Abstract
Background:
To examine the blood flow and detection of the issues related to it by medical ultrasound, it is extremely important to have suitable blood mimicking fluid (BMF) to be used in vitro and to have a movable or portable Doppler flow phantom to use it as a standardizing tool. As known, the main drawbacks of the currently commercial BMF used in the research studies are high in cost and the long time needed for preparation, which is at least 5–7 h. Moreover, there are only two common scatter particles using in BMF as suspension materials such as nylon (Orgasol) and polystyrene. Thus, we need to prepare BMF with both a new mixture fluid and new scatter particle to be as a reflecting factor of ultrasonic waves, for evaluating the speed of sound of the blood flow in the same method like in the research study of ultrasound with relatively low-cost and less consuming time of preparation. However, both the acoustical and physical features of the Doppler flow phantom components (BMF and tissue mimicking material) must correspond the features of the human tissues to make the examination significance. In addition, the BMF must also represent the hemodynamic features of real human blood.
Methods:
In this experiment, a new adequate ternary mixture liquid for preparation of BMF applied and suspended with a new scatter particle material, this scatter particle material called poly (4-methylstyrene), it used to be adequate with the mixture density and for saving neutrally buoyant. This BMF was prepared for use in the test objects or Doppler flow phantom. The poly (4-methylstyrene) particles were applied for suspension in a mixture liquid or fluid based on three items, which were distilled water, propylene glycol (PG), and polyethylene glycol (PEG) (200 Mw). The diameter of poly (4-methylstyrene) particles is 3–8 μm, which determined by specific sieve in a unit of μm, and the density is 1.040 g/ml.
Results:
Speed of sound, viscosity, density, Backscatter power and attenuation features of mixture fluid or liquid which used for preparing a BMF were measured, discussed, and agreed with draft International Electrotechnical Commission values.
Conclusions:
There are three various types of ternary items of mixture fluid (water, PG, and PEG [200 Mw]), and a new type of scatter particle material poly (4-methylstyrene) was utilized for preparing the BMF. The scatter particles and mixture fluid prepared and measured at a temperature that simulates the body temperature 37°C. Moreover, one of the advantages of this new blood that is being cheaper than the commercially available BMF products because the PG and the polyethylene glycol (200 Mw) are much cheaper and more available than glycerol and the Dextran that used usually. In addition, new BMF needs less time for preparation compared to the commercial one.
Keywords: Acoustical properties, blood mimicking fluid, Doppler ultrasound, Physical properties, tissue mimicking material
INTRODUCTION
The liquid or fluid applied in Doppler ultrasound flow phantoms and for testing the object models in vitro must preferably have similar properties and features which mimic real human blood (in vivo).[1,2] Furthermore, for estimating the achievements of Doppler instrument, blood itself may seem to be the best liquid to use. However, there are several difficulties connected with utilizing blood and its ingredients. There is a possible biohazard and care should be taken into consideration to minimize this hazard. The lifespan of blood is finite and red blood cells in vitro are easily deteriorated. This avoids utilizing the blood like a standard liquid in long-term research studies of measurements. The room temperature plays an important role in changing the acoustical and physical properties of blood, especially with a temperature of more than at 37°C.[3]
The nature of human blood is non-Newtonian.[2,4,5,6,7,8,9] In other words, the human viscosity of blood is highly based on shear rate. Thus, the non-Newtonian feature of blood is idea happen in the small or tiny vessels. However, in the large or main vessels, it is proper to consider that the blood is Newtonian.[2,4,5] Thus, the BMF applied in Doppler medical ultrasound (in vitro) must typically have similar features to the real human blood. Then, the ultrasound scanner will receive an equal pulse echo (PE) signal from the blood. Moreover, the details of suitable connected acoustical and physical properties of blood mimicking fluid (BMF) must be considered accurately. The speed of sound, density, attenuation, viscosity, and the particle size are significant factors that must be studied and tested in the first stage of preparing and manufacturing a BMF. Because the vessel cross-sectional region would be measured, the sound velocity is necessary. The BMF viscosity does not depend on shear rate because the BMF is Newtonian. Furthermore, the attenuation is also one of the most important factors influencing on the Doppler signals pulse from several parts of the vessel lumen. In the real human blood, the attenuation is small <0.1 dB. However, The International Electrotechnical Commission (IEC) used and applied as standard and constant values of the acoustical and physical features of BMF for Doppler ultrasound flow phantoms [Table 1].[1,2,3,4,5]
Table 1.
Specifications of the BMF defined as the IEC standard. f is the acoustic frequency (Hz)
| Acoustical and physical properties of BMF | Values |
|---|---|
| Viscosity (×10−3 Pas) | 4.0±0.4 |
| Acoustic speed (m/s) | 1570±30 |
| Attenuation (dB/cm/MHz) | <0.1×10−4×f |
| Density (×103 kg/m3) | 1.050±0.040 |
The particles' items utilized in BMF preparation must be able to stay hanging (suspended), in other words, to avoided float or ascent inside a fluid. That means, it is necessary to stay neutrally buoyant, even the speed is very.[2,3,4,5,6] The item particles' density must nearly close to as possible to the real human blood density which is range between 1.01 and 1.09 g/ml. However, those scatter particles represent or simulate the human red cells (Erythrocytes). The size (diameter) of those particles should close or near as much as possible to the diameter of human red cells which is 7–8 μm.[10] Moreover, particles that must be applied in BMF are essentially spherical in shape to avoid clotting during flow. For instance, microspheres' particles of polystyrene with suitable diameters 10–30 μm.[4,5,6,11] Another example is nylon particles with proper diameters 3–20 μm.[2,3,8,9,12,13] In addition, the speed of sound and attenuation in the BMF must be similar to those of real human blood. The acoustic sound and attenuation coefficient of BMF are typically 1570 ± 30 m/s and 0.1 dB/cm·MHz, respectively, about to the draft IEC 1685 standard.[13,14,15,16] Moreover, one of the significant factors of an item (practical) that suspension inside the BMF is stability and the strength of ultrasound backscatter.[17,18] The backscatter strength must describe when utilizing it in a Doppler wall-less flow phantom. The backscatter power must be defined by the draft IEC 1685.
METHODS
Preparation of a new blood mimicking fluid
In this experimental study, our purpose was to prepare a suitable new BMF with a convenient attenuation, the speed of sound, density, and viscosity which are known and defined as stable values in the IEC standard. However, two diverse types of BMF were chosen for the research study; the first one (BMFa) did not agree with IEC standard requirements, and the second one (BMFb), which appears to be more credible in mimicking blood, was studied and explained in more details in the method and discussion section in this article. The BMFs were prepared by various steps which described and explained in the Appendix 1. As the BMF viscosity is not based on the shear rate, the arbitrary viscosity could be applied.[5]
To get a new proper BMFb, first, a proper mixture fluid which was made of distilled water, propylene glycol (PG), and polyethylene glycol (200 MW) was prepared, dispersed in spherical poly (4-methylstyrene) scatters' particle material was adopted in the BMF. However, in this experiment, we have modified the ratios of PG and polyethylene glycol many times to obtain adequate acoustical and physical features which were known and defined in the IEC standard. Principally, during increasing the ratio of glycol, the average density of mixture fluid will increase[5] because the densities of PG and polyethylene glycol (1.036 and 1.124 g/ml, respectively) are greater than the density of water (0.998 g/ml), then the average speed of sound will rise directly due to its direct proportional relationship to the density. The speed of sound of PG, polyethylene glycol, and water was measured in this experiment; they were 1513, 1612, and 1508 m/s, respectively.
At the beginning of the research study, in the first trial study for preparation of BMFa of ternary mixture liquid, increased the PG and fixed the silicon oil ratio were done. The experimental research study ratios were changed various times [Table 2a-c] to gain more convenient density and speed of sound which must be matched with IEC standard requirements. However, because the silicon oil was not a suitable fluid for mixing with PG, other items with a density larger than water density were used. Thus, in the second trial for preparing BMFb, new items with different ratios were added, which were water, PG, and polyethylene glycol (200 Mw). The concentration of both the PG and polyethylene glycol were increased gradually to obtain proper acoustical and physical properties of mixture fluid. However, to mark which causes more frequent influence on the mixture liquid, the PG or the polyethylene glycol, two extra trial experiments were added. The first one increased PG ratios and fixed the polyethylene glycol ratio [Table 3b]. The second one fixed the ratios of PG and increased the polyethylene glycol ratio [Table 3c].
Table 2a.
Constitutions and physical properties of water, propylene glycol, and polyethylene glycol aqueous solutions (temperature: 37.0 °C)
| Number of samples | Water | Propylene glycol | Silicon oil | Density (g/ml) | Speed of sound (m/s) |
|---|---|---|---|---|---|
| 1 | 100 | 0 | 0 | 0.998 | 1508 |
| 2 | 90 | 9 | 1 | 1.002 | 1480 |
| 3 | 85 | 14 | 1 | 1.003 | 1490 |
| 4 | 80 | 19 | 1 | 1.004 | 1505 |
| 5 | 70 | 29 | 1 | 1.01 | 1517 |
| 6 | 60 | 39 | 1 | 1.012 | 1535 |
| 7 | 50 | 49 | 1 | 1.015 | 1568 |
Table 2c.
Constitutions and physical properties of water, propylene glycol, and polyethylene glycol aqueous solutions (temperature: 37.0 °C)
| Number of samples | Water | Propylene glycol | Silicon oil | Density (g/ml) | Speed of sound (m/s) |
|---|---|---|---|---|---|
| 1 | 100 | 0 | 0 | 0.998 | 1508 |
| 2 | 90 | 5 | 5 | 0.996 | 1500 |
| 3 | 85 | 7.5 | 7.5 | 0.994 | 1497 |
| 4 | 80 | 10 | 10 | 0.993 | 1493 |
| 5 | 70 | 15 | 15 | 0.991 | 1485 |
| 6 | 60 | 20 | 20 | 0.988 | 1478 |
| 7 | 50 | 25 | 25 | 0.986 | 1470 |
Table 3b.
The acoustical and physical features of water, propylene glycol, and polyethylene glycol at increase the concentrations of the propylene glycol, and fixed the polyethylene glycol at temperature 37°C±1°C
| Number of samples | Water | Propylene glycol | Polyethylene glycol | Density (g/ml) | Speed of sound (m/s) |
|---|---|---|---|---|---|
| 1 | 100 | 0 | 0 | 0.998 | 1508 |
| 2 | 90 | 5 | 5 | 1.005 | 1480 |
| 3 | 85 | 10 | 5 | 1.01 | 1490 |
| 4 | 80 | 15 | 5 | 1.013 | 1505 |
| 5 | 70 | 25 | 5 | 1.015 | 1517 |
| 6 | 60 | 35 | 5 | 1.017 | 1535 |
| 7 | 50 | 45 | 5 | 1.019 | 1568 |
Table 3c.
The acoustical and physical features of water, propylene glycol, and polyethylene glycol at fixed concentrations of the propylene glycol, and increase the polyethylene glycol at temperature 37°C±1°C
| Number of sample | Water | Propylene glycol | Polyethylene glycol | Density (g/ml) | Speed of sound (m/s) | Viscosity (mPas) |
|---|---|---|---|---|---|---|
| 1 | 100 | 0 | 0 | 0.998 | 1508 | 0.89 |
| 2 | 90 | 5 | 5 | 1.01 | 1513 | 2.6 |
| 3 | 85 | 5 | 10 | 1.02 | 1544 | 4.8 |
| 4 | 80 | 5 | 15 | 1.03 | 1570 | 7.1 |
| 5 | 70 | 5 | 25 | 1.04 | 1595 | 9.3 |
| 6 | 60 | 5 | 35 | 1.06 | 1645 | 11.5 |
| 7 | 50 | 5 | 45 | 1.08 | 1698 | 14.8 |
Those items or materials were 99% pure and supplies by Sigma-Aldrich. The diameter of poly (4-methylstyrene) particles is 3–8 μm, which determined by specific sieve in a unit of μm. Moreover, the PG and polyethylene glycol were provided and supplied by Sigma-Aldrich with suitable densities and molecular weight. The water in this experiment was obtained from medical physics and radiation science laboratory using a quartz distiller which produced distilled water for better performance.
Setup the ultrasonic A-scan (GAMPT)
In this experimental study, the set-up steps for a preliminary study of mixture fluid and BMF measurement were done by ultrasonic A-scan (GAMPT) technique model 10121. The signal wave transmitted from a transmitter propagates through the fluid sample and reaches a receiver. The electric signal wave amplified from the receiver side is converted to a pulse, and it made triggers a pulse. This process made a uniform oscillation cycle in the GAMPT ultrasonic system. The period time of the oscillation is called the sing-around period time [Figure 1].
Figure 1.

Experimental set-up of ultrasonic A-scan GAMPT technique. (a) A thin Plexiglas plates made of acrylic and filled with water. (b) A frequency 2–5 MHz red color probe connect to the water inside Plexiglas plates without using aquasonic 100 ultrasound transmission gel between them. This probe sends sound wave through the sample (water or liquid) then receive it. (c) A control GAMPT A-scan which work as a function generator to send and receive the electrical signal then convert it to pulse sound signal wave when pass through the probe. (d) The USB wires connecting to the control GAMPT A-scan from backside and with personal computer to display the signal wave, data and result show on the personal computer monitor. (e) The overall steps together of the echoscope and its components
Ultrasonic transducer (probe) used in ultrasonic (GAMPT) scan
The ultrasonic transducers used in this research were red color transducers with frequency 1–5 MHz. The ultrasonic transducers were coupled with powerful snap-in-connectors. The frequency of the transducer is automatically known from the appliance. With the help of the adjustable sending and receiving power [Figure 2a]. A control A-scan GAMPT which work as a function generator to send and receive the electrical signal then convert it to sound wave when pass through the probe.
Figure 2.
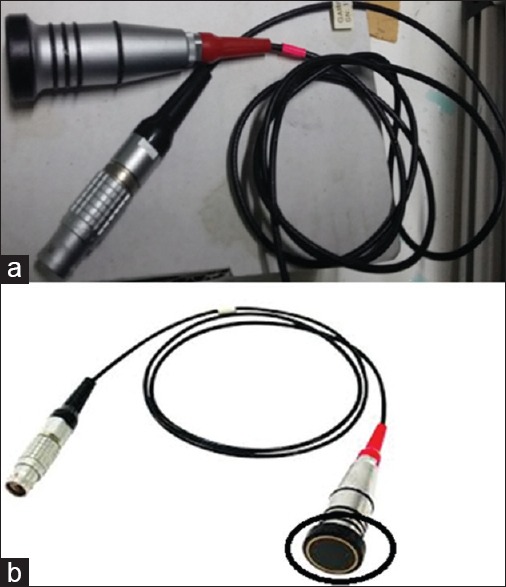
(a) Red color ultrasonic transducer with frequency ranges 1–5 MHz. (b) Red color Ultrasonic transducer with frequency ranges 1–5 MHz with shows the protective layer inside black circle
Measure speed of sound of samples by applying A-scan (GAMPT) technique
The model equation for determining the ultrasound velocity applying PE method was shown in Eq. 1. Hence, there is an ability to measure the speed of ultrasound by utilizing PE method by different methods. Initially, measuring the time of flight (TOF) between the highest two following peaks or between two identical peak signal pulses of transmitted and received a wave and then use the thickness or distance of the sample. Moreover, the average density of the sound measurements both different methods was taken into account. However, there are some influences on the measurements because of the protective layer thickness of the probe. This thickness can effect on ToF [Figure 2b].[11] For accuracy of ultrasonic speed measurement, the thickness of the protective layer must be known first, then measuring the speed of sound of samples with taken this thickness in account by following the Eq. 2.
SoS=2D/T (1)
SoS=(2[D+dpl])/T (2)
Where SoS is the speed of sound, D is the sample depth or distance, dpl is the thickness of r protective layer and it was measured in this article and equal to 1.03 mm, and ultimately T is the TOF.
Measure the attenuation coefficient using ultrasonic A-scan (GAMPT) technique
After measuring the speed sound of the mixture fluid of each sample, insert the value in the ultrasonic (GAMPT) pulse-echo techniques to calculate the attenuation coefficient of the sample by the following equation:
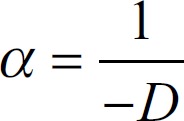 in
in 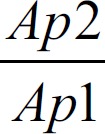 (3)
(3)
Where α is the attenuation coefficient of sample, −D is the difference in distance or depth of sample in mm, Ap1 is the power signal amplitude at frequency f and x, y position of (reference signal) with no presence sample (amplitude of transmitted signal wave), Ap2 is the power signal amplitude at frequency f, and x, y position through the sample (amplitude of received signal wave).
Measure the viscosity and the density of blood mimicking fluid
Samples viscosity of the BMF and mixture fluid was measured using electronic rotational viscometer. Some earlier studies used U-tube viscometer to measure the kinematic viscosity then converted it to dynamic viscosity. A rotational viscometer is an electronic tool used for measuring the dynamic viscosity (mPa.s) directly instead of measuring the kinematic viscosity then converts it to dynamic viscosity (mPa.s). However, on the other hand, the density measurements of the mixture fluid and BMF in general of each sample by a digital tool called Density Meter (DMA35). This tool utilized for measuring the density in a unit of g/ml or in g/cm3, the advantage of this tool that it's able to measure the mixture fluid temperature. In addition, utilizing this tool is better than utilizing the pycnometer because this tool gives more precise result than pycnometer [Figure 3].
Figure 3.

Electronic density meter (DMA 35) for measuring the samples densities
Proportional backscatter strength
The backscatter principle of ultrasound is significant feature of a BMF since it is related to BMF stability. Several of radiofrequency signals were measured and analyzed in this research study to check the backscatter power by calculating the average power spectrum through applying the fast Fourier transform task that built-in A-scan (GAMPT) software at 5 MHz. However, the measuring of backscattering strength of BMF between laboratories display vast variations, thus, and this is discomforted by the loss of a standard level reference backscatter examination.[2] We measured the backscatter power at different concentrations of poly(4-methylstyrene) to check if this BMF behave like real human blood.
RESULTS AND DISCUSSIONS
In the first research experiment, we used water, PG, and silicon oil for preparation of the (BMFa) mixture fluid. First, when the ratio of the PG was increased while the silicon oil was fixed [Table 2a], the result was that the average density and speed of sound slightly increased because the PG density is more than water density about 0.036 g/ml and the silicon oil density less than the water density about 0.09 g/ml. In other words, even with increasing large ratios of both items, the increase of density will still be minimal due to the average density of mixture fluid with using silicon oil decrease with increase the silicon oil concentration [Figure 4]. Thus, the acoustical and physical properties of mixture fluid did not correspond to the IEC standard requirements. Again, we have repeated the experiment with fixed the concentration of PG and increased the silicon oil ratio but also the suitable properties of mixture fluid were not obtained, due to the average density and speed of sound decreased dramatically [Table 2b]. This decrease because the silicon oil density is less than the water and PG densities. Finally, the last experiment of BMFa was prepared by increasing the same ratios of both the PG and silicon oil, the density of mixture fluid samples were decreased gradually, because the difference in density between the silicon oil and the distilled water 0.09 g/ml is slightly larger than the difference in density between the PG and the distilled water 0.036 g/ml. However, the results of the last experiment did not correspond to the IEC standard requirements [Table 2c]. Moreover, there was no measuring of the viscosity of BMFa mixture fluid because the speed of sound and the density were not corresponding to the IEC standard requirements.
Figure 4.
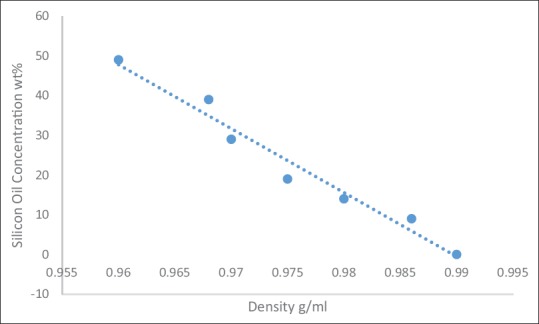
Relationship between the silicon oil concentration and the average density of mixture fluid
Table 2b.
Constitutions and physical properties of water, propylene glycol, and polyethylene glycol aqueous solutions (temperature: 37.0 °C)
| Number of samples | Water | Propylene glycol | Silicon oil | Density (g/ml) | Speed of sound (m/s) |
|---|---|---|---|---|---|
| 1 | 100 | 0 | 0 | 0.99 | 1508 |
| 2 | 90 | 1 | 9 | 0.986 | 1500 |
| 3 | 85 | 1 | 14 | 0.98 | 1497 |
| 4 | 80 | 1 | 19 | 0.975 | 1493 |
| 5 | 70 | 1 | 29 | 0.97 | 1485 |
| 6 | 60 | 1 | 39 | 0.968 | 1478 |
| 7 | 50 | 1 | 49 | 0.96 | 1470 |
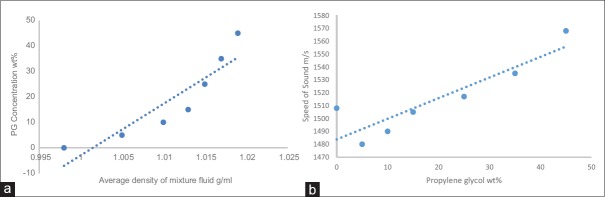
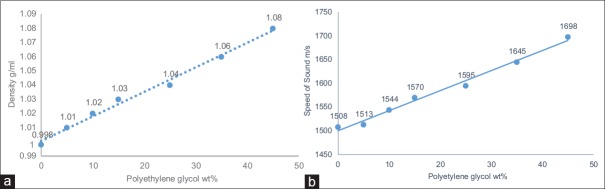
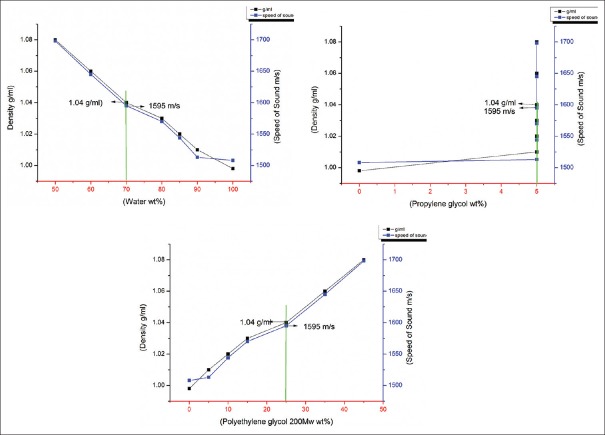
For this reason, the decision of prepare a BMFb that consists of other items such as a PG, polyethylene glycol (200 Mw) and water was done. Because of the densities of PG and polyethylene are slightly above the water density, the density and speed of sound of mixture fluid will increase directly. Furthermore, this mixture fluid of BMF should have a suitable density to be proper with the scatter particle poly (4-methylstyrene) density for keeping neutrally buoyant. Hence, when the concentration of both the PG and polyethylene glycol were increased, the speed of sound and density of mixture fluid were increased [Table 3a]. However, the average density and speed of sound were slightly increased with increased the PG as shown in [Figure 5a and b], whereas rapidly increased with increased the PEG (200 Mw) as shown in Figure [Figure 6a and b], because the density of PEG (1.124 g/ml) larger than the density of blood within 0.074 g/ml, while the density of PG (1.036 g/ml) lower than the density of blood within -0.014 g/ml, and the result of mixing it together was suitable to obtains suitable BMF density (1.05 ± 0.04 g/ml). As shown in Table 3a, the density of mixture fluid at sample number 4 is 1.040 g/ml, but the speed of sound is much high, so this sample cannot be considered as a suitable mixture fluid disperses with (Poly (4-methylstyrene)) particle material. Again, the results of the next experiment [Table 3b], when the ratios of PG, were increased, and the polyethylene glycol ratio was constant, the density of mixture was increased much slightly and was not suitable for IEC standard requirements. Thus, for this reason, the viscosity of this experiment did not measure, and both the acoustical and physical features were not suitable and did not agree with IEC standard requirements. However, to obtain proper density and speed of sound to be suitable with a new scatter particle poly (4-methylstyrene), the ratios of PG were fixed, and the polyethylene glycol ratio was increased, the density of the mixture fluid increased properly, and it was matched with IEC standard values as a density and speed of sound [Table 3c]. From the data which showed at Table 3c, samples' number 5 is suitable to be mixture fluid dispersed poly(4-methylstyrene) because all the physical and acoustical properties (viscosity, density, and speed of sound) were convenient in the sample [Figure 7]. Moreover, the attenuation coefficient of all samples was <0.1 dB/cm/MHz.
Table 3a.
The acoustical and physical features of water, propylene glycol, and polyethylene glycol at increase both the concentrations of the items at temperature 37°C±1°C
| Number of sample | Water | Propylene glycol | Polyethylene glycol | Density (g/ml) | Speed of sound (m/s) | Viscosity (mPas) |
|---|---|---|---|---|---|---|
| 1 | 100 | 0 | 0 | 0.998 | 1508 | 0.890 |
| 2 | 90 | 6 | 4 | 1.008 | 1485 | 2.2 |
| 3 | 85 | 9 | 6 | 1.013 | 1535 | 2.7 |
| 4 | 80 | 12 | 8 | 1.02 | 1550 | 4.2 |
| 5 | 70 | 20 | 10 | 1.03 | 1591 | 6.8 |
| 6 | 60 | 25 | 15 | 1.04 | 1629 | 9.7 |
| 7 | 50 | 30 | 20 | 1.05 | 1660 | 15.0 |
Figure 5.
(a) Relationship between the propylene glycol concentration and the average density of the mixture fluid. (b) Relationship between the propylene glycol concentration and the average speed of sound of the mixture fluid
Figure 6.
(a) Relationship between the polyethylene glycol (200 Mw) concentration and the average density of the mixture fluid. (b) Relationship between the polyethylene glycol (200 Mw) concentration and the average speed of sound of the mixture fluid
Figure 7.
Appropriate density and speed of sound of mixture fluid for blood mimicking fluid at various ratios by wt% of propylene glycol and polyethylene glycol, respectively
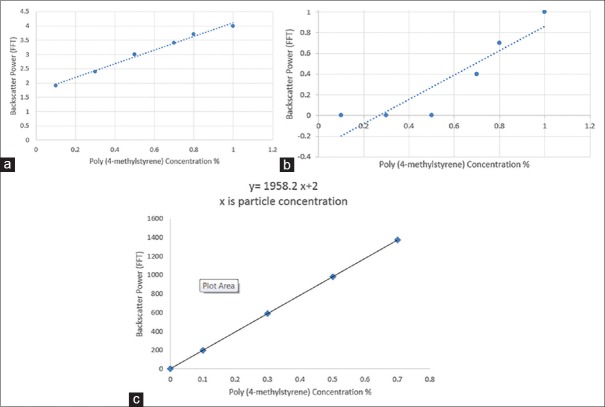
However, the human blood is non-Newtonian which means that the human blood viscosity depends on shear rate. However, because the BMF is Newtonian, the BMF viscosity does not depend on shear rate and can be arbitrary and more than 4.1 mPas.[5] In addition, due to the molecular weight of propylene glycol (76.09 g/mol) is slightly different from the molecular weight of polyethylene glycol (200.0 Ave. g/mol), no big changes in the viscosity with changing of these items [Table 3a and c.], and because the molecular weight of polyethylene glycol is larger than the molecular weight of PG; thus, the changes in the viscosity increase with increasing the polyethylene glycol concentration more than increasing the PG concentration [Table 3a and c]. However, the experimental research outcome of the connection or relationship between both the molecular weight (Mw) and the propylene and polyethylene glycol viscosity is shown in Figure 8. Furthermore, the backscatter strength reliance with poly(4-methylstyrene) particle size at a several particle ratios showed that backscatter was increased with increased particle ratios [Figure 9].[19,20,21,22]
Figure 8.
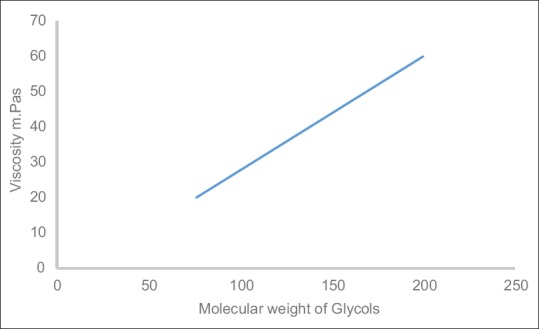
Viscosity in propylene and polyethylene glycol as a task of the glycol molecular weight (76.09 g/mol and 200 g/mol, respectively)
Figure 9.
Backscatter power as a function of particle (poly (4-methylstyrene)) concentration, displaying linear response of the FFT by (GAMPT) ultrasonic backscatter measurement under (a) static (measurements were done directly in the plexiglass) (b) uniform flow (measurements were done over the gear pump tube) and (c) overall particles
CONCLUSION
In this research study, only one type of mixture fluid with a new type of scatter particle material was used for preparing the BMF which is used in test object or flow phantom and it was a BMFb. Initially, the mixture fluid made of PG and silicon oil could not be used because of the values were not consistent with the values defined and known in IEC standard requirements. Thus, The BMFb was used and prepared by mixing the fluid items (distilled water 70.0 wt%, PG 5.0 wt%, and polyethylene glycol 25.0 wt%) with poly (4-methylstyrene) 0.8 wt% as a scatter particle to allow it suspension inside the fluids. The speed of sound and density of the BMFb were 1595 m/s and 1.04 g/ml, respectively. Thus, the experiment values agreed well with the IEC requirements. However, the average densities and speed of sounds were changed with changing the ratio of items because the relationship between the items is not a linear proportional function. Usually, when the ratios of polyethylene glycol are more than the ratios of PG, the mixture fluid density will rise highly and the speed of sound too because of the speed of sound's direct proportional relationship to the density. One type of new blood-mimicking fluid with a speed of sound and density corresponded to IEC requirements. This BMF has been wished for correct wall-less flow phantom by utilizing Doppler ultrasound technique.[22] However, the main advantage of the mixture fluid that was used for BMF preparation that needed only 15 min mixing by stirrer for preparing mixture fluid and to 20 min too for mixing with poly(4-methylstyrene) as a scatter material; thus, this BMF required less consuming time for preparing and with low cost of items compared to other commercial BMF that need 2 h stirring for mixing the items for preparing mixture fluid and 2 h more for mixing the scattered particles (such as nylon and polystyrene) and more time inside vacuum pump. Moreover, the backscatter strength reliance with poly(4-methylstyrene) particle size at a several particle ratio displayed proportional relationship with particle ratios.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This research study was supported by Prof. Dr. Mohammad Zubir Mat Jafri, under the grant number 304/PFIZIK/6315023. We thank our colleagues from (Universiti Sains Malaysia, Medical physics, and Radiation Science department) who provided insight and expertise that greatly assisted the research, although they may not agree with all the interpretations of this paper.
Appendix 1: Regular steps for prepare a new BMF with a novel scatter particle called poly (4-methylstyrene)
The mixture fluid and BMF were prepared in the following method ways:
Utilizing a suitable beaker with size at least two times the volume of required fluids for preparing and make the BMF avert overflow the ingredients during stirring
Weigh all the items components in a safe place such as a fume hood and pouring them into the beaker
Stirring the beaker via the stirrer plate for around 15 min of each sample mixture, and set the room temperature to be 37°C ± 0.7°C
Applying a vacuum pump instrument to remove the gas of the fluid mixture for 30 min
Measure both the physical (density and viscosity) and acoustical (speed of sound and attenuation) features of fluids of each sample
Select the best mixture fluid sample which corresponds to the defined IEC standard. Then, mix it with a proper scattered particles material for around 20 min for poly (4-methylstyrene) particles
Applying a vacuum pump equipment to remove the gas (Degas) the BMF for around 1 h. Then the BMF now is ready for users.
REFERENCES
- 1.Browne JE, Ramnarine KV, Watson AJ, Hoskins PR. Assessment of the acoustic properties of common tissue-mimicking test phantoms. Ultrasound Med Biol. 2003;29:1053–60. doi: 10.1016/s0301-5629(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 2.Ramnarine KV, Nassiri DK, Hoskins PR, Lubbers J. Validation of a new blood-mimicking fluid for use in Doppler flow test objects. Ultrasound Med Biol. 1998;24:451–9. doi: 10.1016/s0301-5629(97)00277-9. [DOI] [PubMed] [Google Scholar]
- 3.Samavat H, Evans JA. An ideal blood mimicking fluid for Doppler ultrasound phantoms. J Med Phys. 2006;31:275–8. doi: 10.4103/0971-6203.29198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka K, Yoshida T, Sato K, Kondo T, Yasukawa K, Miyamoto N, et al. Blood-Mimicking Fluid for Testing Ultrasonic Diagnostic Instrument. Jpn J Appl Phys. 2012;51:7GF18. [Google Scholar]
- 5.Yoshida T, Sato K, Kondo T. Blood-mimicking fluid using glycols aqueous solution and their physical properties. Jpn J Appl Phys. 2014;53:7KF01. [Google Scholar]
- 6.Yoshida T, Tanaka K, Sato K, et al. Ultrasonics Symposium (IUS), 2012 IEEE International. IEEE; 2012. Blood-mimicking fluid for the Doppler test objects of medical diagnostic instruments. [Google Scholar]
- 7.Hoskins PR, Loupas T, McDicken WN. A comparison of the Doppler spectra from human blood and artificial blood used in a flow phantom. Ultrasound Med Biol. 1990;16:141–7. doi: 10.1016/0301-5629(90)90142-y. [DOI] [PubMed] [Google Scholar]
- 8.Oates CP. Towards an ideal blood analogue for Doppler ultrasound phantoms. Phys Med Biol. 1991;36:1433–42. doi: 10.1088/0031-9155/36/11/003. [DOI] [PubMed] [Google Scholar]
- 9.Thorne ML, Poepping TL, Rankin RN, Steinman DA, Holdsworth DW. Use of an ultrasound blood-mimicking fluid for Doppler investigations of turbulence in vitro . Ultrasound Med Biol. 2008;34:1163–73. doi: 10.1016/j.ultrasmedbio.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Udroiu I. Estimation of Erythrocyte Surface area in Mammals. arXiv preprint arXiv. 2014:1403–7660. [Google Scholar]
- 11.Kimme-Smith C, Hussain R, Duerinckx A, Tessler F, Grant E. Assurance of consistent peak-velocity measurements with a variety of duplex Doppler instruments. Radiology. 1990;177:265–72. doi: 10.1148/radiology.177.1.2204967. [DOI] [PubMed] [Google Scholar]
- 12.Raine-Fenning NJ, Nordin NM, Ramnarine KV, Campbell BK, Clewes JS, Perkins A, et al. Determining the relationship between three-dimensional power Doppler data and true blood flow characteristics: An in vitro flow phantom experiment. Ultrasound Obstet Gynecol. 2008;32:540–50. doi: 10.1002/uog.6110. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Kenwright DA, Wang S, Hossack JA, Hoskins PR. Fabrication of two flow phantoms for Doppler ultrasound imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64:53–65. doi: 10.1109/TUFFC.2016.2634919. [DOI] [PubMed] [Google Scholar]
- 14.Browne JE, Watson AJ, Hoskins PR, Elliott AT. Validation of a sensitivity performance index test protocol and evaluation of colour Doppler sensitivity for a range of ultrasound scanners. Ultrasound Med Biol. 2004;30:1475–83. doi: 10.1016/j.ultrasmedbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Sato M, Ishida H, Konno K, Komatsuda T, Furukawa K, Yamada M, et al. Analysis of refractive artifacts by reconstructed three-dimensional ultrasound imaging. J Med Ultrason (2001) 2006;33:11–6. doi: 10.1007/s10396-005-0072-9. [DOI] [PubMed] [Google Scholar]
- 16.Duck FA. Physical Properties of Tissues: A Comprehensive Reference Book. Bath, England: Academic Press; 2013. [Google Scholar]
- 17.Sigelmann RA, Reid JM. Analysis and measurement of ultrasound backscattering from an ensemble of scatterers excited by sine-wave bursts. J Acoust Soc Am. 1973;53:1351–5. [Google Scholar]
- 18.Yang P, Zhu H. Biomedical Engineering and Computer Science (ICBECS), 2010 International Conference. IEEE; 2010. Influence of transducer focus position and signal length in backscatter coefficient measurement for blood mimicking fluid. [Google Scholar]
- 19.Oglat AA, Matjafri M, Suardi N, Oqlat MA, Abdelrahman MA, Oqlat AA. A review of medical doppler ultrasonography of blood flow in general and especially in common carotid artery. Journal of Medical Ultrasound. 2018;26:3. doi: 10.4103/JMU.JMU_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oglat AA, Matjafri M, Suardi N, Oqlat MA, Oqlat AA, Abdelrahman MA. A new blood mimicking fluid using propylene glycol and their properties for a flow phantom test of medical doppler ultrasound. International Journal of Chemistry. Pharmacy & Technology. 2017;2:220–31. [Google Scholar]
- 21.Oglat AA, Matjafri MZ, Suardi N, Oqlat MA, Abdelrahman MA, Oqlat AA, et al. Chemical items used for preparing tissue-mimicking material of wall-less flow phantom for doppler ultrasound imaging. J Med Ultrasound. 2018 doi: 10.4103/JMU.JMU_13_17. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oglat AA, Suardi N, Matjafri Mz, Oqlat MA, Abdelrahman MA, Oqlat AA. A review of suspension-scattered particles used in blood-mimicking fluid for Doppler ultrasound imaging. J Med Ultrasound. 2018 doi: 10.4103/JMU.JMU_1_17. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]