Abstract
Persulfides (R–SSH) have been hypothesized as critical components in sulfur-mediated redox cycles and as potential signaling compounds, similar to hydrogen sulfide (H2S). Hindering the study of persulfides is a lack of persulfide donor compounds with selective triggers that release discrete persulfide species. Herein we report the synthesis and characterization of an ROS-responsive, self-immolative persulfide donor. The donor, termed BDP-NAC, showed selectivity towards H2O2 over other potential oxidative or nucleophilic triggers, resulting in the sustained release of the persulfide of N-acetyl cysteine (NAC) over the course of 2 h, as measured by LCMS. Exposure of H9C2 cardiomyocytes to H2O2 revealed that BDP-NAC mitigated the effects of a highly oxidative environment in a dose-dependent manner over relevant controls and to a greater degree than common H2S donors sodium sulfide (Na2S) and GYY4137. BDP-NAC also rescued cells more effectively than a non-persulfide releasing control compound with a Bpin moiety in concert with common H2S donors and thiols.
Keywords: Hydrogen sulfide, Gasotransmitter, Self-Immolative, Prodrug, Peroxide
Graphical Abstract
Persulfides (R–SSH) have been hypothesized as critical components in sulfur-mediated redox cycles and as potential signaling compounds, similar to hydrogen sulfide (H2S). Hindering the study of persulfides is a lack of persulfide donor compounds with selective triggers that release discrete persulfide species. Herein we report the synthesis and characterization of a redox-responsive, self-immolative persulfide donor. The donor, termed BDP-NAC, shows selectivity towards H2O2.
Hydrogen sulfide (H2S) plays a key signaling role in mammalian biology and has been under investigation as a potential therapeutic via exogenous delivery.[1] To help elucidate its biological roles, chemists have synthesized several types of H2S releasing compounds (termed H2S donors) with a variety of biologically relevant triggers, including water,[2] nucleophiles (e.g., thiols, amines),[3] enzymes,[4] and light[5]. Additionally, compounds that release carbonyl sulfide (COS),[6] and sulfur dioxide (SO2),[7] have recently been reported, allowing for the study of other small molecule sulfur species as potential signaling compounds. These donors aid in our understanding of the physiological roles of H2S and related compounds, and hold potential therapeutic value via exogenous H2S delivery.[8] Interestingly, recent studies into the redox chemistry of sulfur species in the body indicate that persulfides (R–SSH) may have physiological roles similar to H2S, insinuating that some of the physiological effects ascribed to delivery of H2S may actually be derived from persulfides.[9] Further study of persulfides is needed to differentiate between the roles of H2S itself and its biological products. Moreover, a clear description of sulfur redox chemistry in a biological context will allow further development of therapeutics that exploit pathways involved in H2S signaling.
Dean and coworkers first identified persulfides in a biological context in their 1994 report on a protein persulfide intermediate of the cysteine desulfurase NifS.[10] Persulfides are prevalent in mammalian biology, generated via reaction of an oxidized thiol (e.g., a sulfenic acid, R–SOH) with H2S in a process called S-persulfidation.[11] More nucleophilic than thiols, persulfides have pKa values a few units lower than their corresponding thiols,[12] as well as greater reduction potentials,[13] making them highly reactive, transiently stable species. In a biological context, persulfides protect thiols from irreversible oxidation, serve as reactive intermediates in sulfur shuttling,[14] and alter enzymatic activity.[15] Some examples of protein persulfidation and the resulting changes in protein activity include: an increased parkin activity upon S-persulfidation resulting in a decrease in Parkinson’s symptoms,[16] an increase in activity of GAPDH, protecting cells from apoptosis,[17] and H-Ras activation in cardiac tissue, regulating cellular redox signaling.[18] More recently, studies have confirmed the presence of endogenously produced small molecule persulfides (e.g., cysteine persulfide and glutathione persulfide) with reported concentrations as high as 150 μM in human and mouse tissue.[9a] Small molecule persulfides likely play a role in regulating cellular redox balance and mediating cellular signaling.[9c] A major barrier in the study of the biological roles of persulfides is a lack of chemical tools capable of generating well-defined persulfide species in response to specific, biologically relevant triggers. Our understanding of H2S biology has been aided immensely by the synthesis of organic H2S donors; analogous to H2S, persulfide donors will be vital tools for understanding how persulfides fit into the overall web of redox signaling.
Polysulfides (RS–(S)n–SR), such as naturally occurring diallyl trisulfide (DATS), are perhaps the best known type of persulfide donor, but their reactivity in biological systems is complex, leading to generation of other redox-active species, including H2S.[19] As a result, polysulfides are not ideal persulfide donors for use in studying persulfide biology, and the complex product mixture may limit their therapeutic potential. Free persulfides (i.e., R–SSH) have been isolated, but they suffer from poor stability under storage conditions and poor water solubility, and thus have relatively low utility in a practical sense.[19c] Persulfides are also proposed intermediates in several types of H2S donors,[3] but these compounds all require conditions that cause rapid conversion of the persulfide into H2S. To date there exist only two families of compounds capable of generating discrete persulfides: Wang and coworkers developed esterase-triggered persulfide prodrugs capable of releasing either a persulfide or hydrogen persulfide (HSSH) and Galardon and coworkers developed a pH-triggered persulfide analog of the nitrosothiol SNAP.[20] These donors generate persulfides without concomitant generation of H2S and can be viewed as spontaneous persulfide donors due to the ubiquity of esterases in vitro and in vivo.
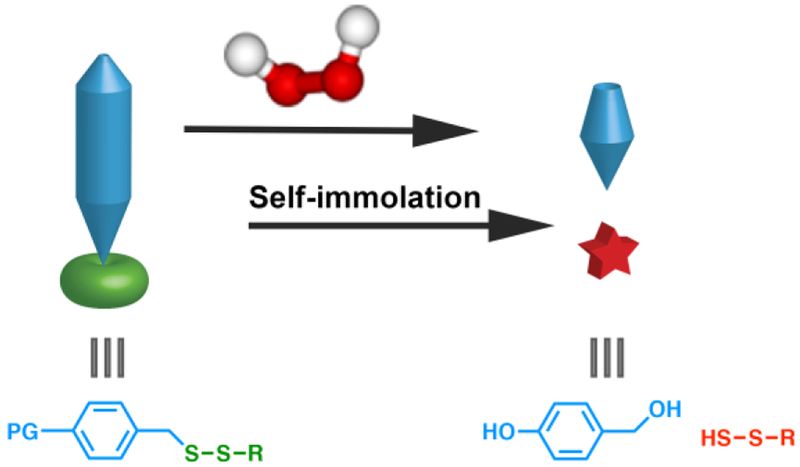
We sought to synthesize a discrete persulfide donor scaffold, inert under normal physiological conditions but capable of self-immolation in response to a specific trigger, revealing a discrete persulfide species (Figure 1). As the triggering moiety and persulfide could be readily tuned, this system would enable persulfide generation in response to many types of triggers, providing a valuable set of laboratory tools similar to the self-immolative COS donors recently reported by Pluth and coworkers.[21] In addition to their use as biological tools to study persulfide reactivity, persulfide prodrugs are exciting from a therapeutic standpoint because the reduction potential of persulfides is higher than that of thiols or H2S, making them prime candidates for scavenging and reducing the harmful effects resulting from high levels of reactive oxygen species (ROS). Therefore, we aimed to synthesize an ROS-responsive persulfide prodrug as a proof of concept. This would allow for a two-stage quenching of ROS: the initial reaction of the ROS with the prodrug to trigger release, followed by the release of the persulfide. We envisioned that such an ROS-triggered persulfide prodrug would be ideal for cytoprotection against harmful levels of ROS.
Figure 1.
Cartoon schematic representing the proposed release of a discrete persulfide species from a generalized self-immolative prodrug (PG = protecting group) in the presence of a trigger (H2O2 shown here).
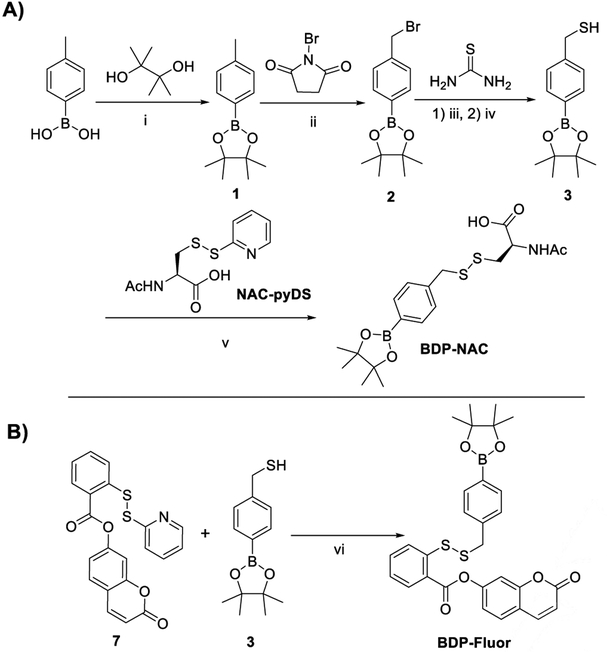
Aryl boronic esters are relatively easy to synthesize, generally biocompatible, and react selectively with ROS in a B–C bond cleavage reaction to reveal the corresponding phenolate. Therefore, we set out to synthesize a self-immolative persulfide donor containing an aryl boronic ester as an ROS-sensitive trigger. The desired persulfide donor (termed BDP-NAC for Bpin-disulfide prodrug-N-acetyl cysteine) was synthesized from commercially available 4-tolylboronic acid in four steps (Scheme 1A). Theoretically, any thiol may be installed on the distal end of the disulfide bond from the trigger/self-immolation moiety. Our choice of N-acetyl cysteine (NAC) was motivated by its biocompatibility as well as its ability to protect cells in vitro in highly oxidative environments.[22]
Scheme 1.
A) Synthetic route to BDP-NAC. Conditions: i) MgSO4, Et2O, rt, 16 h; ii) AIBN, C6H12, reflux, 16 h; iii) 1) EtOH, rt, 4 h; iv) 1 N NaOH, reflux, 45 min; v) NEt3, CHCl3, rt, 3 h; B) Synthetic route to BDP-Fluor. Conditions: vi) CHCl3:MeOH (1:1 v/v), rt, 40 h.
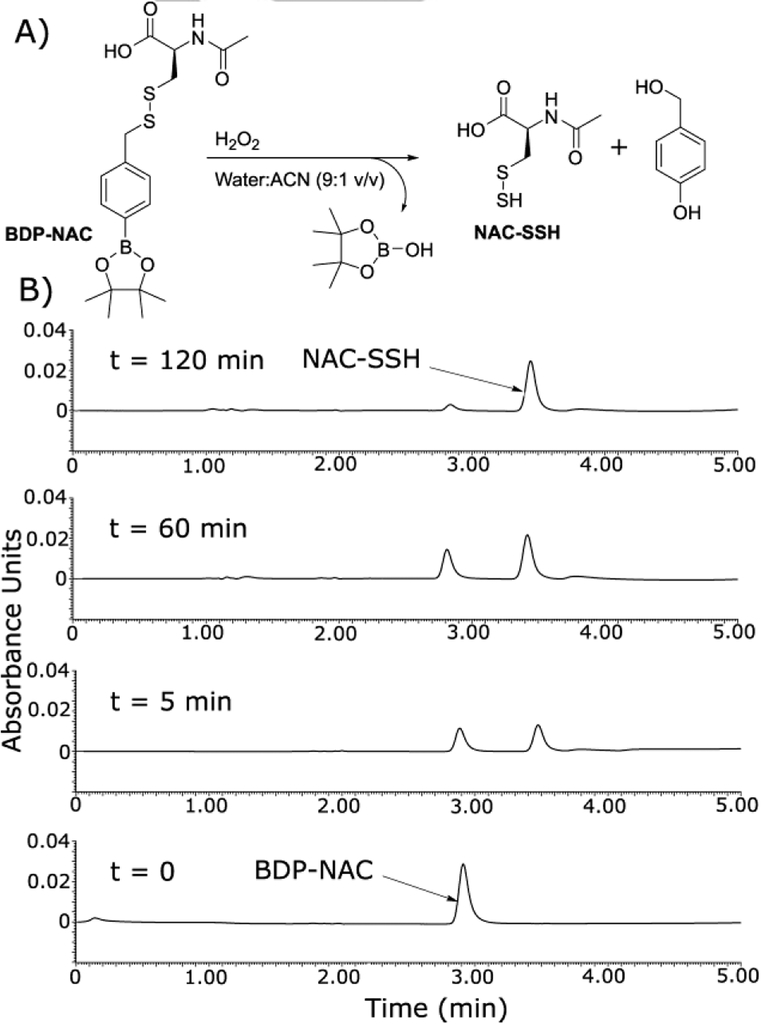
The ability of BDP-NAC to mediate the release of the desired NAC persulfide (NAC-SSH) in response to ROS was analyzed by LCMS (Figure 2). Aliquots of the reaction mixture of BDP-NAC with H2O2 were injected at various time points until the peak attributed to BDP-NAC (2.9 min) had subsided, revealing near complete decomposition of BDP-NAC within 2 h. A peak corresponding to NAC–SSH (3.4 min) increased in intensity over the course of the reaction, consistent with our proposed mechanism of persulfide generation. Mass spectrometry evidence also confirmed the presence of the other byproduct of the reaction, 4-hydroxybenzyl alcohol (a result of addition of water to the quinone methide), but the chromatogram peak was weak, likely due to low absorbance at the monitoring wavelength.
Figure 2.
A) Proposed reaction of BDP-NAC in the presence of H2O2 leading to the release of NAC–SSH. B) LC chromatograms highlighting the conversion of BDP-NAC into NAC persulfide (NAC-SSH) in the presence of H2O2. Timepoints are noted above each LC chromatogram. The peak eluting at 2.9 min corresponds to BDP-NAC, and the peak at 3.4 min corresponds to NAC-SSH (see Figure S20 for corresponding mass spectrometry data).
In addition to LCMS, we also investigated the reaction of BDP-NAC with H2O2 utilizing 1H NMR spectroscopy. Experiments were conducted in DMSO-d6:D2O (9:1 v/v) due to the hydrophobic nature of BDP-NAC and the increased concentration required in NMR spectroscopy compared with LCMS. Shortly after the addition of H2O2 to the BDP-NAC solution, two new sets of peaks in the aryl region of the 1H NMR spectrum appeared. One was consistent with 4-hydroxybenzyl alcohol, and the other was attributed to the slow hydrolysis of the Bpin moiety of BDP-NAC, yielding a boronic acid; boronic acids react with H2O2 in a similar fashion as pinacol boronic esters.[23] The reaction was considerably slower under these conditions than in the LCMS experiments. This retardation in reaction rate is likely a result of the high organic solvent content in the reaction.[24] Further insights into the stability of BDP-NAC as a persulfide prodrug as well as pertinent controls without the Bpin triggering moiety can be found in the SI (Figure S25 and S26).
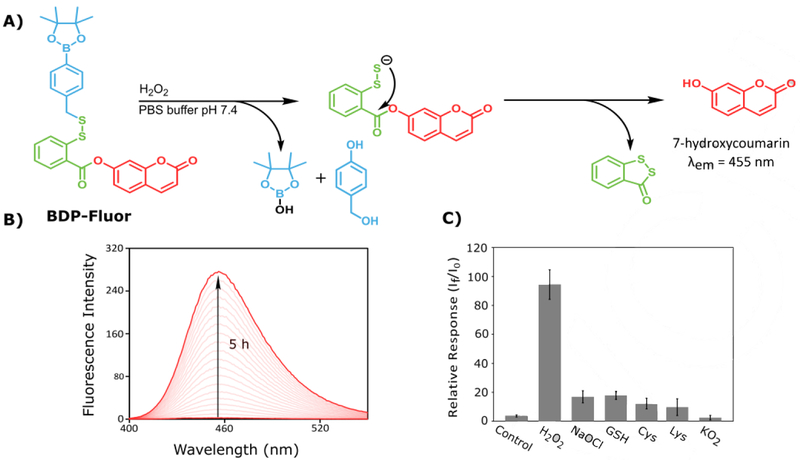
To further evaluate the reactivity and trigger selectivity of BDP-NAC, a profluorophore (BDP-Fluor, Scheme 1B) was synthesized. Drawing inspiration from Xian and coworkers’ turnon fluorescence probe (compound 7) used for detection of sulfane sulfur species (HSSH, RS–(S)n–SR, or S8), the self-immolative BDP-Fluor has the same general structure as BDP-NAC, but with a coumarin-based fluorophore as the distal thiol species.[25] As shown in Figure 3A, we expected self-immolation to trigger release of a discrete persulfide, which would then cyclize to form a 5-membered benzodithiolone species, resulting in the release of 7-hydroxycoumarin. Because BDP-Fluor itself is not fluorescent, an increase in fluorescence at the characteristic emission wavelength of 7-hydroxycoumarin should only result from persulfide release and subsequent intramolecular cyclization, providing secondary confirmation of persulfide release from these self-immolative prodrug systems.
Figure 3.
A) Proposed reaction mechanism for the release of 7-hydroxycoumarin from BDP-Fluor in the presence of H2O2. B) Representative overlay of the fluorescence spectra of BDP-Fluor in the presence of 100-fold excess H2O2 resulting from the release of 7-hydroxycoumarin over the course of 5 h. C) Relative response of BDP-Fluor (3.3 μM) to each potential trigger (330 μM) or control (no trigger added) represented as the ratio of the final fluorescence (If) intensity after 5 h to the initial fluorescence intensity (I0), showing an increased selectivity for H2O2 over other potential triggers.
We tested this design by exposing BDP-Fluor to a variety of potential triggers. BDP-Fluor showed no evidence of self-immolative behavior (i.e., no fluorescence signal) in the absence of a trigger, but addition of H2O2 (100-fold excess) led to a 90-fold increase in fluorescence intensity at the characteristic wavelength of 7-hydroxycoumarin after incubation for 5 h in PBS buffer (Figure 3B and C). When BDP-Fluor was treated with other potential triggers, including sodium hypochlorite (NaOCl), cysteine (Cys), glutathione (GSH), lysine (Lys), and potassium superoxide (KO2), the response was significantly lower, with H2O2 showing a greater than 20-fold response over all of these potential triggers, and a greater than 90-fold response over Lys and KO2.
As the increase in fluorescence response to thiols was unexpected, further investigation indicated that the fluorescence increase may be attributed to nucleophilic attack by cysteine at the aryl ester position, resulting in the release of 7-hydroxycoumarin. For more discussion on the probe response to thiols and relevant controls, see the SI (Figure S22). Taken together, these results confirm release of the desired persulfide species and demonstrate the selectivity of H2O2 as a trigger.
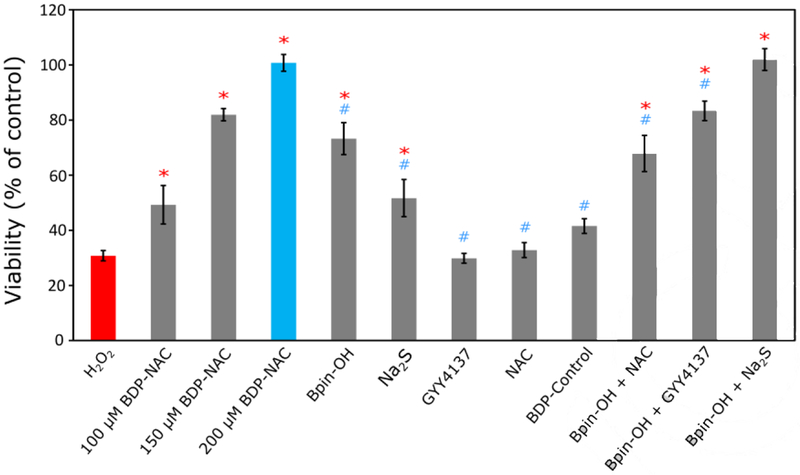
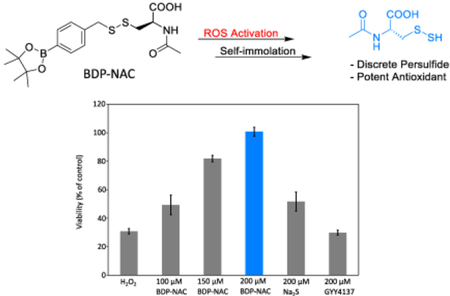
We next aimed to analyze BDP-NAC in a biological context. In vitro cytotoxicity studies on H9C2 cardiomyocytes showed that BDP-NAC is non-toxic up to 200 μM (Figure S27). As mentioned previously, persulfides have greater reducing potential than their corresponding thiols as well as H2S. Thus, we envisioned that BDP-NAC might be effective in rescuing cells under oxidative stress, either via direct reduction of H2O2 or via upregulation of antioxidant pathways mediated by persulfide signaling.[9a, 15a] To this end, we evaluated the protective effects of BDP-NAC on H9C2 cells in culture via exogenous delivery of H2O2, which stresses the cells and promotes apoptosis (Figure 4). In the absence of BDP-NAC, cell viability drastically decreased after exposure to H2O2 (100 μM) for 1 h. However, simultaneous application of BDP-NAC (100–200 μM) with H2O2 showed a dose-dependent increase in cell viability, with no cytotoxicity observed after treatment with 200 μM BDP-NAC. At longer treatment times (2 h), BDP-NAC rescued a similar percentage of cells compared to H2O2-only controls (Figure S29). These results indicate that BDP-NAC can successfully mitigate the deleterious effects of a hyperoxidative environment in culture.
Figure 4.
Viability of H9C2 cardiomyoctyes treated with BDP-NAC or various controls and related compounds concurrent with exposure to H2O2 (100 μM) for 1 h. Each control compound was applied at a concentration of 200 μM (except for Na2S (100 μM) in the Bpin-OH + Na2S treatment group). Quantification of viability was carried out using Cell Counting Kit-8 (CCK-8). Results are expressed as the mean ± SEM (n = 10–15 for each treatment group) with 2–3 independent experiments. *P<0.01 for comparisons with the H2O2 treatment group and #P<0.01 for comparisons with the BDP-NAC (200 μM) treatment group. Group comparisons are indicated as determined by a one-way analysis of variance (ANOVA) with a Student-Newman-Keuls comparisons post-hoc test.
To further ensure that persulfide release imparts protection to the cardiomyocytes in the presence of H2O2, several control studies were carried out. Exposure of the cells to H2O2 with added 4-(hydroxymethyl)benzeneboronic acid pinacol ester (Bpin-OH), a non-persulfide releasing compound with a Bpin moiety, showed an increase in viability compared to H2O2 alone but did not rescue cells to the same extent as BDP-NAC. We also compared BDP-NAC to sodium sulfide (Na2S), a fast-releasing H2S donor, and GYY4137, a slow-releasing H2S donor, under the same experimental conditions. Na2S had a limited ability to rescue cells while GYY4137 had no effect on viability. Interestingly, BDP-NAC was more effective at rescuing cells than Na2S, even while Na2S enhanced H9C2 proliferation in the absence of H2O2 (Figure S28). This provides further evidence that persulfides may serve to maintain redox homeostasis in cells to a greater extent than H2S. NAC, a potential thiol byproduct after reaction of BDP-NAC, also had no effect on viability. To confirm that BDP-NAC derives its activity from ROS-triggered persulfide release, the cardiomyocytes were treated with BDP-Control, which has an identical structure to BDP-NAC, but without the Bpin triggering moiety. BDP-Control also did not rescue cells exposed to H2O2 under identical conditions to the previous experiments.
Finally, to recreate the synergistic effects of the Bpin moiety of BDP-NAC and the resultant persulfide release, cells were treated with Bpin-OH simultaneously with either NAC, GYY4137, or Na2S. Each of these combinations was able to mitigate the effects of H2O2 on cell viability, but not to the same degree as BDP-NAC, with the exception of Bpin-OH + Na2S. We suspect that simultaneous treatment of the cardiomyocytes with Bpin-OH and Na2S gives a greater instantaneous concentration of potential antioxidants than BDP-NAC, considering its sustained release. In a system with continuous generation of ROS, delivery of Bpin-OH and Na2S would likely have a diminished ability to rescue cells compared to sustained release from BDP-NAC.
In summary, we have synthesized a self-immolative prodrug that releases a discrete persulfide species (BDP-NAC) in the presence of H2O2. Persulfide release and trigger specificity were characterized by LCMS, NMR, and fluorescence spectroscopy, demonstrating that sustained release of the persulfide is selectively triggered by H2O2. In vitro studies using H9C2 cardiomyocytes under oxidative stress showed that BDP-NAC mitigates the harmful effects of highly oxidative environments with greater potency than commonly used H2S donors Na2S and GYY4137 as well as relevant controls. BDP-NAC not only shows promise therapeutically, but it also provides a modular system for persulfide donors that may be triggered under a variety of conditions. We envision that a library of persulfide donors based on the BDP-NAC template will enable the study of persulfide biology in greater depth than is currently possible, providing insight into sulfur redox cycles and sulfur-mediated cell signaling.
Supplementary Material
Acknowledgements
This work was supported by the National Science Foundation (DMR-1454754) and the National Institutes of Health (R01GM123508). We also thank 3M for support of this work through a Non-Tenured Faculty Award to JBM. We thank Dr. Tijana Grove and Dr. Webster Santos and their students for experimental assistance as well as Dr. Mehdi Ashraf-Khorassani for help with the LCMS
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1] a).Wang R, FASEB J. 2002, 16, 1792–1798; [DOI] [PubMed] [Google Scholar]; b) Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabó C, Proc. Natl. Acad. Sci. U. S. A 2009, 106, 21972–21977; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mustafa AK, Gadalla MM, Snyder SH, Sci. Signal 2009, 2, re2–re2; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Predmore BL, Lefer DJ, Gojon G, Antioxid. Redox Signal 2012, 17, 119–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2] a).Zhao W, Zhang J, Lu Y, Wang R, EMBO J 2001, 20, 6008–6016 ;b) Nicolau LAD, Silva RO, Damasceno SRB, Carvalho NS, Costa NRD, Aragão KS, Barbosa ALR, Soares PMG, Souza MHLP, Medeiros JVR, Braz. J. Med. Biol. Res 2013, 46, 708–714;11689441 [Google Scholar]; c) Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan C-H, Moore PK, Circulation 2008, 117, 2351–2360; [DOI] [PubMed] [Google Scholar]; d) Zanatta SD, Jarrott B, Williams SJ, Aust. J. Chem 2010, 63, 946–957. [Google Scholar]
- [3] a).Zhao Y, Wang H, Xian M, J. Am. Chem. Soc. 2011, 133, 15–17; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Foster JC, Powell CR, Radzinski SC, Matson JB, Org. Lett 2014, 16, 1558–1561. [DOI] [PubMed] [Google Scholar]
- [4] a).Zheng Y, Yu B, Ji K, Pan Z, Chittavong V, Wang B, Angew. Chem. Int. Ed. Engl 2016, 55, 4514–4518; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li Z, Organ CL, Zheng Y, Wang B, Lefer DJ, Circulation 2016, 134, A17903–A17903; [Google Scholar]; c) Chauhan P, Bora P, Ravikumar G, Jos S, Chakrapani H, Org. Lett 2017, 19, 62–65. [DOI] [PubMed] [Google Scholar]
- [5] a).Devarie-Baez NO, Bagdon PE, Peng B, Zhao Y, Park C-M, Xian M, Org. Lett 2013, 15, 2786–2789; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fukushima N, leda N, Sasakura K, Nagano T, Hanaoka K, Suzuki T, Miyata N, Nakagawa H, Chem. Commun 2014, 50, 587–589. [DOI] [PubMed] [Google Scholar]
- [6] a).Powell CR, Foster JC, Okyere B, Theus MH, Matson JB, J. Am. Chem. Soc 2016, 138, 13477–13480; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Steiger AK, Pardue S, Kevil CG, Pluth MD, J. Am. Chem. Soc 2016, 138, 7256–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7] a).Wang W, Ji X, Du Z, Wang B, Chem. Commun 2017, 53, 1370–1373; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Day JJ, Yang Z, Chen W, Pacheco A, Xian M, ACS Chem. Biol 2016, 11, 1647–1651. [DOI] [PubMed] [Google Scholar]
- [8] a).Zheng Y, Yu B, De La Cruz LK, Roy Choudhury M, Anifowose A, Wang B, Med. Res. Rev 2018, 38, 57–100; [DOI] [PubMed] [Google Scholar]; b) Powell CR, Dillon KM, Matson JB, Biochem. Pharmacol. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhao Y, Biggs TD, Xian M, Chem. Commun 2014, 50, 11788–11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9] a).Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, Akaike T, Proc. Natl. Acad. Sci. U. S. A 2014, 111, 7606–7611; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yadav PK, Martinov M, Vitvitsky V, Seravalli J, Wedmann R, Filipovic MR, Banerjee R, J. Am. Chem. Soc 2016, 138, 289–299; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Cuevasanta E, Möller MN, Alvarez B, Arch. Biochem. Biophys 2017, 617, 9–25; [DOI] [PubMed] [Google Scholar]; d) Filipovic MR, Zivanovic J, Alvarez B, Banerjee R, Chem. Rev 2018, 118, 1253–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zheng L, White RH, Cash VL, Dean DR, Biochemistry 1994, 33, 4714–4720. [DOI] [PubMed] [Google Scholar]
- [11].Filipovic MR, in Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide (Eds.: Moore PK, Whiteman M), Springer International Publishing, Cham, 2015, pp. 29–59. [Google Scholar]
- [12].Cai Y-R, Hu C-H, J. Phys. Chem. B 2017, 121, 6359–6366. [DOI] [PubMed] [Google Scholar]
- [13].Francoleon NE, Carrington SJ, Fukuto JM, Arch. Biochem. Biophys. 2011, 516, 146–153. [DOI] [PubMed] [Google Scholar]
- [14].Flint DH, J. Biol. Chem. 1996, 271, 16068–16074. [PubMed] [Google Scholar]
- [15] a).Millikin R, Bianco CL, White C, Saund SS, Henriquez S, Sosa V, Akaike T, Kumagai Y, Soeda S, Toscano JP, Lin J, Fukuto JM, Free Radic. Biol. Med 2016, 97, 136–147; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mueller EG, Nat. Chem. Biot 2006, 2, 185–194. [DOI] [PubMed] [Google Scholar]
- [16].Vandiver MS, Paul BD, Xu R, Karuppagounder S, Rao F, Snowman AM, Ko HS, Lee YI, Dawson VL, Dawson TM, Sen N, Snyder SH, Nat. Commun. 2013, 4, 1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH, Sci. Signal 2009, 2, ra72–ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, Akaike T, Nat. Chem. Biol. 2012, 8, 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19] a).Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW, Proc. Natl. Acad. Sci. U. S. A 2007, 104, 17977–17982; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cerda MM, Hammers MD, Earp MS, Zakharov LN, Pluth MD, Org. Lett 2017, 19, 2314–2317; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Park C-M, Weerasinghe L, Day JJ, Fukuto JM, Xian M, Mol. Biosyst 2015, 11, 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20] a).Zheng Y, Yu B, Li Z, Yuan Z, Organ CL, Trivedi RK, Wang S, Lefer DJ, Wang B, Angew. Chem. Int. Ed 2017, 56, 11749–11753; [DOI] [PubMed] [Google Scholar]; b) Yu B, Zheng Y, Yuan Z, Li S, Zhu H, De La Cruz LK, Zhang J, Ji K, Wang S, Wang B, J. Am. Chem. Soc 2017; [DOI] [PubMed] [Google Scholar]; c) Artaud I, Galardon E, ChemBioChem 2014, 15, 2361–2364. [DOI] [PubMed] [Google Scholar]
- [21].Zhao Y, Henthorn HA, Pluth MD, J. Am. Chem. Soc 2017, 139, 16365–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22] a).Zafarullah M, Li W, Sylvester J, Ahmad M, Cell. Mol. Life Sci 2003, 60, 6–20; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Murphy NP, Lampe KJ, Biotechnol. Bioeng 2018, 115, 246–256. [DOI] [PubMed] [Google Scholar]
- [23].Kuivila HG, J. Am. Chem. Soc 1954, 76, 870–874. [Google Scholar]
- [24].Schmid KM, Jensen L, Phillips ST, J. Org. Chem 2012, 77, 4363–4374. [DOI] [PubMed] [Google Scholar]
- [25].Chen W, Liu C, Peng B, Zhao Y, Pacheco A, Xian M, Chem. Sci 2013, 4, 2892–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.