery could become more relevant in the future thanks to advances obtained with the introduction of radioenhancers and radiosensitizers.[28,29] Finally, one of the interesting aspects of the present case is the initial lesion shrinkage after steroid therapy. Giant lesion may present an inflammatory component on which steroids could have acted, obtaining a volumetric reduction. Furthermore, the histopathology examination revealed, in 5%–10% of the cells, positivity to anti-ACTH antibodies (polyclonal) and negativity for all other hormones. Therefore, this GPA may have been a silent corticotroph adenoma, very well known for its aggressive behavior and tendency to recur, thus explaining its regrowth after steroid therapy suspension.[13,30]
Table 1.
Surgical approaches with their anatomical and technical limitations and complications
Conclusion
Many factors affect the outcome of patients with GPA, and the management of these lesions should, therefore, be tailored on a case-by-case basis. Although long-term disease control requires adjuvant treatment, its initial treatment consists in maximal surgical resection. Multistaged EEA allows good resection of lesions extending into the CS, ventricular, and clival regions. This approach is more difficult in case of fibrous lesions, with adherent and multilobular configurations, and/or extension beyond the lateral wall of CS. With the exception of CSF leak, the complication rate of EEA remains nearly the same or even lower than that reported for other approaches. The EEA represents a safe and effective treatment for GPA in a setting of multimodal management since surgery alone cures <60% of patients with GPA. Depending on the presence of a surgical residue and the histology/immunohistochemistry characteristics of the lesion, the remaining 40% will require medical therapy and most likely adjuvant radiosurgery/radiotherapy to achieve disease control.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Liu JK, Couldwell WT. Contemporary management of prolactinomas. Neurosurg Focus. 2004;16:E2. doi: 10.3171/foc.2004.16.4.3. [DOI] [PubMed] [Google Scholar]
- 2.Tritos NA, Biller BM, Swearingen B. Management of cushing disease. Nat Rev Endocrinol. 2011;7:279–89. doi: 10.1038/nrendo.2011.12. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal A, Cincu R, Goel A. Current concepts and controversies in the management of non-functioning giant pituitary macroadenomas. Clin Neurol Neurosurg. 2007;109:645–50. doi: 10.1016/j.clineuro.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz TH, Fraser JF, Brown S, Tabaee A, Kacker A, Anand VK, et al. Endoscopic cranial base surgery: Classification of operative approaches. Neurosurgery. 2008;62:991–1002. doi: 10.1227/01.neu.0000325861.06832.06. [DOI] [PubMed] [Google Scholar]
- 5.Frank G, Pasquini E, Farneti G, Mazzatenta D, Sciarretta V, Grasso V, et al. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology. 2006;83:240–8. doi: 10.1159/000095534. [DOI] [PubMed] [Google Scholar]
- 6.Mortini P, Barzaghi R, Losa M, Boari N, Giovanelli M. Surgical treatment of giant pituitary adenomas: Strategies and results in a series of 95 consecutive patients. Neurosurgery. 2007;60:993–1002. doi: 10.1227/01.NEU.0000255459.14764.BA. [DOI] [PubMed] [Google Scholar]
- 7.Chacko G, Chacko AG, Lombardero M, Mani S, Seshadri MS, Kovacs K, et al. Clinicopathologic correlates of giant pituitary adenomas. J Clin Neurosci. 2009;16:660–5. doi: 10.1016/j.jocn.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 8.de Paiva Neto MA, Vandergrift A, Fatemi N, Gorgulho AA, Desalles AA, Cohan P, et al. Endonasal transsphenoidal surgery and multimodality treatment for giant pituitary adenomas. Clin Endocrinol (Oxf) 2010;72:512–9. doi: 10.1111/j.1365-2265.2009.03665.x. [DOI] [PubMed] [Google Scholar]
- 9.Jane JA, Jr, Han J, Prevedello DM, Jagannathan J, Dumont AS, Laws ER, Jr, et al. Perspectives on endoscopic transsphenoidal surgery. Neurosurg Focus. 2005;19:E2. doi: 10.3171/foc.2005.19.6.3. [DOI] [PubMed] [Google Scholar]
- 10.Xue-Fei S, Yong-Fei W, Shi-Qi L, Jing-Song W, Yao Z, Ying M, et al. Microsurgical treatment for giant and irregular pituitary adenomas in a series of 54 consecutive patients. Br J Neurosurg. 2008;22:636–48. doi: 10.1080/02688690802346083. [DOI] [PubMed] [Google Scholar]
- 11.Somma M, Rasio E, Beauregard H, Hardy J. Pituitary adenomas. Surgical treatment by transphenoidal approach. Union Med Can. 1980;109:68–81. [PubMed] [Google Scholar]
- 12.Goel A, Nadkarni T, Muzumdar D, Desai K, Phalke U, Sharma P, et al. Giant pituitary tumors: A study based on surgical treatment of 118 cases. Surg Neurol. 2004;61:436–45. doi: 10.1016/j.surneu.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Karavitaki N, Ansorge O, Wass JA. Silent corticotroph adenomas. Arq Bras Endocrinol Metabol. 2007;51:1314–8. doi: 10.1590/s0004-27302007000800017. [DOI] [PubMed] [Google Scholar]
- 14.Cappabianca P, Cavallo LM, Esposito F, De Divitiis O, Messina A, De Divitiis E, et al. Extended endoscopic endonasal approach to the midline skull base: The evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg. 2008;33:151–99. doi: 10.1007/978-3-211-72283-1_4. [DOI] [PubMed] [Google Scholar]
- 15.Chibbaro S, Cornelius JF, Froelich S, Tigan L, Kehrli P, Debry C, et al. Endoscopic endonasal approach in the management of skull base chordomas – Clinical experience on a large series, technique, outcome, and pitfalls. Neurosurg Rev. 2014;37:217–24. doi: 10.1007/s10143-013-0503-9. [DOI] [PubMed] [Google Scholar]
- 16.Tabaee A, Anand VK, Barrón Y, Hiltzik DH, Brown SM, Kacker A, et al. Endoscopic pituitary surgery: A systematic review and meta-analysis. J Neurosurg. 2009;111:545–54. doi: 10.3171/2007.12.17635. [DOI] [PubMed] [Google Scholar]
- 17.Cappabianca P, Cavallo LM, de Divitiis O, de Angelis M, Chiaramonte C, Solari D, et al. Endoscopic endonasal extended approaches for the management of large pituitary adenomas. Neurosurg Clin N Am. 2015;26:323–31. doi: 10.1016/j.nec.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Gondim JA, Almeida JP, Albuquerque LA, Gomes EF, Schops M. Giant pituitary adenomas: Surgical outcomes of 50 cases operated on by the endonasal endoscopic approach. World Neurosurg. 2014;82:e281–90. doi: 10.1016/j.wneu.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Paluzzi A, Wang EW, Snyderman CH, et al. Endoscopic endonasal surgery for giant pituitary adenomas: Advantages and limitations. J Neurosurg. 2013;118:621–31. doi: 10.3171/2012.11.JNS121190. [DOI] [PubMed] [Google Scholar]
- 20.Sankhla SK, Jayashankar N, Khan GM. Surgical management of selected pituitary macroadenomas using extended endoscopic endonasal transsphenoidal approach: Early experience. Neurol India. 2013;61:122–30. doi: 10.4103/0028-3886.111114. [DOI] [PubMed] [Google Scholar]
- 21.Buchfelder M, Kreutzer J. Transcranial surgery for pituitary adenomas. Pituitary. 2008;11:375–84. doi: 10.1007/s11102-008-0120-8. [DOI] [PubMed] [Google Scholar]
- 22.Dolenc VV. Transcranial epidural approach to pituitary tumors extending beyond the sella. Neurosurgery. 1997;41:542–50. doi: 10.1097/00006123-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Fatemi N, Dusick JR, de Paiva Neto MA, Kelly DF. The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: A 10-year experience. Neurosurgery. 2008;63:244–56. doi: 10.1227/01.NEU.0000327025.03975.BA. [DOI] [PubMed] [Google Scholar]
- 24.Nomikos P, Ladar C, Fahlbusch R, Buchfelder M. Impact of primary surgery on pituitary function in patients with non-functioning pituitary adenomas – A study on 721 patients. Acta Neurochir (Wien) 2004;146:27–35. doi: 10.1007/s00701-003-0174-3. [DOI] [PubMed] [Google Scholar]
- 25.Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–6. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 26.Alobid I, Mason E, Solares CA, Prevedello D, Enseñat J, De Notaris M, et al. Pedicled lateral nasal wall flap for the reconstruction of the nasal septum perforation. A radio-anatomical study. Rhinology. 2015;53:235–41. doi: 10.4193/Rhino14.042. [DOI] [PubMed] [Google Scholar]
- 27.Chibbaro S, Cebula H, Aldea S, Baussart B, Tigan L, Todeschi J, et al. Endonasal endoscopic odontoidectomy in ventral diseases of the craniocervical junction: Results of a multicenter experience. World Neurosurg. 2017;106:382–93. doi: 10.1016/j.wneu.2017.06.148. [DOI] [PubMed] [Google Scholar]
- 28.Ganau M, Foroni RI, Gerosa M, Zivelonghi E, Longhi M, Nicolato A, et al. Radiosurgical options in neuro-oncology: A review on current tenets and future opportunities. Part I: Therapeutic strategies. Tumori. 2014;100:459–65. doi: 10.1700/1636.17912. [DOI] [PubMed] [Google Scholar]
- 29.Ganau M, Foroni RI, Gerosa M, Ricciardi GK, Longhi M, Nicolato A, et al. Radiosurgical options in neuro-oncology: A review on current tenets and future opportunities. Part II: Adjuvant radiobiological tools. Tumori. 2015;101:57–63. doi: 10.5301/tj.5000215. [DOI] [PubMed] [Google Scholar]
- 30.Jahangiri A, Wagner JR, Pekmezci M, Hiniker A, Chang EF, Kunwar S, et al. A comprehensive long-term retrospective analysis of silent corticotrophic adenomas vs.hormone-negative adenomas. Neurosurgery. 2013;73:8–17. doi: 10.1227/01.neu.0000429858.96652.1e. [DOI] [PubMed] [Google Scholar]


