Abstract
Introduction:
Laminectomy is the workhorse of spinal cord tumor surgery. This procedure is not without the debilitating sequelae of postoperative pain and delayed kyphosis. Hemilaminectomy is an alternate option to laminectomy which offers the advantage of preserving the posterior supporting structures of the spine on the contralateral side. In this study, we analyze the outcome of hemilaminectomy clinically with improvement in pain scores and Nurick's grade as well as radiologically by assessing for the development of delayed kyphosis. We also discuss the technique and operative nuances of hemilaminectomy in intradural extramedullary tumors of the spinal cord.
Materials and Methods:
All patients with intradural spinal cord tumors were included in the study. All patients underwent unilateral hemilaminectomy (UHL) depending on the laterality of the tumor on the preoperative magnetic resonance imaging. Preoperative neurologic status was assessed with Nurick's grade for tumors involving the cervicothoracic region tumors, and visual analog scale scores were recorded for tumors of Thoracic, Lumbar and Lumbosacral regions. The postoperative outcomes were assessed by improvement in respective scales on follow-up. The occurrence of delayed spinal deformity was assessed by follow-up X-rays. Any complications whether intraoperative or postoperative were recorded.
Results:
There were a total of 34 cases of intradural extramedullary tumors in this study. Patient population consisted of 11 males and 23 females. Total excision was achieved in 31 patients. In three patients, we were unable to achieve complete removal through UHL. In these patients the procedure was converted to total laminectomy. They were excluded from analysis. The distribution of the tumors was in cervical, cervicothoracic, thoracic, lumbar, and lumbosacral region. All patients presented with pain or varying degrees or neurologic deficits. Sixteen patients underwent UHL from the right side, while 18 from the left. There were no intraoperative complications. The neurological status and pain scores of all patients improved postoperatively at 3 and 6 months of follow-up. There was no radiological evidence of kyphosis of the involved segment.
Conclusion:
With a small learning curve, UHL is a good corridor for the removal of intradural extramedullary spinal cord tumors. This approach offers the advantage of less postoperative pain and no postoperative deformity.
Keywords: Hemilaminectomy, intradural, spinal cord tumors
Introduction
The global burden of spinal cord tumor is unknown. In the united states, the age-adjusted incidence for primary spinal tumors is around 0.76/lakh persons for nonmalignant tumors and 0.22/lakh persons for malignant spinal tumors.[1] Laminectomy performed either at single or multiple levels was the standard of approach in the management of spinal cord tumors. However, this can lead to destabilization of the posterior tension band which is the prime factor in maintaining the stability and primary curvature of the spine. Postoperatively delayed kyphosis is inevitable.[2,3,4] To circumvent this complication, Taylor in 1908 proposed a unilateral corridor to the dural opening which involves leaving most of the vertebral attachments intact. This unilateral hemilaminectomy (UHL) approach soon became the most favored approach for the removal of intradural tumors by the minimal invasive surgeons. In our institution, we routinely perform laminectomy for spinal cord tumors. However, as experience improved, we shifted our focus on less invasive surgeries for their removal. In this retrospective study, we present our experience of UHL in the management of intradural extramedullary tumors.
Materials and Methods
All consecutive patients from 2014 to 2016 who underwent UHL for intradural extramedullary spinal cord tumors in our institution were included in this study. All patients had either back pain or varying degrees of neurologic deficits. Tumor location, pathology, size, levels involved, and laterality were the factors chosen to consider UHL. All patients underwent gadolinium-enhanced magnetic resonance imaging (MRI) preoperatively. The patient and tumor characteristics were entered retrospectively into database (Microsoft Excel). Neurologic status was recorded for cervical and cervicothoracic region tumors, and intensity of pain was recorded on visual analog scale (VAS) for tumors involving other regions. All patients with tumor located in the thoracic, thoracolumbar region had a preoperative marker X-ray with a coin affixed at the site of level of the tumor on the evening before surgery. Intraoperatively, the level was re-confirmed with C-arm. All patients underwent surgery in the prone position. Any intraoperative or postoperative complications were recorded. Patients with large dumbell tumors and giant tumors involving more than 2 segments were excluded from the study because of the difficulty in removing them thorough the restricted corridor of UHL. Patients were usually discharged on the 3rd day after surgery if there were no complications. This study was approved by the ethics committee of our institution and was carried out in accordance with the Helsinki Declaration of 1975 as revised in 2000.
Surgical technique
Preoperative dexamethasone was given 12–16 mg/day to all patients. Localization of the tumor was usually done on the evening before the surgery with a marker X-ray, whereby a coin was affixed at the level of the spine overlying the tumor. No neuromonitoring was used in any of the patients. After intubation, the patient was kept in prone position. A small midline incision was made and deepened to dissect the muscles off the midline to expose the lamina on one side. This subperiosteal elevation of muscles was meticulously done until the desired levels of exposure were attained. The ligaments and tendinous insertions of the contralateral muscles were left undisturbed. The hemilamina at the desired level was removed using Kerrison rongeur or high-speed drill depending on the surgeon's discretion. The ligamentum flavum was excised using the Kerrison rongeur to expose the dura. The dura was opened and held in that position with tack sutures. The arachnoid was cut to expose the tumor. The tumor was then subjected to piecemeal biopsy. Whenever ultrasonic aspirator was available (CUSA Excel, Integra, Inc.,), it was decompressed internally and completely removed. In case of extraforaminal extension of tumor, total/partial facetectomy was done to visualize the extraforaminal part and total excision could be achieved. After achieving resection of the tumor, the dura was closed with 5.0 prolene. Reinforcing fibrin sealant (Tisseel™, Baxter healthcare India) was used as per the discretion of the surgeon.
Follow-up
All patients were followed up on outpatient basis at 2 weeks, 3 months, and 6 months after discharge. The outcome of the patients was assessed by Nurick's grade in cases of cervicothoracic tumors, whereas for thoracic, lumbar and sacral regions, the difference in pain on VAS scale was recorded. A postoperative X-ray of the operated segment of the spine was taken at 3 and 6 months to detect the presence of any kyphotic deformity. Kyphosis was defined as anterior angulation of the spine at the operative segment.
Illustrative case 1
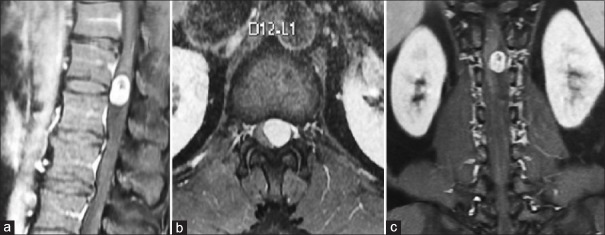
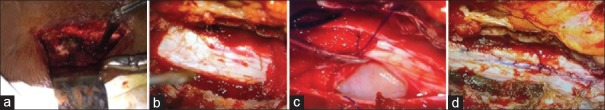
A 27-year-old male presented with severe back pain (VAS 8) and weakness of both lower limbs. MRI [Figure 1] shows well-defined intradural extramedullary tumor in the region of D12-L1. The lesion is contrast enhancing on T1-weighted images (A, B, and C). The tumor is compressing the spinal cord from the left side (C). Intraoperative images show the exposure of the D12-L1 region on the left side [Figure 2a] with Taylor's hemilaminectomy retractor. The extent of UHL and the underlying dura is visible in [Figure 2b]. The dura was opened and the cord gently retracted to visualize the tumor toward the left side of the spinal cord [Figure 2c]. The tumor was debulked with CUSA and the nerve root from which it was arising was cut. After total resection, the dura was closed watertight with 5.0 prolene [Figure 2d]. The patient had marked improvement in the back pain and could walk without difficulty postoperatively.
Figure 1.
(a) Magnetic resonance imaging T1-weighted sagittal image with contrast showing heterogeneously enhancing intradural extramedullary lesion at the level of the D12-L1 vertebrae. (b) Magnetic resonance imaging T1-weighted axial image with contrast showing the lesion which is eccentrically located more toward the left side. (c) Magnetic resonance imaging T1-weighted coronal image with contrast showing the heterogeneously enhancing lesion arising from the left side, which is widening the cerebrospinal fluid spaces above and below the lesion
Figure 2.
Intraoperative photographs. (a) Subperiosteal stripping of muscles done at the level of D12-L1 vertebrae from the left side. (b) Dura exposed after removal of the D12 and L1 hemilamina. (c) Dura is opened to visualize the tumor which is lateralized to the left side. (d) Dura is closed meticulously after total excision of the tumor
Results
There were 34 patients with spinal cord intradural extramedullary tumors who underwent UHL. Among them, three had to be converted to total laminectomy and were excluded from the analysis. The final histology in the remaining 31 cases was schwannoma (17), meningioma (11), and arachnoid cyst (3). There were 10 males and 21 females in the study group. Back pain, radiculopathy, weakness of lower limbs, paraesthesia, and involvement of bowel and bladder were the presenting features in most of the patients. On examination, band-like sensation was present in 5 (16.1%) and sensory loss was apparent in 24 (77.4%) patients. The frequency distribution of symptoms is enumerated in Figure 3. The location of tumors was intradural extramedullary in all cases. The mean size of the tumor was 2.74 cm ± 1.2 cm. The distribution of tumor depending on the location was as follows: 4 cervical (12.9%), 2 cervicothoracic (6.45%), 19 thoracic (61.3%), 2 thoracolumbar (6.45%), 3 lumbar (9.6%), and 1 lumbosacral (3.2%). All patients underwent UHL in the manner described above; the side of approach in each case was decided by the location of tumor on the preoperative MRI. Fifteen tumors had right-sided approach and 16 were approached from the left. The mean operating time was 4.69 h. The operating time was much longer in cases where CUSA was not used and also in the beginning of the series during the learning curve of this technique. Majority of patients underwent two-level laminectomies 19 (61.2%), 7 (22.5%) patients had single-level laminectomy, while three patients (9.6%) had three-level and two patients (6.4%) had single-level plus additional half of the adjacent removed. There were two cases of dumbbell tumors in the thoracic region who underwent facetectomy in addition to UHL for the removal of the extraforaminal part. Thirty-one cases had total removal of tumors, whereas three cases where total removal was not feasible UHL were converted to total laminectomy. There were no intraoperative complications. All patients were observed in Intensive Care Unit for 1 day and later to progressive care. Postoperative complications that occurred included one incidence of cerebrospinal fluid (CSF) leak, urinary retention (2), postoperative fever (1), upper respiratory tract infection (1), and new-onset low back ache in one patient. All complications were managed successfully except the urinary retention. The mean time of discharge among the patients was 5 days ± 2 days. The patients were followed up in outpatient unit regularly. The improvement in Nurick's grade was recorded for cases of cervical and cervicothoracic region, and the improvement of pain on VAS scales was charted for cases of tumors in other regions. All six patients with cervical/cervicothoracic region tumors had an improvement in their Nurick's grade postoperatively on follow-up at 6 months. Two patients in Grade 4 had improved to Grade 3 on follow-up while four patients had improved from Grade 3 to Grade 2 on Nurick's scale [Figure 4]. In the remaining 27 patients, who had tumors in the thoracic and lumbar region, the preoperative VAS was 7.5 ± 0.7 and on follow-up showed a significant decline with mean score of 1.6 ± 0.5 [Figure 5]. There was no evidence of kyphosis on the postoperative X-ray lateral view taken at 3 months and 6 months for the involved region.
Figure 3.
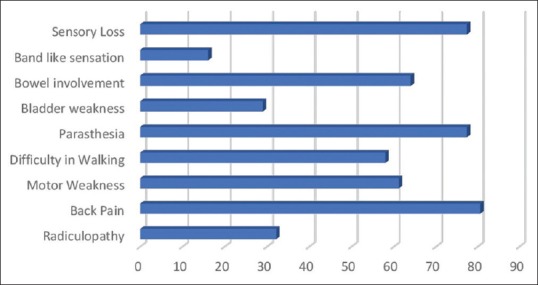
Bar chart demonstrating the symptomatology of the patients presented with intradural spinal cord tumors
Figure 4.
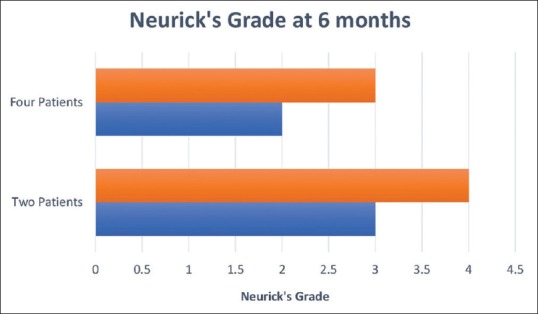
Graph demonstrating outcome of cervical and cervicothoracic region tumors as assessed by Nurick's grade preoperatively (brown) and at 6-month follow-up (blue). Four patients had improvement from Grade 3 to Grade 2. Two patients had improvement from Grade 4 to Grade 3
Figure 5.
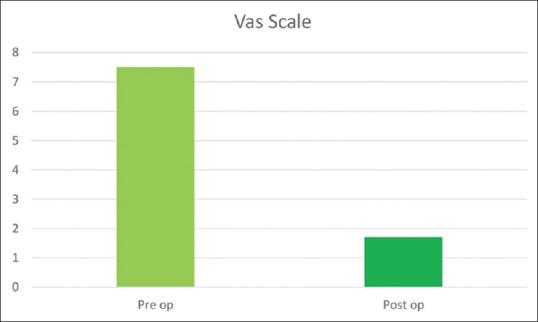
Visual Analogue Scale (VAS) in patients with Thoracic and Lumbar region tumors. There was significant reduction in pain scores as seen in the postoperative (1.6 + 0.5) (dark green) compared to preoperative (7.5 + 0.7) (light green). Pre-op – Preoperative. Post op – Post operative
Discussion
UHL was the initial step toward minimal invasive spine surgery developed by Taylor in 1908.[5] The superiority of this approach has been utilized by various authors effectively in the removal of spinal cord tumors in large series of patients comparing it to conventional laminectomy.[6,7] Total laminectomy involves subperiosteal stripping of the paravertebral muscles, removal of the ligaments of the posterior arch, and excision of the lamina and the ligamentum flavum. This would, in turn, jeopardize the stability of the spine as the muscles and ligaments of the posterior arch form an integral part in maintaining the stability of the spinal column.[8,9] Instability and deformity in the form of kyphosis are frequently associated with laminectomy.[3,10] Chronic subaxial pain due to epidural fibrosis and myelopathy secondary to kyphotic deformity are delayed sequelae of total laminectomy. The need for a second operation for instability following this approach has been reported in the range of 60% by Ogden et al.[11] In an order to circumvent this, many modifications of posterior spinal explorations, namely, laminoplasty, laminotomy, interlaminar fenestrations have been described by different authors.[12,13] However, these modifications involve paravertebral stripping of muscles bilaterally. Hemilaminectomy represents an approach which perfectly balances the biomechanics as well as the appropriateness of corridor for both visibility and removal of the intraspinal lesions. However, not all authors agree to this approach for all types of intradural tumors. Gu et al. suggested that only tumors with transverse diameter of <2 cm and limited to two spinal segments are best approached by UHL.[14] It only involves unilateral stripping of the muscles at a single or at the most double levels which hardly compromises the stability. The histology of tumors both intramedullary and extramedullary managed by this modality of approach can be vivid. Most of the tumors in our series were located in the thoracic region. Among them, there were two cases of dumbbell tumors. This necessitated partial removal of the facet to visualize the extraforaminal part and performing this maneuver aided in the total excision. Because of the ribcage giving additional support to the spine at this level, there was no instrumentation used. This strategy has been adopted successfully in thoracic subset of large series of intraspinal tumors undergoing excision with UHL.[15] For cases involving similar tumors in the cervical and lumbar regions, the spine should be stabilized using instrumentation following facetectomy for the removal of the extraspinal compartment. There were 17 cases of schwannomas in our series all of which were totally excised. The use of CUSA significantly reduced the operating time in multisegmental tumors. The tumor arising from the nerve root was usually cut to ensure total excision. There were 11 cases of meningioma of which 9 had total excision. For the remaining, 2 cases in which total excision was not possible UHL were converted to total laminectomy. All efforts were made to ensure a total excision whenever possible. In cases of ventrally located tumors, a liberal lateral cut was made on the dura and it was sutured to the fascia at the base of the ipsilateral facet aided in better visibility and resection. This technique was suggested by Yeo et al. in their series of 25 cases of spinal cord tumors.[9] The denticulate ligament was cut and the cord was gently rotated in cases which required additional room. This was not required in all cases as the internal debulking itself will provide the remaining of the tumor to fall into the field of view facilitating adequate extirpation. The dural attachment of the tumor was coagulated in all cases. This amounted to Simpson's Grade 2 excision and has shown equally good recurrence rates and overall morbidity compared to Grade 1 removal in long-term follow-up.[16] There is alternative technique described for spinal meningiomas which includes dural splitting and excision of inner layer of dura along with the tumor.[17] However, due to unfamiliarity of this technique and the fear of inadvertent tear which can lead to CSF leak and added morbidity, this method was not employed by us. In spite of this cautious approach, we had one case of CSF leak. This particular patient had a dorsolateral meningioma where the dura was thinned out, and there was a minimal rent during the coagulation which was sutured. However, this complication subsided with conservative management. There were three cases of arachnoid cyst in our series all of which were successfully excised. The cyst wall was totally excised and fenestration into the subarachnoid space was done in all three cases. Various authors have reported similar strategies and successful outcomes following hemilaminectomy for excision of arachnoid cysts.[18,19,20]
All the patients did exceptionally well in our series barring two who had retention of urine and were catheter dependent for long time. These patients had minimal bladder involvement preoperatively. The mean operating time in our series was 4.6 ± 0.2 h. This varied vividly in the beginning of the series to the end. After attaining reasonable experience, the duration of the operating hours came down significantly. There were two instances in our series where the tumor was not found at the location, but an adjacent level exploration revealed the lesion. This leads to the increase in the operating time. Turel et al. had suggested preoperative MRI of the patient in the same position as the surgery in the MRI gantry marking the level with cod liver capsules to avoid this complication.[21] However, this was not feasible in our series as we have only one MRI machine in our institution which caters an overwhelming number of cases. The availability of CUSA also did affect the duration of the surgery beneficially. The mean duration of discharge in our series was 5 days ± 2 days. Mostly patients were discharged on the 3rd or 4th day except in complicated cases. This comes close to the recently published series of UHL involving 97 cases.[22] UHL has shown to significantly mitigate the burden of economics on the patients by lowering the duration of hospital stay.[22,23]
All patients in the cervical and cervicothoracic group had considerable improvement in their Nurick's grade on follow-up. Two patients who were ambulant only with support were able to walk independently without support at 6 months. Four patients who had difficulty in walking enough to prevent employment were able to walk without support with only slight difficulty. Patients who had thoracic and lumbar tumors had considerable relief of pain. Patients who had radiculopathy were totally cured whereas those who presented with low back pain had remarkable improvement of pain on the VAS scores.
The follow-up X-rays at 3 and 6 months showed no evidence of kyphosis. We generally do not recommend early X-rays on the first visit after 2 weeks as there would be some straightening due to the paravertebral spasm of the muscles. A follow-up MRI was not done in patients who underwent UHL as we think it was not necessary because the clinical examination showed marked improvement in them. Till 6 months on follow-up, there has not been a single case which showed deterioration in motor power. However, as this is a short time, further follow-up is required to ascertain the recurrence rates.
Limitations of the study
The lack of a control group is a gross limitation of the study. The follow-up of the patients was 6 months which was too early to assess the postoperative kyphosis. A long-term follow-up of these patients is essential to accurately determine the recurrence rates as well as development of delayed kyphosis.
Conclusion
UHL offers an alternative method of operating on patients with spinal cord tumors. This approach involves minimal stripping of muscle and bone removal with preservation of posterior ligamentous bands which offers added advantage of preserving the stability. There is a learning curve which would enable the surgeons to perform this operation in short duration compared to conventional method. All these factors contribute to early healing and discharge from the hospital alleviating pecuniary burden on patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Duong LM, McCarthy BJ, McLendon RE, Dolecek TA, Kruchko C, Douglas LL, et al. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004-2007. Cancer. 2012;118:4220–7. doi: 10.1002/cncr.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert TJ, Vacarro A. Postlaminectomy kyphosis. Spine (Phila Pa 1976) 1998;23:2738–45. doi: 10.1097/00007632-199812150-00014. [DOI] [PubMed] [Google Scholar]
- 3.Papagelopoulos PJ, Peterson HA, Ebersold MJ, Emmanuel PR, Choudhury SN, Quast LM, et al. Spinal column deformity and instability after lumbar or thoracolumbar laminectomy for intraspinal tumors in children and young adults. Spine (Phila Pa 1976) 1997;22:442–51. doi: 10.1097/00007632-199702150-00019. [DOI] [PubMed] [Google Scholar]
- 4.Yasuoka S, Peterson HA, MacCarty CS. Incidence of spinal column deformity after multilevel laminectomy in children and adults. J Neurosurg. 1982;57:441–5. doi: 10.3171/jns.1982.57.4.0441. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AS. X. Unilateral laminectomy. Ann Surg. 1910;51:529–33. doi: 10.1097/00000658-191004000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zong S, Zeng G, Xiong C, Wei B. Treatment results in the differential surgery of intradural extramedullary schwannoma of 110 cases. PLoS One. 2013;8:e63867. doi: 10.1371/journal.pone.0063867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie T, Qian J, Lu Y, Chen B, Jiang Y, Luo C, et al. Biomechanical comparison of laminectomy, hemilaminectomy and a new minimally invasive approach in the surgical treatment of multilevel cervical intradural tumour: A finite element analysis. Eur Spine J. 2013;22:2719–30. doi: 10.1007/s00586-013-2992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panjabi MM. Clinical spinal instability and low back pain. J Electromyogr Kinesiol. 2003;13:371–9. doi: 10.1016/s1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 9.Yeo DK, Im SB, Park KW, Shin DS, Kim BT, Shin WH, et al. Profiles of spinal cord tumors removed through a unilateral hemilaminectomy. J Korean Neurosurg Soc. 2011;50:195–200. doi: 10.3340/jkns.2011.50.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mobbs RJ, Maharaj MM, Phan K, Rao PJ. Unilateral hemilaminectomy for intradural lesions. Orthop Surg. 2015;7:244–9. doi: 10.1111/os.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogden AT, Bresnahan L, Smith JS, Natarajan R, Fessler RG. Biomechanical comparison of traditional and minimally invasive intradural tumor exposures using finite element analysis. Clin Biomech (Bristol, Avon) 2009;24:143–7. doi: 10.1016/j.clinbiomech.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Iplikcioglu AC, Hatiboglu MA, Ozek E, Dinc C, Erdal M. Surgical removal of spinal mass lesions with open door laminoplasty. Cent Eur Neurosurg. 2010;71:213–8. doi: 10.1055/s-0030-1249044. [DOI] [PubMed] [Google Scholar]
- 13.Xie T, Qian J, Wu X, Lu Y, Hu G, Luo C, et al. Unilateral, multilevel, interlaminar fenestration in the removal of a multisegment cervical intramedullary ependymoma. Spine J. 2013;13:747–53. doi: 10.1016/j.spinee.2013.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Gu R, Liu JB, Xia P, Li C, Liu GY, Wang JC, et al. Evaluation of hemilaminectomy use in microsurgical resection of intradural extramedullary tumors. Oncol Lett. 2014;7:1669–72. doi: 10.3892/ol.2014.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turel MK, D’Souza WP, Rajshekhar V. Hemilaminectomy approach for intradural extramedullary spinal tumors: An analysis of 164 patients. Neurosurg Focus. 2015;39:E9. doi: 10.3171/2015.5.FOCUS15170. [DOI] [PubMed] [Google Scholar]
- 16.Boström A, Bürgel U, Reinacher P, Krings T, Rohde V, Gilsbach JM, et al. A less invasive surgical concept for the resection of spinal meningiomas. Acta Neurochir (Wien) 2008;150:551–6. doi: 10.1007/s00701-008-1514-0. [DOI] [PubMed] [Google Scholar]
- 17.Saito T, Arizono T, Maeda T, Terada K, Iwamoto Y. A novel technique for surgical resection of spinal meningioma. Spine (Phila Pa 1976) 2001;26:1805–8. doi: 10.1097/00007632-200108150-00017. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi RH, German JW. Minimally invasive approach for the treatment of intradural spinal pathology. Neurosurg Focus. 2013;35:E5. doi: 10.3171/2013.5.FOCUS13163. [DOI] [PubMed] [Google Scholar]
- 19.Shibata T, Nakamura H, Yamano Y. Intradural arachnoid cyst associated with thoracic spinal compression fracture: 7-year follow up after surgery. Spinal Cord. 2001;39:599–601. doi: 10.1038/sj.sc.3101217. [DOI] [PubMed] [Google Scholar]
- 20.Endo H, Takahashi T, Shimizu H, Tominaga T. Thoracic intradural arachnoid cyst associated with surgical removal of epidural hematoma – Case report. Neurol Med Chir (Tokyo) 2004;44:607–10. doi: 10.2176/nmc.44.607. [DOI] [PubMed] [Google Scholar]
- 21.Turel MK, Rajshekhar V. Magnetic resonance imaging localization with cod liver oil capsules for the minimally invasive approach to small intradural extramedullary tumors of the thoracolumbar spine. J Neurosurg Spine. 2014;21:882–5. doi: 10.3171/2014.9.SPINE14199. [DOI] [PubMed] [Google Scholar]
- 22.Pompili A, Caroli F, Crispo F, Giovannetti M, Raus L, Vidiri A, et al. Unilateral laminectomy approach for the removal of spinal meningiomas and schwannomas: Impact on pain, spinal stability, and neurologic results. World Neurosurg. 2016;85:282–91. doi: 10.1016/j.wneu.2015.09.099. [DOI] [PubMed] [Google Scholar]
- 23.Zong S, Zeng G, Du L, Fang Y, Gao T, Zhao J, et al. Treatment results in the different surgery of intradural extramedullary tumor of 122 cases. PLoS One. 2014;9:e111495. doi: 10.1371/journal.pone.0111495. [DOI] [PMC free article] [PubMed] [Google Scholar]