Abstract
Introduction
Scutellaria baicalensis is commonly used in Asia as an herbal medicine to treat a variety of ailments, including cancer. Wogonoside, one major constituent of Scutellaria baicalensis, can be primarily converted to wogonin through deglycosylation via enteric microbiome metabolism.
Materials and Methods
The antiproliferative effects of the glycoside (wogonoside) and its deglycosylated compound (wogonin) on a panel of human cancer cell lines from the most common solid tumors were evaluated using the MTS method. Cell cycle and apoptosis were determined using flow cytometry. Enzymatic activities of caspases were measured, and the interactions of wogonin and caspases were explored by a docking analysis.
Results
Wogonoside did not have obvious antiproliferative effects on the cancer cells. In contrast, wogonin showed significant antiproliferative activities on all the tested cancer cells. Wogonin arrested the cells in the G1 phase and significantly induced cell apoptosis. The compound also activated the expression of caspases 3 and 9. The docking results suggest that the compound forms hydrogen bonds with Phe250 and Ser251, and π-π interactions with Phe256 in caspase 3, and with Asp228 in caspase 9.
Conclusions
After wogonoside deglycosylation, wogonin significantly enhanced its anticancer potential as a potent anticancer compound derived from S. baicalensis.
Keywords: Deglycosylation, wogonoside, wogonin, apoptosis, receptor-ligand docking
INTRODUCTION
Scutellaria baicalensis is widely used in the traditional medical systems of China and Japan as an herbal medicine to treat a variety of ailments, including cancer.[1–4] The major constituents of this botanical are a group of flavonoid glycosides, including wogonoside, baicalin, and oroxylin A-7-O-glucuronide.[5] S. baicalensis is most commonly administered orally.[6,7] Thus, the constituents of the herb inevitably come into contact with intestinal microbiota after oral ingestion. Many of these constituents can be transformed by the intestinal bacteria before being absorbed in the gastrointestinal tract.[8] For natural glycosides, the most common metabolic pathway is the deglycosylation reaction induced by intestinal bacteria via the stepwise cleavage of the sugar moieties.[9–11] After deglycosylation, the newly-formed compounds may have more potent anticancer activities.[12,13]
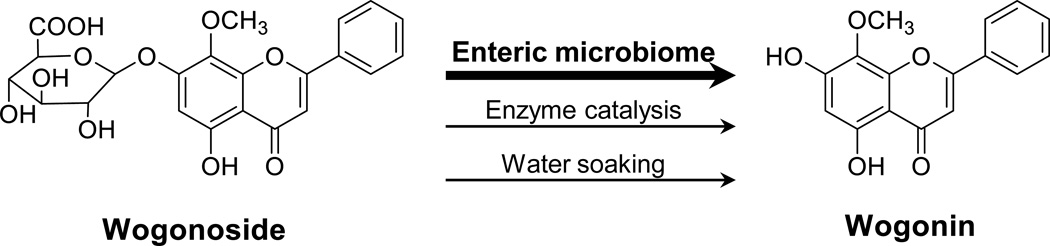
After the oral ingestion of S. baicalensis, enteric microbiota play a major role in converting the parent compound (wogonoside) to the deglycosylated metabolite (wogonin).[8] In addition, wogonoside can also be catalyzed by enzymes or hydrolyzed during the herb’s processing or storage [Figure 1].[12,14] Although attempts have been made to evaluate these two compounds’ anticancer activities,[15,16] a chemopreventive effect comparison between wogonoside and wogonin, linked to the deglycosylation process, has not been performed.
Figure 1.
Chemical structures of wogonoside and wogonin. The deglycosylation of wogonoside and conversion to wogonin can be achieved through enteric microbiome transformation, enzyme catalysis, or herbal processing, such as water soaking.
In this study, we firstly compared the antiproliferative effects of wogonoside and wogonin using six cancer cell lines from three common solid tumors. We selected SW-480 colon cancer cells, which are very sensitive to wogonin treatment, for further mechanistic observations. Levels of caspase expression were subsequently determined. Finally, the possible binding modes of wogonin at the catalytic domains of caspases 3 and 9 were simulated using the receptor-ligand docking analysis.
MATERIALS AND METHODS
Chemicals and materials
The MTS assay kit, CellTiter 96 Aqueous Solution Cell Proliferation Assay, was obtained from Promega (Madison, WI, USA). The annexin V-FITC apoptosis detection kit was obtained from BD Biosciences (Rockville, MD, USA). Caspase 3, 8, 9 kits were obtained from BioVison (Mountain View, CA, USA). Wogonoside and wogonin were obtained the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Both wogonoside and wogonin were of biochemical-reagent grade with > 90% purity as confirmed by HPLC. Other chemicals have been described previously.[17]
Cell lines and cultures
The human colorectal cancer cell lines SW-480, HCT-116, HT-29, human breast cancer cell lines MCF-7, MDA-MB-231, and NSCLC non-small cell lung cancer cells (DMEM) were obtained from American Type Culture Collection (Manassas, VA, USA). The cells were grown in the indicated medium supplemented with 10% FBS and 50 IU penicillin/streptomycin in a humidified atmosphere with 5% CO2 at 37°C.
Cell proliferation, cell cycle and apoptotic analyses
Cells were seeded in 96-well plates (1×104 cells/well). After 24 h, the indicated concentrations of drugs were added to the wells. The final concentration of the drug solvent DMSO was 0.5%. Controls were exposed to culture medium containing 0.5% DMSO without drugs. Following the indicated incubation period, cell proliferation was evaluated using an MTS assay according to the manufacturer’s instructions [18]. Since 0.5% DMSO did not influence the proliferation of six cell lines, results were expressed as the percent of control (DMSO vehicle set at 100%). Cell cycle and apoptosis were determined using flow cytometry.[17] For each measurement, at least 20,000 cells were counted.
Caspases 3, 8 and 9 analyses
SW-480 cells were seeded in 6-well tissue culture plates. After 24 h, the medium was changed and wogonin was added. After treatment for 24 h, cell lysates were collected and expression levels of caspases 3, 8, and 9 were determined by ELISA analysis.[13]
Receptor docking analysis
The possible binding modes of wogonin at the catalytic domains of human caspase 3 and caspase 9 were predicted using the docking program Surflex-Dock (Tripos, St. Louis, MO, USA). The structure of wogonin was generated (through Ligand model in Sybyl), and protein crystal structures were obtained (PDB code 3H0E for caspase 3 and 2AR9 for caspase 9). Intermolecular interactions between wogonin and caspases were analyzed, and the key pharmacophore in the ligand was identified.[19,20]
Statistical analysis
Data are presented as a mean ± standard error (S.E.) (n=3). A one-way ANOVA was employed to determine the statistical significance of the results. In some cases, a Student’s t-test was used to compare two groups. The level of statistical significance was set at P < 0.05.
RESULTS
Antiproliferative effects of wogonoside and wogonin
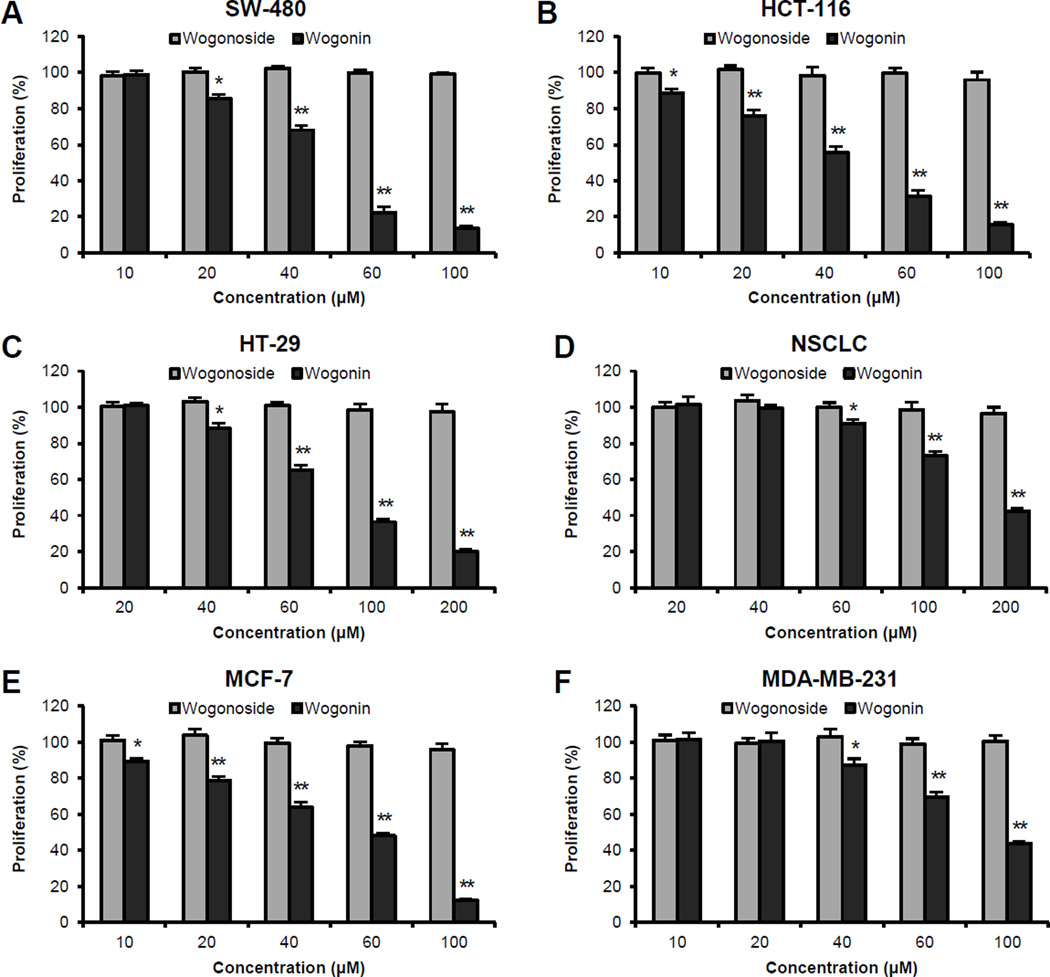
As shown in Figure 2A–C, while 48 h treatment with wogonoside did not inhibit cancer cell growth, wogonin caused concentration-dependent growth suppression in all three colorectal cancer cell lines. At 60 µM, wogonin inhibited cancer cell growth by 77.5 ± 2.9% in SW-480, 68.4 ± 2.8% in HCT-116, and 34.9 ± 2.6% in HT-29 cells, respectively (all P < 0.01 vs. control). Among the three cell lines, wogonin showed the most potent antiproliferative effects in SW-480 and HCT-116 cells with IC50 values of 47.8 and 44.6 µM, respectively.
Figure 2.
Effects of wogonoside and wogonin on the proliferation of different human cancer cell lines were assayed by the MTS method. The cell lines used include colorectal cancer (SW-480, HCT-116, HT-29), non-small cell lung cancer cells (NSCLC) and breast cancer (MCF-7, MDA-MB-231). Cells were treated with 10–200 µM of tested compounds for 48 h. * P<0.05; and ** P<0.01 vs. control (100%).
Similar effects were observed in breast cancer cells. At levels as high as 200 µM, wogonoside did not show antiproliferative effects on either cancer cell lines. Treatment with 60 µM of wogonin inhibited cancer cell growth by 52.0 ± 1.4% in MCF-7 and 30.4 ± 2.7% in MDA-MB-231 cells, respectively (all P < 0.01 vs. control) [Figure 2E, F]. On the other hand, NSCLC cells showed more resistance to wogonin treatment, while cell growth was inhibited by only 9.0 ± 2.3% when treated with 60 µM of wogonin. However, dose-dependent effects were also observed when the treatment concentration increased to 100–200 µM [Figure 2D]. Our data suggested that wogonoside did not show any antiproliferative effects within the tested concentrations. The deglycosylated compound, wogonin, showed significant antiproliferative effects in different human cancer cell lines [Figure 2].
Effects of wogonoside and wogonin on the cell cycle
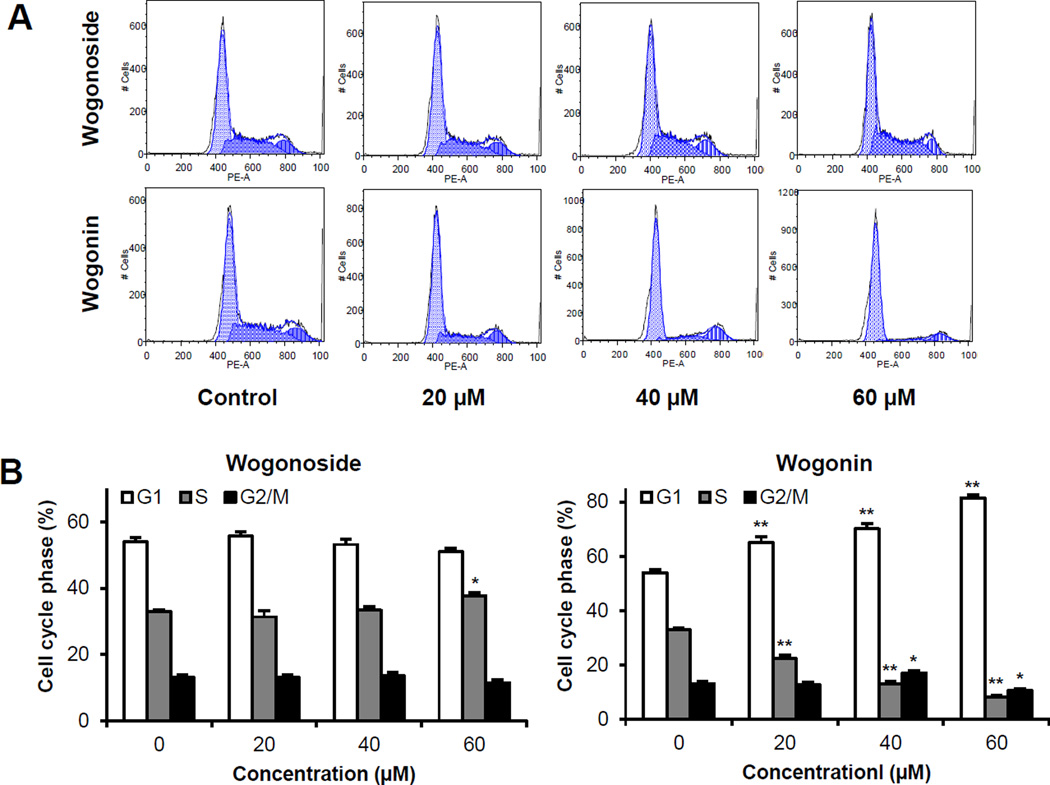
Since SW-480 colorectal cancer cells were very sensitive to wogonin treatment, we selected this cell line for further mechanistic evaluations. As shown in Figure 3, compared to the control, the effects of wogonin on the cell cycle profile were observed at concentrations as low as 20 µM. Treatment of SW-480 cells with 20, 40 and 60 µM wogonin for 48 h increased G1 phase to 65.1%, 70.1% and 81.5%, respectively, compared to 54.0% in vehicle treated cells (all P < 0.01). The 20, 40 and 60 µM wogonin treatment also decreased G2/M phase to 22.3%, 12.9% and 8.0%, respectively, compared to 32.9% in vehicle treated cells (all P < 0.01). Thus, wogonin significantly arrested cancer cells in G1 phase.
Figure 3.
Cell cycle analysis using flow cytometry after staining with propidium iodide (PI). SW-480 cells were treated with 20–60 µM of wogonoside or wogonin for 48 h. (A) Typical cell cycle profiles. (B) Data are presented as the means ± SE of triplicate experiments. *P<0.05, and **P<0.01, vs. control.
Effects of wogonoside and wogonin on apoptosis
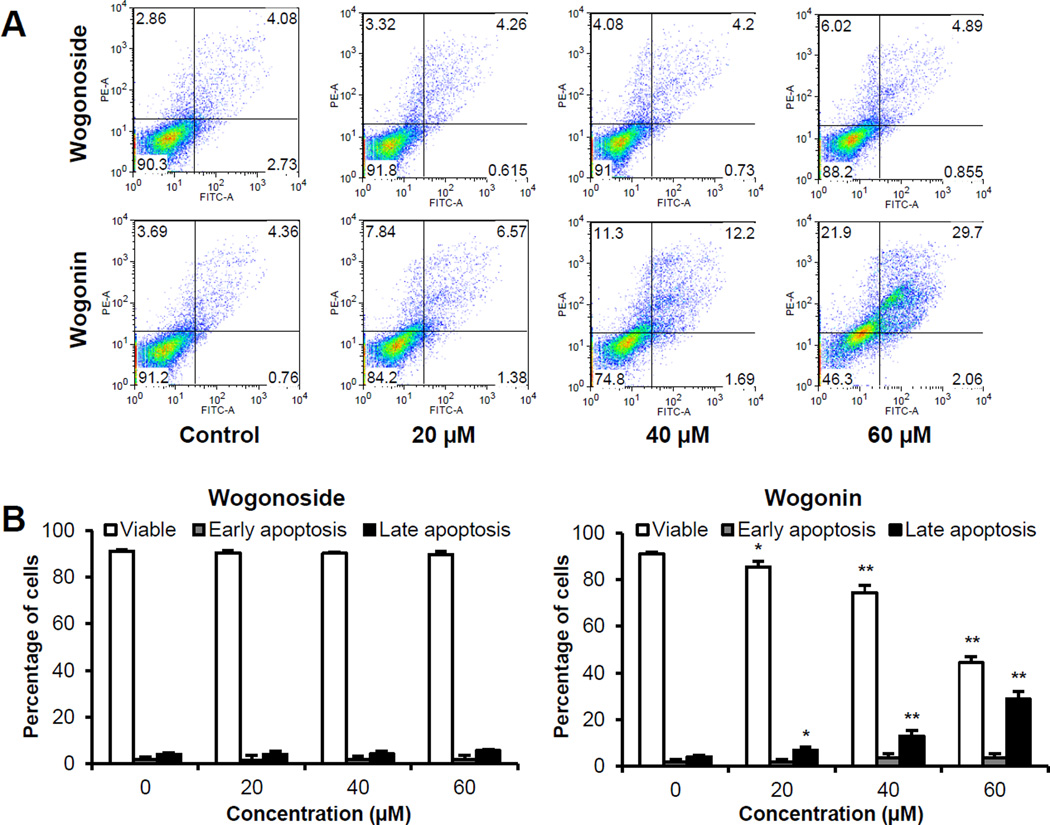
The apoptotic effects of wogonoside and wogonin were evaluated by flow cytometry after staining with annexin V and PI. Annexin V can be detected in both early and late stages of apoptosis, whereas PI stains cells can only be detected in late apoptosis or necrosis. Early apoptotic cells were positive for annexin V and negative for PI (lower right quadrant); late apoptotic cells were stained for both annexin V and PI (upper right quadrant). As shown in Figure 4, following treatment with 20, 40 and 60 µM of wogonin for 48 h, compared to the control (4.0%), the percentage of late apoptotic SW-480 cells increased to 7.0%, 13.0% and 28.9%, respectively (P < 0.05, P < 0.01 and P < 0.01). In contrast, wogonoside at the same concentrations did not induce apoptosis. The data demonstrate that only wogonin significantly induces cell apoptosis.
Figure 4.
Apoptosis assay using flow cytometry after staining with annexin V-FITC/propidium iodide (PI). SW-480 cells were treated with 20–60 µM of wogonoside or wogonin for 48 h. (A) Representative scatter plots of PI (y-axis) vs. annexin V (x-axis). (B) Percentage of viable, early apoptotic and late apoptotic cells. Data are presented as the means ± SE of triplicate experiments. *P<0.05, and **P<0.01 vs. control.
Effects wogonin on the activities of caspases 3, 8 and 9
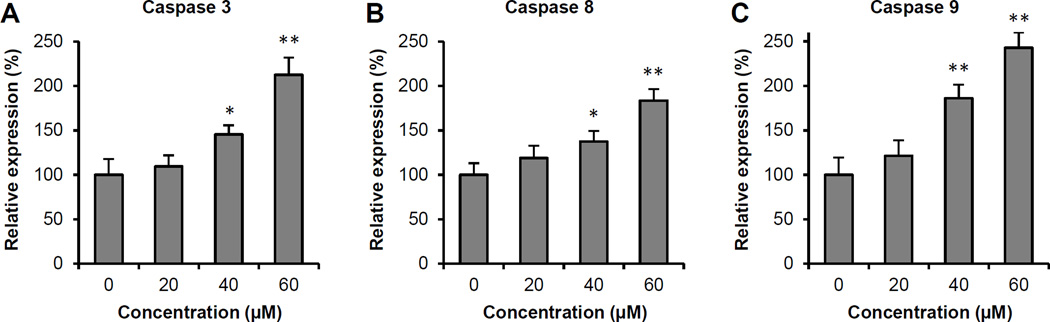
To further characterize the potential mechanism of wogonin’s anticancer activity, we assayed the activities of several caspases since wogonin increased cancer cell apoptosis. As shown in Figure 5, treatment of SW-480 cells with 40 µM wogonin for 24 h signficantly up-regulated the activity of caspases 3, 8 and 9 activities. These activities were further enhanced by 60 µM of wogonin, which increased the activity of caspases 3, 8, 9 to 112.6 ± 19.5%, 83.4 ± 12.9%, and 142.9 ± 17.0% above vehicle treated cells, respectively (all P < 0.01). Our results suggested that between the three caspases, wogonin showed more potent effects on caspases 3 and 9.
Figure 5.
Effects of wogonin on caspases 3, 8, and 9 activities in SW-480 cells. After treatment with 20–60 µM of wogonin for 24 h, cell lysates were prepared and enzymatic activities were measured by a colorimetric assay for (A) Caspase 3, (B) Caspase 8, and (C) Caspase 9. Results are normalized to each control in percentage and expressed as the means ± SE of triplicate experiments. *P<0.05, and **P<0.01 vs. control.
Molecular modeling of caspases 3 and 9 and the binding mode of wogonin
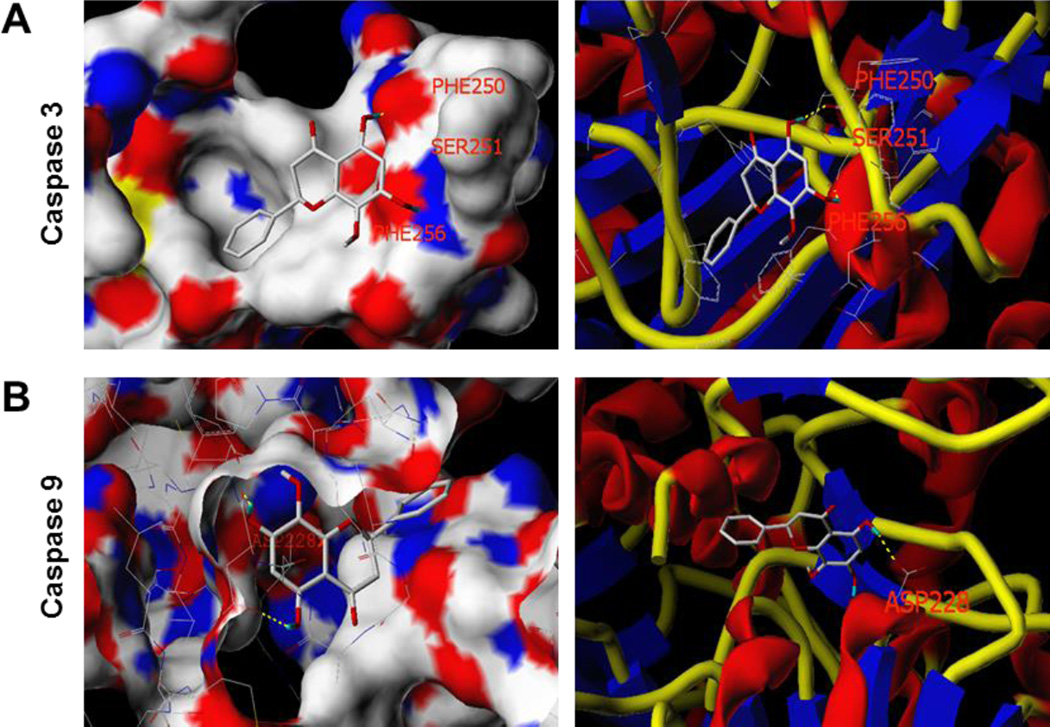
We performed a docking analysis to characterize the physical interactions of wogonin with human caspase 3 (PDB code: 3H0E) and human caspase 9 (PDB code: 2AR9).[21] The Surflex-Dock program was used to determine the binding sites of wogonin to caspases 3 and 9. The in silico modeling suggested that wogonin forms hydrogen bonds with residues Phe250 and Ser251, and has π-π interactions with a Phe256 amino acid residue at the active site of caspase 3, while wogonin forms hydrogen bonds with a residue Asp228 in caspase 9 through its hydroxyl groups [Figure 6]. In addition, wogonin is predicted to show significant binding affinity for caspase 3 (CScore 2.97) and caspase 9 (CScore 3.61), suggesting that wogonin may directly interact with these caspases.
Figure 6.
Three-dimensional docking model of wogonin at the binding site of human caspase 3 and caspase 9 proteins. The possible binding modes of wogonin at the catalytic domains of caspases were predicted using the docking program Surflex-Dock. (A) Wogonin docked with caspase 3 through hydrogen bonds with Phe250 and Ser251, and π-π interactions with Phe256. (B) Wogonin docked with caspase 9 through hydrogen bond interactions with residue Asp228. In (A) and (B), surface views are shown in the left panel, and stick-ribbon models shown on the right.
DISCUSSION
S. baicalensis is a potent herb that has been popularly used in Asia to treat various diseases including cancer. Wogonoside is a main constituent of the herb [2,3,6]. In this study, using a panel of human cancer cell lines selected from three of the most common human cancers, including colorectal cancer (SW-480, HCT-116 and HT-29), non-small cell lung cancer (NSCLC), and breast cancer (MCF-7 and MDA-MB-231), we observed that wogonoside did not show obvious antiproliferative effects. In contrast, wogonin showed significant antiproliferative effects. These results suggested that the deglycosylation of wogonoside significantly increased its antiproliferative effects.
We observed that wogonin markedly induced cancer cell apoptosis. Our results suggest that wogonin’s inhibitory effect on cancer cell growth was predominantly mediated by the induction of apoptosis. Apoptosis is a highly regulatory process of programmed cell death, in which the caspase protease family is considered to be a key factor.[21] We assayed the activities of caspases 3, 8 and 9, and observed that wogonin significantly up-regulated the activities of these caspases, especially caspases 3 and 9. Our docking analysis further explored binding sites between wogonin and caspase 3 and caspase 9. Apoptosis is considered to be an important mechanism in the inhibition of cancer cells, and many cancer chemotherapeutic agents are strong inducers of apoptotic against cancer cells. Caspases 3 and 9 are two key proteins in the caspase family of proteases, which are situated at critical points in apoptotic pathways and they play an important role in apoptosis signal transduction.[22,23] Our docking analysis suggested the existence of interaction sites between wogonin and caspases 3 and 9, contributing to the wogonin-induced apoptosis.
Comparisons of the structural differences and antiproliferative activities of wogonoside and wogonin, show that the elimination of a sugar molecule in wogonoside can increase its anticancer effects. The presence of a sugar moiety reduces the hydrophobic character of the glycoside and decreases its ability to permeate cell membranes. Similar results were also observed in our recent ginsenoside studies.[13,17,24] Our data demonstrated that deglycosylation is a promising approach to increase S. baicalensis glycosides’ anticancer potential.
In conclusion, the antiproliferative activity of the wogonoside-wogonin pair of flavonoids was compared. Wogonin showed significant antiproliferative activities, induced G1 phase arrest, apoptosis and caspase activation. After wogonoside deglycosylation, wogonin showed a significantly enhanced anticancer potential. Wogonin is a potent anticancer compound derived from S. baicalensis.
Acknowledgments
This study was supported in part by the NIH/NCCAM grants K01 AT005362 and P01 AT004418.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
The manuscript has been read and approved by all the authors.
REFERENCES
- 1.Kim EH, Shim B, Kang S, Jeong G, Lee JS, Yu YB, et al. Anti-inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and MAP kinase signaling molecules. J Ethnopharmacol. 2009;126:320–331. doi: 10.1016/j.jep.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 2.Arweiler NB, Pergola G, Kuenz J, Hellwig E, Sculean A, Auschill TM. Clinical and antibacterial effect of an anti-inflammatory toothpaste formulation with Scutellaria baicalensis extract on experimental gingivitis. Clin Oral Investig. 2011;15:909–913. doi: 10.1007/s00784-010-0471-1. [DOI] [PubMed] [Google Scholar]
- 3.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Chen HM, Liou SF, Hsu JH, Chen TJ, Cheng TL, Chiu CC, et al. Baicalein inhibits HMGB1 release and MMP-2/-9 expression in lipopolysaccharide-induced cardiac hypertrophy. Am J Chin Med. 2014;42:785–797. doi: 10.1142/S0192415X14500505. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Zhou L, Lin G, Zuo Z. Contents of major bioactive flavones in proprietary traditional Chinese medicine products and reference herb of radix Scutellariae. J Pharm Biomed Anal. 2009;50:298–306. doi: 10.1016/j.jpba.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Huang HH, Shao ZH, Li CQ, Vanden Hoek TL, Li J. Baicalein protects cardiomyocytes against mitochondrial oxidant injury associated with JNK inhibition and mitochondrial Akt activation. Am J Chin Med. 2014;42:79–94. doi: 10.1142/S0192415X14500050. [DOI] [PubMed] [Google Scholar]
- 7.Hong T, Jin G-B, Cho S, Cyong J-C. Evaluation of the Anti-Inflammatory Effect of Baicalein on Dextran Sulfate Sodium-Induced Colitis in Mice. Planta Med. 2002;68:268–271. doi: 10.1055/s-2002-23143. [DOI] [PubMed] [Google Scholar]
- 8.Shi R, Zhou H, Liu Z, Ma Y, Wang T, Liu Y, et al. Influence of coptis Chinensis on pharmacokinetics of flavonoids after oral administration of radix Scutellariae in rats. Biopharm Drug Dispos. 2009;30:398–410. doi: 10.1002/bdd.674. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa H. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Metabolic activation of ginsenoside: Deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- 10.Liu HF, Yang JL, Du FF, Gao XM, Ma XT, Huang YH, et al. Absorption and Disposition of Ginsenosides after Oral Administration of Panax notoginseng Extract to Rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- 11.Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 12.Yu C, Zhang Z, Zhang H, Zhen Z, Calway T, Wang Y, et al. Pretreatment of baicalin and wogonoside with glycoside hydrolase: a promising approach to enhance anticancer potential. Oncol Rep. 2013;30:2411–2418. doi: 10.3892/or.2013.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CZ, Du GJ, Zhang Z, Wen XD, Calway T, Zhen Z, et al. Ginsenoside compound K, not Rb1, possesses potential chemopreventive activities in human colorectal cancer. Int J Oncol. 2012;40:1970–1976. doi: 10.3892/ijo.2012.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan JY, Liu P, Wang HY, Qi LW, Wang CZ, Li P, et al. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J Chromatogr A. 2013;1286:83–92. doi: 10.1016/j.chroma.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Zou M, Hu C, Qin Y, Song X, Lu N, et al. Wogonoside induces autophagy in MDA-MB-231 cells by regulating MAPK-mTOR pathway. Food Chem Toxicol. 2013;51:53–60. doi: 10.1016/j.fct.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Kim MS, Bak Y, Park YS, Lee DH, Kim JH, Kang JW, et al. Wogonin induces apoptosis by suppressing E6 and E7 expressions and activating intrinsic signaling pathways in HPV-16 cervical cancer cells. Cell Biol Toxicol. 2013;29:259–272. doi: 10.1007/s10565-013-9251-4. [DOI] [PubMed] [Google Scholar]
- 17.Wang CZ, Zhang Z, Wan JY, Zhang CF, Anderson S, He X, et al. Protopanaxadiol, an active ginseng metabolite, significantly enhances the effects of fluorouracil on colon cancer. Nutrients. 2015;7:799–814. doi: 10.3390/nu7020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang CZ, Li XL, Wang QF, Mehendale SR, Yuan CS. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine. 2010;17:63–68. doi: 10.1016/j.phymed.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain AN. Morphological similarity: a 3D molecular similarity method correlated with protein-ligand recognition. J Comput Aided Mol Des. 2000;14:199–213. doi: 10.1023/a:1008100132405. [DOI] [PubMed] [Google Scholar]
- 20.Giganti D, Guillemain H, Spadoni JL, Nilges M, Zagury JF, Montes M. Comparative evaluation of 3D virtual ligand screening methods: impact of the molecular alignment on enrichment. J Chem Inf Model. 2010;50:992–1004. doi: 10.1021/ci900507g. [DOI] [PubMed] [Google Scholar]
- 21.Kim R, Tanabe K, Uchida Y, Emi M, Inoue H, Toge T. Current status of the molecular mechanisms of anticancer drug-induced apoptosis. The contribution of molecular-level analysis to cancer chemotherapy. Cancer Chemother Pharmacol. 2002;50:343–352. doi: 10.1007/s00280-002-0522-7. [DOI] [PubMed] [Google Scholar]
- 22.Sudhakar C, Jain N, Swarup G. Sp1-like sequences mediate human caspase-3 promoter activation by p73 and cisplatin. FEBS J. 2008;275:2200–2213. doi: 10.1111/j.1742-4658.2008.06373.x. [DOI] [PubMed] [Google Scholar]
- 23.Wurstle ML, Laussmann MA, Rehm M. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res. 2012;318:1213–1220. doi: 10.1016/j.yexcr.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Wang CZ, Zhang Z, Anderson S, Yuan CS. Natural products and chemotherapeutic agents on cancer: prevention vs. treatment. Am J Chin Med. 2014;42:1555–1558. doi: 10.1142/S0192415X1420002X. [DOI] [PubMed] [Google Scholar]