Abstract
Deep brain stimulation (DBS) is a new alternative treatment for treatment-resistant major depression (MD) and obsessive-compulsive disorder (OCD). Various DBS targets were defined for MD and OCD. Nucleus accumbens (NAcc) comes out among the other targets in patients with MD and comorbid OCD when physiopathology and limited side effects are taken into account. We report a 27-year-old male with MD and OCD who was treated by bilateral NAcc-DBS. The aim of this study is to discuss NAcc as a DBS target in patients with MD and OCD and to report the first case of a psychiatric disorder treated with DBS in Turkey.
Keywords: Deep brain stimulation, major depression, nucleus accumbens, obsessive-compulsive disorder
Introduction
Obsessive-compulsive disorder (OCD) and major depression (MD) are common and disabling psychiatric disorders.[1,2] Patients with MD or OCD may not response satisfactory to pharmacological, psychotherapeutic, or somatic treatments.[3] After nonresponse to two competent treatment steps, a patient is diagnosed with treatment-resistant depression, which is associated with illness chronicity, a reduced quality of life, and a higher risk for suicide.[4]
The majority of neurosurgical procedures to treat psychiatric illnesses were targeted to disconnect the frontal lobe from the basal ganglia or to intercept the anatomic pathways within the limbic system. In recent years, deep brain stimulation (DBS) has been intended in the treatment of MD and OCD. DBS aims to modulate dysfunctional neuronal networks involved in depression. Three targets were defined in a hypothesis-driven way for MD: nucleus accumbens (NAcc), subgenual cingulate cortex (Cg25), and the anterior limb of the capsula interna. Between supposed targets, NAcc is especially interesting because of its major involvement in the pathophysiology of both OCD and MD. Among three regions, DBS to the NAcc has supplied relieved antidepressant effects over up to 4 years.[5,6]
In this article, we present the case of a patient who was diagnosed as MD and OCD and was treated with NAcc-DBS. To the best of our knowledge, this is the first case of a psychiatric disorder treated with DBS in Turkey.
Case Report
Our patient was a 27-year-old male who had suffered from severe MD with mild anxiety and OCD since 2006. His symptoms were depressed mood, profound anhedonia, decreased sleep, decreased appetite, fatigue, significant psychomotor retardation, feelings of worthlessness and guilt, and poor concentration. He had attempted suicide three times. He has been hospitalized six times since he was 12 years old. He was administered electroconvulsive therapy four times. His psychiatric diagnosis was a severe depressive episode without psychotic symptoms, moderate generalized anxiety disorder, and moderate OCD according to Tenth Revision of the International Statistical Classification of Diseases and Related Health Problems, when he was referred to us. His Hamilton Depression Rating Scale (HDRS) score was 35 and Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) score was 20. He was under follow-up with venlafaxine 300 mg/day and psychotherapy. His MD was not controlled with various antidepressants, electroconvulsive therapy, transcranial magnetic stimulation, and cognitive behavioral therapy.
In August 2015, surgical treatment with DBS was performed. The surgical procedure was performed under local anesthesia and sedation using the Leksell (Elekta, Stockholm, Sweden) stereotactic frame with ring system. The surgical targets were bilateral NAcc. Their positions were localized anatomically by direct visualizing on T2-weighted and inversion recovery magnetic resonance (MR) imaging. FrameLink 2.0 software (Medtronic, Inc., Minneapolis, MN, USA) was used for the application of computed tomography-MR fusion technique and reformatted the MR image to the anterior commissure-posterior commissure (AC-PC) plane to yield the calculated coordinates for the NAcc, with the aid of Schaltenbrand and Wahren stereotactic atlas [Figure 1a–c]. Quadripolar electrodes (Model 3389, Medtronic, Inc.) were implanted in the bilateral NAcc in a single session. Only macrostimulation was performed without microelectrode recording (MER) for both NAcc. The patient had smiled and his breathing speed had accelerated during the macrostimulation of right NAcc. He told that he could not stop smiling. Although no side effects were seen during the macrostimulation of left NAcc, the patient told that he had a feeling and he was unable to define that it was not negative. The final stereotactic X, Y, Z coordinates were 87.7, 102.8, 103, respectively, for right NAcc and 106.2, 102.8, 100.6, respectively, for left NAcc. A dual-channel pulse generator (Kinetra Model 7428, Medtronic, Inc.) was connected to the leads and implanted subclavicularly in the right chest wall during the same surgical procedure. No complication occurred during or after the surgery. The activation of the electrodes took place 7 days after the surgery. The stimulation parameters were amplitude 2V, pulse width 90 ms, and frequency 130 Hz with permanent pulse-train square wave stimulation.
Figure 1.
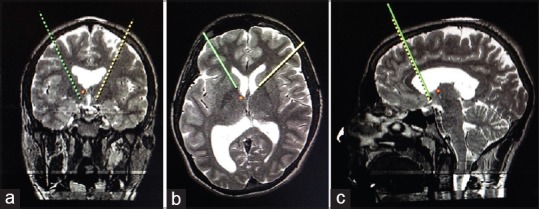
(a-c) Stereotactic targeting images of bilateral nucleus accumbens
In the 6-month follow-up after surgery, his psychological symptoms were improved. He was able to express his self-better. He had turned back to his old school and had a new girlfriend. He was still under antidepressant medication. There was reduction of the obsessions and compulsions. His HDRS score was 15 and Y-BOCS score was 8, 10 months after surgery.
Consent of the patient was taken to have his data published. This publication is approved by our Institution's Ethics Committee.
Discussion
MD is a common and potentially life-threatening disorder characterized by various symptoms, including depressed mood, anhedonia, fatigue, hopelessness, psychomotor retardation, anxiety, apathy, cognitive deficits, and suicidal tendency. Nearly 20% of patients with MD do not respond to pharmacotherapy and psychotherapy.[7]
DBS is a useful alternative treatment for treatment-resistant MD.[8] Different targets have been used in the treatment of behavior disorders, including the temporal lobe amygdala, hypothalamus, subcaudate white matter, NAcc, Cg25, and cingulate gyrus.[9] Among these targets, NAcc is an important intersection point in the pathophysiology of both MD and OCD.
It is hypothesized that the NAcc plays an important role in the abnormal reward process and motivations in MD.[10] NAcc takes projections from anterior cingulate cortex, agranular insular cortex, and orbitofrontal cortex. Since the center of NAcc receives input from all of these areas, the pod receives input mainly from subgenual and pregenual cingulate cortices. NAcc then projects to the dorsomedial nucleus of thalamus by the ventral tegmental area, ventral pallidum, and substantia nigra – which in turn projects back to the prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, amygdala, and hypothalamus; this figures the limbic ring of basal ganglia. The association of the NAcc with areas affected in depression makes it an appropriate target for DBS.[11] It is shown that NAcc-DBS modulates NAcc activity and frontostriatal connectivity, and thereby reverses disease related hyperactivity of the cortical–striatal–thalamo–cortico loop in OCD patients.[1] On the other hand, no serious adverse effects due to stimulation of NAcc was reported. In the guidance of these previous studies, we decided NAcc as our target for this patient with MD and OCD.
There is not a defined MER pattern for NAcc, so emotional and motor responses in macrostimulation are important for confirmation of the target as in our case. Smiling, palpitation, and acceleration of breathing speed were motor side effects of NAcc for this patient.
Conclusion
Various studies showed that DBS is a promising alternative nonablative treatment for MD and OCD. On the other hand, further research with larger groups of patients with MD and comorbid OCD are still needed to take place in standard treatment algorithms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kohl S, Gruendler TO, Huys D, Sildatke E, Dembek TA, Hellmich M, et al. Effects of deep brain stimulation on prepulse inhibition in obsessive-compulsive disorder. Transl Psychiatry. 2015;5:e675. doi: 10.1038/tp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 3.Bewernick B, Schlaepfer TE. Update on neuromodulation for treatment-resistant depression. F1000Res. 2015;4:pii:F1000 Rev-1389. doi: 10.12688/f1000research.6633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, et al. STAR*D: Revising conventional wisdom. CNS Drugs. 2009;23:627–47. doi: 10.2165/00023210-200923080-00001. [DOI] [PubMed] [Google Scholar]
- 5.Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: Evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–85. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aouizerate B, Cuny E, Bardinet E, Yelnik J, Martin-Guehl C, Rotge JY, et al. Distinct striatal targets in treating obsessive-compulsive disorder and major depression. J Neurosurg. 2009;111:775–9. doi: 10.3171/2009.2.JNS0881. [DOI] [PubMed] [Google Scholar]
- 7.Cleary DR, Ozpinar A, Raslan AM, Ko AL. Deep brain stimulation for psychiatric disorders: Where we are now. Neurosurg Focus. 2015;38:E2. doi: 10.3171/2015.3.FOCUS1546. [DOI] [PubMed] [Google Scholar]
- 8.Millet B, Jaafari N, Polosan M, Baup N, Giordana B, Haegelen C, et al. Limbic versus cognitive target for deep brain stimulation in treatment-resistant depression: Accumbens more promising than caudate. Eur Neuropsychopharmacol. 2014;24:1229–39. doi: 10.1016/j.euroneuro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez F, Nicolini H, Lozano AM, Piedimonte F, Salín R, Velasco F. Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg. 2013;80:S30.e17–25. doi: 10.1016/j.wneu.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Morishita T, Fayad SM, Higuchi MA, Nestor KA, Foote KD. Deep brain stimulation for treatment-resistant depression: Systematic review of clinical outcomes. Neurotherapeutics. 2014;11:475–84. doi: 10.1007/s13311-014-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauptman JS, DeSalles AA, Espinoza R, Sedrak M, Ishida W. Potential surgical targets for deep brain stimulation in treatment-resistant depression. Neurosurg Focus. 2008;25:E3. doi: 10.3171/FOC/2008/25/7/E3. [DOI] [PubMed] [Google Scholar]


