Abstract
Glioblastoma multiforme (GBM) has the highest rate of vascular proliferation among solid tumors. Angiogenesis is the central feature of rapid tumor growth in GBM and therefore remains an appealing therapeutic target in the treatment of these highly malignant tumors. Antiangiogenic therapy is emerging as an important adjuvant treatment. Multiple antiangiogenic agents targeting various sites in vascular endothelial growth factor (VEGF) and integrin pathways have been tested in clinical trials of newly diagnosed and recurrent GBMs. These include bevacizumab, enzastaurin, aflibercept, cediranib, and cilengitide. In this review, we discuss the current status and challenges facing clinical application of antiangiogenic treatment including anti-VEGF therapy and integrin pathway agents’ therapy in glioblastoma. Here, we highlight a strong biologic rationale for this strategy, also focusing on integrin pathways. PubMed-indexed clinical trials published in English on antiangiogenic treatment of glioblastomas in the past 5 years were reviewed. The results of the current clinical trials of these agents are presented.
Keywords: Antiangiogenic therapy, cilengitide, glioblastoma multiforme, integrin, vascular endothelial growth factor
Introduction
Glioblastoma multiforme (GBM) accounts for 17% of primary central nervous system tumors. Survival rates remain poor despite advancements in surgical technique and chemotherapies. The median progression-free survival (PFS) is 6.9 months, with overall survival (OS) of 14.6 months.[1] The standard of care for newly diagnosed GBM is surgical resection and postoperative chemoradiation with temozolomide. Despite this aggressive regimen, survival has only improved by 2–7 months at best and options for salvage chemotherapy remain limited.[2] The poor prognosis is partly secondary to rapid tumor growth from angiogenesis and aggressive tumor cell invasion.[3,4] Upregulation of vascular endothelial growth factor (VEGF) from intratumoral hypoxia and dysregulation of growth factor signaling play important roles in the pathophysiology of tumor resistance and recurrence.[3,5] As a result, these pathways have led to the development of new pharmaceutical targets for the management of GBM with mixed success.[6]
Pathophysiology
Tumor growth relies on four major processes: neovascularization, tumor cell invasion, migration, and resistance to apoptosis. Angiogenesis is stimulated by hypoxia. Highly aggressive tumors such as GBM rapidly outgrow their blood supply, resulting in hypoxia and thus activating the cascade for upregulation of VEGF, a tyrosine kinase inhibitor (TKI) on the vascular endothelial cell surface. Downstream signal transduction promotes angiogenesis and mitogenesis [Figure 1].[3] While VEGF plays a crucial role in normal and pathologic neovascularization, there are other molecules that are equally involved.
Figure 1.
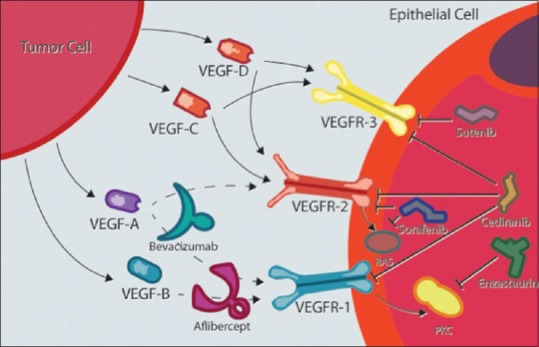
Suppression of vascular endothelial growth factor receptor signaling can be achieved at different steps. Blocking the ligand-binding site of vascular endothelial growth factor receptor with monoclonal antibodies, synthetic peptides, or the tyrosine kinase activation site with small-molecule inhibitors. Cediranib, sunitinib, and sorafenib are vascular endothelial growth factor receptor inhibitors. Enzastaurin prevents downstream vascular endothelial growth factor receptor signaling by inhibiting protein kinase C beta. Aflibercept has a high affinity for vascular endothelial growth factor receptor 1 acting as a decoy protein to prevent ligand binding and activation. Bevacizumab is a monoclonal antibody that inhibits vascular endothelial growth factor receptor 1 and 2 on the vascular endothelial cell
Integrins are transmembrane receptor proteins with intracellular and extracellular activity that are composed of alpha and beta chains. There are 18 alpha subtypes and 8 beta subtypes. The combination of various subtypes determines extracellular ligand binding and downstream signaling responsible for tumor angiogenesis, invasion, and migration.[7,8,9,10,11] The avB3 and avB5 subtypes are upregulated in tumor cells and tumor vasculature, thus serving as a target for multiple pathways of tumor growth.[11,12,13] The avb3 and avb5 integrins specifically recognize the arginine-glycine-aspartic acid sequence of extracellular ligands. On binding to ligand, integrins aggregate and recruit focal adhesion kinase resulting in signaling through the PI3K and mitogen-activated protein kinase cascade.[8,9,10] Through this pathway, growth factors are activated which promote cell cycle progression and suppress apoptosis, leading to increased tumor burden.[14]
Antiangiogenic Agents in Vascular Endothelial Growth Factor Pathways
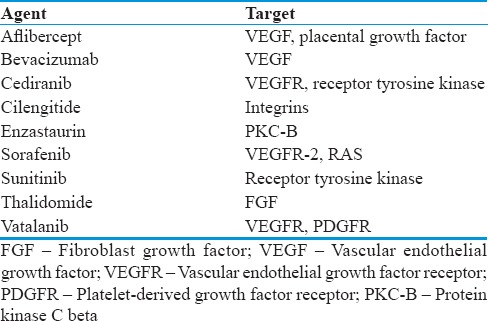
A number of drugs have targeted different steps in the pathway of angiogenesis through VEGF-signaling cascades [Table 1 and Figure 1]. The therapeutic approaches to suppression of VEGF receptor (VEGFR) signaling can be achieved by intervening at multiple steps in the activation cascade. As illustrated in Figure 1, those include blocking the ligand-binding site of VEGFR with either monoclonal antibodies or synthetic peptides or blocking the tyrosine kinase activation site of VEGFR with small-molecule inhibitors (TKIs). Multiple receptors of TKIs have been under investigation for the treatment of recurrent GBM. These multi-kinase VEGFR inhibitor agents include cediranib, sunitinib, pazopanib, vandetanib, and sorafenib. The potency of these molecules against the VEGFR family is variable and each agent inhibits multiple other potentially relevant receptors, including KIT, platelet-derived growth factor receptor, rearranged during transfection, RAF, and epidermal growth factor receptor. The most studied of these pharmaceutical agents in GBM include cediranib, enzastaurin, aflibercept, and bevacizumab.
Table 1.
Antiangiogenic agents and targets
Bevacizumab
Bevacizumab is a recombinant humanized monoclonal antibody that directly targets VEGF and has been studied in both recurrent and newly diagnosed GBM.[15] In recurrent GBM, there is clear radiographic response of tumor regression. The standard of care for newly diagnosed GBM, temozolomide, adds little benefit to patients with recurrent GBM and those with unmethylated O(6) methylguanine-DNA methyltransferase (MGMT).[16,17] On the other hand, Phase II trials have shown encouraging results measuring the PFS and OS benefits of bevacizumab therapy, alone or combined with topoisomerase inhibitor irinotecan in the subgroup of MGMT-negative GBM. Notably, an initial Phase II trial investigating combination therapy of bevacizumab and irinotecan showed 6-month PFS (PFS-6) rate of 46% and OS of 9.7 months.[18,19] The BRAIN trial then showed PFS-6 rates of 42.6% and 50.3% for single and combined therapy, respectively, and median OS of 9.2 and 8.7 months.[20] This strong evidence for PFS improvement led the US FDA to approve the drug, while the lack of OS advantage prompted EU refusal for GBM-indicated approval, underscoring the enduring contention that surrounds bevacizumab. Still, in recurrent GBM, the use of bevacizumab, alone or combined with irinotecan, produces favorable radiographic response rates.[21] In a meta-analysis compiling data from 12 Phase II trials comparing bevacizumab use alone versus in combination with irinotecan, patients in the combination therapy arm showed a statistically higher PFS-6, objective radiographic response, and rate of treatment discontinuation.[21]
Phase III trials investigating bevacizumab in newly diagnosed GBM patients focused primarily on evaluating patient outcomes echo previous concerns for the drug. The AVAglio Phase III trial, investigating the role of bevacizumab in conjunction with temozolomide and radiation therapy (RT) for patients with newly diagnosed GBM, demonstrated that this combination can increase PFS-6 by as much as 36% but had no significant increase in OS and there was no particular subgroup of patients who benefited more.[22] Similar results were found in the Phase III RTOG trial, including an increased burden of symptoms, worse quality of life, and neurocognitive decline.[23] The study allowed patients to crossover at event of disease progression with unblinding and initiation of bevacizumab if in placebo group, ruling out any potential survival benefit of first-line therapy compared with salvage.[23] The results were not all discouraging, however, as patients enrolled in the AVAglio trial showed stable quality of life during PFS period as opposed to the deteriorating state seen in the RTOG trial.[22,23] The RTOG trial excluded patients with unresected (biopsy-only) GBM upon enrollment, which could possibly contribute to this difference.[23] Overall, neither study indicated that bevacizumab therapy increases OS or provides strong PFS improvement, mirroring results from the past studies and discouraging hopes for direct translation of the drug's promising results in other cancers.[22,23] There are other factors complicating the use of bevacizumab as a preferred therapy.
The side effects of bevacizumab can lead to significantly increased morbidity. These include venous thromboembolism, bleeding, and high-grade congestive heart failure. These risks are increased with combination chemotherapy compared to monotherapy.[24,25,26,27] Furthermore, there is some evidence to suggest that bevacizumab has promoted tumor resistance to future treatment, complicating indications for use.[28,29]
Cediranib and related agents
Cediranib is a VEGFR2 inhibitor that was postulated to decrease tumor growth by inhibiting endothelial cell proliferation and survival, thereby preventing neovascularization.[30] Despite promising results from preclinical models, this drug did not lead to increased PFS in the Phase III REGAL trial for recurrent GBM when compared to the treatment with alkylating nitrosourea agent lomustine.[31] Similar agents such as TKIs sunitinib and sorafenib have been explored and received FDA approval for use in advanced renal cell carcinoma.[32] Sunitinib recently underwent Phase II testing in GBM patients, showing minimal antiglioblastoma activity and high toxicity.[33] Likewise, both sorafenib monotherapy and combination therapy with standard of care chemotherapeutic agent temozolomide showed only limited results – PFS-6 of 25% and 26%, respectively, in GBM patients, and considerable toxicity.[34,35] Addition of radiotherapy to sorafenib and temozolomide combination therapy generated similarly moderate outcome results as compared to standard therapy alone, as well as significantly increased adverse events, discouraging further investigation of the drug in GBM.[36]
Enzastaurin
Enzastaurin is an acyclic bisindolylmaleimide that inhibits VEGF-induced signaling through inhibition of protein kinase C beta.[37] Inhibition of VEGF-driven vessel proliferation and growth along with decreased tumor microvessel density in cell line and murine xenografts provides the foundational evidence for the drug's antiangiogenic effects.[38,39] Phase II trials of enzastaurin in 85 patients with recurrent malignant glioma showed objective radiographic responses in only 16% of patients and a median PFS of 5 months.[40] Another study combining enzastaurin with radiotherapy and temozolomide in newly diagnosed GBM showed low toxicity profile, spurring a Phase III trial. However, this was soon terminated due to nonsuperiority of the drug as compared to lomustine.[41,42]
Aflibercept
Aflibercept is a decoy protein made up of the extracellular domains of VEGF fused to the Fc portion of immunoglobulin G1. Thus, it binds with high affinity to both VEGFR stimulating tumor angiogenesis.[43,44] Aflibercept showed promising results in prolonging survival in both initial and advanced stages of glioma tumor development in animal models.[43,45] Nevertheless, Phase II trials in human patients with recurrent GBM resulted in 25% of patients (14) removed from the study due to toxicity, including CNS ischemia and systemic hemorrhage, and only demonstrated a PFS-6 of 7.7%.[46]
Antiangiogenic Agents in Integrin Pathways-cilengitide
Angiogenesis consists of three steps: blood vessel degradation, disruption of the basement membrane, and endothelial cell migration.[3] Endothelial cell migration and adhesion are facilitated and enhanced by the integrin avb3.[7,8,9,10,11,12,13] Tumor cell invasion similarly requires multiple steps: invading cells detach from the tumor mass, adhere to the extracellular matrix (ECM), which then breaks down to allow for cell penetration.[3,4] Integrins are responsible for cell attachment to the ECM. Cilengitide (CIL) is a cyclic arginine-glycine-aspartic acid pentapeptide inhibitor of avb3 and avb5 integrins [Figure 2].[5,12,47,48] By blocking these critical steps, CIL has the potential to reduce tumor burden [Figure 3].[10] Unlike previous antiangiogenic chemotherapy agents, CIL simultaneously targets tumor angiogenesis, invasion, and migration.[12] Due to this multifaceted approach, there is less potential for developing resistance and relapse.
Figure 2.
Cilengitide is a cyclic arginine-glycine-aspartic acid pentapeptide inhibitor of avb3 and avb5 integrins
Figure 3.
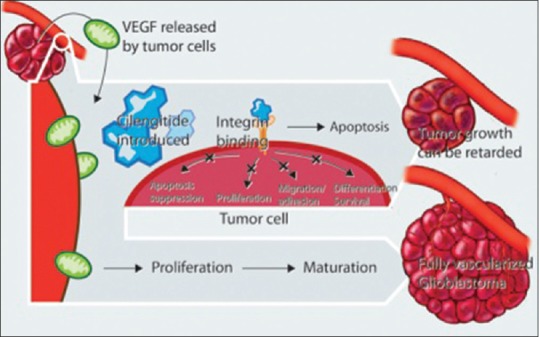
Tumor growth occurs secondary to suppression of apoptosis, cell proliferation cell differentiation, and angiogenesis. Neovascularization requires endothelial cell adhesion and migration. Integrins play a partial role in all of these processes through the mitogen-activated protein kinase signaling cascade. Cilengitide, an integrin antagonist, provides a multifaceted approach to preventing tumor progression
Preclinical models have demonstrated that cells treated with CIL upregulate caspase 8, leading to increased apoptosis.[49] While murine models suggest a role for CIL as a single-agent therapy, there is even greater preclinical evidence to support a synergistic relationship when combined with radiotherapy.[50,51] As a result, the efficacy and safety profile of CIL in recurrent and newly diagnosed GBM are being investigated in numerous clinical trials summarized below and in Table 2.
Table 2.
Current and ongoing clinical trials of cilengitide in recurrent and newly diagnosed glioblastoma multiforme
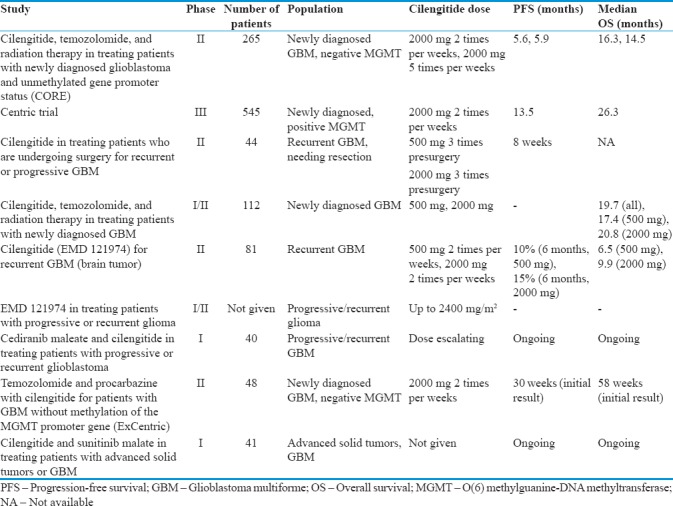
Results of clinical trials of cilengitide in recurrent glioblastoma multiforme
Multiple Phase I trials have examined the role of CIL monotherapy in recurrent adult and pediatric malignant glioma. These trials provided insight into potential dose-limiting toxicities, pharmacokinetics, and biological measures of response. A Phase I trial of 37 patients who received an escalating dose of CIL infusions between 30 and 1600 mg/m2 over 1 h twice a week did not reveal any toxicity in that range.[52] Another Phase 1 trial administering doses between 600 and 1200 mg/m2 twice weekly supported the safety profile of CIL between these dosage levels.[47] A subsequent Phase I trial of 51 patients with recurrent malignant glioma evaluated the safety of doses between 120 and 2400 mg/m2. Although it did not consistently demonstrate dose-limiting toxicity (DLT) and maximum tolerated dosage (MTD), one patient reported thrombosis at the dose of 120 mg/m2. One other patient reported grade 4 joint and bone pain at dose of 480 mg/m2. One patient experienced thrombocytopenia at dose of 600 mg/m2. At 1800 mg/m2 anorexia, hypoglycemia and hyponatremia were reported in one patient. Importantly, none of the patients developed intracranial hemorrhage.[53] A similarly designed dose escalation Phase I trial in the pediatric population of 31 patients with recurrent malignant glioma had more equivocal results. Patients were administered CIL twice weekly starting at a dose of 120 mg/m2 and increased to 2400 mg/m2 over a period of 52 weeks. Three of 13 patients who received doses of 2400 mg/m2 developed intracranial hemorrhage. Of these, however, two were asymptomatic and all three were self-limiting.[54] These results indicate that CIL has a low side effect profile and is safe to use up to 2400 mg/m2 twice weekly.[53,54] In addition to providing insight into the DLT and MTD of CIL in recurrent malignant glioma, these studies investigated the tumor response by measuring specific biological markers. While tumor vessel density, endothelial cell apoptosis, gene expression profiling, and serum angiogenic factor levels proved not to be reliable indicators, perfusion magnetic resonance imaging demonstrated decreased tumor blood flow at increased doses of CIL correlating with clinical response.[53]
Given the early success of the Phase I trials, a Phase II trial was administered with the purpose of measuring the efficacy and drug delivery into the tumor in patients who developed recurrent or progressive GBM and required surgical resection. Patients were randomized to receive either 500 or 2000 mg of intravenous (IV) CIL 8, 4, and 1 day before resection. Blood samples were taken to compare plasma levels of CIL to the drug level within the tumor. In the postsurgery period, patients were given 2000 mg IV CIL twice weekly. The primary outcome was PFS, and the secondary outcome was measurement of intratumoral drug uptake. The PFS-6 rate was 12% with median PFS of 8 weeks. At the time of resection, on an average, there was a 3-fold higher concentration of drug within the tumor as compared to the plasma level in the 500 mg dose group and 4-fold higher in the 2000 mg dose group. CIL showed little efficacy as a single agent to extend PFS, but effective delivery to the tumor was demonstrated.[55]
These results conflict with a second multicenter, Phase II trial instituted with the purpose of measuring the efficacy and safety of CIL in GBM patients at first recurrence, which showed moderate efficacy. Patients were randomized to two arms to receive either 500 or 2000 mg IV CIL twice weekly and assessed every 4 weeks. The primary outcome was PFS-6; secondary outcomes included OS and radiographic response. In the 500 mg/day arm, the PFS-6 rate was 10% and OS was 6.5 months. In the 2000 mg/day arm, the PFS-6 rate was 15% and OS was 9.9 months. The authors concluded that CIL monotherapy had moderate efficacy in recurrent GBM patients.[56] Overall, these studies suggest that CIL is safe and probably effective as a single-agent therapy in recurrent malignant glioma. Considering the inconsistent findings of efficacy in these trials, larger sample sizes are needed to better power the studies.
Results of clinical trials of cilengitide in newly diagnosed glioblastoma multiforme
The safety and efficacy of CIL as part of multi-agent therapy in newly diagnosed GBM are still under investigation through ongoing trials; however, recently presented results have shown mixed findings. A phase I/II trial measuring safety and efficacy of CIL administered with temazolamide and RT for newly diagnosed GBM patients randomized subjects to 2 arms. In Arm I, patients received RT and temozolomide as in the Phase I initiation course and CIL at 500 mg doses. In Arm 2, patients received RT and temozolomide as in the Phase I initiation course and CIL at 2000 mg doses. The primary outcomes were MTD and OS. The secondary outcomes included separate failure rates, frequency of grade 3 or 4 toxicities, and changes in radiological features such as tumor blood volume, blood flow, and permeability. Patient samples were also tested for MGMT promoter methylation status. OS for all patients was 19.7 months (OS in 500 mg dose group: 17.4 months, OS in 2000 mg dose group: 20.8 months). The unadjusted hazard ratio (HR) of death for 2000 mg group versus 500 mg was 0.83, and adjusted HR was 0.8. Median OS for methylated, unmethylated, and unknown MGMT status was 30, 19.1, and 17.4 months, respectively. A total of 48 grade 3 or 4 adverse events occurred in the Arm 1 group and 35 in Arm 2 group. The authors concluded that CIL may improve survival for newly diagnosed patients regardless of MGMT status and should be used at the 2000 mg dose.[57] However, these findings are not consistent with the recently presented results of the CENTRIC and CORE trials.
The CENTRIC study was a multicenter, open-label, randomized controlled Phase III trial with the purpose of measuring the safety and efficacy of CIL therapy in patients with newly diagnosed GBM and methylated MGMT gene promoter. Patients were randomized 1:1 either to control treatment group of temozolomide and RT followed by maintenance therapy with temozolomide or to the group with CIL regimen of 2000 mg IV CIL 2 times/week in addition to temozolomide + RT followed by maintenance temozolomide. The primary outcome was OS. Secondary outcomes included PFS and safety. Median OS was 26.3 months in both arms. Median PFS per investigator read was 13.5 months in the CIL arm and 10.7 months in the control arm. No new safety concerns were identified. However, CIL did not seem to prolong PFS or OS in newly diagnosed patients with methylated MGMT gene promoter status.[58]
The CORE study was a multicenter, open-label, randomized controlled Phase II trial with the purpose of measuring the safety and efficacy of CIL therapy in patients with newly diagnosed GBM and unmethylated MGMT gene promoter. Patients were randomized 1:1:1 either to the control group with conventional treatment of temozolomide + RT followed by maintenance temozolomide, or to a standard CIL regimen of 2000 mg of IV CIL 2 times/week in addition to temozolomide + RT followed by maintenance temozolomide, or finally to an intensified CIL regimen of 2000 mg IV CIL 5 times/week in addition to temozolomide + RT followed by maintenance temozolomide. The primary outcome was OS. Secondary outcomes included PFS and safety. The median OS was 13.4 months in the control arm, 16.3 months in the CIL arm, and 14.5 months in the CIL intensified dose arm. Respective median PFS per independent read was 4.1, 5.6, and 5.9 months. No new safety concerns were identified. CIL appeared to increase OS in newly diagnosed patients with unmethylated MGMT gene promoter status but only with the standard regimen and not with the intensified regimen.[59]
Ongoing trials of combination of anti-vascular endothelial growth factor therapy with cilengitide in glioblastoma multiforme
While the use of CIL as monotherapy in recurrent GBM and in combination with conventional treatment in newly diagnosed GBM has been studied, the role of multi-antiangiogenic therapy has not been fully elucidated. Ongoing Phase I trials are investigating this exact question.
Cediranib maleate and CIL in treating patients with progressive or recurrent glioblastoma is a Phase I trial with the purpose of finding the optimal dose of cediranib maleate when given with CIL to treat patients with progressive or recurrent GBM. Patients will be initially enrolled in a dose-finding study (Part A) where cediranib maleate will be given orally daily together with IV CIL on days 1, 4, 8, 11, 15, 18, 22, and 25. In the second part of the study (Part B), patients will be grouped on prior anti-VEGF therapy status and will be given cediranib maleate at the dose determined in Part A along with IV CIL as in Part A. The primary outcome is the safety profile of cediranib maleate based on the incidence of DLT. Secondary outcomes include OS, radiographic responses, PFS, and change in markers[60]
CIL and sunitinib malate in treating patients with advanced solid tumors or glioblastoma multiform is a Phase I trial studying the efficacy of CIL together with sunitinib malate for patients with advanced solid tumors or GBM. Patients received oral sunitinib malate for 2 weeks and then randomized to 2 arms. Arm 1 patients receive CIL IV twice weekly for 2 weeks and Arm 2 patients do not receive treatments for 2 weeks. Both arms receive a second course of sunitinib malate for 2 weeks followed by CIL twice weekly for 2 weeks with this treatment repeating every 4 weeks. The primary outcome is changes in serum VEGFR2 during the withdrawal phase from Sunitinib in course 1. Secondary outcomes include comparison of serum VEGFR2 in course 1 versus 2 and toxicity[61]
Temozolomide and procarbazine with CIL for patients with GBM without methylation of the MGMT promoter gene (ExCentric) is a Phase II trial investigating the safety and efficacy of the combination of temozolomide, procarbazine, RT, and CIL in newly diagnosed GBM patients with unmethylated MGMT promoter status. Patients receive CIL 2000 mg 2 times per week for 18 months, with 6 weeks of RT started on week 2 with daily temozolomide and procarbazine followed by cycles of these two drugs given on days 1–20 every 28 days. The primary outcome is 12-month OS, and secondary outcomes include PFS-6 and toxicity. Initial results confirm the safety profile of the treatment combination and the current median OS is 58 weeks and current median PFS is 30 weeks.[62]
Analysis of mixed response to cilengitide in clinical trials
While Phase II trials of CIL in other cancers have failed to demonstrate significant antitumor activity, early clinical trials in GBM have suggested that it is a safe single agent therapy for recurrent GBM that leads to modest improvement in OS. When combined with radiation and temozolomide for newly diagnosed GBM, CIL improved OS in appropriately selected patients in the Phase II CORE trial. However, the Phase III CENTRIC trial did not demonstrate any benefit to OS or PFS in newly diagnosed GBM patients with methylated MGMT status. This subsequently led to the manufacturer halting drug development, which begs the question of why favorable Phase II trials of CIL did not translate into a positive Phase III trial. Numerous explanations are possible. First, CIL has a dose-dependent effect on angiogenesis. At low doses, the drug actually promotes angiogenesis, whereas at high doses, it inhibits it. The dosing regimen used in clinical trials was based on preclinical data and generally included a low-intermediate dose of 500 mg intravenously twice a week or an intermediate-high dose of 2000 mg intravenously twice a week. The latter of which was used in the CENTRIC trial. Considering the short half-life of 2-4 h, a twice-weekly schedule may be less favorable than a continuous infusion, at the cost of convenience to the patient. Second, despite radiographic and biopsy-proven evidence that CIL reaches tumor cells, there is no reliable biomarker to trace the signal of antitumor activity, a long-standing issue for many of the antiangiogenic agents. Third, the trial focused on MGMT positive patients given prior evidence of favorable prognosis in this subgroup; however, there is little known about the pathophysiologic underpinning and interaction between MGMT methylation and integrin signaling.
Conclusions and Future Directions
The angiogenesis pathway has been heavily targeted in recent investigations of GBM therapy. VEGF is one of the most important regulators of angiogenesis in glioblastoma. Multiple strategies involving targeting the VEGF/VEGFR pathway, including VEGF sequestration, vascular disruption, and suppression of VEGFR signaling using TKIs, are being actively explored in various clinical trials. Bevacizumab continues to be the most studied antiangiogenic agent in the treatment of newly diagnosed and recurrent GBM. Although bevacizumab has yet to conclusively demonstrate durable response and survival benefit in patients with GBM, its inhibitory effect on peritumoral edema contributing to transient clinical and radiographic response is important in reducing disabling neurological symptoms. Further studies are required to understand the highly complex process of angiogenesis in relation to mechanisms of actions and resistance to VEGF/VEGFR therapies to optimize the treatment protocols in case of newly diagnosed and recurrent GBM.
In addition to the various drugs acting along the VEGF signaling cascade, CIL acting on integrin pathways offers a novel cancer therapy that simultaneously prevents angiogenesis, invasion, and migration while promoting apoptosis. Although the favorable Phase II trials of CIL did not translate into a positive Phase III trial, this may be due to multiple reasons such as dose dependency, lack of reliable biomarker for assessment of tumor activity, and a bias of using in MGMT-positive patients during the trial. Taking these factors into consideration, we believe that it is too early to give up on CIL and future investigations taking into account the unique pharmacokinetics of the drug are needed to explore the therapeutic potential of the drug.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Sweet JA, Feinberg ML, Sherman JH. The role of avastin in the management of recurrent glioblastoma. Neurosurg Clin N Am. 2012;23:331–41. doi: 10.1016/j.nec.2012.02.001. x. [DOI] [PubMed] [Google Scholar]
- 3.Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: Biology and molecular pathophysiology. Brain Pathol. 2005;15:297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: An integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17:1225–35. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onishi M, Kurozumi K, Ichikawa T, Date I. Mechanisms of tumor development and anti-angiogenic therapy in glioblastoma multiforme. Neurol Med Chir (Tokyo) 2013;53:755–63. doi: 10.2176/nmc.ra2013-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beal K, Abrey LE, Gutin PH. Antiangiogenic agents in the treatment of recurrent or newly diagnosed glioblastoma: Analysis of single-agent and combined modality approaches. Radiat Oncol. 2011;6:2. doi: 10.1186/1748-717X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 8.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 9.Stupack DG, Cheresh DA. Get a ligand, get a life: Integrins, signaling and cell survival. J Cell Sci. 2002;115(Pt 19):3729–38. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- 10.Buerkle MA, Pahernik SA, Sutter A, Jonczyk A, Messmer K, Dellian M. Inhibition of the alpha-nu integrins with a cyclic RGD peptide impairs angiogenesis, growth and metastasis of solid tumours in vivo. Br J Cancer. 2002;86:788–95. doi: 10.1038/sj.bjc.6600141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, et al. Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery. 2001;48:151–7. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Tabatabai G, Weller M, Nabors B, Picard M, Reardon D, Mikkelsen T, et al. Targeting integrins in malignant glioma. Target Oncol. 2010;5:175–81. doi: 10.1007/s11523-010-0156-3. [DOI] [PubMed] [Google Scholar]
- 13.Gladson CL. Expression of integrin alpha v beta 3 in small blood vessels of glioblastoma tumors. J Neuropathol Exp Neurol. 1996;55:1143–9. doi: 10.1097/00005072-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Aguzzi MS, Giampietri C, De Marchis F, Padula F, Gaeta R, Ragone G, et al. RGDS peptide induces caspase 8 and caspase 9 activation in human endothelial cells. Blood. 2004;103:4180–7. doi: 10.1182/blood-2003-06-2144. [DOI] [PubMed] [Google Scholar]
- 15.Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]
- 16.Norden AD, Bartolomeo J, Tanaka S, Drappatz J, Ciampa AS, Doherty LM, et al. Safety of concurrent bevacizumab therapy and anticoagulation in glioma patients. J Neurooncol. 2012;106:121–5. doi: 10.1007/s11060-011-0642-1. [DOI] [PubMed] [Google Scholar]
- 17.Norden AD, Lesser GJ, Drappatz J, Ligon KL, Hammond SN, Lee EQ, et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol. 2013;15:930–5. doi: 10.1093/neuonc/not040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 19.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 20.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Huang S, Wang Z. A meta-analysis of bevacizumab alone and in combination with irinotecan in the treatment of patients with recurrent glioblastoma multiforme. J Clin Neurosci. 2012;19:1636–40. doi: 10.1016/j.jocn.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Chinot OL, Wick W, Cloughesy T. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:2049. doi: 10.1056/NEJMc1403303. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert MR, Sulman EP, Mehta MP. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:2048–9. doi: 10.1056/NEJMc1403303. [DOI] [PubMed] [Google Scholar]
- 24.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA. 2008;300:2277–85. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 25.Qi WX, Fu S, Zhang Q, Guo XM. Bevacizumab increases the risk of severe congestive heart failure in cancer patients: An up-to-date meta-analysis with a focus on different subgroups. Clin Drug Investig. 2014;34:681–90. doi: 10.1007/s40261-014-0222-1. [DOI] [PubMed] [Google Scholar]
- 26.Odia Y, Shih JH, Kreisl TN, Fine HA. Bevacizumab-related toxicities in the National Cancer Institute malignant glioma trial cohort. J Neurooncol. 2014;120:431–40. doi: 10.1007/s11060-014-1571-6. [DOI] [PubMed] [Google Scholar]
- 27.Nghiemphu PL, Green RM, Pope WB, Lai A, Cloughesy TF. Safety of anticoagulation use and bevacizumab in patients with glioma. Neuro Oncol. 2008;10:355–60. doi: 10.1215/15228517-2008-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–5. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boockvar JA, Tsiouris AJ, Hofstetter CP, Kovanlikaya I, Fralin S, Kesavabhotla K, et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article. J Neurosurg. 2011;114:624–32. doi: 10.3171/2010.9.JNS101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brave SR, Ratcliffe K, Wilson Z, James NH, Ashton S, Wainwright A, et al. Assessing the activity of cediranib, a VEGFR-2/3 tyrosine kinase inhibitor, against VEGFR-1 and members of the structurally related PDGFR family. Mol Cancer Ther. 2011;10:861–73. doi: 10.1158/1535-7163.MCT-10-0976. [DOI] [PubMed] [Google Scholar]
- 31.Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31:3212–8. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein MN, Flaherty KT. CCR drug updates: Sorafenib and sunitinib in renal cell carcinoma. Clin Cancer Res. 2007;13:3765–70. doi: 10.1158/1078-0432.CCR-06-2844. [DOI] [PubMed] [Google Scholar]
- 33.Hutterer M, Nowosielski M, Haybaeck J, Embacher S, Stockhammer F, Gotwald T, et al. A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01-07) Neuro Oncol. 2014;16:92–102. doi: 10.1093/neuonc/not161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassler MR, Ackerl M, Flechl B, Sax C, Wöhrer A, Widhalm G, et al. Sorafenib for patients with pretreated recurrent or progressive high-grade glioma: A retrospective, single-institution study. Anticancer Drugs. 2014;25:723–8. doi: 10.1097/CAD.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 35.Zustovich F, Landi L, Lombardi G, Porta C, Galli L, Fontana A, et al. Sorafenib plus daily low-dose temozolomide for relapsed glioblastoma: A phase II study. Anticancer Res. 2013;33:3487–94. [PubMed] [Google Scholar]
- 36.Hottinger AF, Aissa AB, Espeli V, Squiban D, Dunkel N, Vargas MI, et al. Phase I study of sorafenib combined with radiation therapy and temozolomide as first-line treatment of high-grade glioma. Br J Cancer. 2014;110:2655–61. doi: 10.1038/bjc.2014.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faul MM, Gillig JR, Jirousek MR, Ballas LM, Schotten T, Kahl A, et al. Acyclic N-(azacycloalkyl)bisindolylmaleimides: Isozyme selective inhibitors of PKCbeta. Bioorg Med Chem Lett. 2003;13:1857–9. doi: 10.1016/s0960-894x(03)00286-5. [DOI] [PubMed] [Google Scholar]
- 38.Keyes KA, Mann L, Sherman M, Galbreath E, Schirtzinger L, Ballard D, et al. LY317615 decreases plasma VEGF levels in human tumor xenograft-bearing mice. Cancer Chemother Pharmacol. 2004;53:133–40. doi: 10.1007/s00280-003-0713-x. [DOI] [PubMed] [Google Scholar]
- 39.Teicher BA, Alvarez E, Menon K, Esterman MA, Considine E, Shih C, et al. Antiangiogenic effects of a protein kinase Cbeta-selective small molecule. Cancer Chemother Pharmacol. 2002;49:69–77. doi: 10.1007/s00280-001-0386-2. [DOI] [PubMed] [Google Scholar]
- 40.Fine HA, Kim L, Royce C, Draper I, Haggarty H, Ellinzano P, et al. Results from phase II trial of enzastaurin (LY317615) in patients with recurrent high grade gliomas. J Clin Oncol. 2005;23:1504. [Google Scholar]
- 41.Wick W, Steinbach JP, Platten M, Hartmann C, Wenz F, von Deimling A, et al. Enzastaurin before and concomitant with radiation therapy, followed by enzastaurin maintenance therapy, in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation. Neuro Oncol. 2013;15:1405–12. doi: 10.1093/neuonc/not100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butowski N, Chang SM, Lamborn KR, Polley MY, Pieper R, Costello JF, et al. Phase II and pharmacogenomics study of enzastaurin plus temozolomide during and following radiation therapy in patients with newly diagnosed glioblastoma multiforme and gliosarcoma. Neuro Oncol. 2011;13:1331–8. doi: 10.1093/neuonc/nor130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–56. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Manzano C, Holash J, Fueyo J, Xu J, Conrad CA, Aldape KD, et al. VEGF Trap induces antiglioma effect at different stages of disease. Neuro Oncol. 2008;10:940–5. doi: 10.1215/15228517-2008-061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Groot JF, Lamborn KR, Chang SM, Gilbert MR, Cloughesy TF, Aldape K, et al. Phase II study of aflibercept in recurrent malignant glioma: A North American Brain Tumor Consortium study. J Clin Oncol. 2011;29:2689–95. doi: 10.1200/JCO.2010.34.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hariharan S, Gustafson D, Holden S, McConkey D, Davis D, Morrow M, et al. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol. 2007;18:1400–7. doi: 10.1093/annonc/mdm140. [DOI] [PubMed] [Google Scholar]
- 48.Yamada S, Bu XY, Khankaldyyan V, Gonzales-Gomez I, McComb JG, Laug WE. Effect of the angiogenesis inhibitor Cilengitide (EMD 121974) on glioblastoma growth in nude mice. Neurosurgery. 2006;59:1304–12. doi: 10.1227/01.NEU.0000245622.70344.BE. [DOI] [PubMed] [Google Scholar]
- 49.Taga T, Suzuki A, Gonzalez-Gomez I, Gilles FH, Stins M, Shimada H, et al. alpha v-Integrin antagonist EMD 121974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int J Cancer. 2002;98:690–7. doi: 10.1002/ijc.10265. [DOI] [PubMed] [Google Scholar]
- 50.Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R, et al. Inhibition of alpha(v)beta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005;11:6270–9. doi: 10.1158/1078-0432.CCR-04-1223. [DOI] [PubMed] [Google Scholar]
- 51.Wick W, Wick A, Schulz JB, Dichgans J, Rodemann HP, Weller M. Prevention of irradiation-induced glioma cell invasion by temozolomide involves caspase 3 activity and cleavage of focal adhesion kinase. Cancer Res. 2002;62:1915–9. [PubMed] [Google Scholar]
- 52.Eskens FA, Dumez H, Hoekstra R, Perschl A, Brindley C, Böttcher S, et al. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer. 2003;39:917–26. doi: 10.1016/s0959-8049(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 53.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–7. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacDonald TJ, Stewart CF, Kocak M, Goldman S, Ellenbogen RG, Phillips P, et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric Brain Tumor Consortium Study PBTC-012. J Clin Oncol. 2008;26:919–24. doi: 10.1200/JCO.2007.14.1812. [DOI] [PubMed] [Google Scholar]
- 55.Scaringi C, Minniti G, Caporello P, Enrici RM. Integrin inhibitor cilengitide for the treatment of glioblastoma: A brief overview of current clinical results. Anticancer Res. 2012;32:4213–23. [PubMed] [Google Scholar]
- 56.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–7. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 57.Nabors LB, Mikkelsen T, Hegi ME, Ye X, Batchelor T, Lesser G, et al. A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306) Cancer. 2012;118:5601–7. doi: 10.1002/cncr.27585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–8. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 59.Nabors LB, Fink KL, Mikkelsen T, Grujicic D, Tarnawski R, Nam DH, et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-labl, controlled, randomized phase II CORE study. Neuro Oncol. 2015;17:708–17. doi: 10.1093/neuonc/nou356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cediranib Maleate and Cilengitide in Treating Patients with Progressive or Recurrent Glioblastoma – Full Text View. [Last accessed on 2016 Aug 14]. Available from: http://www.clinicaltrials.gov/show/NCT00979862 .
- 61.Cilengitide and Sunitinib Malate in Treating Patients with Advanced Solid Tumors or Glioblastoma Multiforme – Full Text View. [Last accessed on 2016 Aug 14]. Available from: http://www.clinicaltrials.gov/show/NCT01122888 .
- 62.Temozolomide and Procarbazine with Cilengitide for Patients with Glioblastoma Multiforme without Methylation of the MGMT Promoter Gene – Full Text View. [Last accessed on 2016 Aug 14]. Available from: http://www.clinicaltrials.gov/show/NCT01124240 .


