Abstract
Hypomagnesemia is postulated as one of the important determinants of outcome following traumatic brain injury (TBI) through its effect on secondary injuries to neurons.
Aims and Objective:
The aim of this study was to determine the relationship between serum magnesium level and neurological outcome in patients admitted with severe head injury.
Materials and Methods:
In this prospective study, patients admitted with severe TBI were recruited and dichotomized into low serum magnesium group and normal serum magnesium group based on the initial serum magnesium level. Data were collected regarding age, sex, and Glasgow Coma Scale at admission. Neurological outcome of the patients in these groups was assessed using Glasgow Outcome Scale at 6 months.
Results:
Seventy-two patients (male = 50, female = 22) with a mean (±standard deviation) age of 42.5 (±12.7) years were studied. Forty-two (58%) patients had low serum magnesium level (<1.3 mEq/L) at admissions. At 6-month follow-up, 81% of patients with poor neurological outcome had low serum magnesium as compared to 19% of patients with good outcome (P = 0.01). Hypomagnesemia was associated with poor neurological outcome (odds ratio = 2.1, P = 0.04, 95% confidence interval = 1.0–8.8) on regression analysis.
Conclusion:
Hypomagnesemia appears to be an independent prognostic marker in patients with severe TBI.
Keywords: Glasgow Outcome Scale, head injury, serum magnesium
Introduction
Traumatic brain injury (TBI) is one of the leading causes of mortality, morbidity, and disability in our country. As patients are usually young individuals, this leads to a huge impact on socioeconomic status. Despite the presence of organized trauma care system and adaptation of head injury guidelines, the outcome of severe head injury is suboptimal. Studies have clearly shown that it is not just the initial trauma which decides the outcome; instead, there are so many biochemical imbalances which lead to substantial neuronal deaths hours after the trauma.[1]
There are multiple biomechanical pathways which get activated on severe head injury, and it is the imbalances in these pathways which lead to secondary injuries to the neurons. Many studies have focused on rebalancing these pathways to preserve the neurons from deaths and apoptosis with replacement therapies including antioxidants, hormones, and ions. Among these serum magnesium levels have been shown to have a crucial impact on the outcome of head injury.[2] The effect of alterations in serum magnesium in severe head injury has been scarcely studied in humans, but it is thought to have an important role in cytotoxic and reperfusion pathways of secondary brain injury.[1,3] A rat TBI model demonstrated that Mg2+ deficiency exacerbates neurologic dysfunction and increases mortality in rats. This study also showed an improvement in neurological outcome on pretreatment with magnesium.[4] These findings were also reproduced later by other experimental studies.[5,6] In this study, we attempted to study the relationship between serum magnesium levels and the neurological outcome in severe head injury patients.
Materials and Methods
This was a prospective study conducted over a period of 1 year from October 2010 to September 2011 at Bangur Institute of Neurosciences, Kolkata, India. This study was approved by the Institutional Review Board. Adult patients with severe TBI admitted to the Bangur Institute of Neurosciences who met the inclusion criteria were recruited after obtaining written informed consent. The inclusion criteria were age between 18 and 75 years, postresuscitative Glasgow Coma Scale (GCS) score of ≤8, not under the influence of pharmacologic agents or alcohol, and admission within 12 h of injury. The patients with significant multiorgan injury, bilateral absent pupillary light reflex, hypotension (systolic blood pressure <90) for 10 min, and the patients who lost the follow-up were excluded from the study.
Standard care of the patients included ventilation, phenytoin for seizure prophylaxis, ceftriaxone for antibiotic prophylaxis, ranitidine for gastric ulcer prophylaxis, and urinary catheterization. Mannitol was added when computerized tomography (CT) brain showed a focal mass effect or diffuses cerebral edema. The decision of surgical decompression was taken according to the mass effect noted in the CT brain and was individualized to each patient. The data were collected with regard to age, gender, and GCS score.
Blood sample was collected for serum magnesium assessment. The serum magnesium measurement was carried out in a fully automated computerized microanalyzer (Hitachi model 911; Boehringer Mannheim, Mannheim, Germany). The initial serum magnesium was assessed within 24 h of admission to our hospital. Patients were dichotomized into two arms based on the initial serum magnesium level: low serum magnesium group with serum magnesium level <1.3 mEq and normal group with serum magnesium level value of 1.3–2.1 mEq. Magnesium sulfate (MgSO4) replacement was instituted for all patients with the initial serum [Mg2−] levels <1.3 mEq/L as a part of routine clinical care.
Outcomes
Glasgow Outcome Scale (GOS) was assessed at 6 months either directly, over telephone, or through letters. Good recovery or moderate disability (GOS 1 and 2) was considered as favorable outcome, and severe disability, persistent vegetative state, or death (GOS 3, 4, and 5, respectively) was considered as unfavorable outcome.
Statistical analysis
The data were expressed as a mean and standard deviation (SD). Proportions were compared using Chi-square tests or Fisher's exact test wherever appropriate. Student's t-test was used to compare the means of two continuous variables which were normally distributed, and nonparametric tests were used if their distribution was not normal. Multivariate analyses were conducted with logistic regression adjusting for well-known prognostic factors such as age and GCS. Two-sided significance tests were used throughout, and the significance level was kept at P = 0.05. Statistical analysis was performed using the SPSS 11 software package (Chicago, Illinois, United States).
Results
A total of 198 patients of severe TBI were admitted within the study period, of which 72 patients (male = 50, female = 22) who met the inclusion criteria were recruited in the study. The mean (±SD) age of the study patients was 42.5 (±12.7) years. The mean GCS at admission was 5.4 ± 1.7. The mean (±SD) serum magnesium level at admission was 1.14 ± 0.35 mEq/L with lowest being 0.5 mEq/L and highest of 1.9 mEq/L. Forty-two (58%) patients had low serum magnesium level (<1.3 mEq/L) at admissions with remaining thirty patients (42%) having normal serum magnesium levels. The outcome of the study patients assessed at 6 months using GOS showed that 51 patients (71%) had a good outcome and 21 (29%) had a poor outcome.
There was no difference in age (43.1 ± 12.6 vs. 41.7 ± 13.2, P = 0.66) and initial GCS (5.2 ± 1.7 vs. 5.7 ± 1.7, P = 0.17) in patients with low serum magnesium as compared to those with normal serum magnesium [Figure 1].
Figure 1.
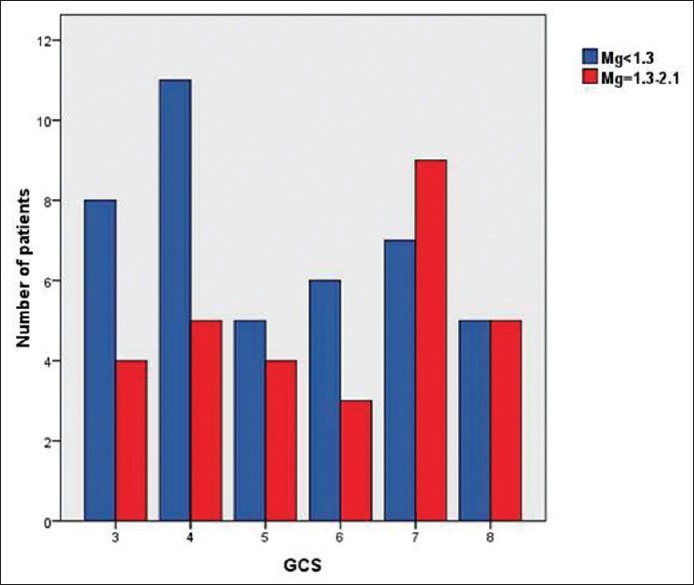
Bar diagram showing that there was no significant association between the initial Glasgow Coma Scale and serum magnesium level (5.2 ± 1.7 vs. 5.7 ± 1.7, P = 0.17)
At 6-month follow-up, 58 patients (81%) with poor outcome had low serum magnesium as compared to 14 patients (19%) with good outcome (P = 0.01) as assessed by GOS [Figure 2].
Figure 2.
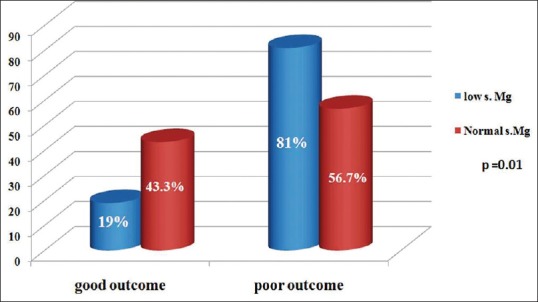
Comparison of serum magnesium levels in patients with good and poor outcome based on Glasgow Outcome Scale
Using a binary logistic regression model, low serum magnesium (mg <1.3 mmol/L) was associated with poor outcome (odds ratio = 2.1, P = 0.04, 95% confidence interval = 1.0–8.8), whereas age above 40 years and initial GCS score <5 were not statistically significant.
Discussion
Magnesium is a second most abundant intracellular cation after potassium found primarily in bone (53%) and soft tissues (46%), with the remaining 1% found in the blood, either in the free ionized form (54%–65%) or bound to proteins (27%–34%) and anions (8%–12%). It is a cofactor for >300 enzymatic reactions.[7]
Magnesium plays a very important role in the secondary brain injury by its effects on numerous biomechanical functions including neurotransmitter release, ion changes, oxidative stress, protein synthesis, and energy metabolism.[8] Only the free, ionized form of magnesium [Mg2−] is physiologically active.
Low serum magnesium is usually seen in critically ill patients including severe head injury patients.[9] In a study by Dhandapani et al., serum ionic Mg2+ was significantly lower in patients with severe TBI as compared to normal controls (mean serum ionic Mg2+: 0.37 + 0.04 mmol/L vs. 0.44 + 0.05 mmol/L, P < 0.01).[10] Although the mechanism of magnesium depletion in head injury patients is largely unknown, a possible explanation could be the increased lipolysis triggered by the stress-induced catecholamine surge leading to increase in the free fatty acids which bind to Mg2+ and thus increase the excretion of Mg2− in the urine.[11,12]
Depletion of intracellular free magnesium in the cortex of brain-injured rats by 70% and its correlation with the functional outcome was demonstrated by Vink et al.,[13] using 31P magnetic resonance spectroscopy, within the 1st h of injury.[14] Experimental studies supported this by demonstrating the exacerbation of neurologic dysfunction and increased mortality rate due to hypomagnesemia in a rat TBI animal model.[15]
Free and total serum [Mg2+] levels have been shown to behave differently in head injury patients. Mendez et al. showed decreased total serum [Mg2+] in all groups of head injury (mild, moderate, and severe tuberculosis), whereas ionized serum [Mg2+] was only decreased in severe head injury patients. Interestingly, the ionized [Mg2+] level normalized within 24 h, whereas total serum [Mg2+] remained abnormally low, thereby suggesting ionic magnesium as a biochemical marker of severe TBI.[16]
Experimental studies have shown that role of magnesium in the activation of N-methyl-D-aspartate receptor,[17] inhibition of glutamate release,[18] opening of calcium channels,[19] lipid peroxidation, production of free radicals, edema formation, and the opening of the mitochondrial permeability transition pores responsible for apoptosis.[3] The neuroprotective effects of Mg2+ may also be a result of its ability to inhibit cell apoptosis-related proteins such as p53 and Bax, primarily in cortical neurons after experimental TBI.[20] The suspected protective role of Mg2+ in TBI may be a result of various cellular factors that inhibit secondary neuronal injury.
This study showed that severe head injury patients with hypomagnesemia at the initial admission had significantly poor outcome at 6-month follow-up. Among the severe head injury patients with hypomagnesemia at admission, 81% of patients with poor outcome had low serum magnesium as compared to 19% of patients with good outcome (P = 0.01) as assessed by GOS at 6-month follow-up. Serum hypomagnesemia can be considered as an independent marker for poor neurological outcome in TBI.[21] Larger studies are required to confirm the outcomes of the present study and to study the exact mechanisms responsible for hypomagnesemia and its role in secondary neuronal injury in the severe head injury.
Conclusion
The neurological outcome of TBI correlates well with the serum magnesium concentration. Low serum magnesium may be related to the initial stress response of the severe head injury. Hypomagnesemia appears to be an independent prognostic marker in patients with severe TBI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Reilly PL. Brain injury: The pathophysiology of the first hours.’Talk and die revisited’. J Clin Neurosci. 2001;8:398–403. doi: 10.1054/jocn.2001.0916. [DOI] [PubMed] [Google Scholar]
- 2.van den Heuvel C, Vink R. The role of magnesium in traumatic brain injury. Clin Calcium. 2004;14:9–14. [PubMed] [Google Scholar]
- 3.Vink R, Nimmo AJ, Cernak I. An overview of new and novel pharmacotherapies for use in traumatic brain injury. Clin Exp Pharmacol Physiol. 2001;28:919–21. doi: 10.1046/j.1440-1681.2001.03548.x. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh TK, Juhler M, Wieloch T. Novel pharmacologic strategies in the treatment of experimental traumatic brain injury: 1998. J Neurotrauma. 1998;15:731–69. doi: 10.1089/neu.1998.15.731. [DOI] [PubMed] [Google Scholar]
- 5.Smith DH, Okiyama K, Gennarelli TA, McIntosh TK. Magnesium and ketamine attenuate cognitive dysfunction following experimental brain injury. Neurosci Lett. 1993;157:211–4. doi: 10.1016/0304-3940(93)90739-8. [DOI] [PubMed] [Google Scholar]
- 6.Bareyre FM, Saatman KE, Helfaer MA, Sinson G, Weisser JD, Brown AL, et al. Alterations in ionized and total blood magnesium after experimental traumatic brain injury: Relationship to neurobehavioral outcome and neuroprotective efficacy of magnesium chloride. J Neurochem. 1999;73:271–80. doi: 10.1046/j.1471-4159.1999.0730271.x. [DOI] [PubMed] [Google Scholar]
- 7.Fox C, Ramsoomair D, Carter C. Magnesium: Its proven and potential clinical significance. South Med J. 2001;94:1195–201. [PubMed] [Google Scholar]
- 8.Ozgurtas T, Kahraman S. State of the art of new data on the role of magnesium in brain injury: Clinical interest of measurements of total and ionized magnesium. Magnes Res. 2004;17:327–34. [PubMed] [Google Scholar]
- 9.Polderman KH, Bloemers FW, Peerdeman SM, Girbes AR. Hypomagnesemia and hypophosphatemia at admission in patients with severe head injury. Crit Care Med. 2000;28:2022–5. doi: 10.1097/00003246-200006000-00057. [DOI] [PubMed] [Google Scholar]
- 10.Dhandapani SS, Gupta A, Vivekanandhan S, Mahapatra AK, Mehta VS. Serum ionic magnesium in traumatic brain injury. Indian J Neurotrauma. 2005;2:103–6. [Google Scholar]
- 11.Sakamoto T, Takasu A, Saitoh D, Kaneko N, Yanagawa Y, Okada Y. Ionized magnesium in the cerebrospinal fluid of patients with head injuries. J Trauma Acute Care Surg. 2005;58:1103–9. doi: 10.1097/01.ta.0000169950.51735.c4. [DOI] [PubMed] [Google Scholar]
- 12.McKee JA, Brewer RP, Macy GE, Borel CO, Reynolds JD, Warner DS. Magnesium neuroprotection is limited in humans with acute brain injury. Neurocrit Care. 2005;2:342–51. doi: 10.1385/NCC:2:3:342. [DOI] [PubMed] [Google Scholar]
- 13.Vink R, McIntosh TK, Demediuk P, Faden AI. Decrease in total and free magnesium concentration following traumatic brain injury in rats. Biochem Biophys Res Commun. 1987;149:594–9. doi: 10.1016/0006-291x(87)90409-8. [DOI] [PubMed] [Google Scholar]
- 14.Vink R, McIntosh TK, Demediuk P, Weiner MW, Faden AI. Decline in intracellular free Mg2 + is associated with irreversible tissue injury after brain trauma. J Biol Chem. 1988;263:757–61. [PubMed] [Google Scholar]
- 15.McIntosh TK, Faden AI, Yamakami I, Vink R. Magnesium deficiency exacerbates and pretreatment improves outcome following traumatic brain injury in rats: 31P magnetic resonance spectroscopy and behavioral studies. J Neurotrauma. 1988;5:17–31. doi: 10.1089/neu.1988.5.17. [DOI] [PubMed] [Google Scholar]
- 16.Mendez DR, Corbett R, Macias C, Laptook A. Total and ionized plasma magnesium concentrations in children after traumatic brain injury. Pediatr Res. 2005;57:347–52. doi: 10.1203/01.PDR.0000150803.36315.FF. [DOI] [PubMed] [Google Scholar]
- 17.Smith DA, Connick JH, Stone TW. Effect of changing extracellular levels of magnesium on spontaneous activity and glutamate release in the mouse neocortical slice. Br J Pharmacol. 1989;97:475–82. doi: 10.1111/j.1476-5381.1989.tb11975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JW, Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-D-aspartate-activated channels. Biophys J. 1990;57:1085–90. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iseri LT, French JH. Magnesium: Nature's physiologic calcium blocker. Am Heart J. 1984;108:188–93. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Han YM, Yoo DS, Choi SJ, Choi BH, Kim JH, et al. A molecular basis for the efficacy of magnesium treatment following traumatic brain injury in rats. J Neurotrauma. 2004;21:549–61. doi: 10.1089/089771504774129883. [DOI] [PubMed] [Google Scholar]
- 21.Stippler M, Fischer MR, Puccio AM, Wisniewski SR, Carson-Walter EB, Dixon CE, et al. Serum and cerebrospinal fluid magnesium in severe traumatic brain injury outcome. J Neurotrauma. 2007;24:1347–54. doi: 10.1089/neu.2007.0277. [DOI] [PubMed] [Google Scholar]


