Abstract
Intracranial aneurysms may cause embolic stroke. Medical or surgical management is selected on an individual basis, as the optimal treatment strategy has not been established. A 79-year-old woman with a large cavernous carotid aneurysm suffered repeated embolic stroke after enlargement and partial thrombosis of the aneurysm, in spite of antiplatelet therapy. Coil embolization of the primitive trigeminal artery and ligation of the internal carotid artery (ICA) at the cervical portion followed by high-flow bypass from the cervical external carotid artery to the middle cerebral artery were performed. The aneurysm was thrombosed, and prevention of further stroke was achieved. Acute enlargement and thrombosis of large or giant cavernous carotid aneurysm may cause repeated embolic stroke, and requires emergent exclusion of the aneurysm from circulation by proximal ICA occlusion together with distal revascularization before devastating embolic stroke occurs.
Keywords: Cavernous carotid aneurysm, embolic stroke, high-flow bypass, proximal occlusion, recurrence
Introduction
Intracranial aneurysms, especially large and giant ones, may manifest as ischemic stroke,[1,2] possibly resulting from parent artery occlusion due to local extension of the luminal thrombus, aneurysms ejecting emboli into the distal arteries or increased mass effect.[3] No optimal treatment strategy for these aneurysms has been established, so medical or surgical management depends on the characteristics of each case.
We describe a case of large intracavernous internal carotid artery (ICA) aneurysm at the bifurcation with the primitive trigeminal artery (PTA) which caused repeated embolic strokes after enlargement and partial thrombosis of the aneurysm. The strokes recurred in spite of medical treatment but were successfully prevented by high-flow bypass using radial artery graft (RAG) following coil embolization of the PTA.
Case Report
History
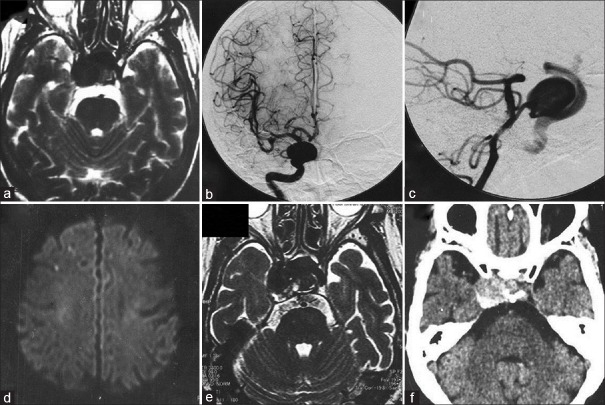
A 76-year-old woman with medical history of cervical lymphoma presented to our hospital with diplopia and ptosis on the right caused by right oculomotor nerve palsy. Head T2-weighted magnetic resonance (MR) imaging disclosed a large intracavernous ICA aneurysm, and cerebral digital subtraction angiography revealed a large aneurysm at the ICA-PTA bifurcation with a maximum diameter of 18 mm [Figure 1a–c]. PTA originated at cavernous portion of ICA and terminated at basilar artery, below the level of the superior cerebral artery and above the union. Because she refused to undergo an operation, follow-up examination was continued.
Figure 1.
T2-weighted magnetic resonance image showing a large intracavernous internal carotid artery aneurysm (a), and cerebral digital subtraction angiograms revealing a large aneurysm at the internal carotid artery-primitive trigeminal artery bifurcation (b and c), with maximum diameter of 18 mm. Three years later, diffusion-weighted magnetic resonance image showing scattered cerebral infarctions in the right hemisphere (d), and T2-weighted magnetic resonance image demonstrating enlargement and partial thrombosis of the aneurysm, with maximum diameter of 22 mm (e). Computed tomography scan showing partial thrombosis in the aneurysm sac (f)
Three years later, she complained of a headache in addition to the right oculomotor nerve palsy. Head diffusion-weighted and T2-weighted MR imaging disclosed scattered cerebral infarctions in the right hemisphere and enlargement of the aneurysm to the maximum diameter of 22 mm with intra-aneurysmal thrombosis [Figure 1d and e]. Computed tomography (CT) also disclosed partial thrombosis in the aneurysm sac [Figure 1f]. We considered the cause of the infarction to be embolism from the aneurysm, and treatment with aspirin was started. She also refused to have a surgical procedure at this time.
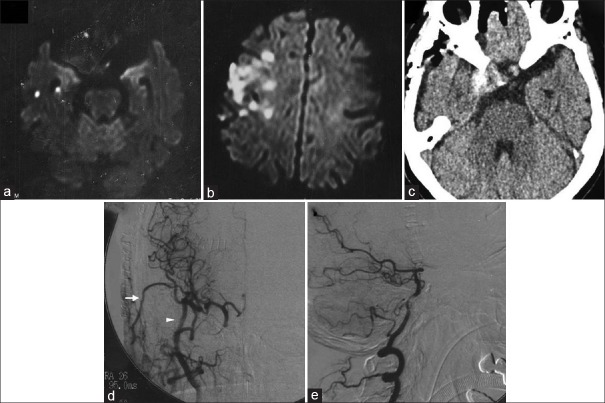
One week later, her headache and diplopia worsened, and she was admitted to our hospital. Right abducens nerve palsy was detected as well as preexisting oculomotor nerve palsy. Head diffusion-weighted MR imaging revealed recurrence of embolic infarction [Figure 2a], and oral administration of cilostazol was added. Echocardiography and electrocardiography monitoring showed no apparent abnormalities indicating the presence of cardiac embolic sources. Cervical MR angiography did not show any carotid or vertebral artery stenosis that would cause stroke. In spite of the dual antiplatelet therapy, she developed left hemiparesis 6 days later, and diffusion-weighted MR imaging disclosed recurrence of cerebral infarction [Figure 2b], so intravenous heparin was added. The surgery was planned to prevent further cerebral infarction and aneurysm rupture under the agreement of the patient, first coil embolization of the PTA, then high-flow bypass from the cervical external carotid artery (ECA) to the middle cerebral artery (MCA) using RAG followed by ligation of the cervical portion of the ICA on the next day.
Figure 2.
Diffusion-weighted magnetic resonance image showing new infarctions in the right temporal lobe (a). Six days later, diffusion-weighted magnetic resonance image revealing recurrence of infarction in the right hemisphere (b). Computed tomography scan on the day after the operation showing thrombosis of the aneurysm (c). Cerebral digital subtraction angiogram of the right carotid artery 5 days after the operation demonstrating good patency of bypasses (arrow: superficial temporal artery, arrowhead: radial artery graft) and disappearance of anterograde flow to the aneurysm (d). Digital subtraction angiogram of the right vertebral artery also demonstrating disappearance of anterograde flow to the aneurysm through the primitive trigeminal artery (e)
Operation
Coil embolization of the primitive trigeminal artery
Under local anesthesia, systemic heparinization was initiated, and a 6 Fr Envoy XB catheter (Johnson and Johnson, Miami, FL) was placed in the right vertebral artery. An Excelsior SL-10 preshaped 45° catheter (Stryker, Kalamazoo, MI) was navigated into the PTA, and the PTA was occluded using a PRESIDIO Spherical 10 CERECYTE 4 mm × 11.5 cm coil, and two ULTIPAQ Helical 10 CERECYTE 2 mm × 8 cm coils (Johnson and Johnson). Throughout the procedure, care was taken to prevent coil protrusion into the aneurysm, ICA, and basilar artery. After tight packing of the PTA was obtained, the procedure was ended.
High-flow bypass followed by internal carotid artery ligation
On the next day, high-flow bypass was conducted as described previously.[4,5,6] Neuroanesthesia was induced under the monitoring of somatosensory evoked potentials (SSEPs) of the left extremities. The right cervical carotid bifurcation was exposed, a curvilinear frontotemporal skin incision was made, and the superficial temporal artery (STA) was meticulously prepared under the operating microscope. The RAG was harvested concurrently by another neurosurgeon. Frontotemporal craniotomy was performed, and a subzygomatic tunnel was formed for the RAG. The Sylvian fissure was split under the operating microscope, and the M2 and M3 portions of the MCA were exposed. First, an “insurance” STA-M3 bypass was made distal to the M2 portion for RAG anastomosis. Then, the harvested RAG was gently pulled through the subzygomatic tunnel, that is, between the lateral pterygoid muscle and the temporal muscle from the cranium to the neck through the lateral corridor of the stylohyoid muscle and the posterior belly of the digastric muscle toward the ECA.[7] The distal end of the RAG was anastomosed to the M2 of the MCA, and the proximal end was anastomosed to the ECA. Microvascular Doppler flowmetry confirmed the patency of the anastomosis. The cervical ICA was permanently ligated after confirming no change in the SSEPs 5 min after temporary ligation. Microvascular Doppler flowmetry confirmed anterograde bypass flow from the STA and RAG. No significant SSEP changes were observed throughout the operation.
Postoperative course
CT obtained the day after the operation disclosed thrombosis of the entire aneurysm sac and cerebral angiography performed at 5 days after the operation demonstrated good patency of the bypasses and disappearance of anterograde flow to the aneurysm [Figure 2c–e]. Diffusion-weighted MR imaging revealed no new infarction. She gradually recovered with rehabilitation without new cerebral infarction over 8 months and was transferred to another hospital with modified Rankin scale 4.
Discussion
Ischemic strokes or transient ischemic attacks attributable to emboli from the aneurysm sac have been observed in 3.3% of patients with unruptured aneurysms.[2] Large or giant aneurysms contain varying amounts of endoluminal thrombus, and partially thrombosed aneurysms are considered dynamic and unstable structures that may grow, rupture, or give rise to thromboembolic stroke as this present case.[8,9,10]
Medical and surgical treatment of aneurysms believed to be the cause of stroke is selected on an individual basis because no optimal treatment for these aneurysms has been defined. Antiplatelet therapy is the most commonly used medical treatment to reduce the risk of future ischemic stroke and is considered to prevent the process of platelet aggregation inside the aneurysm sac. Treatment with aspirin has led to a low incidence of recurrent ischemic stroke.[1,2] Our patient suffered repeated stroke 6 years after diagnosis of the aneurysm. Head MR imaging revealed enlargement and thrombosis of the aneurysm, and the cause of the stroke was considered as embolism due to this thrombosis in the aneurysm sac. However, further medical treatment with aspirin, with added dual antiplatelet therapy with cilostazol, could not prevent subsequent stroke, so surgical treatment was considered necessary.
Surgical treatment mostly involves clipping or trapping rather than coiling, because endovascular manipulation may result in further embolism by disturbing the thrombus in the aneurysm sac. Partially, thrombosed intracranial aneurysms can be treated by preventing blood flow from the lesion of the pathogenic segment of the parent artery rather than obliteration of the remnant perfused aneurysm sac.[11] Moreover, endovascular coil embolization with or without stenting for large and giant aneurysms has not been successful with high recanalization and re-treatment rates.[12] Recently, flow diverting stents have offered potential treatment for formidable and complex aneurysms, but previous trials have shown complete aneurysm occlusion rates of 73.6% at 6 months and 86.8% at 1 year, and major complications such as major ipsilateral thrombosis, intraparenchymal hemorrhage, and neurologic death occurred in 5.6% of cases.[13,14]
Direct and indirect open surgical methods are available for treating large ICA aneurysms of the cavernous sinus. Direct options, such as neck clipping or trapping, offer definitive treatment of the aneurysms, but are technically challenging and carry higher risks of cranial nerve palsy and compromising the ICA.[15] Indirect surgical treatments, such as proximal ligation of the ICA with or without construction of a bypass to the MCA, avoid violation of the cavernous sinus.[16,17,18] High-flow bypass with ICA ligation has achieved aneurysm obliteration with acceptable rates of morbidity and mortality, as well as graft patency.[7]
In the present case, the aneurysm was 22 mm in maximum diameter, originated at the ICA-PTA bifurcation, and was located at the cavernous sinus. PTA is the most common primitive carotid-basilar anastomosis with an incidence of 0.2%–0.34% in large case series, and the aneurysms of a PTA are very rare.[19,20,21] Occlusion of both the ICA and PTA was necessary to prevent anterograde blood flow into the aneurysm with the risk of further stroke. Therefore, we first performed coil embolization of the PTA, then proximal ICA ligation at the cervical portion, followed by high-flow bypass using RAG from the cervical ECA to the MCA. These interventions prevented anterograde flow into the aneurysm from ICA and PTA, and bypass flow perfused intradural ICA territory, and subsequently achieved prevention of further stroke and rupture of the aneurysm without complication [Figure 3].
Figure 3.
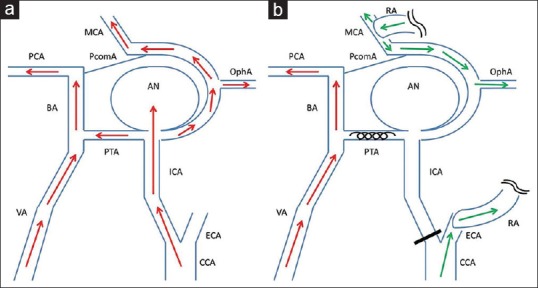
Schematic drawing of the surgery. Preoperative (a) and postoperative (b) hemodynamics are shown. The aneurysm was located at internal carotid artery-primitive trigeminal artery bifurcation, and received anterograde flow from internal carotid artery. Coil embolization of primitive trigeminal artery and ligation of internal carotid artery at cervical portion followed by construction of the external carotid artery-radial artery-M2 bypass prevented anterograde blood flow into the aneurysm from both internal carotid artery and primitive trigeminal artery. Intradural internal carotid artery territory including ophthalmic artery (OphA) was perfused by bypass flow. Red arrows: anterograde blood flow from internal carotid artery and vertebral artery, green arrows: Blood flow from external carotid artery-radial artery-M2 bypass, AN aneurysm, BA: Basilar artery, CCA: Common carotid artery, MCA: Middle cerebral artery, PCA: Posterior cerebral artery, PcomA: Posterior communicating artery
The balloon occlusion test (BOT) is commonly used to assess tolerance to ligation of the ICA. However, there is no universal standard for this procedure, the accuracy is complicated and controversial, and sometimes, complications result such as cerebral infarction.[16,22] For these reasons, we employed the graft bypass without performing the preoperative BOT. We chose the radial artery as the graft, not the saphenous vein because the RAG is associated with long-term patency,[23,24] and the Allen test was positive.
Large to giant intracavernous ICA aneurysm frequently cause intracavernous cranial nerve compression during enlargement, manifesting as oculomotor, and abducens nerve palsy. However, acute aneurysm enlargement accompanied by rapid intraaneurysmal thrombosis and resultant repeated emboli transmission into the distal intracranial ICA territory might be a rare clinical manifestation of large cavernous ICA aneurysm. However, such a rare occurrence requires urgent exclusion of the aneurysm from the circulation by proximal ICA occlusion together with distal revascularization before the patient suffers devastating embolic stroke. Therefore, an emergent clinical manifestation of acute enlargement and thrombosis of large or giant intracavernous ICA aneurysm in conjunction with evidence of repeated embolic diffusion requires immediate surgical intervention.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mokin M, Darkhabani Z, Binning MJ, Levy EI, Siddiqui AH. Small unruptured partially thrombosed aneurysms and stroke: Report of three cases and review of the literature. J Neurointerv Surg. 2012;4:e6. doi: 10.1136/neurintsurg-2011-010026. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Mohammad Y, Yahia AM, Luft AR, Sharma M, Tamargo RJ, et al. Ischemic events associated with unruptured intracranial aneurysms: Multicenter clinical study and review of the literature. Neurosurgery. 2000;46:282–9. doi: 10.1097/00006123-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Arauz A, Patiño-Rodríguez HM, Chavarría-Medina M, Becerril M, Merino JG, Zenteno M, et al. Embolic stroke secondary to spontaneous thrombosis of unruptured intracranial aneurysm: Report of three cases. Interv Neuroradiol. 2016;22:196–200. doi: 10.1177/1591019915618169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono H, Inoue T, Kunii N, Tanishima T, Tamura A, Saito I, et al. Giant cavernous carotid aneurysm causing pituitary dysfunction: Pituitary function recovery with high-flow bypass. Surg Neurol Int. 2017;8:180. doi: 10.4103/sni.sni_178_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ono H, Inoue T, Suematsu S, Tanishima T, Tamura A, Saito I, et al. Middle cerebral artery dissection causing subarachnoid hemorrhage and cerebral infarction: Trapping with high-flow bypass preserving the lenticulostriate artery. Surg Neurol Int. 2017;8:157. doi: 10.4103/sni.sni_154_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ono H, Inoue T, Tanishima T, Tamura A, Saito I, Saito N, et al. High-flow bypass with radial artery graft followed by internal carotid artery ligation for large or giant aneurysms of cavernous or cervical portion: Clinical results and cognitive performance. Neurosurg Rev. 2018;41:655–65. doi: 10.1007/s10143-017-0911-3. [DOI] [PubMed] [Google Scholar]
- 7.Ishishita Y, Tanikawa R, Noda K, Kubota H, Izumi N, Katsuno M, et al. Universal extracranial-intracranial graft bypass for large or giant internal carotid aneurysms: Techniques and results in 38 consecutive patients. World Neurosurg. 2014;82:130–9. doi: 10.1016/j.wneu.2013.02.063. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JE, Itshayek E, Gomori JM, Grigoriadis S, Raphaeli G, Spektor S, et al. Spontaneous thrombosis of cerebral aneurysms presenting with ischemic stroke. J Neurol Sci. 2007;254:95–8. doi: 10.1016/j.jns.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Schaller B, Lyrer P. Focal neurological deficits following spontaneous thrombosis of unruptured giant aneurysms. Eur Neurol. 2002;47:175–82. doi: 10.1159/000047978. [DOI] [PubMed] [Google Scholar]
- 10.Whittle IR, Dorsch NW, Besser M. Spontaneous thrombosis in giant intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1982;45:1040–7. doi: 10.1136/jnnp.45.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang K, Park JC, Ahn JS, Kwon DH, Kwun BD, Kim CJ, et al. Characteristics and outcomes of varied treatment modalities for partially thrombosed intracranial aneurysms: A review of 35 cases. Acta Neurochir (Wien) 2014;156:1669–75. doi: 10.1007/s00701-014-2147-0. [DOI] [PubMed] [Google Scholar]
- 12.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 13.Becske T, Potts MB, Shapiro M, Kallmes DF, Brinjikji W, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg. 2017;127:81–8. doi: 10.3171/2015.6.JNS15311. [DOI] [PubMed] [Google Scholar]
- 14.Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology. 2013;267:858–68. doi: 10.1148/radiol.13120099. [DOI] [PubMed] [Google Scholar]
- 15.Dolenc VV. Extradural approach to intracavernous ICA aneurysms. Acta Neurochir Suppl. 1999;72:99–106. doi: 10.1007/978-3-7091-6377-1_9. [DOI] [PubMed] [Google Scholar]
- 16.Barnett DW, Barrow DL, Joseph GJ. Combined extracranial-intracranial bypass and intraoperative balloon occlusion for the treatment of intracavernous and proximal carotid artery aneurysms. Neurosurgery. 1994;35:92–7. doi: 10.1227/00006123-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656–65. doi: 10.3171/jns.1994.81.5.0656. [DOI] [PubMed] [Google Scholar]
- 18.Gelber BR, Sundt TM., Jr Treatment of intracavernous and giant carotid aneurysms by combined internal carotid ligation and extra- to intracranial bypass. J Neurosurg. 1980;52:1–10. doi: 10.3171/jns.1980.52.1.0001. [DOI] [PubMed] [Google Scholar]
- 19.Kim MJ, Kim MS. Persistent primitive trigeminal artery: Analysis of anatomical characteristics and clinical significances. Surg Radiol Anat. 2015;37:69–74. doi: 10.1007/s00276-014-1318-2. [DOI] [PubMed] [Google Scholar]
- 20.Uchino A, Saito N, Okada Y, Kozawa E, Mizukoshi W, Inoue K, et al. Persistent trigeminal artery and its variants on MR angiography. Surg Radiol Anat. 2012;34:271–6. doi: 10.1007/s00276-011-0848-0. [DOI] [PubMed] [Google Scholar]
- 21.Vasović L, Jovanović I, Ugrenović S, Vlajković S, Jovanović P, Stojanović V, et al. Trigeminal artery: A review of normal and pathological features. Childs Nerv Syst. 2012;28:33–46. doi: 10.1007/s00381-011-1622-7. [DOI] [PubMed] [Google Scholar]
- 22.Field M, Jungreis CA, Chengelis N, Kromer H, Kirby L, Yonas H, et al. Symptomatic cavernous sinus aneurysms: Management and outcome after carotid occlusion and selective cerebral revascularization. AJNR Am J Neuroradiol. 2003;24:1200–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Cao C, Ang SC, Wolak K, Peeceeyen S, Bannon P, Yan TD, et al. A meta-analysis of randomized controlled trials on mid-term angiographic outcomes for radial artery versus saphenous vein in coronary artery bypass graft surgery. Ann Cardiothorac Surg. 2013;2:401–7. doi: 10.3978/j.issn.2225-319X.2013.07.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houkin K, Kamiyama H, Kuroda S, Ishikawa T, Takahashi A, Abe H, et al. Long-term patency of radial artery graft bypass for reconstruction of the internal carotid artery. Technical note. J Neurosurg. 1999;90:786–90. doi: 10.3171/jns.1999.90.4.0786. [DOI] [PubMed] [Google Scholar]