Abstract
Objective and Background:
Data on intraoperative neurophysiological monitoring (IOM) during surgery of spinal dural arteriovenous fistulas (SDAVFs) are lacking. The purpose of this study was to evaluate the role of IOM during microsurgery for SDAVFs.
Materials and Methods:
From March 2007 to March 2013, 12 patients had microsurgery with IOM for SDAVFs. The IOM included somatosensory-evoked potentials, motor-evoked potentials (MEPs), and – in selected cases – D-Waves. All patients were evaluated at admission and at follow-up (6, 12, and 24 months) with Aminoff–Logue Disability Scale for Gait-Aminoff–Logue Disability Scale (G-ALS) and Micturition-Aminoff–Logue Disability Scale (M-ALS).
Statistical Analysis Used:
Logistic regression was used for detecting the clinical risk factors influencing neurological functions after the treatment.
Results:
During surgery, we registered the absence of significant modifications of evoked potentials in nine cases (75%), while improvement of MEPs occurred in three cases (25%). No false-negative case was registered, and IOM predicted the absence of new postoperative neurological deficit in all patients. At 24-month follow-up, nine patients improved their overall neurological status, while three patients remained stable. At univariate analysis, Aminoff–Logue Disability Scales for Gait and Micturition (G + M-ALS) score at 24-month follow-up was directly associated with the duration of symptom before the surgery (P = 0.024), preoperative G-ALS (P = 0.02), M-ALS (P = 0.022), and G + M-ALS scores (P = 0.045), and improvement of IOM after occlusion of the fistula (P = 0.025).
Conclusions:
In our series, no significant worsening of evoked potentials occurred and subsequently the surgical strategy was not changed by IOM. However, no false-negative case was registered, and IOM predicted the absence of new postoperative neurological deficit in all patients. Patients with improvement of IOM parameters after occlusion of the fistula had greater chances of postsurgical improvement at the univariate analysis.
Keywords: D-Waves, intraoperative neurophysiological monitoring, motor-evoked potential, somatosensory-evoked potentials, spinal dural arteriovenous fistulas, surgery
Introduction
Spinal dural arteriovenous fistulas (SDAVFs) are rare but potentially reversible cause of progressive myelopathy.[1] SDAVFs constitute approximately 70% of spinal arteriovenous malformations and affect about 5–10 cases per million people annually.[2] SDAVFs are seen more frequently in males than females, with a ratio of 4:1; commonly these lesions occur in adults, with a peak incidence in the fourth and fifth decades of life.[3]
The lesion consists of an abnormal connection between a meningeal branch of a segmental artery and radiculomedullary vein (without an intervening nidus) within the dural sleeve of a nerve root. Its venous drainage is toward the perimedullary coronal venous plexus. The resulting venous hypertension has been considered to be responsible for progressive myelopathy.[4,5]
If not diagnosed and treated early on, SDAVFs can produce significant neurological impairment over time.[6] Aminoff and Logue reported the progression of disability from 19% at 6 months to 50% after 3 years, whereas the same group had 56% of patients with no restriction of activity at 6 months which had increased to 91% at 3 years.[7]
Surgical disconnection of SDAVFs is a straightforward procedure with a high success rate and virtually no risk of recurrence or incomplete treatment.[6,8,9,10,11,12,13,14,15,16,17,18,19,20] Besides, neurological deterioration after surgery was reported by several authors and constitutes still an unsolved problem.[20,21,22]
In this scenario, several authors reported the use of intraoperative neurophysiological monitoring (IOM) during surgery for SDAVFs to assess the functional integrity of sensory and motor pathways under general anesthesia. However, in the same series, the results and clinical relevance of these monitoring were not provided.[16,21,23]
The purpose of this study was to evaluate the role of IOM during surgery of SDAVFs both in terms of modification of the surgical strategy and in terms of predictor of functional outcome. Moreover, IOM results were compared with the other variables influencing the outcome.
Materials and Methods
Patient population
From March 2007 to March 2013, we performed IOM during 12 surgical procedures for SDAVF. Clinical and IOM data of these 12 patients with SDAVF were retrospectively collected in a database and analyzed.
Demographic and clinical data including age, gender, level of shunting, initial symptom, duration from initial symptom to validated diagnosis by digital subtraction angiography (DSA), pre- and post-operative neurological state classified according to Aminoff–Logue Disability Scales for Gait (G-ALS), and Aminoff–Logue Disability Scales for Micturition (M-ALS), and Aminoff–Logue Disability Scales for Gait and Micturition (G + M-ALS) [Table 1], preoperative neurophysiological evaluation, surgical technique, IOM findings during surgery, and complications were obtained and summarized in Table 2. All SDAVFs were initially detected by magnetic resonance imagining (MRI). Diagnosis of SDAVF was verified in all cases using conventional spinal DSA.
Table 1.
Aminoff–Logue Disability Scale for Gait and Aminoff–Logue Disability Scale for Micturition
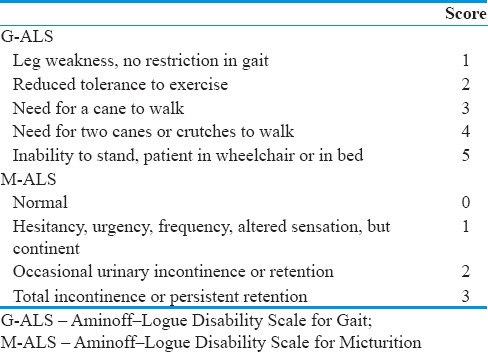
Table 2.
Demographic and clinical data including age, gender, level of shunting, initial symptom, duration, pre- and post-operative neurological state classified according to the Aminoff–Logue Disability Scale for Gait, Aminoff–Logue Disability Scale for Micturition, and Aminoff–Logue Disability Scale for Gait and Micturition, preoperative neurophysiological evaluation, surgical technique, intraoperative neurophysiological findings during surgery, and complications
All patients granted their permission for this study before surgery. The risk to participants is minimal. The research data analysis has no effect on the participants and their medical care.
Intraoperative neurophysiological monitoring
Our standardized protocol for IOM has been described in detail elsewhere[24] and include: pre-, intra-, and post-operative somatosensory-evoked potentials (SEPs) and motor-evoked potentials (MEPs), intraoperative D-Waves (in cervical and thoracic lesions), free-running electromyography (fEMG), and bulbocavernosus reflex for cauda or filum terminale procedures. For stimulation and recording, the ISIS system was used (Inomed Co., Emmendingen, Germany). During surgery, IOM was subdivided into postinduction baseline, intraoperative period, and closure. A brief description of our protocol is as follows.
Somatosensory-evoked potentials
SEPs were elicited by stimulation of the median nerve at the wrist and the posterior tibial nerve at the ankle (intensity, 40 mA; duration, 0.2 ms; repetition rate, 4.3 Hz). Recordings were ensured through corkscrew (CS)-like electrodes inserted in the scalp at CZ/-FZ (legs) and C3/C4/-FZ (arms), according to the International 10–20 system of electrode placement.
Motor-evoked potentials and D-Wave
As described previously in literature,[25] transcranial electrical stimulation (TES) with multipulse technique was used to elicit muscle MEPs and a single TES stimulus was applied to elicit a D-Wave. TES with multipulse technique includes short trains of five square wave stimuli (single pulse duration, 0.5 ms; interstimulus interval, 4 ms; at a rate of 2 Hz) through CS electrodes placed at C1/C2 (lower limbs) and C3/C4 (upper limbs) scalp sites, according to the 10–20 system. A constant current stimulator with a maximum output of 200 mA was applied. MEPs were recorded through needle electrodes inserted into the upper and lower extremity muscles. We usually monitor muscle MEPs from the abductor pollicis brevis and the extensor digitorum longus for superior limbs and the vastus lateralis, tibialis anterior, and the abductor hallucis for inferior limbs.
D-Wave was monitored in patients harboring vascular lesions in the cervical and thoracic spine. A single TES stimulus of 0.5 ms duration was applied to elicit a D-Wave, recorded by an electrode placed in the epidural or subdural space cranial and caudal to the fistula, after laminectomy or laminotomy. The electrode cranial to the fistula serves as a control recording, to discriminate whether or not an intraoperative neurophysiological event is related to surgical maneuvers or general influences such as anesthesia or cardiovascular factors. The electrode used in this study is FSR-03 (Inomed Co., Emmendingen, Germany). This electrode in platino-iridio has three cylinders for registration of 3 mm in length, 1.3 mm in diameter, and 18 mm in length, with recording surfaces of about 12.3 mm2.
Free-running electromyography
Monitoring fEMG during SDAVFs surgery is potentially useful because its high-frequency discharges are likely to be associated with injury. Compression or stretch of a nerve as well as hypothermia and ischemia can produce depolarization of the axons resulting in the appearance of spontaneous action potentials. These action potentials subsequently produce contractions of muscle fibers that can be recorded by electrodes placed in the muscle. Potential irritation to and/or compression of nerve roots can be monitored using fEMG.
fEMG events can result from transient traction on the corticospinal and other descending motor tracts, from vascular compromise to the cord during fistula occlusion or direct root compression during placement of clips.
Surgical procedure and interpretation of intraoperative neurophysiological monitoring
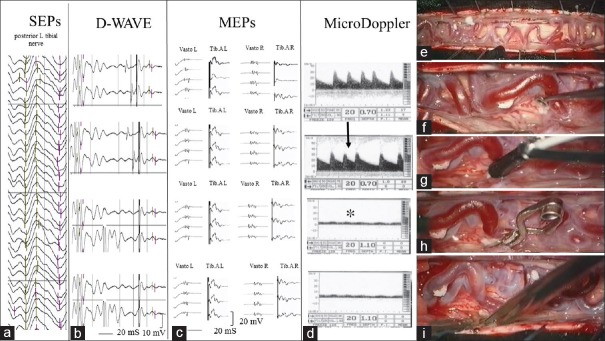
All surgical procedures were performed under permanent control of SEPs and MEPs and whenever possible D-Wave [Figure 1a–i].
Figure 1.
(a) Intraoperative left inferior limb somatosensory-evoked potential monitoring stable during all surgical steps. (b and c) Intraoperative lower limbs motor-evoked potential (VastL.: Left vastus lateralis; T.A.L.: Left tibialis anterior, VastR.: Right vastus lateralis; T.A.R.: Right tibialis anterior) and D-Wave monitoring results stable during all surgical steps. (d) Microvascular Doppler findings. After dura opening, intraoperative micro-Doppler monitoring detected an arterialized, high resistance, and pulsatile flow on the redundant dorsal perimedullary vein. Then, once identified the intradural draining vein, intraoperative micro-Doppler monitoring confirmed the location of radicular fistulous link, characterized by high flow velocity (black arrow). Finally, after temporary clipping of dural arteriovenous fistula draining vein, a nonpulsatile flow with low resistance was registered at the perimedullary veins, referable to a normal venous pattern (asterisk). (e-i) Intraoperative images of the various surgical steps. (f) Following laminectomy (or hemilaminectomy), the dura was opened in a standard longitudinal fashion, and the congested perimedullary plexus was clearly seen. (e) The intradural arterialized vein was identified near the nerve root where the former goes out through the dura. (g) Intraoperative micro-Doppler monitoring confirmed the location of radicular fistulous link. (h) Temporary clipping of dural arteriovenous fistula draining vein. (i) If no loss or substantial reduction of evoked potentials occurred, the intradural draining vein was cauterized with bipolar forceps and sharply divided
Tailored hemilaminectomy, laminoplasty, or laminectomy was performed according to spinal anatomy and the location of the fistula point and extended to the side of the SDAVF. Following laminectomy (or hemilaminectomy/laminoplasty), the dura was opened in a standard longitudinal fashion, and the congested perimedullary plexus was clearly seen [Figure 1e]. Then, the intradural arterialized vein was identified near the nerve root where the former goes out through the dura [Figure 1f]; obviously, preoperative MRI and DSA were often checked to match radiological and anatomical findings [Figure 2a–c]. At this step, the micro-Doppler was useful to confirm the arterialized intradural draining vein and the radicular fistulous link, characterized by a high-flow velocity [Figure 1d and g].[26]
Figure 2.
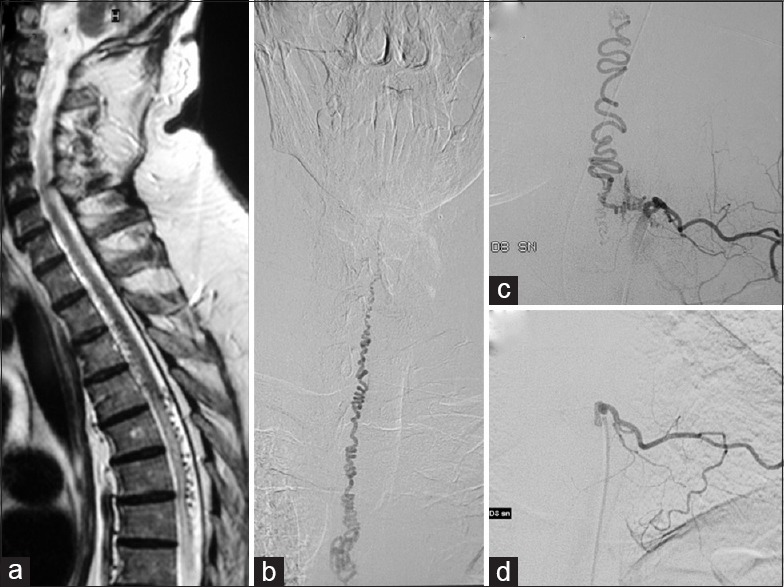
(a) Preoperative sagittal T2-weighted magnetic resonance image demonstrating high intramedullary signal intensity, associated with subarachnoid serpiginous flow voids hinting at the presence of an spinal dural arteriovenous fistula. (b and c) Preoperative selective spinal angiography revealing a dural arteriovenous fistula at the T8–T9 level, supplied by a left T8 radicular artery, as well as a tortuous and enlarged venous plexus, developing upward to the thoracic and cervical regions. (d) At 1-year postoperative follow-up, angiogram of the left T8 intercostal artery demonstrating complete disappearance of dural arteriovenous fistula
Then, sharp dissection of the vein was carried out. When the entire vein was mobilized, a temporary aneurysm clip was placed on the fistulous point under IOM [Figure 1h]. The temporary clipping was maintained at least for 20 min to obtain a stable IOM evaluation of sensory and motor pathways after the closure of pathological microcirculation of the spinal cord [Figure 1a–c]. At this moment, the micro-Doppler was again used to check the reappearance of a nonpulsatile flow with low resistances at the perimedullary veins, referable to a normal venous pattern [Figure 1d].
If no loss or substantial reduction of evoked potentials occurred, the intradural draining vein was cauterized with bipolar forceps and sharply divided [Figure 1a–c and i]. Next, inspection and cauterization of the inner dural layer was usually the final step. The epidural feeding artery was also coagulated if possible.
On the basis of data published in literature,[24,25] the following IOM criteria were used to adjust the surgical strategy:
A persistent amplitude loss of at least 50% of cortical SEPs was used as warning criteria
A persistent MEPs’ loss was considered significant; however, the surgeon was warned if there were persistent amplitude decrements of more than 50% of baseline values
A decrease of more than 50% of the baseline amplitude for D-Wave was considered a warning criterion
Moreover, during surgery, a significant improvement of MEPs included a persistent MEPs and/or D-Wave amplitude increments of more than 30% of baseline values.
Follow-up
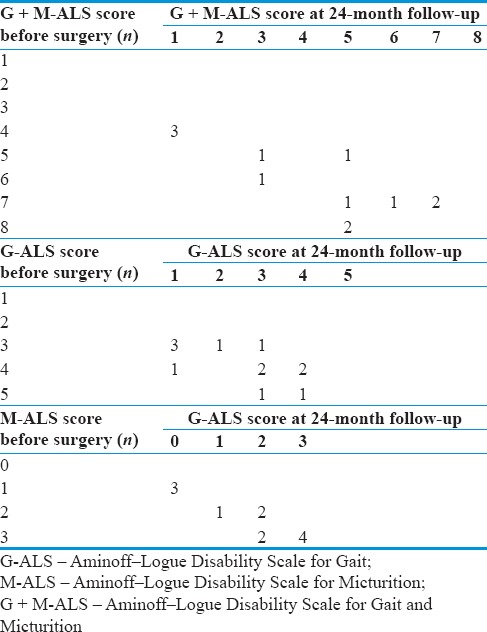
All patients received a clinical follow-up examination at the neurosurgical outpatient department 6, 12, and 24 months after therapy. Motor, sphincter, and motor + sphincter impairments immediately before fistula occlusion, at 6, 12, and 24-month follow-up and at last follow-up were classified according to the G + M-ALS [Table 3].
Table 3.
Change in Aminoff–Logue Disability Scale class of disability for Gait, Aminoff–Logue Disability Scale for Micturition, and Aminoff–Logue Disability Scale for Gait and Micturition between presentation and 24-month follow-up
At our institution, the first six treated patients had undergone DSA after the intervention for confirmation of fistula disconnection [Figure 2d]. In the last six patients, follow-up imaging included a MRI examination 3, 6, 12, and 24 months after surgery [Figure 3d]. In these cases, angiographic confirmation was planned in which broad magnetic resonance (MR) T2-weighted intensity changes and the abnormal venous dilatation were not improved 3 months after the intervention. All the six patients showed improvement in broad MR T2-weighted intensity changes. The monitorability and the sensitivity and specificity of the IOM were also evaluated.
Figure 3.
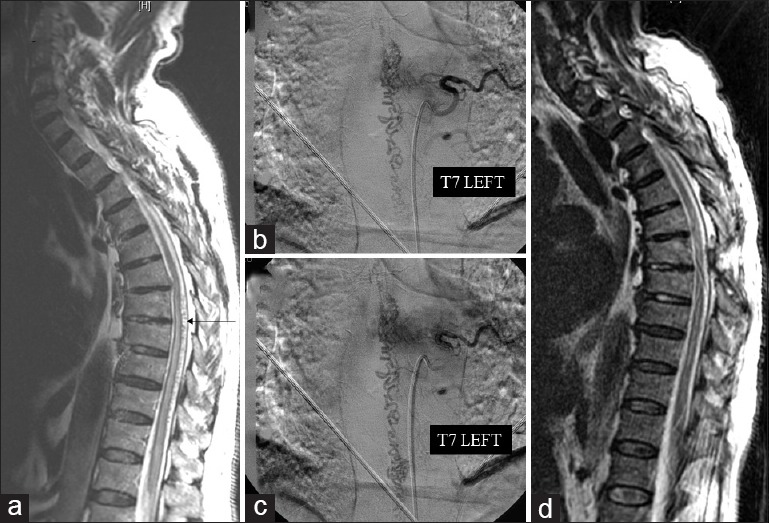
(a) Preoperative sagittal T2-weighted magnetic resonance image showing high intramedullary signal intensity, associated with subarachnoid serpiginous flow voids. (b and c) Preoperative selective spinal angiography revealing a dural arteriovenous fistula supplied by a left T7 radicular artery, as well as a tortuous and enlarged venous plexus, developing downward to the thoracic region. (d) Postoperative sagittal T2-weighted magnetic resonance image showing disappearance of abnormal venous dilatation and improvement of high intramedullary signal intensity
Finally, we performed postoperative and 6-month follow-up neurophysiological examinations with SEP, MEP, and electromyography for all patients.
Statistical analysis
Data were summarized using descriptive statistics, including frequencies and percentages for categorical data, and means ± standard deviations (SDs) for continuous data. Logistic regression was used for detecting the clinical risk factors influencing neurological functions after the treatment. SAS system (SAS Inc., Cary, NC, USA) was used for all analyses. P < 0.05 was considered statistically significant.
Results
There were a total of 12 patients (10 males and 2 females), with a mean age of 63.91 years (range: 51–79 years, SD ± 8.72) from 2007 to 2013 at our department. The level of SDAVFs was thoracic in eight patients and lumbar in four patients (all patients presented one feeder). In terms of initial symptom, paralysis including bladder bowel dysfunction was the first symptom in ten cases whereas sensory disorder was the initial symptom in two cases. The mean duration from initial symptom to validated diagnosis was 16.33 months (range: 7–37 months, SD ± 9.32).
Preoperative MRI signal abnormality was present in all patients except one with a mean extension of 6.83 levels (range: 2–12, SD ± 3.66).
Average Aminoff–Logue Disability Score for Gait (G-ALS) at presentation was 3.75 ± 0.73, average Aminoff–Logue Disability Score for Micturition (M-ALS) score was 2.25 ± 0.89, and G + M-ALS was 6.00 ± 1.53.
Preoperative neurophysiological assessment including SEPs and MEPs was pathological in all cases.
Monitorable D-Waves was achieved in all thoracic SDAVFs, except in one patient with severe neurological deficits before surgery (patient (n = 1) with G + M-ALS = 8). SEP and MEP monitoring, at least unilaterally, could be performed in all patients. The overall monitorability was 95.58%, where at least two of the three modalities were applicable in all the 12 surgical procedures.
The surgical procedure with IOM and micro-Doppler described above resulted in complete occlusion of the fistula in all the 12 patients, as showed by postoperative and follow-up imaging (DSA in six patients and serial follow-up spinal MRI with disappearance of abnormal venous dilatation and improvement of intramedullary edema).
Two different IOM patterns were observed during surgery: absence of modifications of evoked potentials (nine cases) and improvement of at least one neurophysiological parameter (three cases). No patient presented during surgery, and in particular during the temporary clipping and the final disconnection of the fistulous link, a decrease and/or significant loss of evoked potentials. Among patients with improvement of IOM parameters after temporary clipping of intradural draining vein, we registered the improvement of bilateral lower limb MEPs alone in one case, simultaneous improvement of bilateral lower limb MEPs and D-Wave in another case, and finally bilateral lower limb MEPs and SEPs in the last cases. These neurophysiological data correlated with immediate postoperative improvement of motor function.
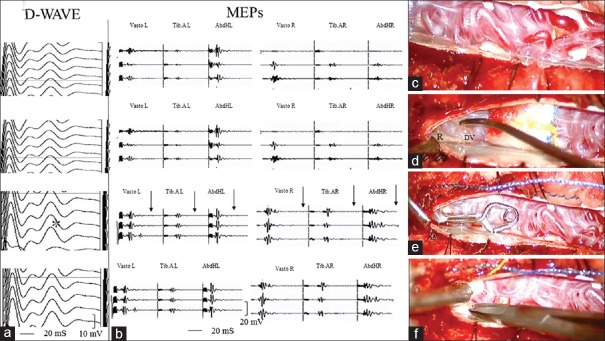
For example, the second patient among those with improvement of IOM presented a SDAVF supplied by a left T7 radicular artery [Figure 3a–d]. During temporary clipping of intradural draining vein (for about 20 min), we observed a significant improvement in the amplitude of bilateral lower limb MEPs and D-Wave [Figure 4a–f]. These neurophysiological data were confirmed by postoperative clinical improvement (preoperative G-ALS 3 vs. postoperative G-ALS 1).
Figure 4.
(a and b) Intraoperative lower limb motor-evoked potentials (VastL.: Left vastus lateralis; T.A.L.: Left tibialis anterior; AbdhL: Left abductor halluces; VastR.: Right vastus lateralis; T.A.R.: Right tibialis anterior; AbdhR: Right abductor hallucis) and D-Wave monitoring results showed, after temporary clipping of intradural draining vein (for about 20 min), a significant improvement in the amplitude of bilateral lower limb motor-evoked potentials and D-Wave. (c-f) Intraoperative images of the various surgical steps. (c) The congested perimedullary plexus. (d) The intradural arterialized vein (DV) was identified near the nerve root (R) where the former goes out through the dura. (e) Temporary clipping of dural arteriovenous fistula draining vein. (f) Permanent occlusion of the fistula by means of coagulation, and division was performed if potentials remained stable and the draining vein was no longer arterialized
On the basis of IOM results, in neither patient, the surgical strategy was modified. However, no false-negative case was registered, and IOM predicted the absence of postoperative neurological worsening in all cases.
Regarding postoperative complications in our series, we observed one case of cerebrospinal fluid leakage and one case of wound infection treated both conservatively. In our series, we did not observe any postoperative instability of the spine and we had no postoperative mortality.
At 24-month follow-up, nine patients improved their overall neurological status assessed with G + M-ALS, while three patients remained stable [Table 3]. The mean score of G + M-ALS before surgery was 6.00 ± 1.53, whereas after surgery there was a statistically significant improvement of 1.75 ± 1.12 (P = 0.0003). At this follow-up, a significant improvement of average G-ALS score to 2.5 ± 1.24 compared to the preoperative G-ALS was observed (P = 0.0005). At the same follow-up, average M-ALS score improved to 1.75 ± 1.16 (P = 0.0034).
A long-term neurological follow-up was also available in all patients with a mean follow-up latency of 49.66 ± 18.10 months (range: 25–82 months). At this follow-up, the G-ALS, M-ALS, and G + M-ALS scores were stable for each patient compared to the values of 24-month follow-up.
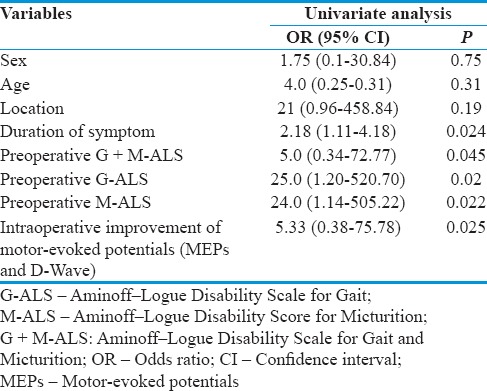
We conducted a univariate analysis using a logistic regression model, in which age, sex, the level of SDAVFs, duration of symptom, preoperative G-ALS, M-ALS, and G + M-ALS were correlated to the final outcome (G + M-ALS 24 months after treatment) for identifying the factors that influence the neurological outcome.
The G + M-ALS score at 24-month follow-up was directly associated with the duration of symptom before the surgery (P = 0.024), preoperative G-ALS (P = 0.02), M-ALS (P = 0.022), and G + M-ALS scores (P = 0.045). Patients with improvement of IOM parameters after temporary and final occlusion of the fistula have greater chances of postsurgical improvement (P = 0.025). Age, sex, and location of the fistula were not associated with functional outcome in our cohort [Table 4]. The same associations with very similar P values were found when using G-ALS score—rather than the composite G + M-ALS—as an outcome measure.
Table 4.
Results of univariate analysis using a logistic regression model
Discussion
This retrospective study was performed to evaluate the role of IOM during SDAVFs surgery both in terms of modification of the surgical strategy and in terms of predictors of functional outcome. Moreover, the IOM results were compared with the other variables influencing the outcome.
The success of the SDAVFs treatment, whether embolization or surgery, is closely related to the interruption of the flow within the dural fistula and the reappearance of normal venous flow at the level of the perimedullary coronal plexus. In the recent years, endovascular treatment has emerged as a safe and effective alternative for the treatment of SDAVF.[12,13,14,15] However, not all fistulas are amenable to endovascular therapy (which may be precluded by arterial feeders too small to catheterize or a common origin of the artery of Adamkiewicz, or not uncommonly a small posterior spinal artery, from the same segmental artery as the feeder), and long-term shunt occlusion rates may not be as high as those obtained with surgery.[16,17] A recent meta-analysis of the recurrence rates of SDAVFs after surgical and endovascular treatment (reported since 2004) found occlusion rates of 96.6% and 72.2% for surgically and endovascularly treated fistulas, respectively.[18] In fact, surgical disconnection of SDAVFs is a straightforward procedure with a high success rate and virtually no risk of recurrence or incomplete treatment. For this reason, many authors state a preference for surgical treatment since it is easy, safe, and effective to permanently exclude the fistula microneurosurgically.[6,19,20]
Besides, “unexplainable” neurological deterioration after surgery was reported by several authors and constitutes an unsolved problem.[20,21,22]
In a large series of 154 consecutive patients treated with surgery as the sole or primary treatment modality over a 23-year period published by Saladino et al.,[20] an unexplainable neurological deterioration has been described in 11 (7.1%) patients. A possible reason for this worsening postulated by the authors is that patients with an SDAVF are hemodynamically fragile and the balance between normal vascularization/drainage and ischemia/edema can be negatively affected by any increase in intra-abdominal pressure (such as during surgery in the prone position), which may further increase resistance to venous drainage.[27] In this series, intraoperative electrophysiological monitoring was not routinely used.
More recently, Özkan et al.[21] reported an interdisciplinary neurosurgical/neuroradiological management strategy of SDAVFs in 32 patients who were evaluated retrospectively. Clinical outcome was good in general in this series, with 30/32 patients improved and only 2 (6.3%) patients worsened neurologically after surgery without further improvement. Both patients had already deteriorated in the early postoperative period despite regular results of postoperative MRI and spinal DSA. These authors suggested another possible explanation for the early neurological deterioration. They postulated that the occurrence of partial thrombosis of the venous component may cause acute onset of spinal cord ischemia. Indirect support for this theory stems from endovascular series in patients who always receive effective anticoagulation with heparin after embolization and in those where such early neurological deterioration was not observed.[28] Really interesting is the fact that in this series all surgeries were performed under permanent control of SEPs and MEPs and that the authors did not report the results of IOM during surgery in these two patients.
Another two authors reported the use of IOM during surgery for SDAVFs, respectively, in seven patients[23] and thirty patients.[16] In the first series, the authors adopted SEPs and MEPs for avoiding an ischemic spinal cord disorder under general anesthesia, while in the second series, only SEPs was used. Both the authors did not report the results of evoked potentials during surgery.
In our series of 12 patients, all microsurgical obliterations of SDAVFs were performed under multimodal IOM, including SEPs, MEPs, and D-Wave. To the best of our knowledge, none of the previous series on the surgical treatment of SDAVFs reported the use of D-Wave. The overall monitorability was 95.58%, where at least two of the three modalities were applicable in all the 12 surgical procedures. Monitorable D-Waves was achieved in all thoracic SDAVFs, except in one patient with severe neurological deficits before surgery (patient (n = 1) with G + M-ALS = 8). SEP and MEP monitoring, at least unilaterally, could be performed in all patients.
Two different IOM patterns were observed during surgery: absence of modifications of evoked potentials (three cases) and improvement of at least one neurophysiological parameter (three cases). No patients presented during surgery, and in particular during the temporary clipping and the final disconnection of the fistulous link, a decrease and/or significant loss of evoked potentials.
On the basis of IOM results, in neither patient, the surgical strategy was modified. However, no false-negative case was registered, and IOM predicted the absence of postoperative neurological worsening in all cases. In fact, at 24-month follow-up, nine patients improved their overall neurological status assessed with G + M-ALS, while three patients remained stable. A long-term neurological follow-up was also available in all patients with a mean follow-up latency of 49.66 ± 18.10 months (range: 25–82 months). At this follow-up, the G-ALS, M-ALS, and G + M-ALS scores were stable for each patient compared to the values of 24-month follow-up.
In this type of surgery, characterized by a rate of neurological decline of about 6% (unpredictable before and during surgery), the IOM offers a unique opportunity for the investigation of hemodynamic patterns in the spinal cord and the subsequent rearrangement of the vascular flow caused by temporary and then final occlusion of fistula.[29] Then, the use of IOM can be considered an important tool to predict the neurological outcome.
Moreover, we compared the IOM results with the other variables influencing the outcome. In agreement with most of the studies in the literature,[20,23,26] in our series, duration of symptomatology before treatment (P = 0.024), higher preoperative G-ALS (P = 0.02), M-ALS (P = 0.022), and G + M ALS scores (P = 0.045) were the major factors determining the outcome. However, as reported by Muralidharan et al.,[30] although worse preoperative G + M-ALS scores were associated with worse long-term functional outcome, patients with more severe deficits before surgery also had greater chances of improvement after the intervention. In fact, among six patients with 8 or 7 G + M-ALS scores, 4 (66.6%) improved after surgery.
Only Özkan et al.[21] did not observe an influence of duration of symptomatology before treatment with late outcome.
In our series, patients with improvement of MEPs after temporary and final occlusion of the fistula had greater chances of postsurgical improvement (P = 0.025). The observation of MEPs and D-Wave improvement after disconnection of SDAVFs is not previously reported, and the prognostic role of MEPs during these procedures should be supported by a large series and a longer follow-up. Nevertheless, the prognostic role of MEPs during spinal cord surgery has been well documented and we might expect a similar correlation for SDAVFs.[24,31]
Conclusions
In our series, no significant worsening of evoked potentials occurred and subsequently the surgical strategy was not changed by IOM. However, no false-negative case was registered, and IOM predicted the absence of new postoperative neurological deficit in all patients. Patients with improvement of IOM parameters after temporary and final occlusion of the fistula had greater chances of postsurgical improvement at the univariate analysis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fugate JE, Lanzino G, Rabinstein AA. Clinical presentation and prognostic factors of spinal dural arteriovenous fistulas: An overview. Neurosurg Focus. 2012;32:E17. doi: 10.3171/2012.1.FOCUS11376. [DOI] [PubMed] [Google Scholar]
- 2.Thron A. Spinal dural arteriovenous fistulas. Radiologe. 2001;41:955–60. doi: 10.1007/s001170170031. [DOI] [PubMed] [Google Scholar]
- 3.Aminoff MJ, Logue V. Clinical features of spinal vascular malformations. Brain. 1974;97:197–210. doi: 10.1093/brain/97.1.197. [DOI] [PubMed] [Google Scholar]
- 4.Takai K, Komori T, Taniguchi M. Microvascular anatomy of spinal dural arteriovenous fistulas: Arteriovenous connections and their relationships with the dura mater. J Neurosurg Spine. 2015;23:526–33. doi: 10.3171/2014.11.SPINE14786. [DOI] [PubMed] [Google Scholar]
- 5.Narvid J, Hetts SW, Larsen D, Neuhaus J, Singh TP, McSwain H, et al. Spinal dural arteriovenous fistulae: Clinical features and long-term results. Neurosurgery. 2008;62:159–66. doi: 10.1227/01.NEU.0000311073.71733.C4. [DOI] [PubMed] [Google Scholar]
- 6.Tacconi L, Lopez Izquierdo BC, Symon L. Outcome and prognostic factors in the surgical treatment of spinal dural arteriovenous fistulas. A long-term study. Br J Neurosurg. 1997;11:298–305. doi: 10.1080/02688699746078. [DOI] [PubMed] [Google Scholar]
- 7.Aminoff MJ, Logue V. The prognosis of patients with spinal vascular malformations. Brain. 1974;97:211–8. doi: 10.1093/brain/97.1.211. [DOI] [PubMed] [Google Scholar]
- 8.McCutcheon IE, Doppman JL, Oldfield EH. Microvascular anatomy of dural arteriovenous abnormalities of the spine: A microangiographic study. J Neurosurg. 1996;84:215–20. doi: 10.3171/jns.1996.84.2.0215. [DOI] [PubMed] [Google Scholar]
- 9.Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage. Case report and review of the literature. J Neurosurg. 2004;100(4 Suppl):385–91. doi: 10.3171/spi.2004.100.4.0385. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto T, Yoshida S, Basugi N. Dural arteriovenous malformation in the cervical spine presenting with subarachnoid hemorrhage: Case report. Neurosurgery. 1992;31:118–20. doi: 10.1227/00006123-199207000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Afshar JK, Doppman JL, Oldfield EH. Surgical interruption of intradural draining vein as curative treatment of spinal dural arteriovenous fistulas. J Neurosurg. 1995;82:196–200. doi: 10.3171/jns.1995.82.2.0196. [DOI] [PubMed] [Google Scholar]
- 12.Nichols DA, Rufenacht DA, Jack CR, Jr, Forbes GS. Embolization of spinal dural arteriovenous fistula with polyvinyl alcohol particles: Experience in 14 patients. AJNR Am J Neuroradiol. 1992;13:933–40. [PMC free article] [PubMed] [Google Scholar]
- 13.Niimi Y, Berenstein A, Setton A, Neophytides A. Embolization of spinal dural arteriovenous fistulae: Results and follow-up. Neurosurgery. 1997;40:675–82. doi: 10.1097/00006123-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Song JK, Vinuela F, Gobin YP, Duckwiler GR, Murayama Y, Kureshi I, et al. Surgical and endovascular treatment of spinal dural arteriovenous fistulas: Long-term disability assessment and prognostic factors. J Neurosurg. 2001;94(2 Suppl):199–204. doi: 10.3171/spi.2001.94.2.0199. [DOI] [PubMed] [Google Scholar]
- 15.Guillevin R, Vallee JN, Cormier E, Lo D, Dormont D, Chiras J. N-butyl 2-cyanoacrylate embolization of spinal dural arteriovenous fistulae: CT evaluation, technical features, and outcome prognosis in 26 cases. AJNR Am J Neuroradiol. 2005;26:929–35. [PMC free article] [PubMed] [Google Scholar]
- 16.Chibbaro S, Gory B, Marsella M, Tigan L, Herbrecht A, Orabi M, et al. Surgical management of spinal dural arteriovenous fistulas. J Clin Neurosci. 2015;22:180–3. doi: 10.1016/j.jocn.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Steinmetz MP, Chow MM, Krishnaney AA, Andrews-Hinders D, Benzel EC, Masaryk TJ, et al. Outcome after the treatment of spinal dural arteriovenous fistulae: A contemporary single-institution series and meta-analysis. Neurosurgery. 2004;55:77–87. doi: 10.1227/01.neu.0000126878.95006.0f. [DOI] [PubMed] [Google Scholar]
- 18.Bakker NA, Uyttenboogaart M, Luijckx GJ, Eshghi OS, Mazuri A, Metzemaekers JD, et al. Recurrence rates after surgical or endovascular treatment of spinal dural arteriovenous fistulas: A meta-analysis. Neurosurgery. 2015;77:137–44. doi: 10.1227/NEU.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 19.Symon L, Kuyama H, Kendall B. Dural arteriovenous malformations of the spine. Clinical features and surgical results in 55 cases. J Neurosurg. 1984;60:238–47. doi: 10.3171/jns.1984.60.2.0238. [DOI] [PubMed] [Google Scholar]
- 20.Saladino A, Atkinson JL, Rabinstein AA, Piepgras DG, Marsh WR, Krauss WE, et al. Surgical treatment of spinal dural arteriovenous fistulae: A consecutive series of 154 patients. Neurosurgery. 2010;67:1350–7. doi: 10.1227/NEU.0b013e3181ef2821. [DOI] [PubMed] [Google Scholar]
- 21.Özkan N, Kreitschmann-Andermahr I, Goerike SL, Wrede KH, Kleist B, Stein KP, et al. Single center experience with treatment of spinal dural arteriovenous fistulas. Neurosurg Rev. 2015;38:683–92. doi: 10.1007/s10143-015-0645-z. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki O, Yajima N, Ichikawa A, Yamashita S, Nakamura K. Deterioration after surgical treatment of spinal dural arteriovenous fistula associated with spinal perimedullary fistula. Neurol Med Chir (Tokyo) 2012;52:516–20. doi: 10.2176/nmc.52.516. [DOI] [PubMed] [Google Scholar]
- 23.Wakao N, Imagama S, Ito Z, Ando K, Hirano K, Tauchi R, et al. Clinical outcome of treatments for spinal dural arteriovenous fistulas: Results of multivariate analysis and review of the literature. Spine (Phila Pa 1976) 2012;37:482–8. doi: 10.1097/BRS.0b013e31822670df. [DOI] [PubMed] [Google Scholar]
- 24.Ghadirpour R, Nasi D, Iaccarino C, Giraldi D, Sabadini R, Motti L, et al. Intraoperative neurophysiological monitoring for intradural extramedullary tumors: Why not? Clin Neurol Neurosurg. 2015;130:140–9. doi: 10.1016/j.clineuro.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: A review focus on the corticospinal tracts. Clin Neurophysiol. 2008;119:248–64. doi: 10.1016/j.clinph.2007.09.135. [DOI] [PubMed] [Google Scholar]
- 26.Padovani R, Farneti M, Maida G, Ghadirpour R. Spinal dural arteriovenous fistulas: The use of intraoperative microvascular Doppler monitoring. Br J Neurosurg. 2003;17:519–24. doi: 10.1080/02688690310001627740. [DOI] [PubMed] [Google Scholar]
- 27.Hassler W, Thron A, Grote EH. Hemodynamics of spinal dural arteriovenous fistulas. An intraoperative study. J Neurosurg. 1989;70:360–70. doi: 10.3171/jns.1989.70.3.0360. [DOI] [PubMed] [Google Scholar]
- 28.Kirsch M, Berg-Dammer E, Musahl C, Bäzner H, Kühne D, Henkes H. Endovascular management of spinal dural arteriovenous fistulas in 78 patients. Neuroradiology. 2013;55:337–43. doi: 10.1007/s00234-013-1134-0. [DOI] [PubMed] [Google Scholar]
- 29.Sala F, Beltramello A, Gerosa M. Neuroprotective role of neurophysiological monitoring during endovascular procedures in the brain and spinal cord. Neurophysiol Clin. 2007;37:415–21. doi: 10.1016/j.neucli.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Muralidharan R, Mandrekar J, Lanzino G, Atkinson JL, Rabinstein AA. Prognostic value of clinical and radiological signs in the postoperative outcome of spinal dural arteriovenous fistula. Spine (Phila Pa 1976) 2013;38:1188–93. doi: 10.1097/BRS.0b013e31828b2e10. [DOI] [PubMed] [Google Scholar]
- 31.Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: A historical control study. Neurosurgery. 2006;58:1129–43. doi: 10.1227/01.NEU.0000215948.97195.58. [DOI] [PubMed] [Google Scholar]