Abstract
Cyanobacteria produce harmful toxins that have been associated with several acute conditions and chronic human diseases, like gastroenteritis, non-alcoholic liver disease, and amyotrophic lateral sclerosis. Aerosol from waterbodies appears to be a likely mechanism for exposure. We conducted a study of human biospecimens focused on the cyanobacterial aerosilization process by evaluating the extent to which cyanobacteria can invade the human respiratory tract. Our study suggests that humans routinely inhale aerosolized cyanobacteria, which can be harbored in the nostrils and the lungs. Using PCR, cyanobacteria were found at high frequencies in the upper respiratory tract (92.20%) and central airway (79.31%) of our study subjects. Nasal swabs were not predictive of bronchoalveolar lavage (BAL) when detecting inhaled cyanobacteria. Interestingly, we found no evidence that time of year was a significant factor for cyanobacteria positivity (BAL cytology p = 1.0 and PCR p = 1.0); (nasal swab cytology p = 0.051 and PCR p = 0.65). Additionally, we found that proximity to a waterbody was not a significant factor for cyanobacteria positivity in BAL and nasal swabs collected during cyanobacteria bloom season [May - October] (p = 0.46 and p = 0.38). These data suggest that cyanobacteria exposure may be a prevalent and chronic phenomenon not necessarily restricted to waterbodies alone. Sources of indoor exposure warrant future investigation. Given the widespread prevalence of cyanobacterial exposure in the airway, investigation of the aerosol spread of cyanotoxins, more specifically, is warranted. Our findings are consistent with the hypothesis that aerosol is a significant route for cyanobacteria exposure, and thus a likely route of transmission for cyanotoxin-associated human Diseases
Keywords: Cyanobacteria, aerosol exposure, respiration, environmental toxin, human health
1. Introduction
Evidence suggests that humans may be exposed to cyanobacteria, also known as blue-green algae, and their harmful toxins through a variety of mechanisms. Cyanobacteria are ubiquitous organisms that can be present not only in cyanobacterial harmful algal blooms (CHABs), but also all year round in the benthic zone of waterbodies (Berdalet et al. 2015; Berger et al. 2006). Two well-documented routes of exposure include incidental consumption of contaminated water (Carmichael and Boyer 2016; el Saadi et al. 1995; Falconer et al. 1983; van Apeldoorn et al. 2007) and direct dermatological contact through recreational activities, such as swimming (Carmichael and Boyer 2016; Drobac et al. 2013; van Apeldoorn et al. 2007). Other routes include dietary consumption of cyanotoxin-contaminated fish (Cazenave et al. 2005; Cazenave et al. 2006; Magalhaes et al. 2001; van Apeldoorn et al. 2007; Xie et al. 2005) or even through, supposedly beneficial, blue-green algae supplements (Dietrich and Hoeger 2005; Drobac et al. 2013; Gilroy et al. 2000; Roy-Lachapelle et al. 2017). More recently, attention has shifted towards understanding cyanobacteria aerosilization, which could make widespread exposure possible and thus be one of the most significant mechanisms with respect to human health (Backer et al. 2010; Lewandowska et al. 2017; May et al. 2018).
Acute cyanotoxin poisoning may present in a clinically diverse fashion. In cases of direct skin contact, clinical findings may include rashes, hives, and blisters (Drobac et al. 2013). Allergic reactions such as cough, runny nose, and sore throat may also occur (Drobac et al. 2013). Swallowing toxic cyanobacteria may also cause gastrointestinal (GI) distress in the form of gastroenteritis, nausea, diarrhea, pain, vomiting, in addition to associated symptoms like fever and headache (Drobac et al. 2013; el Saadi et al. 1995). Exposure to large quantities of cyanotoxins may also cause acute liver damage and even death, as was the case in the Brazilian hemodialysis center in 1996 (Azevedo et al. 2002; Carmichael et al. 2001; Hilborn et al. 2005; Jochimsen et al. 1998). Studies have documented other serious health concerns associated with cyanotoxin exposure including a significantly increased risk for hepatocellular carcinoma (Fleming et al. 2002) and non-alcoholic liver disease (Zhang et al. 2015).
Recent evidence also suggests that living within 0.5 miles of a waterbody affected by frequent CHABs appears to be a significant risk factor for amyotrophic lateral sclerosis (ALS) in Northern New England (Caller et al. 2009; Caller et al. 2012; Caller et al. 2013; Stommel et al. 2013; Torbick et al. 2014; Torbick et al. 2018). Chronic exposure to the cyanobacteria-derived toxin, β-Methylamino-L-alanine (BMAA), has been implicated as a significant risk factor for developing neurodegenerative disease, like ALS, in genetically predisposed individuals (Al-Chalabi et al. 2014; Andrew et al. 2017; Banack and Cox 2003; Banack et al. 2010; Banack et al. 2015; Bradley et al. 2013; Dunlop et al. 2013; Field et al. 2013; Michaelson et al. 2017; Murch et al. 2004a; Murch et al. 2004b; Pablo et al. 2009; Riancho et al. 2018).
A noteworthy and particular route of interest is through aerosol exposure. This mechanism could potentially explain how individuals nearby a cyanobacteria source are exposed to acute and chronic toxicities. A recent ecological study examined the chemical and biological composition of particulate lake spray aerosol (LSA) produced using freshwater samples from Lakes Michigan and Erie, both of which have experienced an increase in CHAB intensity and frequency (May et al. 2018). Blue-green algae was found to be present in individual freshwater LSA particles suggesting that cyanobacteria may be aerosolized through freshwater wave breaking (May et al. 2018). In a related study, Lewandowska and colleagues found that respirable bioaerosols collected over the Baltic Sea, as well as hundreds of meters inland, harbored cyanobacteria and other related microalgae species at very high frequencies (Lewandowska et al. 2017). The authors suggest that microorganism-infested bioaerosols may present a ubiquitous exposure risk for human health, and further studies are needed in characterizing the ability of aerosolized microorganism in carrying other potential toxins like heavy metals and pesticides (Lewandowska et al. 2017).
In the Florida “red tides,” brevetoxins produced by a similarly toxic marine dinoflagellate, Karenia brevis, were detected in aerosol samples as far as 4.2km from the beach of origin (Kirkpatrick et al. 2010) as well as in nasal swab specimens of exposed individuals (Backer et al. 2003; Backer et al. 2005). Respiratory symptoms and inflammatory responses were common among those exposed (Backer et al. 2003). An additional study by Backer and colleagues recruited children and adults who had gone to two California lakes to engage in recreational activities, such as swimming and water skiing, and attempted to detect the cyanotoxin, Microcystin (MC), in plasma and nasal swab specimens (Backer et al. 2010). They found that although plasma MCs were all below detectable limits (using MC-specific enzyme-linked immunosorbent assay [ELISA]), levels in nasal specimens increased from pre-recreational to post-recreational sampling. This suggests that aerosol inhalation is a potentially significant route of exposure (Backer et al. 2010). The authors encouraged future studies to collect nasal swab specimens in order to assess upper respiratory tract susceptibility to cyanotoxin infiltration.
The purpose of our study was to expand upon the cyanobacteria aerosol mechanism by investigating cyanobacterial presence in bio-specimens from both the upper respiratory tract and the central airway. Previous studies have primarily used MC-ELISA to detect exposure to cyanotoxins (Backer et al. 2010; Hilborn et al. 2005); however, in a novel method we aimed to identify the bacteria itself and the extent to which it may invade the human respiratory system. We accomplished this by analyzing not only nasal swab specimens but also bronchoalveolar lavage (BAL) fluid from consenting research participants. Our primary objectives were to: a) identify cyanobacteria in respiratory samples, b) study the efficacy of using nasal swab specimens as surrogates to bronchoscopy when identifying cyanobacteria, and c) determine if residential proximity to a waterbody correlates with cyanobacteria identification. Factors such as sample collection time of year and significant medical contributing factors (pulmonary disease, smoking status, etc.) were studied as secondary measures.
2. Materials and Methods
2.1. Patient Recruitment
Institutional review board (IRB) approval was obtained in March 2015 (Center for the Protection of Human subjects (CPHS) at Dartmouth, IRB #20843) with yearly approval in 2015 through 2018. Patients were recruited from the fall of 2016 through the late winter of 2018 via a collaborative effort between the Departments of Neurology and Pulmonary and Critical Care Medicine at Dartmouth-Hitchcock Medical Center (DHMC). Subjects were identified on the DHMC pulmonology service who were either, a) set for participation in an already ongoing research study involving bronchoscopy (CPHS at Dartmouth, IRB #22781), or b) having a bronchoscopy for diagnostic/ clinical work-up. All research participants from the pulmonology service were off antibiotics for ≥2 weeks before the study and were in the hospital for less than 4 hours before recruitment and sample collection. Additional study participants were recruited from the Neurology clinic during outpatient visits to achieve a higher sample size in order to study a broader prevalence of exposure. Access to medical record information was requested from each participant and was used to identify pertinent demographic information, such as a current residential address, smoking status, and medical history. Study participants were provided written informed consent and all questions were addressed at the time of enrollment.
Current residential address at the time of sample collection was ascertained from each patient’s electronic medical record and was plotted using Google Earth. Distance to the closest waterbody was measured for each participant. Waterbody was defined as any lake, river, or private pond, and excluded estuaries. Depending on when recruitment occurred, participants were assigned to either the “May-October” or “November-April” group for season-based analyses.
2.2. Sample Collection
2.2.1. Bronchoscopy and BAL Retrieval
Bronchoscopies were performed by experienced providers at our institution. The procedure was performed under conscious sedation using intravenous fentanyl and midazolam. All subjects received local anesthesia of the posterior pharynx and vocal chords using 2% lidocaine. A flexible, fiberoptic bronchoscope was advanced into the right upper lobe (RUL) for lavage and we obtained ≤15 mL of BAL. Participant’s vitals were monitored throughout the procedure. The lavage was done using sterile, normal saline (0.9% NaCl), preheated to body-temperature (37°C) to help prevent coughing and to increase cellular yield. After completion of the procedure, participants were monitored in the recovery room until deemed clinically stable for discharge.
2.2.2. Nasal Swab Specimens
The nasal swab collection technique was based on the method used by Backer and colleagues (Backer et al. 2003; Backer et al. 2010). Samples were obtained by rotating a culture swab seven times along the anterior nasal mucosa, one culture swab per nostril (BD BBL™ CultureSwab™ Transport Systems: Liquid Amies, Single Swab (polyester), BD 220146). After collection, each culture swab was returned to its vesicle until processing (see section 2.3.2). In some cases, participants were selectively excluded from nasal swab specimen collection if they were already on supplemental oxygen at the time of consent or if they had pre-existing nasal cavity damage/ pain.
2.3. Sample Processing
2.3.1. BAL Processing
BAL samples were processed within two hours of collection. All handling of BAL was performed under a laminar flow culture hood to minimize contamination issues. Multiple original BAL samples (250μL each) were reserved and stored at −80°C until analysis (section 2.4). The concentration of cells (cells/ mL) in the BAL sample was determined using a manual hemacytometer (Sigma Aldrich Bright-Line™ Hemacytometer, Z359629). Thereafter, enough BAL (in 1 mL 0.9% normal, isotonic saline) to equilibrate to a final concentration of approximately 5,500 cells/ mL was cytocentrifuged (Shandon Cytospin 3 Cell Preparation System) at 500RPM for 5 minutes onto two charged microscope slides. Slides were fixed in methanol for 6 minutes. One slide was preserved as is, unstained, while the other slide was stained for 30 minutes in 1:20 deionized water (diH20): Giemsa Stain (Sigma Giemsa stain, modified, G S500) solution. The stained slide was washed gently in diH20, allowed to air dry, and sealed with a coverslip with permount before storing at room temperature away from light.
Two forms of controls were prepared. Cultured Microcystis sp. (Carolina Biological Supply; Microcystis sp., Living, Item #151840) was used as the positive control and Escherichia coli K-12 strain (Carolina Biological Supply; Escherichia coli K-12, Living, Item #155068) was used as the negative control. Both cultures were processed by cytocentrifugation as described in this section, however done so at 2000RPM to account for small size and weight such that a compact, uniform cellular field of ~5500 cells/ mL was established.
2.3.2. Nasal Swab Processing
Nasal swab specimens were gently smeared on two charged microscope slides. Thereafter, one slide was preserved as is, unstained, while the other slide was stained for 30 minutes in 1:20 diH20: Giemsa Stain (Sigma Giemsa stain, modified, G S500) solution. Slides were stored at room temperature away from light. Original swabs were stored at −18°C until analysis.
2.4. Polymerase Chain Reaction (PCR)
DNA was extracted from the original sample using a Qiagen DNeasy Blood and Tissue kit (Qiagen, Netherlands). Nested PCR using cyano-specific 16S primers CYA359F (GGG GAA TTT TCC GCA ATG GG), CYA781R(a) (GAC TAC TGG GGT ATC TAA TCC CAT T), CYA781R(b) (GAC TAC AGG GGT ATC TAA TCC CTT T), and 16SUR (5’-GTA TTA CCG CGG CTG CTG G-3’) was performed on isolated DNA (IDT, USA). β-Globin was used as a positive, human samples control. For the primary reaction, 0.2 μM CYA359F and 0.2 μM of equimolar CYA781Ra and CYA781Rb were added to 100 ng of sample DNA and SsoFast ready mixed reagents (Bio-Rad Laboratories, USA). Reactions were amplified in a Bio-Rad SI000 cycler with the following protocol: 95°C for 60 seconds, and 40 cycles of 95°C for 10 seconds and 60°C for 30 seconds. Primers were then digested in the primary reaction using ExoSAP-IT (Affymetrix, USA) per the manufacturer’s recommendation. 1 μL of the primary PCR was then amplified using CYA359F and 16SUR using the same reactions conditions as in the primary PCR. Amplified DNA was examined by electrophoresis of 20 μL of the PCR product on 2% agarose E-Gels, and visualization with an E-Gel imager (Thermoscientific, USA).
2.5. Fluorescence Microscopy (FM)/ Cytology
An Olympus IX73 Inverted Fluorescence Microscope was used to examine slides for the presence of phycocyanobilin, a protein present in the cyanobacteria-specific pigment, phycocyanin (excitation 575nm (Chroma, ET575/22x)/emission 650nm [Chroma, ET650/75m]). Microscope magnification was ubiquitously set at 10x. Giemsa-stained slides were scanned for fluorescent particles, which, at the aforementioned wavelengths, appeared red in color. BAL slides were scanned within the focal area of cells created by the cytocentrifuge process and nasal swab slides were scanned over the entire area of the smear. Images were taken of fluorescent signals and bright-field images were also captured and used for quality control.
2.6. Fiji/ImageJ Image Analysis
Fiji (also known as “Image J”) imaging software was used to analyze BAL and nasal swab fluorescence images for the presence of cyanobacteria. Fluorescent signals on the images were measured for size parameters (area and diameter) using established macros and plug-ins developed into a unique method. Control Microcystis and E. coli were imaged via FM as described in section 2.5 and analyzed by Fiji/ ImageJ for the same size parameters.
2.7. Statistical Analyses
Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at DHMC (Harris et al. 2009). We performed univariate statistical analyses using t-tests for continuous measures and Fisher’s exact tests or chi-square tests for categorical variables. We calculated Pearson correlation coefficients to assess relationships between two continuous variables. P-values <0.05 were considered statistically significant.
3. Results
3.1. Patient Characteristics
During the study period of October 2016 – February 2018, BAL samples were collected from 29 participants, 23 of which were swabbed for nasal specimens (Table 1). Reasons for not collecting nasal specimens included: a) the participant was already on supplemental oxygen at the time of consent (n = 5), and b) pre-existing nasal cavity damage/ pain (n = 1). Study participants included 19 healthy controls, 8 individuals with Cystic Fibrosis (CF), 1 individual with pulmonary lung nodules and asthma, and 1 participant with Chronic Obstructive Pulmonary Disease (COPD). Females (n=17) outnumbered male (n=12) bronchoscopy participants (p=0.57). The mean age for bronchoscopy participants was 35.59 ± 14.56 years, and was not statistically different by gender (Table 1). Smoking status revealed a majority (79.3%) of “never” smokers and 13.8% of non-user/ “former” smokers. A small minority (6.9%) were “current” smokers (Table 1).
Table 1.
Overall Characteristics of Bronchoscopy and Nasal Swab Participants
| Characteristics | Bronchoscopy Participants |
Nasal Swab Participants |
P- value | |
|---|---|---|---|---|
| Gender | Total, n (%) Female, n(%) Male, n (%) |
29/29 (100%) 17(58.62%) 12 (41.38%) |
77/81* (92.8%) 44 (57.1%) 33 (42.9%) |
- Reference 0.69 |
| Age | Total, mean ±SD Female, mean ± SD Male, mean ± SD |
35.59 ±14.56 35.84 ± 15.03 35.23 ± 13.86 |
54.96 ±18.64 56.53 ± 19.18 52.76 ± 17.93 |
- Reference 0.28 |
|
Smoking Status |
Never, n (%) Former, n(%) Current, n (%) Unavailable, n (%) |
23 (79.3%) 4 (13.8%) 2 (6.9%) - |
46 (59.7%) 26 (33.8%) 3 (3.9%) 2 (2.6%) |
0.40 - - - |
Includes 23 participants from Bronchoscopy group that contributed nasal swabs in addition to BAL
In order to appreciate a broader understanding about the prevalence of cyanobacteria exposure, we recruited an additional 54 participants from the Neurology clinic during outpatient visits. Thus, we collected nasal swab specimens on 77/83 total study participants (92.8% participation rate) (Table 1). Female participants (n = 44) outnumbered male participants (n = 33) (Table 1). The average age for all nasal swab subjects was 54.96 ± 18.64 years, and was not statistically different by gender (Table 1). Smoking status revealed a majority (59.7%) of “never” smokers and significant proportion of non-user/ “former” smokers (33.8%) (Table 1). A small minority were “current” smokers (3.9%) or report “unavailable” (2.6%) (Table 1).
3.2. Cyanobacteria are found in the human respiratory tract
3.2.1. BAL
Cyano-specific 16S rDNA PCR revealed 23/29 (79.31%) cyanobacteria positive specimens (Table 2). Age was not a significant factor for cyanobacteria PCR positivity (p = 0.79), however samples from male participants were all positive versus 6 negative samples from female participants; p = 0.028 (Table 2). Successful BAL cytopreparation (for cytological analysis) was performed on 26/29 specimens. Minor, early study protocol issues/ sample retrieval was the only reason for not having processed these three specimens. BAL cytology identified cyanobacteria in 10 (38.46%) specimens and did not differ based on age or gender; p = 0.28 and p = 0.69 respectively (Figure 1; Table 2). Lung diseases (CF, COPD, asthma) were unrelated to BAL cytology for cyanobacteria (Fishers exact, p=1.0, data not reported). Smoking status was also not significant (data not reported).
Table 2.
Bronchoalveolar Lavage (BAL) Patient Characteristics, Cyanobacterial Cytology, and PCR Findings
| Characteristics | BAL Cytology | BAL PCR | |||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | P-value* | Negative | Positive | P-value* | ||
| Gender | Female, n (%) Male, n (%) |
10 (62%) 6 (38%) |
5 (50%) 5 (50%) |
- 0.69 |
6 (100%) 0 (0%) |
11 (48%) 12 (52%) |
- 0.028 |
| Age | Mean ± SD Range (min-max) |
30.5 ±9.6 19.0–56.2 |
29.6 ±4.7 21.8–34.7 |
0.28 | 55.93 ± 17.44 24.39–75.9 |
54.88 ± 18.98 21.82–86.38 |
0.79 |
Gender P-value determined by Chi-square
Age P-value determined by T-test
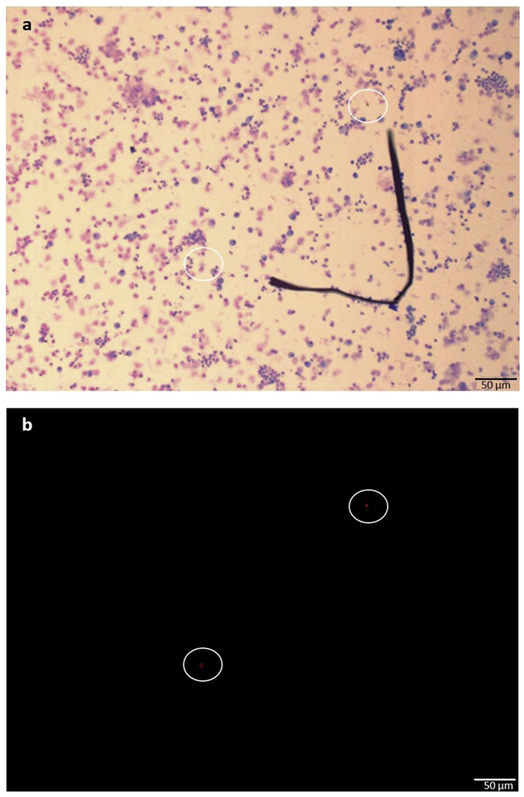
Figure 1. Representative sample preparation of normal human BAL cells at a concentration of ~5,500 cells/mL.
This sample was identified as PCR+ for cyanobacteria. (a) Giemsa stain (purple/ pink) was used to label nucleated human lavage cells. (b) Two cyanobacteria cells expressing phycocyanobilin pigments are seen (10x, 50um scale) using an Olympus IX73 Inverted Fluorescence Microscope. A phycocyanobilin cube with the excitation wavelength of 572nm and emission wavelength of 640nm was used. Images were enhanced to increase cell visibility using a standard procedure applied to all images.
Epifluorescent signals in FM positive images were measured using Fiji/ImageJ for diameter and area. Control Microcystis and E. coli were imaged and measured using the same methods (Figure 2). Independent samples t-tests found that the measured mean diameter of BAL cyanobacteria cells (mean 3.17μm; 95% CI 2.17 - 4.30μm) was in agreement with the mean diameter of standard Microcystis cells (mean 3.19μm; 95% CI 2.43 - 4.06μm); p = 0.94 (Table 3). Similarly, the mean area of BAL cyanobacteria (mean 6.22μm2; 95% CI 2.96 - 8.82μm2) were found to be similar to the mean area of standard Microcystis (mean 5.82μm2; 95% CI 3.11 - 8.19μm2); p = 0.70 (Table 3). Negative control, E. coli did not produce any epifluorescence signals and Fiji/ ImageJ analysis calculated no size measurements (Figure 2; Table 3).
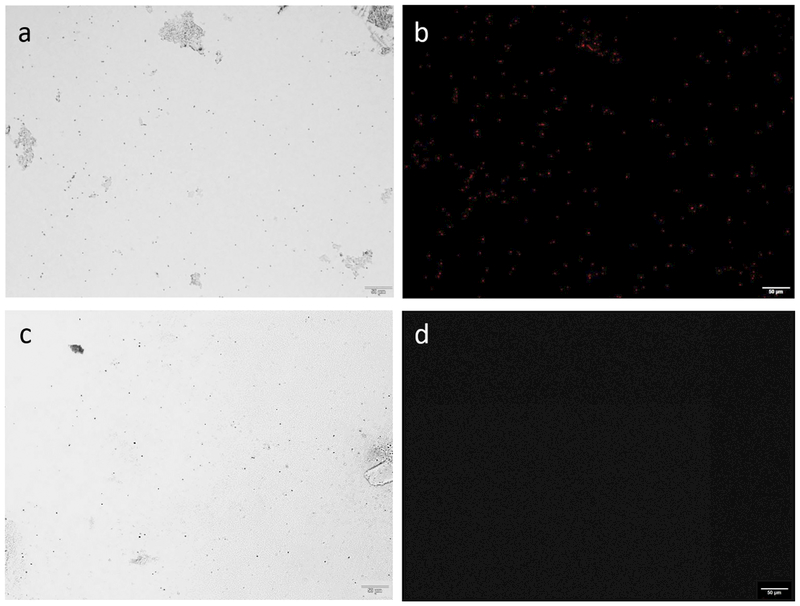
Figure 2. Control microbes.
Preparations (at ~5,500 cells/mL) were made for the two control microbes. (a)/ (b) Positive Control: brightfield microscopy shows a population of Microcystis sp. cells and fluorescence microscopy depicts these cells globally expressing phycocyanobilin pigments (c)/ (d) Negative Control: brightfield microscopy shows a population of Escherichia coli K-12 strain cells while fluorescence microscopy do not show any cells expressing phycocyanobilin pigments. Performed using an Olympus IX73 Inverted Fluorescence Microscope (10x, 50um scale). A phycocyanobilin cube with the excitation wavelength of 572nm and emission wavelength of 640nm was used. Images were enhanced to increase cell visibility using a standard procedure applied to all images.
Table 3.
Diameter and Area Measurements for Epifluorescent Cyanobacteria Detected in BAL and Nasal Swab samples versus Microcystis sp. and E. coli
| Parameter | Mean | Min | Max | S.D. | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Microcystis sp. Diameter (μm) | 3.19 | 0.62 | 4.92 | 0.77 | 2.43, 4.06 | Ref. |
| BAL Cyanobacteria Diameter (μm) | 3.17 | 0.98 | 4.40 | 1.00 | 2.17, 4.30 | 0.94 |
| Nasal Swab Cyanobacteria Diameter (μm) | 2.47 | 0.62 | 5.38 | 1.37 | 2.24, 2.71 | <0.001 |
| E. Coli Diameter (μm) | - | - | - | - | - | - |
| Microcystis sp. Area (μm) | 5.82 | 0.19 | 14.12 | 2.71 | 3.11, 8.19 | Ref. |
| BAL Cyanobacteria Area (μm) | 6.22 | 0.39 | 11.80 | 3.25 | 2.96, 8.82 | 0.70 |
| Nasal Swab Cyanobacteria Area (μm) | 4.28 | 0.19 | 16.25 | 4.32 | 3.54, 5.03 | <0.001 |
| E. Coli Area (μm) | - | - | - | - | - | - |
3.2.2. Nasal Swabs
Cyano-spceific 16S rDNA PCR revealed 71/77 (92.2%) cyanobacteria positive specimens and did not differed based on age or gender; p = 0.89 and p = 0.69 (Table 4). Two nasal swab specimens could not be prepared for cytology. Nasal swab cytology identified cyanobacteria in 48/75 (64.0%) smears and did not differ based on age or gender; p = 0.28 and p = 0.63 respectively (Figure 3; Table 4). Smoking status was also not significant via nasal cytology (Fishers exact, p=0.40, data not reported).
Table 4.
Nasal Swab Patient Characteristics, Cyanobacterial Cytology, and PCR Findings
| Characteristics | Nasal Swab Cytology | Nasal Swab PCR | |||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | P-value* | Negative | Positive | P-value* | ||
| Gender | Female, n (%) Male, n (%) |
17 (63%) 10 (37%) |
27 (56%) 21 (44%) |
- 0.63 |
3 (50%) 3 (50%) |
42 (59%) 29 (41%) |
- 0.69 |
| Age | Mean ± SD Range (min-max) |
58.0 ± 17.8 24.7 – 84.0 |
53.3 ± 19.3 21.82– 86.4 |
0.28 | 55.93 ± 17.44 24.39–75.9 |
54.88 ± 18.98 21.82–86.38 |
0.89 |
Gender P-value determined by Chi-square
Age P-value determined by T-test
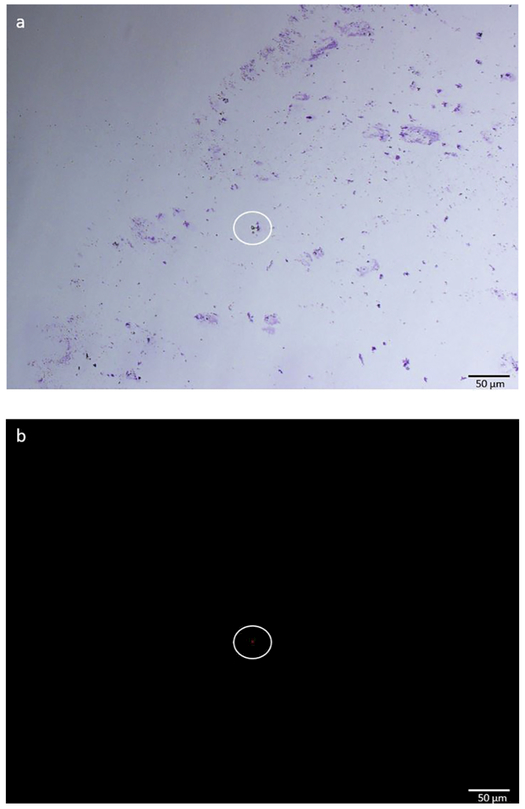
Figure 3. Representative sample preparation of a nasal swab smear.
This sample was identified as PCR+ for cyanobacteria (a) Giemsa stain (purple/ pink) was used to label nucleated cells. (b) A singular cyanobacteria cell expressing phycocyanobilin pigments is seen (10x, 50um scale) using an Olympus IX73 Inverted Fluorescence Microscope. A phycocyanobilin cube with the excitation wavelength of 572nm and emission wavelength of 640nm was used. Images were enhanced to increase cell visibility using a standard procedure applied to all images.
Independent samples t-tests found that the measured mean diameter of nasal swab cyanobacteria cells (mean 2.47μm; 95% CI 2.24 - 2.71μm) was statistically smaller than the mean diameter of standard Microcystis cells (mean 3.19μm; 95% CI 2.43 - 4.06μm); p< 0.001 (Table 3). Similarly, the mean area of nasal swab cyanobacteria (mean 4.28μm2; 95 % CI 3.54 - 5.03μm2) was statistically smaller than the mean area of Microcystis (mean 5.82μm2; 95% CI 3.11 - 8.19μm2); p< 0.001 (Table 3).
3.3. Cyanobacteria Detection by Season
Given that CHABs do not occur during the winter in northern New England, analysis was dichotomized to differentiate between samples that were collected during the warmer months of May - October, versus samples collected the colder months of November - April (Table 5). Nasal swab cytology revealed a sensitivity of 0.67 and specificity of 0.89 for detecting cyanobacteria in May - Oct samples, which was borderline not significant by season; p = 0.051 (Table 5). Nasal swab PCR determined a sensitivity of 0.75 and specificity of 0.67 for detecting cyanobacteria in samples collected during May - Oct, and was not significantly different versus the winter; p = 0.65 (Table 5). BAL cytology and PCR sensitivity and specificity analyses did not exceed 0.50, and were also not significant based on season; p = 1.0 and p = 1.0 respectively (Table 5). Interestingly, nasal swabs (90%) and BAL (81%) collected during Nov - Apr were found to be positive for cyanobacteria at high frequencies (Table 5). Cytology Pearson correlation analysis of nasal swab samples revealed that cyanobacteria cell counts were also not significantly different between samples collected during Nov - Apr vs. May - Oct; R2 = 0.18, p = 0.45 and R2 = 0.23, p = 0.090.
Table 5.
Cyanobacteria detection by season
|
Nasal cytology |
BAL cytology |
|||||||||||
| Negative (n) |
% | Positive (n) |
% | p-value* | Sensitivity/ Specificity |
Negative (n) |
% | Positive (n) |
% | p-value* | Sensitivity/ Specificity |
|
| May - Oct Nov - Apr |
24 3 |
43% 16% |
32 16 |
57% 84% |
0.051 |
0.67/0.89 | 7 9 |
64% 60% |
4 6 |
36% 40% |
1.0 |
0.40/0.44 |
|
Nasal PCR |
BAL PCR |
|||||||||||
| Negative (n) |
% | Positive (n) |
% | p-value* | Sensitivity/ Specificity |
Negative (n) |
% | Positive (n) |
% | p-value* | Sensitivity/ Specificity |
|
| May - Oct Nov - Apr |
4 2 |
7% 10% |
53 18 |
93% 90% |
0.65 |
0.75/0.67 | 3 3 |
23% 19% |
10 13 |
77% 81% |
1.0 |
0.43/0.50 |
Fishers exact two-tailed
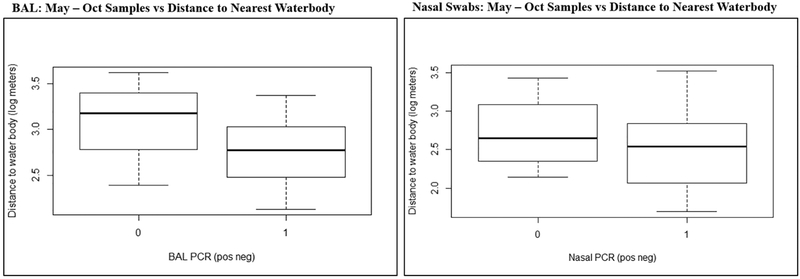
We then restricted data analysis to only those samples collected from May - Oct in order to determine if, when controlling for CHAB seasonality, residential proximity to the nearest waterbody was a significant factor. Distance measurement data was log transformed to normalize distribution. Using a two-samples independent t-test, we found that May - Oct bronchoscopy participants with PCR positive BAL (n = 12) did not live significantly closer to the nearest waterbody than did participants with negative specimens (n = 3) (Mean POS = 3.06 log meters, Mean NEG = 2.81 log meters); p = 0.384 (Figure 4). Similarly, May - Oct nasal swab samples alone revealed that participants with PCR positive nasal samples (n = 55) did not live a significantly closer distance to the nearest waterbody when compared with participants with PCR negative samples (n = 3) (Mean POS = 2.72 log meters, Mean NEG= 2.49 log meters); p = 0.46 (Figure 4).
Figure 4. May - Oct Samples vs Distance to Nearest Waterbody:
(a) BAL: Two-samples independent t-test was used to determine if cyanobacteria positive BAL samples collected during May-October came from subjects who lived a closer mean log distance to the nearest waterbody. (b) Nasal Swabs: Two-samples independent T-test was used to determine if cyanobacteria positive nasal swab samples collected during May-October came from subjects who lived a closer mean log distance to the nearest waterbody.
3.4. Determining the Efficacy of using Nasal Swabs as a surrogate to Bronchoscopy for Cyanobacteria Identification
One of the main goals of our study was to determine the efficacy of using nasal swab collection, a non-invasive and clinically relevant screening method, as a surrogate for bronchoscopy when gauging aerosolized cyanobacteria exposure. Positive predictive value (PPV) and negative predictive value (NPV) parameters, as well as sensitivity and specificity were measured to address this goal. When evaluating nasal swab cytology to BAL cytology, we detected a sensitivity of 1.0, specificity of 0.40, with a PPV of 0.50 and a NPV of 1.0 (Table 6). Nasal swab PCR revealed a sensitivity to BAL PCR of 0.83, specificity of 0.00, with a PPV of 1.0 and a NPV of 0.00 (Table 6).
Table 6.
Assessing the efficacy of using nasal swabs as a surrogate to bronchoscopy when detecting inhaled Cyanobacteria
| BAL cytology |
|||||||
| Negative | Positive | ||||||
| Nasal cytology |
n | n | Sensitivity/ Specificity |
PPV/NPV | |||
| Negative | 3 | 60% | 0 | 0% | |||
| Positive | 2 | 40% | 3 | 100% | 1.0/0.40 | 0.50/1.0 | |
| BAL PCR |
|||||||
| Negative | Positive | ||||||
| Nasal PCR |
Negative | 0 | 0 | ||||
| Positive | 4 | 19 | 0.83 / 0.00 | 1.0 / 0.0 |
Positive Predictive Value (PPV)
Negative Predictive Value (NPV)
4. Discussion
We identified cyanobacteria that infiltrated not only the upper respiratory tract (nasal cavity), but also the central airway (lung: right upper lobe) suggesting that aerosilization may be a significant route of human exposure. The findings from our study suggest a broad prevalence of cyanobacteria exposure in both male and female subjects of varying ages, regardless of season, smoking status, and pulmonary pathology.
Among the other goals in this study, we aimed to determine if time of year and/ or residential proximity to a waterbody correlated with cyanobacteria identification. We hypothesized that due to the seasonality of CHABs and the likelihood of persons being outdoors in the warmer months, we would detect a significant relationship between time of year and cyanobacteria. One of the more interesting discoveries from this study was that we did not find that samples collected during the months of May - October differed in cyanobacteria presence than those collected during November - April. In fact, we found a surprisingly high preponderance of cyanobacteria in both forms of respiratory tract samples collected during the colder months. Similarly, we did not find that residential proximity to a waterbody, regardless of season, was a significant factor. Likewise, Backer et al. reported that subjects had MC levels of 0.2 ±0.1 ng in “pre-exposure” nasal swabs (before swimming/ water skiing), as well as identical MC content in an “unexposed” control group (Backer et al. 2010). These values were proposed to represent a “baseline” amount of cyanobacteria among the 81 study participants (Backer et al. 2010). This “baseline,” constitutive amount of cyanobacteria exposure likely explains why sample collection time of year or participant residential proximity to random waterbodies were not significant factors in our study.
We determined that there is a low efficacy for using nasal swabs as a surrogate to bronchoscopy when detecting cyanobacteria. Previous studies have documented a similar variability in sensitivity/ specificity analyses, as well as PPV/ NPV between throat culture and BAL when identifying other respiratory microbes, like Pseudomonas aeruginosa and Staphylococcus aureus (Seidler et al. 2016). Although we determined that it is not efficacious to screen for cyanobacteria using nasal swabs as a surrogate for BAL, each sample may alone be an important tools for determining risk for cyanotoxin-associated diseases associated with cyanobacteria exposure.
Our findings of a broad prevalence of cyanobacterial exposure are especially interesting in light of a recent comprehensive topographic assessment study of the healthy lung. Bacteria may enter the lung via “microaspiration” during which bacteria are harbored in micro-droplets of saliva originating from the mouth - where upper respiratory tract inhalation and bacterial introduction occurs (Dickson et al. 2017). The bacteria-filled saliva droplets can then penetrate the central airways by gravity-dependent topography (i.e. being in an upright position) (Dickson et al. 2017). “Microaspiration” supports our hypothesis that inhaled cyanobacteria-filled aerosol could be a significant source of human exposure.
We used a phycocyanobilin cube (excitation 572nm, emission 640nm) to illuminate an epifluorescent protein present in the cyanobacteria-specific pigment, phycocyanin, to capture images via FM. As is seen in Figure 1A, particulate matter did not produce fluorescence, evidence that the signals we imaged via FM were specific for cyanobacteria. A study by Dang et al. defined many characteristics of freshwater cyanobacterium (Microcysits aeruginosa) and found the average cellular diameter to be 4.1 ± 0.07 μm, 3.8 ± 0.01 μm, 3.8 ± 0.05 μm, and 3.8 ± 0.01 μm between four different cultures grown at varying dilution rates (Dang et al. 2012). These data agree with our image analysis (using Fiji/ ImageJ) of the positive control Microcysits sp. (average diameter of 3.19 ± 0.77 μm) (Dang et al. 2012).
The BAL cyanobacteria average diameter and area measurements were in statistical agreement with control Microcystis, however, cyanobacteria detected in the nasal swab samples, were statistically smaller with respect to both diameter and area measurements. Reasons as to why the make-up of cyanobacteria populations may differ between these two regions of the respiratory tract/ types of bio-specimens remains unclear. It is possible that a mixture of symbiotic freshwater cyanobacteria species populated our nasal swab samples. A study comparing morphological features between five species of both symbiotic and non-symbiotic cyanobacteria, from two genera concluded that comparing cell size differences was valuable when distinguishing between closely related species found in the same water column (Usher et al. 2006). A mixture of different cyanobacteria species in our nasal swab samples might account for the size incongruences. In future studies complete 16S sequencing/ taxonomy could be performed to characterize the species distribution of these samples. Other confounding factors, such as mucous and debris content, may also be considered.
Furthermore, it is understood that inhaled particles (i.e. dust, silica, inhalers, etc.) deposit deeper into the respiratory tree dependent on particle size - where smaller objects tend to deposit the furthest (Heyder 2004). However, we must make the distinction between inhaled particles vs. inhaled organisms. It is important to keep in mind that organism deposition is vastly different and more complex. Organisms, like cyanobacteria, have the capacity to grow and express complex behavior, like biofilm formation (Rossi and De Philippis 2015). Thus, it is conceivable that smaller cyanobacteria are inhaled, which then grow and form colonies, potentially living both dormant and symbiotic in a biofilm once deep into the respiratory tract. This is one potential explanation and warrants future investigation.
Although our findings suggest that proximity to a waterbody was not significant, this is not to say that waterbodies are not a notable source of cyanobacteria and cyanotoxin exposure. Previous literature has provided strong evidence to support that cyanobacteria can be aerosolized from a waterbody and travel in LSA or other respirable particles (Lewandowska et al. 2017; May et al. 2018). Additionally, Backer and colleagues identified MC in the nares of persons who visited a waterbody with known cyanobacteria blooms (Backer et al. 2010). Furthermore, they described how pre-recreational to post-recreational nasal sampling appreciated an increase in MC concentration (Backer et al. 2010). Thus, targeted sampling of bloom areas is necessary to study exposure via CHAB-related aerosolization.
Are there other significant cyanobacteria sources, in addition to waterbodies, that could pose a health risk? As an aside, consider the gram-negative bacterium, Legionella pneumonia that is responsible for causing legionellosis (also known as, Legionnaire’s disease) (Prussin et al. 2017). In Legionnaire’s disease, primary infection occurs via inhaling L. pneumonia-containing aerosol particles (Allegra et al. 2016; Prussin et al. 2017). Transmission of aerosolized Legionella can originate from a multitude of water sources such as air conditioning units (cooling towers) and hot tubs, to name a few (Hamilton et al. 2017; Hamilton et al. 2018; Prussin et al. 2017). Recently, a group from Yale has identified a relationship between sporadic legionellosis and spatial distribution with water systems in Connecticut (Cassell et al. 2018). In a study by Allegra et al., the authors discovered that inhaled bioaerosols (on the range of 1 – 10 μm) were capable of carrying pathogenic Legionella, 93.4% of which was of a particle size conducive to aerosilization (Allegra et al. 2016). Out of the total airborne Legionella DNA sequenced, approximately 44% of the experimentally aerosolized (or 7% of the original bacterial culture) had the potential to reach the alveoli of the human lung (Allegra et al. 2016).
During the winter of 2003 – 2004, an outbreak of Legionnaire’s disease occurred in northern France that resulted in 18 deaths out of 86 reported cases (Mathieu et al. 2006). The authors remarked that the pathogenic L. pneumonia source was discovered to be cooling towers and water treatment basins (Mathieu et al. 2006). This outbreak and bacterial transmission occurred during the winter months and through air conditioning units. Association with cyanobacteria species was not investigated, however is possible based on previous literature, particularly in aerosols (Berendt 1981; Tison et al. 1980). Similar outbreaks have been reported in New Zealand in 2015 (Thornley et al. 2017), Memphis, TN, USA (Dondero et al. 1980), the Netherlands (Den Boer et al. 2002), Stafford General Hospital, United Kingdom (OMahony et al. 1990), an Australian aquarium (Greig et al. 2004), and more. Outbreaks are monitored annually by the Centers for Disease Control and Prevention (CDC) in the United States (CDC 2017).
Environmental microbiology studies dating back decades have documented that L. pneumonia growth is associated with cyanobacteria, and that temperature, pH, and colony nutrition survival requirements may not be as significant as once thought (Tison et al. 1980). Similarly, cyanobacteria (Fischerealla sp.) has been shown to facilitate L. pneumonia growth and survival in stringent conditions, and has been proposed to promote L. pneumonia survival in bio-aerosols from lakes and air conditioning systems (Berendt 1981; Tison et al. 1980). Cyanobacteria tend to colonize the same water coolant systems as L. pneumonia (Tison et al.1980), and have also been found in biofilms of the upper walls of cooling towers where sunlight is plentiful (Hauer 2010). Cyanobacteria are unique in that they can perform all three of photosynthesis, respiration, and nitrogen fixation (Vermaas 2001). This diversity in metabolic processes has allowed cyanobacteria to evolve and survive in many different, and often harsh, conditions. In fact, photosynthesis and respiration are regulated in cyanobacteria, allowing for processes conversions based on light availability (Vermaas 2001). This might explain why cyanobacteria can be found in coolant systems as well as temporarily in the human lung.
Furthermore, a study from the Malaysian city of Kuala Lumpur discovered that airborne cyanobacteria were detected at every level within the indoor environment of an office building in this city, as well as in the surrounding areas (Chu et al. 2013). The authors suggest that the outdoor environment may introduce indoor cyanobacteria into the air coolant system of the office building, as there was a high-to-low gradient of bacteria concentration from the outdoor environment to indoors (Chu et al. 2013). It is important to note that this is a tropical environment that does not experience winters comparable to those of northern New England and that the dominant cyanobacteria species found in the building was Phormidium cmgustissima (Chu et al. 2013). Cyanobacteria have also been found on buildings, walls, and other architectural surfaces (Gaylarde et al. 2005; Rindi and Guiry 2004; Uher et al. 2004). The literature on indoor cyanobacteria sources is remarkably scant; however, the study by Chu et al. along with analogous L. pneumonia literature, namely the winter Legionnaire’s outbreak, warrants future investigation into significant sources of indoor cyanobacteria and emphasizes the importance of speciation.
Although cyanobacteria are found in aerosol and thus may be respirable, an alternative explanation of cyanobacteria in the lung is via a symbiotic or commensal association with the lung microbiome. Erb-Downward and colleagues reported the existence of a “high frequency,” “core” lung microbiome in healthy, smoking individuals (Erb-Downward et al. 2011). They discovered that the healthy lung microbiome consists primarily of Pseudomonas, Streptococcus, Prevotella, Fusobacterium, Haemophilus, Veillonella, and Porphyromonas (Erb-Downward et al. 2011). Cyanobacteria (Microcystis sp. and other related species) were not among the microbes detected via 16S pyrosequencing, perhaps due to their photosynthetic requirements necessary for extended survival.
Numerous studies, primarily in the fields of CF (Fancello et al. 2011), COPD (Pragman et al. 2012), and asthma (Huang et al. 2011), have studied the diversity of the lung microbiome and how diseased states alter this diversity (Dickson et al. 2013; ODwyer et al. 2016). Cyanobacteria were not classified in the COPD or asthma populations, however did appear in an investigation of the CF virome (Fancello et al. 2011). In this study, sputum was analyzed for specific coding sequences associated with antimicrobial resistance in the CF virome. Upon closer examination, the finding of relevance for our purposes was that one (out of nine detected) cyanobacteria β-lactamase-encoding open reading frame gene was identified in one-out-of-five CF patient samples (Fancello et al. 2011). This is not sufficient evidence to suggest that cyanobacteria are commonly found in the CF microbial flora. Accordingly, we can deduce that cyanobacteria are not commonly found in the lungs of healthy individuals or those with lung disease.
A study by Frank et al. found that the majority of sequences from the nares of healthy individuals contained bacterial phyla like corynebacteria (68%) and staphylococci (27%), while cyanobacteria accounted for only 0.08% of all bacterial sequences in the nares of healthy individuals and 0.02% of in-patient, hospitalized individuals (Frank et al. 2010). Oh and colleagues investigated the changing microbiome among individuals ages 2 - 40 years old and the authors did not report the presence of cyanobacteria on the skin or in the nares via phylum-, genus-, or species-level analyses (Oh et al. 2012). We also could consider that the skin may pose a route of incidental cyanobacteria exposure, be it through epithelial cell shedding, onychophagy (nail biting), or even rhinotillexis (nose-picking). However, in two comprehensive reviews by Byrd et al. and Grade et al., cyanobacteria were not reported to inhabit any significant portion of the skin surface, even after accounting for interpersonal variation among four different participants (Byrd et al. 2018; Grice and Segre 2011).
Taken together, previous literature suggests that although the human microbiome is vast and diverse in the lungs, nares, and skin, cyanobacteria do not appear to be a fundamental member of the human microbial flora in these regions, suggesting that the cyanobacteria we observed in the BAL are via environmental exposure. Thus, aerosol, albeit outdoor from waterbodies or from indoor sources, may be a significant mechanism for cyanobacteria transmission. The ubiquity of these exposures indicates that it will be critical to more specifically differentiate the cyanotoxin secreting species from benign organisms, the situations that induce this toxin release e.g. “blooms,” and the acute and chronic health effects of toxin exposure.
Cyanotoxin exposure has been correlated with a significantly increased risk for hepatocellular carcinoma (Fleming et al. 2002), cyanobacterial pneumonia (Turner et al. 1990), and non-alcoholic liver disease (Falconer et al. 1983; Zhang et al. 2015). For instance, in an ecological study by Zhang and colleagues, the authors reported a 0.3% increased risk for nonalcoholic liver disease for every 1% CHAB coverage in disease-clustered counties (Zhang et al. 2015). The aerosol mechanism might explain how cyanobacteria and cyanotoxins are introduced into the body and promote disease. Lung macrophages, which are critical in the innate immune system, have been quantifiably detected (via flow cytometry) to uptake environmental particulate matter as a part of the defense mechanism (Stringer et al. 1995). An immunological study by Landsman et al. characterized that alveolar macrophages trace linage to blood monocytes, requiring a lung macrophage intermediate (Landsman and Jung 2007). This suggests that environmental particles inhaled into the lungs and taken up by lung macrophages may be able to enter the blood stream via barrier-crossing immune cells.
Our current study showed a high prevalence of cyanobacteria in the nasal cavity of our study participants, however it is unclear whether there is mechanism for direct cyanotoxin exposure from the nasal cavity that would not require transport to the lung. Dating back decades, studies have shown that inhaled substances have the ability to be transported to the brain via the olfactory bulb and subsequently have functional influence on neurons in central nervous system (CNS) (Miwa et al. 1998; Shipley and Adamek 1984; Shipley et al. 1985). Future work should assess whether aerosols containing cyanobacteria and cyanotoxins, might have olfactory bulb retrograde transport potential directly to the CNS. Aerosilization may be a significant mechanism for introducing cyanotoxins such as BMAA into the CNS, potentiating the pathophysiology of BMAA-induced protein misfolding in neurodegenerative diseases, like ALS or Alzheimer’s disease (Banack et al. 2010; Caller et al. 2018; Dunlop et al. 2013; Lobner et al. 2007; Michaelson et al. 2017; Pablo et al. 2009; Rao et al. 2006; Rush et al. 2012; Yin et al. 2014).
There are limitations with our study. First, based on our methods we identified cyanobacteria using a binary (positive or negative) approach via PCR. Although this answers the question regarding if cyanobacteria are present in these samples, it does not necessarily provide data on levels. We attempted to account for this by performing manual cell counting on prepared slides. However, this method is imperfect and potential for error exists. Similarly, this method may not represent the presence of microbes on the swab as a whole. A more precise and quantifiable method (i.e. quantitative PCR) should prove useful. Our second limitation is that we could not account for an association with known CHAB events. State databases do not comprehensively record the timing of blooms. Although our analyses showed that time of year and residential proximity were not major factors for cyanobacteria presence, we could not determine if samples collected around the time of a bloom were positive at a higher rate or in larger cyanobacteria quantity (using cytology or suggested qPCR approach). Future work might consider combining analytical data with geographical information systems modeling to identify the influence of known CHAB events with cyanobacteria in the respiratory tract.
We did not attempt to classify (sequence) the specific species of cyanobacteria present in positive samples. According to van Apeldoorn et al., the cyanobacteria class contains 150 genera and roughly 2000 species, where an estimated 40 genera are responsible for producing cyanotoxins (van Apeldoorn et al. 2007). The primary toxin producing cyanobacteria include Anabaena, Aphanizomenon, Cylindrospermopsis, Lyngbya, Microcystis, Nostoc and Oscillatoria (Carmichael et al. 2001; van Apeldoorn et al. 2007). Thus, we cannot make a claim regarding any specific population of cyanobacterium in our samples or their potential harmful nature. Future classification of cyanobacterial species in human bio-samples may provide insight into clinically relevant aerosolized cyanobacteria with cyanotoxin exposure and the potential for associated human disease.
5. Conclusion
Our study suggests that humans inhale aerosolized cyanobacteria which can be harbored in the nostrils and the lungs. Moreover, we have shown that this is a prevalent and chronic phenomenon, occurring in northern New England year-round. This is consistent with the hypothesis that aerosol may be a significant route of cyanobacteria transmission to humans. Both nasal swabs and BAL may individually be useful in screening for cyanobacteria exposure, though speciation and cyanotoxin production of these aerosolized cyanobacteria will be important for any linkage with health effects. Sources of significant indoor cyanobacteria exposure must be explored further and associations with L. pneumonia should be considered. This study provides evidence of human exposure to aerosolized cyanobacteria, motivating the more specific assessments of the cyanotoxin-secreting species and situations, which will be critical for mitigating cyanotoxin-associated diseases.
Highlights.
Humans routinely inhale aerosolized cyanobacteria, into the nostrils and lungs.
Samples collected during the winter were positive at surprisingly high frequencies.
Proximity to a waterbody was not a significant factor.
Sources of indoor exposure warrant future investigation.
Aerosol is a likely route of transmission for cyanotoxin-associated human diseases.
Acknowledgements
Thank you to all of our study subjects for participating and donating bio-specimens, the Translational Research Core at Dartmouth for coordinating bronchoscopy sample collection (NIH [P30GM106394]), and to Dr. Walter Bradley for his assistance with revisions.
Funding
This work was supported by the Research Development Award from the Diamond Endowment Program at Dartmouth-Hitchcock Medical Center, Lebanon, NH; the National Institutes of Health [NIHR01HL122372]; and recruitment and enrollment of human subjects was achieved with the assistance of the Translational Research Core at Dartmouth which is funded by the National Institutes of Health [P30GM106394].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Chalabi A, Calvo A, Chio A, Colville S, Ellis CM, Hardiman O, et al. 2014. Analysis of amyotrophic lateral sclerosis as a multistep process: A population-based modelling study. The Lancet Neurology 13:1108–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra S, Leclerc L, Massard PA, Girardot F, Riffard S, Pourchez J. 2016. Characterization of aerosols containing legionella generated upon nebulization. Scientific reports 6:33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew AS, Caller TA, Tandan R, Duell EJ, Henegan PL, Field NC, et al. 2017. Environmental and occupational exposures and amyotrophic lateral sclerosis in new england. Neuro-degenerative diseases 17:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo SM, Carmichael WW, Jochimsen EM, Rinehart KL, Lau S, Shaw GR, et al. 2002. Human intoxication by microcystins during renal dialysis treatment in caruaru-brazil. Toxicology 181-182:441–446. [DOI] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng Y-S, Benson J, Pierce RH, et al. 2003. Recreational exposure to aerosolized brevetoxins during florida red tide events. Harmful algae 2:19–28. [Google Scholar]
- Backer LC, Kirkpatrick B, Fleming LE, Cheng YS, Pierce R, Bean JA, et al. 2005. Occupational exposure to aerosolized brevetoxins during florida red tide events: Effects on a healthy worker population. Environmental health perspectives 113:644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer LC, McNeel SV, Barber T, Kirkpatrick B, Williams C, Irvin M, et al. 2010. Recreational exposure to microcystins during algal blooms in two california lakes. Toxicon : official journal of the International Society on Toxinology 55:909–921. [DOI] [PubMed] [Google Scholar]
- Banack SA, Cox PA. 2003. Biomagnification of cycad neurotoxins in flying foxes: Implications for als-pdc in guam. Neurology 61:387–389. [DOI] [PubMed] [Google Scholar]
- Banack SA, Caller TA, Stommel EW. 2010. The cyanobacteria derived toxin beta-n-methylamino-l-alanine and amyotrophic lateral sclerosis. Toxins 2:2837–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banack SA, Caller T, Henegan P, Haney J, Murby A, Metcalf JS, et al. 2015. Detection of cyanotoxins, beta-n-methylamino-l-alanine and microcystins, from a lake surrounded by cases of amyotrophic lateral sclerosis. Toxins 7:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdalet E, Fleming LE, Gowen R, Davidson K, Hess P, Backer LC, et al. 2015. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. Journal of the Marine Biological Association of the United Kingdom Marine Biological Association of the United Kingdom 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt RF. 1981. Influence of blue-green algae (cyanobacteria) on survival of legionella pneumophila in aerosols. Infection and immunity 32:690–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Ba N, Gugger M, Bouvy M, Rusconi F, Coute A, et al. 2006. Seasonal dynamics and toxicity of cylindrospermopsis raciborskii in lake guiers (senegal, west africa). FEMS microbiology ecology 57:355–366. [DOI] [PubMed] [Google Scholar]
- Bradley WG, Borenstein AR, Nelson LM, Codd GA, Rosen BH, Stommel EW, et al. 2013. Is exposure to cyanobacteria an environmental risk factor for amyotrophic lateral sclerosis and other neurodegenerative diseases? Amyotrophic lateral sclerosis & frontotemporal degeneration 14:325–333. [DOI] [PubMed] [Google Scholar]
- Byrd AL, Belkaid Y, Segre JA. 2018. The human skin microbiome. Nature reviews Microbiology 16:143–155. [DOI] [PubMed] [Google Scholar]
- Caller T, Henegan P, Stommel E. 2018. The potential role of bmaa in neurodegeneration. Neurotoxicity research 33:222–226. [DOI] [PubMed] [Google Scholar]
- Caller TA, Doolin JW, Haney JF, Murby AJ, West KG, Farrar HE, et al. 2009. A cluster of amyotrophic lateral sclerosis in new hampshire: A possible role for toxic cyanobacteria blooms. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases 10 Suppl 2:101–108. [DOI] [PubMed] [Google Scholar]
- Caller TA, Field NC, Chiμman JW, Shi X, Harris BT, Stommel EW. 2012. Spatial clustering of amyotrophic lateral sclerosis and the potential role of bmaa. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases 13:25–32. [DOI] [PubMed] [Google Scholar]
- Caller TA, Chiμman JW, Field NC, Stommel EW. 2013. Spatial analysis of amyotrophic lateral sclerosis in northern new england, USA, 1997–2009. Muscle & nerve 48:235–241. [DOI] [PubMed] [Google Scholar]
- Carmichael WW, Azevedo SM, An JS, Molica RJ, Jochimsen EM, Lau S, et al. 2001. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environmental health perspectives 109:663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael WW, Boyer GL. 2016. Health impacts from cyanobacteria harmful algae blooms: Implications for the north american great lakes. Harmful algae 54:194–212. [DOI] [PubMed] [Google Scholar]
- Cassell K, Gacek P, Warren JL, Raymond PA, Cartter M, Weinberger DM. 2018. Association between sporadic legionellosis and river systems in connecticut. The Journal of infectious diseases 217:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave J, Wunderlin DA, de Los Angeles Bistoni M, Ame MV, Krause E, Pflugmacher S, et al. 2005. Uptake, tissue distribution and accumulation of microcystin-rr in corydoras paleatus, jenynsia multidentata and odontesthes bonariensis. A field and laboratory study. Aquatic toxicology 75:178–190. [DOI] [PubMed] [Google Scholar]
- Cazenave J, Bistoni Mde L, Pesce SF, Wunderlin DA. 2006. Differential detoxification and antioxidant response in diverse organs of corydoras paleatus experimentally exposed to microcystin-rr. Aquatic toxicology 76:1–12. [DOI] [PubMed] [Google Scholar]
- CDC. 2017. Waterborne disease & outbreak surveillance reporting. Available: https://www.cdc.gov/healthywater/surveillance/index.html 2018].
- Chu W-L, Tneh S-Y, Ambu S. 2013. A survey of airborne algae and cyanobacteria within the indoor environment of an office building in kuala lumpur, malaysia. Grana 52:207–220. [Google Scholar]
- Dang TC, Fujii M, Rose AL, Bligh M, Waite TD. 2012. Characteristics of the freshwater cyanobacterium microcystis aeruginosa grown in iron-limited continuous culture. Applied and environmental microbiology 78:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Boer JW, Yzerman EP, Schellekens J, Lettinga KD, Boshuizen HC, Van Steenbergen JE, et al. 2002. A large outbreak of legionnaires’ disease at a flower show, the netherlands, 1999. Emerging infectious diseases 8:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP, Huang YJ, Martinez FJ, Huffnagle GB. 2013. The lung microbiome and viral-induced exacerbations of chronic obstructive pulmonary disease: New observations, novel approaches. American journal of respiratory and critical care medicine 188:1185–1186. [DOI] [PubMed] [Google Scholar]
- Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, et al. 2017. Bacterial topography of the healthy human lower respiratory tract. mBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich D, Hoeger S. 2005. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): A reasonable or misguided approach? Toxicology and applied pharmacology 203:273–289. [DOI] [PubMed] [Google Scholar]
- Dondero TJ Jr., Rendtorff RC, Mallison GF, Weeks RM, Levy JS, Wong EW, et al. 1980. An outbreak of legionnaires’ disease associated with a contaminated air-conditioning cooling tower. The New England journal of medicine 302:365–370. [DOI] [PubMed] [Google Scholar]
- Drobac D, Tokodi N, Simeunovic J, Baltic V, Stanic D, Svircev Z. 2013. Human exposure to cyanotoxins and their effects on health. Arhiv za higijenu rada i toksikologiju 64:119–130. [DOI] [PubMed] [Google Scholar]
- Dunlop RA, Cox PA, Banack SA, Rodgers KJ. 2013. The non-protein amino acid bmaa is misincorporated into human proteins in place of l-serine causing protein misfolding and aggregation. PloS one 8:e75376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Saadi OE, Esterman AJ, Cameron S, Roder DM. 1995. Murray river water, raised cyanobacterial cell counts, and gastrointestinal and dermatological symptoms. The Medical journal of Australia 162:122–125. [DOI] [PubMed] [Google Scholar]
- Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, et al. 2011. Analysis of the lung microbiome in the “healthy” smoker and in copd. PloS one 6:e16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer IR, Beresford AM, Runnegar MT. 1983. Evidence of liver damage by toxin from a bloom of the blue-green alga, microcystis aeruginosa. The Medical journal of Australia 1:511–514. [DOI] [PubMed] [Google Scholar]
- Fancello L, Desnues C, Raoult D, Rolain JM. 2011. Bacteriophages and diffusion of genes encoding antimicrobial resistance in cystic fibrosis sputum microbiota. The Journal of antimicrobial chemotherapy 66:2448–2454. [DOI] [PubMed] [Google Scholar]
- Field NC, Metcalf JS, Caller TA, Banack SA, Cox PA, Stommel EW. 2013. Linking beta-methylamino-l-alanine exposure to sporadic amyotrophic lateral sclerosis in annapolis, md. Toxicon : official journal of the International Society on Toxinology 70:179–183. [DOI] [PubMed] [Google Scholar]
- Fleming LE, Rivero C, Burns J, Williams C, Bean JA, Shea KA, et al. 2002. Blue green algal (cyanobacterial) toxins, surface drinking water, and liver cancer in florida. Harmful algae 1:157–168. [Google Scholar]
- Frank DN, Feazel LM, Bessesen MT, Price CS, Janoff EN, Pace NR. 2010. The human nasal microbiota and staphylococcus aureus carriage. PloS one 5:e10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylarde μm, Crispim CA, Neilan BA, Gaylarde CC. 2005. Cyanobacteria from brazilian building walls are distant relatives of aquatic genera. Omics : a journal of integrative biology 9:30–42. [DOI] [PubMed] [Google Scholar]
- Gilroy DJ, Kauffman KW, Hall RA, Huang X, Chu FS. 2000. Assessing potential health risks from microcystin toxins in blue-green algae dietary supplements. Environmental health perspectives 108:435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig JE, Carnie JA, Tallis GF, Ryan NJ, Tan AG, Gordon IR, et al. 2004. An outbreak of legionnaires’ disease at the melbourne aquarium, april 2000: Investigation and case-control studies. The Medical journal of Australia 180:566–572. [DOI] [PubMed] [Google Scholar]
- Grice EA, Segre JA. 2011. The skin microbiome. Nature reviews Microbiology 9:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KA, Ahmed W, Toze S, Haas CN. 2017. Human health risks for legionella and mycobacterium avium complex (mac) from potable and non-potable uses of roof-harvested rainwater. Water research 119:288–303. [DOI] [PubMed] [Google Scholar]
- Hamilton KA, Hamilton MT, Johnson W, Jjemba P, Bukhari Z, LeChevallier M, et al. 2018. Health risks from exposure to legionella in reclaimed water aerosols: Toilet flushing, spray irrigation, and cooling towers. Water research 134:261–279. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (redcap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer T 2010. Phototrophic biofilms on the interior walls of concrete iterson-type cooling towers. [Google Scholar]
- Heyder J 2004. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc 1:315–320. [DOI] [PubMed] [Google Scholar]
- Hilborn ED, Carmichael WW, Yuan M, Azevedo SM. 2005. A simple colorimetric method to detect biological evidence of human exposure to microcystins. Toxicon : official journal of the International Society on Toxinology 46:218–221. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, et al. 2011. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. The Journal of allergy and clinical immunology 127:372–381 e371–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochimsen EM, Carmichael WW, An JS, Cardo DM, Cookson ST, Holmes CE, et al. 1998. Liver failure and death after exposure to microcystins at a hemodialysis center in brazil. The New England journal of medicine 338:873–878. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Pierce R, Cheng YS, Henry MS, Blum P, Osborn S, et al. 2010. Inland transport of aerosolized florida red tide toxins. Harmful algae 9:186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L, Jung S. 2007. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. Journal of immunology 179:3488–3494. [DOI] [PubMed] [Google Scholar]
- Lewandowska AU, Sliwinska-Wilczewska S, Wozniczka D. 2017. Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the southern baltic sea. Marine pollution bulletin 125:30–38. [DOI] [PubMed] [Google Scholar]
- Lobner D, Piana PM, Salous AK, Peoples RW. 2007. Beta-n-methylamino-l-alanine enhances neurotoxicity through multiple mechanisms. Neurobiology of disease 25:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes VF, Soares RM, Azevedo SM. 2001. Microcystin contamination in fish from the jacarepagua lagoon (rio de janeiro, brazil): Ecological implication and human health risk. Toxicon : official journal of the International Society on Toxinology 39:1077–1085. [DOI] [PubMed] [Google Scholar]
- Mathieu L, Robine E, Deloge-Abarkan M, Ritoux S, Pauly D, Hartemann P, et al. 2006. Legionella bacteria in aerosols: Sampling and analytical approaches used during the legionnaires disease outbreak in pas-de-calais. The Journal of infectious diseases 193:1333–1335. [DOI] [PubMed] [Google Scholar]
- May NW, Olson NE, Panas M, Axson JL, Tirella PS, Kirpes RM, et al. 2018. Aerosol emissions from great lakes harmful algal blooms. Environmental science & technology 52:397–405. [DOI] [PubMed] [Google Scholar]
- Michaelson N, Facciponte D, Bradley W, Stommel E. 2017. Cytokine expression levels in als: A potential link between inflammation and bmaa-triggered protein misfolding. Cytokine & growth factor reviews 37:81–88. [DOI] [PubMed] [Google Scholar]
- Miwa T, Horikawa I, Uramoto N, Ishimaru T, Yamamoto K, Furukawa M, et al. 1998. Trka expression in mouse olfactory tract following axotomy of olfactory nerves. Acta otolaryngologica Supplementum 539:79–82. [DOI] [PubMed] [Google Scholar]
- Murch SJ, Cox PA, Banack SA. 2004a. A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in guam. Proceedings of the National Academy of Sciences of the United States of America 101:12228–12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murch SJ, Cox PA, Banack SA, Steele JC, Sacks OW. 2004b. Occurrence of beta-methylamino-l-alanine (bmaa) in als/pdc patients from guam. Acta neurologica Scandinavica 110:267–269. [DOI] [PubMed] [Google Scholar]
- O’Dwyer DN, Dickson RP, Moore BB. 2016. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. Journal of immunology 196:4839–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony MC, Stanwell-Smith RE, Tillett HE, Harper D, Hutchison JG, Farrell ID, et al. 1990. The stafford outbreak of legionnaires’ disease. Epidemiology and infection 104:361–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Conlan S, Polley EC, Segre JA, Kong HH. 2012. Shifts in human skin and nares microbiota of healthy children and adults. Genome medicine 4:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, Bradley WG, et al. 2009. Cyanobacterial neurotoxin bmaa in als and alzheimer’s disease. Acta neurologica Scandinavica 120:216–225. [DOI] [PubMed] [Google Scholar]
- Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. 2012. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PloS one 7:e47305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin AJ, Schwake DO, Marr LC. 2017. Ten questions concerning the aerosolization and transmission of legionella in the built environment. Building and Environment 123:684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SD, Banack SA, Cox PA, Weiss JH. 2006. Bmaa selectively injures motor neurons via ampa/kainate receptor activation. Experimental neurology 201:244–252. [DOI] [PubMed] [Google Scholar]
- Riancho J, Bosque-Varela P, Perez-Pereda S, Povedano M, de Munain AL, Santurtun A. 2018. The increasing importance of environmental conditions in amyotrophic lateral sclerosis. International Journal of Biometeorology. [DOI] [PubMed] [Google Scholar]
- Rindi F, Guiry MD. 2004. Composition and spatial variability of terrestrial algal assemblages occurring at the bases of urban walls in europe. Phycologia 43:225–235. [Google Scholar]
- Rossi F, De Philippis R. 2015. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life (Basel) 5:1218–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Lachapelle A, Solliec M, Bouchard MF, Sauve S. 2017. Detection of cyanotoxins in algae dietary supplements. Toxins 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush T, Liu X, Lobner D. 2012. Synergistic toxicity of the environmental neurotoxins methylmercury and beta-n-methylamino-l-alanine. Neuroreport 23:216–219. [DOI] [PubMed] [Google Scholar]
- Seidler D, Griffin M, Nymon A, Koeppen K, Ashare A. 2016. Throat swabs and sputum culture as predictors of p. Aeruginosa or s. Aureus lung colonization in adult cystic fibrosis patients. PloS one 11:e0164232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Adamek GD. 1984. The connections of the mouse olfactory bulb: A study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain research bulletin 12:669–688. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Halloran FJ, de la Torre J. 1985. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain research 329:294–299. [DOI] [PubMed] [Google Scholar]
- Stommel EW, Field NC, Caller TA. 2013. Aerosolization of cyanobacteria as a risk factor for amyotrophic lateral sclerosis. Medical hypotheses 80:142–145. [DOI] [PubMed] [Google Scholar]
- Stringer B, Imrich A, Kobzik L. 1995. Flow cytometric assay of lung macrophage uptake of environmental particulates. Cytometry 20:23–32. [DOI] [PubMed] [Google Scholar]
- Thornley CN, Harte DJ, Weir RP, Allen LJ, Knightbridge KJ, Wood PRT. 2017. Legionella longbeachae detected in an industrial cooling tower linked to a legionellosis outbreak, new Zealand, 2015; possible waterborne transmission? Epidemiology and infection 145:2382–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tison DL, Pope DH, Cherry WB, Fliermans CB. 1980. Growth of legionella pneumophila in association with blue-green algae (cyanobacteria). Applied and environmental microbiology 39:456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbick N, Hession S, Stommel E, Caller T. 2014. Mapping amyotrophic lateral sclerosis lake risk factors across northern new england. International journal of health geographics 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbick N, Ziniti B, Stommel E, Linder E, Andrew A, Caller T, et al. 2018. Assessing cyanobacterial harmful algal blooms as risk factors for amyotrophic lateral sclerosis. Neurotoxicity research 33:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PC, Gammie AJ, Hollinrake K, Codd GA. 1990. Pneumonia associated with contact with cyanobacteria. Bmj 300:1440–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher B, Aboal M, Kovacik L. 2004. Cyanobacteria and algae on monuments and buildings in region of murcia (spain). [Google Scholar]
- Usher KM, Kuo J, Fromont J, Toze S, Sutton DC. 2006. Comparative morphology of five species of symbiotic and non-symbiotic coccoid cyanobacteria. European Journal of Phycology 41:179–188. [Google Scholar]
- van Apeldoorn ME, van Egmund HP, Speijers GJA, Bakker GJI. 2007. Toxins of cyanobacteria. Molecular Nutritional Food Research 51:53. [DOI] [PubMed] [Google Scholar]
- Vermaas WFJ. 2001. Photosynthesis and respiration in cyanobacteria. ENCYCLOPEDIA OF LIFE SCIENCES:7. [Google Scholar]
- Xie L, Xie P, Guo L, Li L, Miyabara Y, Park HD. 2005. Organ distribution and bioaccumulation of microcystins in freshwater fish at different trophic levels from the eutrophic lake chaohu, china. Environmental toxicology 20:293–300. [DOI] [PubMed] [Google Scholar]
- Yin HZ, Yu S, Hsu CI, Liu J, Acab A, Wu R, et al. 2014. Intrathecal infusion of bmaa induces selective motor neuron damage and astrogliosis in the ventral horn of the spinal cord. Experimental neurology 261:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Lee J, Liang S, Shum CK. 2015. Cyanobacteria blooms and non-alcoholic liver disease: Evidence from a county level ecological study in the united states. Environmental health : a global access science source 14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]