Abstract
Angiomyolipoma is one of the renal benign neoplasms. The most of the angiomyolipomas are asymptomatic and found incidentally with ultrasound. They are more prevalent in patients with tuberous sclerosis. It is very important to make differential diagnosis from other renal neoplasm such as renal cell carcinoma. Growth rate is higher among pregnant women suggest that the hormones may play a role in the stimulation of angiomyolipoma. The most common serious presentation is rupture and hemorrhage, and sometimes it can lead to shock. Adequate diagnosis, treatment, and follow-up are very important in the face of renal angiomyolipoma.
Keywords: Angiomyolipoma, benign, kidney
INTRODUCTION
As the availability and convenience of image studies, more renal masses are found incidentally during health examination or the evaluation of other diseases. Most of these incidental renal masses are malignant, and a great deal of these tumors is renal cell carcinomas (RCCs). Among the benign neoplasms of kidney, angiomyolipoma (AML) is the most common seen and easily diagnosed with renal ultrasonography.[1]
Renal angiomyolipoma
Renal AML is not a common neoplasm. A screen study using ultrasound was performed on 17,941 healthy Japanese adults without any signs suggestive of urinary tract malignancies which revealed only 24 (0.13%) angiomyolipomas.[2] The prevalence of these AML demonstrates a female-to-male ratio of 2:1.[2,3] In sporadic patients, angiomyolipoma is usually solitary, small (<4 cm), and asymptomatic.[4,5] In cases of multifocal, bilateral, bigger renal AML, and tuberous sclerosis complex (TSC) should be considered. Tuberous sclerosis is an autosomal dominant disorder, and patients may have features of epilepsy, mental retardation, facial angiofibromas, shagreen patch, etc.[6] Up to 55%–90% TSC patients have renal AML, which is prone to growth.[7] Therefore, renal evaluation for these TSC patients is much more important.
Composition and image studies of renal angiomyolipoma
Renal AML is a triphasic tumor composed of abnormal blood vessels, special spindle cells, and mature adipocyte.[8] Most patients have no specific symptoms. More than 80% of AMLs are discovered incidentally by abdominal image studies. About 10% patients have retroperitoneal hematoma and even hypovolemic shock as initial presentation.[4] Ultrasound showed them to be usually hyperechoic and homogeneous due to the presence of macroscopic fat [Figure 1]. Although AML has relatively characteristic finding on ultrasound, they can closely mimic RCC because some RCC can also be hyperechoic[9] [Figure 2]. Therefore, computed tomography (CT) scan is recommended when hyperechoic lesion is found by ultrasound. CT has excellent sensitivity, specificity, and reproducibility regarding renal masses in general. CT scan is better in making differential diagnosis with other renal tumors. Even in small masses, CT can identify macroscopic fat in AML as low-density area of −10 HU or lower.[10] Ultrasonography is useful as an initial approach and following patients with AML. However, it is difficult to make the diagnosis of lipid-poor renal AML with ultrasound because of the lack of macroscopic fat[11] [Figure 3].
Figure 1.
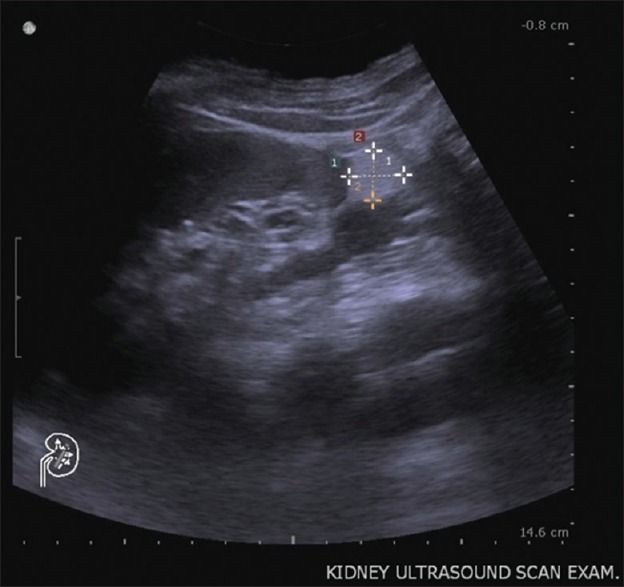
Kidney ultrasound. Ultrasound reveals a homogeneous, well-defined, hyperechoic lesion in the left lower kidney, which demonstrates the presence of macroscopic fat. Renal angiomyolipoma is the most likely diagnosis
Figure 2.
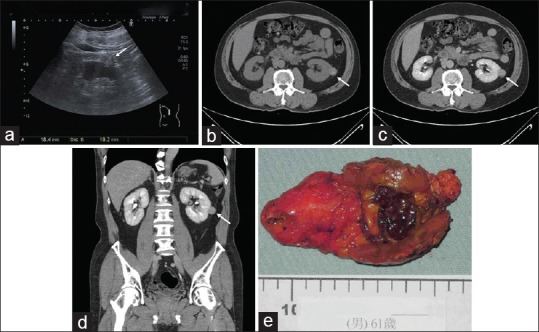
Image studies of a renal mass. (a) A hyperechoic left renal tumor (arrow) in a 61-year-old man. (b) Abdominal computed tomography scan without contrast revealed a hyperdensity renal mass. (c) The tumor was not enhanced on computed tomography scan. (d) Coronal view of the abdominal computed tomography demonstrated the renal mass. (e) Robotic-assisted laparoscopic partial nephrectomy was performed, and the pathology is papillary renal cell carcinoma
Figure 3.
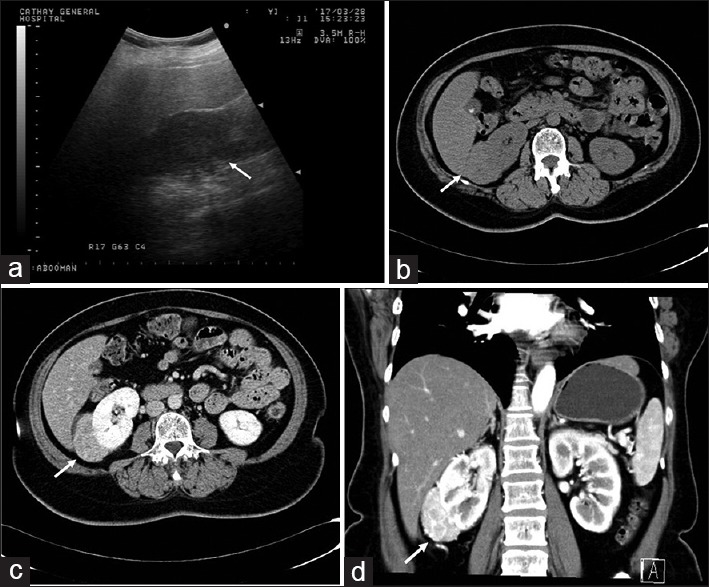
Lipid-poor angiomyolipoma (Epithelioid angiomyolipoma) in a 55-year-old woman. (a) An isoechoic right renal mass. (Arrow). (b) Abdominal computed tomography revealed a hyperdensity right renal mass. No obvious fat component was found within the tumor. (c) The mass was enhanced after contrast injection. (d) Coronal view of the abdominal computed tomography (late arterial phase) showed the hypervascularity of the tumor
Magnetic resonance imaging (MRI) can be made through noncontrast enhance manner. It can also diagnose renal AML in patients with compromised renal function or contrast allergy. Renal AML can be similar with some RCC and hemorrhagic cysts in the MRI study, and fat suppression techniques may be helpful in the differentiation between hemorrhage and macroscopic fat.[12] The disadvantages of MRI are lower availability, higher cost, and time-consuming comparing to ultrasound or CT.
Management
Most of the AMLs can be managed by active surveillance. Until now, there are no indications about the frequency of follow-up images. Intervention should be considered in patients with suspicious malignancy, symptomatic lesion larger than 4 cm, and women in childbearing age.[10,13,14] A study revealed the average growth rate is 0.19 cm every year in sporadic AML and 1.25 cm in TSC patients.[5] Therefore, doctors should be more aggressive to treat AML in TSC patients. Besides, the risk of rupture and bleeding is also increased in AML >4 cm and intralesional aneurysm <5 mm.[15,16] Several case reports[17,18,19,20] showed that AML growth and rupture during pregnancy and the massive bleeding can be fatal. On the other hand, estrogen receptor expression is found ubiquitously in renal AML,[21] we should well explain how to follow and deal with AML in females with desire of pregnancy.
The aim to manage renal AML is avoidance of tumor growth and rupture and to preserve normal renal parenchyma as possible. Nephron-sparing surgery (NSS) should be considered first when feasible, and it can be performed by traditional open surgery or minimal invasive manners such as laparoscopic or robotic-assisted surgery.[22,23,24,25] The common complications after NSS include urine leak, fistula, hemorrhage, and ileus.
The mainstream of renal AML management is selective embolization, especially in ruptured or postembolization rebleeding AMLs.[26] Embolization can be performed as selectively as possible to preserve renal function, and hospital stay is much shorter compared to that of surgery.[27] Higher recurrence rate after embolization is the disadvantage,[15,28,29] and long-term follow-up is necessary. Besides, the complications and postembolization syndrome, including fever, flank pain, leukocytosis, vascular injury, and renal infarction, are common.[26,30,31,32] Most of these complications can be treated conservatively. Tumor ablation therapy, such as microwave, radiofrequency, and cryoablation, can be performed percutaneously or laparoscopically.[33,34] This technique is mostly applied on smaller tumors.
Mammalian target of rapamycin (mTOR) inhibitors is recommended as the first-line medical treatment of renal angiomyolipoma. Everolimus, a rapamycin derivative, inhibits the mTOR pathway by acting on the mTOR complex 1 and is commonly used for TSC-associated renal AML.[35,36] TSC is caused by mutations in either TSC1 or TSC2 suppressor genes, resulting in increased mTOR activity.[37] If mTOR pathway is uncontrolled, it may lead to protein synthesis and cell growth problems. Everolimus is also approved by the Food and Drug Administration for treatment of adult patients with renal AML and TSC.
Active surveillance
Active surveillance is introduced as the safest option for low-risk or nonemergent renal AML.[38] There is no standard monitoring protocol, and some author recommends a physical examination and CT imaging at 6 months, 12 months, and then annually. For high-risk patients, such as larger tumor size, symptomatic, or TSC-associated AML, close follow-up is necessary. Ultrasonography plays an important role in the follow-ups of renal AML. It does not use any ionizing radiation and is a widely available, easy-to-use, safe, noninvasive, and relatively inexpensive image study. Abdominal CT, MRI, or angiography is required in the identification and intervention of renal AML.[1]
CONCLUSION
Renal AML can be found incidentally in abdominal image studies. Ultrasound is important in the diagnosis, management, and follow-up. CT scan is required for definite diagnosis and pretreatment evaluation. When renal AML is found, active treatment should be considered in large size and symptomatic tumors to avoid rupture, bleeding, and shock. Female patients in childbearing age should be more vigilant to this disease. Close follow-up is necessary after treatment, especially in postembolization patients. Medical treatment with mTOR inhibitor is the treatment of choice for TSC-associated AML. Further, follow-up protocol, treatment medication, and intervention techniques remain as areas of the future study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M, et al. Renal angiomyolipoma: A radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014;39:588–604. doi: 10.1007/s00261-014-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujii Y, Ajima J, Oka K, Tosaka A, Takehara Y. Benign renal tumors detected among healthy adults by abdominal ultrasonography. Eur Urol. 1995;27:124–7. doi: 10.1159/000475142. [DOI] [PubMed] [Google Scholar]
- 3.Fittschen A, Wendlik I, Oeztuerk S, Kratzer W, Akinli AS, Haenle MM, et al. Prevalence of sporadic renal angiomyolipoma: A retrospective analysis of 61,389 in- and out-patients. Abdom Imaging. 2014;39:1009–13. doi: 10.1007/s00261-014-0129-6. [DOI] [PubMed] [Google Scholar]
- 4.Sooriakumaran P, Gibbs P, Coughlin G, Attard V, Elmslie F, Kingswood C, et al. Angiomyolipomata: Challenges, solutions, and future prospects based on over 100 cases treated. BJU Int. 2010;105:101–6. doi: 10.1111/j.1464-410X.2009.08649.x. [DOI] [PubMed] [Google Scholar]
- 5.Seyam RM, Bissada NK, Kattan SA, Mokhtar AA, Aslam M, Fahmy WE, et al. Changing trends in presentation, diagnosis and management of renal angiomyolipoma: Comparison of sporadic and tuberous sclerosis complex-associated forms. Urology. 2008;72:1077–82. doi: 10.1016/j.urology.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 6.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–68. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 7.Patel A, Watchko S, Nellesen D, Herbst F, Neary M. The natural history and burden of illness of epilepsy in tuberous sclerosis complex (TSC): A systematic literature review. [Last accessed on 2018 Feb 14];Eur J Paediatr Neurol. 2017 21:e186–7. Available from: http://www.ejpn-journal.com/article/S1090-3798(17)31032-2/abstract . [Google Scholar]
- 8.Flum AS, Hamoui N, Said MA, Yang XJ, Casalino DD, McGuire BB, et al. Update on the diagnosis and management of renal angiomyolipoma. J Urol. 2016;195:834–46. doi: 10.1016/j.juro.2015.07.126. [DOI] [PubMed] [Google Scholar]
- 9.Kim JK, Kim SH, Jang YJ, Ahn H, Kim CS, Park H, et al. Renal angiomyolipoma with minimal fat: Differentiation from other neoplasms at double-echo chemical shift FLASH MR imaging. Radiology. 2006;239:174–80. doi: 10.1148/radiol.2391050102. [DOI] [PubMed] [Google Scholar]
- 10.Davenport MS, Neville AM, Ellis JH, Cohan RH, Chaudhry HS, Leder RA, et al. Diagnosis of renal angiomyolipoma with hounsfield unit thresholds: Effect of size of region of interest and nephrographic phase imaging. Radiology. 2011;260:158–65. doi: 10.1148/radiol.11102476. [DOI] [PubMed] [Google Scholar]
- 11.Hakim SW, Schieda N, Hodgdon T, McInnes MD, Dilauro M, Flood TA, et al. Angiomyolipoma (AML) without visible fat: Ultrasound, CT and MR imaging features with pathological correlation. Eur Radiol. 2016;26:592–600. doi: 10.1007/s00330-015-3851-8. [DOI] [PubMed] [Google Scholar]
- 12.Halpenny D, Snow A, McNeill G, Torreggiani WC. The radiological diagnosis and treatment of renal angiomyolipoma-current status. Clin Radiol. 2010;65:99–108. doi: 10.1016/j.crad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Zapardiel I, Delafuente-Valero J, Bajo-Arenas JM. Renal angiomyolipoma during pregnancy: Review of the literature. Gynecol Obstet Invest. 2011;72:217–9. doi: 10.1159/000329328. [DOI] [PubMed] [Google Scholar]
- 14.Raft J, Lalot JM, Meistelman C, Longrois D. Influence of pregnancy on renal angiomyolipoma. Gynecol Obstet Fertil. 2005;33:898–906. doi: 10.1016/j.gyobfe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K, et al. Renal angiomyolipoma: Relationships between tumor size, aneurysm formation, and rupture. Radiology. 2002;225:78–82. doi: 10.1148/radiol.2251011477. [DOI] [PubMed] [Google Scholar]
- 16.Rimon U, Duvdevani M, Garniek A, Golan G, Bensaid P, Ramon J, et al. Large renal angiomyolipomas: Digital subtraction angiographic grading and presentation with bleeding. Clin Radiol. 2006;61:520–6. doi: 10.1016/j.crad.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Hatakeyama S, Habuchi T, Ichimura Y, Akihama S, Terai Y, Kakinuma H, et al. Rapidly growing renal angiomyolipoma during pregnancy with tumor thrombus into the inferior vena cava: A case report. Nihon Hinyokika Gakkai Zasshi. 2002;93:48–51. doi: 10.5980/jpnjurol1989.93.48. [DOI] [PubMed] [Google Scholar]
- 18.Ao L, Ogasahara E, Okuda Y, Hirata S. Spontaneous rupture of renal angiomyolipoma during pregnancy. BMJ Case Rep 2017. 2017. [Last accessed on 2018 Feb 14]. Available from: http://casereports.bmj.com/content/2017/bcr-2016-217284 . [DOI] [PMC free article] [PubMed]
- 19.Liu J, Meng T, Yang X, Zhao G, Li B. Spontaneous rupture of renal angiomyolipoma in the third trimester. Taiwan J Obstet Gynecol. 2015;54:788–90. doi: 10.1016/j.tjog.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Orywal AK, Zeile M, Brüning R, Gross AJ, Netsch C. Rupture of renal angiomyolipoma during childbirth. Urology. 2015;85:e19–20. doi: 10.1016/j.urology.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Boorjian SA, Sheinin Y, Crispen PL, Lohse CM, Kwon ED, Leibovich BC, et al. Hormone receptor expression in renal angiomyolipoma: Clinicopathologic correlation. Urology. 2008;72:927–32. doi: 10.1016/j.urology.2008.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kara O, Akca O, Zargar H, Andrade HS, Maurice MJ, Ramirez D, et al. Robotic partial nephrectomy in the treatment of renal angiomyolipoma. J Endourol. 2016;30:275–9. doi: 10.1089/end.2015.0624. [DOI] [PubMed] [Google Scholar]
- 23.Heidenreich A, Hegele A, Varga Z, von Knobloch R, Hofmann R. Nephron-sparing surgery for renal angiomyolipoma. Eur Urol. 2002;41:267–73. doi: 10.1016/s0302-2838(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 24.Lin CY, Yang CK, Ou YC, Chiu KY, Cheng CL, Ho HC, et al. Long-term outcome of robotic partial nephrectomy for renal angiomyolipoma. [Last accessed on 2018 Feb 14];Asian J Surg. 2018 41:187–91. doi: 10.1016/j.asjsur.2016.11.003. Available from: http://www.easianjournalsurgery.com/article/S1015-9584(16)30238-X/fulltext . [DOI] [PubMed] [Google Scholar]
- 25.Msezane L, Chang A, Shikanov S, Deklaj T, Katz MH, Shalhav AL, et al. Laparoscopic nephron-sparing surgery in the management of angiomyolipoma: A single center experience. J Endourol. 2010;24:583–7. doi: 10.1089/end.2009.0330. [DOI] [PubMed] [Google Scholar]
- 26.Lenton J, Kessel D, Watkinson AF. Embolization of renal angiomyolipoma: Immediate complications and long-term outcomes. Clin Radiol. 2008;63:864–70. doi: 10.1016/j.crad.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Faddegon S, So A. Treatment of angiomyolipoma at a tertiary care centre: The decision between surgery and angioembolization. Can Urol Assoc J. 2011;5:E138–41. doi: 10.5489/cuaj.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kothary N, Soulen MC, Clark TW, Wein AJ, Shlansky-Goldberg RD, Crino PB, et al. Renal angiomyolipoma: Long-term results after arterial embolization. J Vasc Interv Radiol. 2005;16:45–50. doi: 10.1097/01.RVI.0000143769.79774.70. [DOI] [PubMed] [Google Scholar]
- 29.Lee W, Kim TS, Chung JW, Han JK, Kim SH, Park JH, et al. Renal angiomyolipoma: Embolotherapy with a mixture of alcohol and iodized oil. J Vasc Interv Radiol. 1998;9:255–61. doi: 10.1016/s1051-0443(98)70266-0. [DOI] [PubMed] [Google Scholar]
- 30.Murray TE, Doyle F, Lee M. Transarterial embolization of angiomyolipoma: A systematic review. J Urol. 2015;194:635–9. doi: 10.1016/j.juro.2015.04.081. [DOI] [PubMed] [Google Scholar]
- 31.Ramon J, Rimon U, Garniek A, Golan G, Bensaid P, Kitrey ND, et al. Renal angiomyolipoma: Long-term results following selective arterial embolization. Eur Urol. 2009;55:1155–61. doi: 10.1016/j.eururo.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz MJ, Smith EB, Trost DW, Vaughan ED., Jr Renal artery embolization: Clinical indications and experience from over 100 cases. BJU Int. 2007;99:881–6. doi: 10.1111/j.1464-410X.2006.06653.x. [DOI] [PubMed] [Google Scholar]
- 33.Castle SM, Gorbatiy V, Ekwenna O, Young E, Leveillee RJ. Radiofrequency ablation (RFA) therapy for renal angiomyolipoma (AML): An alternative to angio-embolization and nephron-sparing surgery. BJU Int. 2012;109:384–7. doi: 10.1111/j.1464-410X.2011.10376.x. [DOI] [PubMed] [Google Scholar]
- 34.Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT, Jr, Brace CL. Percutaneous tumor ablation tools: Microwave, radiofrequency, or cryoablation – What should you use and why? Radiographics. 2014;34:1344–62. doi: 10.1148/rg.345140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817–24. doi: 10.1016/S0140-6736(12)61767-X. [DOI] [PubMed] [Google Scholar]
- 36.Budde K, Gaedeke J. Tuberous sclerosis complex-associated angiomyolipomas: Focus on mTOR inhibition. Am J Kidney Dis. 2012;59:276–83. doi: 10.1053/j.ajkd.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Huang J, Manning BD. The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem J. 2008;412:179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouzaid I, Autorino R, Fatica R, Herts BR, McLennan G, Remer EM, et al. Active surveillance for renal angiomyolipoma: Outcomes and factors predictive of delayed intervention. BJU Int. 2014;114:412–7. doi: 10.1111/bju.12604. [DOI] [PubMed] [Google Scholar]


