ABSTRACT
Introduction
Hepatitis C virus genotype 3 represents a unique entity within HCV treatment and multiple studies have documented that HCV genotype 3 infection is associated with more rapid disease progression than other genotypes, resulting in increased risk of cirrhosis, hepatocellular carcinoma, and all-cause mortality. In the current study, we further evaluated the real-world effectiveness of 12 weeks of ledipasvir/sofosbuvir ± ribavirin (LDV/SOF ± RBV) and sofosbuvir + daclatasvir (SOF + DCV) for treatment-naive or treatment-experienced patients infected with HCV genotype 3, with or without cirrhosis.
Material and methods
Retrospective and observational study carried out in a third level hospital. Study period: April 2015 to January 2016. Inclusion criteria: Patients with HCV genotype-3 infection treated either with LDV/SOF ± RBV or with SOF + DCV during study period treated for 12 weeks. The patients that were treated during 24 weeks were excluded and those treated with peg-interferon. The main endpoint measured was the sustained virologic response (SVR) at 12 weeks (SVR12) and the secondary endpoint was SVR at 24 weeks (SVR24).
Results
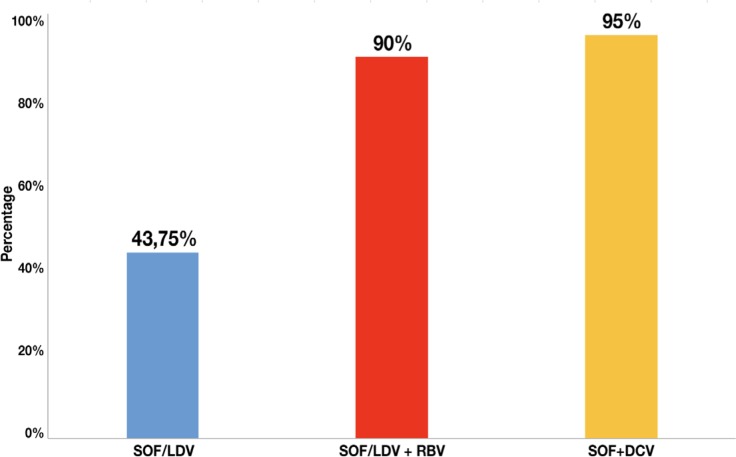
During the study period, 603 patients were treated in our hospital: 71 with genotype 3. We included 46 patients who were treated with LDV/SOF ± RBV or SOF + DCV for 12 weeks. A 43.75% (7/16) of all patients treated with LDV/SOF achieved SVR12, 90% (9/10) of the patients treated with LDV/SOF+RBV achieved SVR12 and 95% (19/20) of the patients treated with SOF+DCV achieved SVR12. There was statistically significant difference (p=0.001) between LDV/SOF respect to SOF+DCV and between LDV/SOF with regard to LDV/SOF +RBV (p=0.018) used to treat HCV genotype 3 infection.
Conclusions
In conclusion, in our cohort of patients, the combination of SOF + DCV followed by LDV/SOF + RBV 12 weeks were the most effective in patients with HCV genotype 3 and with cirrhosis (SVR12 90% and 80%, respectively) and in those without cirrhosis (SVR12 100% in both combinations). All patients who achieved SVR12 also achieved SVR24, regardless of the regimen received.
Keywords: HCV, genotype 3, Interferon-free treatments
RESUMEN
Introducción
El virus de la hepatitis C (VHC) genotipo 3 representa una entidad única dentro del tratamiento de la hepatitis C y múltiples estudios sugieren que la infección del VHC genotipo 3 está asociada a una progresión más rápida de la enfermedad comparado con otros genotipos, resultando en un mayor riesgo de cirrosis, carcinoma hepatocelular y mortalidad. En el presente estudio se evaluó la efectividad del tratamiento ledipasvir/sofosbuvir ± ribavirina (LDV/SOF ± RBV) y sofosbuvir + daclatasvir (SOF + DCV) durante 12 semanas en pacientes con VHC genotipo 3 naive o pre-tratados, con o sin cirrosis.
Material y métodos
Se realizó un estudio observacional, retrospectivo en un hospital de tercer nivel. El periodo de estudio comprendió 9 meses (abril 2015-enero 2016). Criterios de inclusión: pacientes con hepatitis C y genotipo 3 que fueron tratados con LDV/SOF ± RBV o con SOF + DCV durante 12 semanas. Los pacientes que fueron tratados durante 24 semanas fueron excluidos así como aquellos que se trataron con peginteferon. La variable principal fue la respuesta viral sostenida (RVS) a semana 12 (RVS12) y la variable secundaria fue RVS24.
Resultados
En el periodo de estudio se trataron en nuestro hospital 603 pacientes: 71 con genotipo 3. Se incluyeron en el análisis 46 pacientes, que fueron tratados con LDV/SOF ± RBV o SOF + DCV durante 12 semanas. El 43,75% (7/16) de todos los pacientes tratados con LDV/SOF alcanzaron RVS12, el 90% (9/10) de los pacientes tratados con LDV/SOF + RBV consiguieron RVS12 y el 95% (19/20) de los pacientes tratados con SOF + DCV. Se obtuvieron diferencias significativas (p=0,001) entre LDV/SOF y SOF + DCV y entre LDV/SOF + RBV y LDV/SOF (p=0,018).
Conclusiones
En nuestra cohorte de pacientes, las combinaciones de SOF + DCV y LDV/SOF + RBV, administrados durante 12 semanas, fueron las más efectivas tanto en pacientes con cirrosis (RVS12 90% y 80%, respectivamente), como en pacientes sin cirrosis (RVS12 100% en ambos casos). Todos los pacientes que alcanzaron RVS12, también alcanzaron RVS24 independientemente del tratamiento recibido.
Palabras clave: VHC, genotipo 3, tratamiento libre interferon
INTRODUCTION
Chronic hepatitis C (CHC) is a worldwide cause of liver-related morbidity and mortality. It affects over 185 million people, approximately 2–3% of the world’s population. While a prevalence of 2–3% may be relatively low overall, prevalence varies by age group and is typically much higher in cohorts between the ages of 45 and 75. For example, in Central and East Asia, the prevalence peaks at 8.8–8.9% for those aged 55–64 [1].
Over the last several years, the management of CHC has been revolutionized by the development of cell-mediated targeted therapies [direct-acting antiviral agents (DAAs)] against hepatitis C virus (HCV). Indeed, we are at the beginning of a new era of HCV management, which is a boon to patients and clinicians alike. Left behind is a treatment regimen fraught with side effects, quality of life (QOL) impairment and high treatment failure rates. The new regimens are simple, safe, effective regimens of short duration with minimal side effects [2].
Six different genotypes of hepatitis C virus HCV (genotypes 1, 2, 3, 4, 5, 6) have been identified [3]. Genotype 1, specifically 1b, is the most common subtype worldwide affecting 42% of HCV-infected individuals [3]. This is followed by geno-type 3 (26%), most commonly found in Pakistan and India, and genotype 4 (14%) which is most common in North Africa and the Middle East. In the US, genotype 1a is the most common, accounting for 58% of HCV infected individuals. Genotype 1b accounts for 21%, genotype 2 accounts for 15% and genotype 3 accounts for 5% [3]. The genotype is clinically relevant given that the majority of current DAAs do not have pangenotypic efficacy. In addition, each genotype is associated with a different sustained virologic response (SVR) rate [2].
With respect to genotype 3, it represents a unique entity within HCV treatment and multiple studies have documented that HCV genotype 3 infection is associated with more rapid disease progression than other genotypes, resulting in increased risk of cirrhosis, hepatocellular carcinoma, and all-cause mortality [4]. Also, it is remarkable that rates of cure have lagged behind for HCV-3 in the modern DAA era and new therapies are being developed in order to close this treatment gap [5].
In 2015, the Ministry of Health, Equality and Social Policy set out a therapeutic strategy for treatment of CHC [6]. It pointed out that the available treatments for genotype 3 were sofosbuvir + daclatasvir (SOF + DCV) with or without ribavirin (RBV), a fixed-dose combination (FDC) tablet of ledipasvir and sofosbuvir (LDV/SOF) or sofosbuvir +peg-interferon/ribavirin (SOF +Peg-IFN + RBV) which were clearly suboptimal for cirrhotic patients with HCV genotype 3 infection with SVR between 58-69%.
Recommended regimens for treating HCV genotype 3 include DCV, an NS5A inhibitor, in combination with SOF, with or without RBV depending on the presence of cirrhosis. DCV has a reported EC50 (50% effective concentration) against genotype 3 HCV replicons ranging from of 0.004 nM to 0.52 nM. ALLY-3 study [7] evaluated 12-week regimen of SOF+DCV in genotype 3 infection achieving SVR12 rates of 90% in treatment-naive patients and 86% in treatment-experienced patients, with an overall SVR12 rate of 89% [4].
In the ELECTRON-2 study [8], treatment-naive patients with genotype 3 infection were randomized to receive 12 weeks of LDV/SOF with or without RBV. In the arm without RBV, 16 of 25 (64%) achieved SVR12 whereas all 26 patients randomized to receive therapy with RBV achieved SVR12, including 6 patients with compensated cirrhosis. Also, 50 treatment-experienced patients with HCV genotype 3 received 12 weeks of LDV/SOF + RBV, only 41 (82%) of them achieved SVR12. The rate of SVR12 was 73% and 89% in those with and without cirrhosis, respectively. SOF is a pangenotypic nucleotide polymerase inhibitor with potent activity against all 6 HCV genotypes in both in vitro replicon assays and extensive clinical use. LDV is a potent and well-tolerated NS5A inhibitor with activity against replicons of genotypes 1a, 1b, 4, 5, and 6, with EC50 values ranging from 0.006 nM (genotype 1b) to 1.1 nM (genotype 6a). However, LDV is much less active against HCV genotype 3a in vitro, with an average EC50 of 168 nM against wild-type virus, although LDV seems to be more active against HCV genotype 3 than predicted based on the replicon data alone [4].
In the current study, we further evaluated the effectiveness of 12 weeks of LDV/SOF ± RBV and SOF + DCV for treatment-naive or treatment-experienced patients infected with HCV genotype 3, with or without cirrhosis.
MATERIAL AND METHODS
Retrospective and observational study carried out in a third level hospital. Study period: April 2015 to January 2016. Inclusion criteria: Patients with HCV genotype-3 infection treated either with LDV/SOF ± RBV or with SOF + DCV during study period treated for 12 weeks. This variety of treatment regimens used reflects the evolution of HCV therapy in clinical practice. The choice of treatment and the use or not of concomitant RBV was entirely at the discretion of the treating physician. It was made in accordance, of the majority of the cases, with the product label, the European Association for the Study of the Liver clinical practice guidelines and the National Hepatitis C Plan developed by the Spanish Ministry of Health, giving priority to the treatment of patients with significant liver fibrosis (F2-F4) [9,10].
Exclusion Criteria: patients from whom adequate clinical and/or analytical information was not available for further analysis. The patients that were treated during 24 weeks were excluded and those treated with peg-interferon.
The information was obtained from the electronic clinical/medical records and dispensing records from outpatient software (Cafydim® and ATHOS-Prisma®) Pharmacy Service.
Outcomes collected: Demographic variables: age and sex. Clinical data: basal viral load (VL), SVR at week 12 (SVR12), defined as HCV RNA titres less than 15 IU/mL 12 weeks after stopping study drug, SVR at week 24 (SVR24), defined as HCV RNA titres lower than 15 IU/mL 24 weeks after stopping study drug. HCV-RNA levels were measured by the COBAS TaqMan HCV Test v2.0 (RCTM) (Roche Molecular Diagnostics) with lower limit quantification (LLOQ) of 15 IU/ml. Respect to fibrosis grade, patients were categorized depending on the fibrosis grade according to METAVIR scale (F0-F4). F4 patients were considered as cirrhotic. Fibrosis stage was determined by non-invasive device: Fibroscan®. Other variables picked up were: platelet levels (cel/μL), albumin concentration (g/dl), transaminases hepatic levels (IU/L): aspartate transaminase (AST) and alanine transaminase (ALT) and bilirubin concentration (mg/dl). We also have assessed whether patients had had liver transplant, had HIV co-infection or had been treated previously for HCV.
The main endpoint measured was the SVR12 and the secondary endpoint was SVR24.
Adherence variable: The calculation was made with the following formula:
Percentage of adherence= number of units of total DAAs agents medication dispensed/number of units of planned DAAs agents medication.
Planned units were considered necessary to comply with the treatment on the days included from the first dispensation to the last in the period of time considered for the calculation.
In the event that one of the patients had an admission to our hospital, the Pharmacy Service provided the DAA agents during the entire hospitalization period. According to this, the adherence calculation also took into account the registration of dispensed medication by unit dose to hospitalized patients.
Statistical analysis. All statistical analysis was performed using SPSS, version 17. Shapiro Wilk test was used to assess whether data were likely from a normal distribution. We used one way ANOVA or Kruskal Wallis test to compare means depending on whether data were from a normal distribution or not. Categorical data were compared by chi-square tests. Here p values < 0.05 were considered statistically significant.
RESULTS
In the study period, in our hospital, were treated 603 HCV patients. We have genotype data of 97.18% (N=586). The genotypic distribution of all patients is summarised in table 1.
Table 1.
Genotypic distribution of different patients treated from April 2015 to January 2016.
| Genotypic distribution | Number of patients |
|---|---|
| Genotype 1 | 431 (73.54%) |
| Genotype 2 | 10 (1.7%) |
| Genotype 3 | 71 (12.11%) |
| Genotype 4 | 74 (12.62%) |
| Total | 586 |
Forty-six patients genotype 3 were included: 20 treated with SOF+DCV, 16 with LDV/SOF and 10 with LDV/SOF+RBV. All were treated during 12 weeks.
Baseline characteristics
a) Patients treated with ledipasvir/sofosbuvir (n=16).
Demographic variables: Age: 53.05 ± 9.05 and 12/16 (75%) were males. One patient had received liver transplant, four patients had been treated with RBV + Peg-IFN previously but they did not achieve SVR12 and only one was VIH co-infected being treated with boosted protease inhibitor such as darunavir plus ritonavir tablets (800/100 mg) once daily. Regarding to fibrosis stage, seven patients had F4 fibrosis, 6 patients were F3, 2 of them F2 and only one F1 and six patients with baseline viral load exceeding 800,000 IU/mL. A further point is that, we have measured other serum biomarkers (mean ± standard deviation) related to stage of liver fibrosis and liver function such as platelet, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and also bilirubin [8]. The mean platelet count was 135,571 ± 50,554 cel/µl, the mean albumin was 3.91 ± 0.47 g/dL. The mean AST, ALT and total bilirubin were 107.64 ± 70.66 IU/L, 115 ± 126 IU/L and 0.93 ± 0.53 mg/dL, respectively.
b) Patients treated with ledipasvir/sofosbuvir plus ribavirin (n=10)
Demographic variables: mean age: 55.83 ± 6.7 years; 8/10 males (80%). One had liver transplantation before starting their treatment regimens. Two patients were treated with RBV + Peg-IFN, formerly, without achieving SVR12. Also, two patients were VIH co-infected being treated one of them with two nucleoside reverse-transcriptase inhibitors (NRTIs) plus non-nucleoside reverse-transcriptase inhibitor (NNRTI) (abacavir/lamiudina/rilpivirina) and other patient with one NRTI plus boosted protease inhibitor (lamivudine/danuravir/cobicistat). Regarding to fibrosis stage, five patients had F4, four patients had F3 and only 1 patient underwent from fibrosis F2. Three patients had basal viral load (BVL) upper than 800,000 UI/ml. Measured serum biomarkers, expressed as mean±standard deviation, were: platelets (132,000 ± 69,739 cel/µl), albumin (3.9 ± 0.55g/dL), bilirubin (0.77 ± 0.28mg/dL), AST (126.16 ± 53.73 IU/L), ALT (146.16 ± 82.11 IU/L).
c) Patients treated with daclatasvir plus sofosbuvir (n=20).
Demographic variables: mean age: 55.78 ± 10.21; 7/20 were females (35%). Nobody had received liver transplant, however, five were treated with RBV +Peg-IFN previously, without achieving SVR12. Four patients were VIH co-infected. Three of them were treated with two NRTIs plus NNRTIs or an integrase strand transfer inhibitor (INSTI): abacavir/lamivudine+rilpìvirin or raltegravir respectively. One patient was treated with two NRTIs plus boosted protease inhibitor (emtricitabine/tenofovir/fosamprenavir/ritonavir).
Regarding to fibrosis stage, 10 were F4 (50%), four F3, four F2 and only two were F1. Seven patients had a basal viral load upper than 800,000 IU/ml. Measured serum biomarkers: platelets (148,850 ± 61,816 cel/µl), albumin (4.10 ± 0.43g/dL), bili-rubin (0.62 ± 0.23mg/dL), AST (107.40 ± 68.71 IU/L), ALT (139.9 ± 87.46 IU/L).
In all groups 100% of patients were adherent to therapy.
There were no statistically significant differences among the different variables analysed (p>0.05). Therefore, there were no important clinical differences respects to baseline characteristics of the patients included. The characteristics of all enrolled patients are summarised in table 2.
Table 2.
The characteristics of all enrolled patients
| Patients treated with sofosbuvir/ledipasvir (n=16) | Patients treated with sofosbuvir/ledipasvir+ribavirin (n=10) | Patients treated with sofosbuvir+daclatasvir (n=20) | P value | |
|---|---|---|---|---|
| Age (years) | 53.05 ± 9.05 | 55.83 ± 6.70 | 55.78±10.21 | |
| Sex | 0.648 | |||
| Male | 12 | 8 | 13 | |
| Female | 4 | 2 | 7 | |
| Stage of fibrosis | 0.853 | |||
| F4 | 7 | 5 | 10 | |
| F3 | 6 | 4 | 4 | |
| F2 | 2 | 1 | 4 | |
| F1 | 1 | - | 2 | |
| Liver transplant | 1 | 1 | 0 | 0.234 |
| Previously treated | 4 | 2 | 5 | 0.917 |
| VIH co-infected | 1 | 2 | 4 | 0.324 |
| Basal viral load > 800,000 U/ml | 6 | 3 | 7 | 0.389 |
| Platelet | 135,571 ± 50,554 cel/µl | 132,000 ± 69,739 cel/µl | 148,850 ± 61,816 cel/µl | 0.742 |
| Albumin | 3.91 ± 0.47 g/dL | 3.9 ± 0.55g/dL | 4.10 ± 0.43g/dL | 0.416 |
| AST | 107.64 ± 70.66 IU/L | 126.16 ± 53.73 IU/L | 107.40 ± 68.71 IU/L | 0.960 |
| ALT | 115 ± 126 IU/L | 146.16 ± 82.11 IU/L | 139.9 ± 87.46 IU/L | 0.9 |
| Bilirrubin | 0.93 ± 0.53 mg/dL | 0.77 ± 0.28mg/dL | 0.62 ± 0.23mg/dL | 0.065 |
Sustained virologic response (SVR)
Only 43.75% (7/16) of all patients treated with LDV/SOF achieved SVR12, 90% (9/10) of the patients treated with LDV/SOF + RBV achieved SVR12 and 95% (19/20) of the patients treated with SOF + DCV achieved SVR12. Therefore, there was statistically significant difference (p=0.001) between LDV/SOF respect to SOF+DCV and between LDV/SOF with regard to LDV/SOF + RBV (p=0.018) used to treat HCV genotype 3 infection (figure 1).
Figure 1.
Percentage of patients who have achieved SVR12 with the different treatments analysed.
a) Ledipasvir/sofosbuvir (n=16)
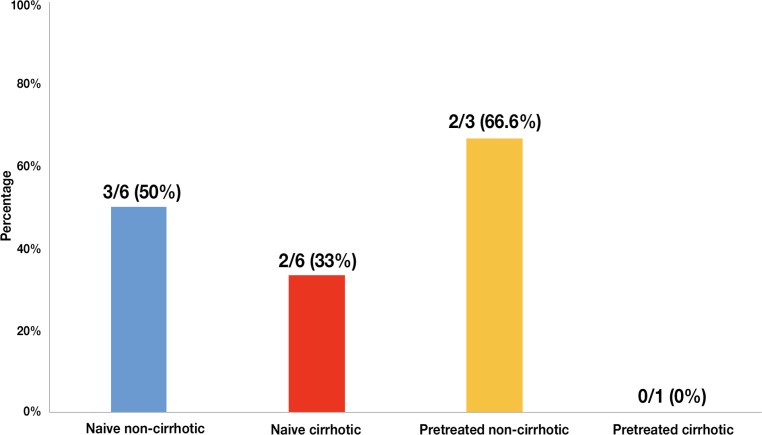
If we analyze the different subgroups of patients we observe that: 50% (n=3) naive-non cirrhotic patients achieved SVR12; 33.33% (n=2) of naive cirrhotic achieved SVR12; 66.66% (n=2) of pre-treated-non cirrhotic patients achieved SVR12 and nobody of pre-treated cirrhotic patients achieved SVR12 (figure 2).
Figure 2.
Percentage of different subgroups of patients (n = 16) who have achieved SVR12 with sofosbuvir/ledipasvir (SOF/LDV).
b) Ledipasvir/sofosbuvir plus ribavirina (n=10).
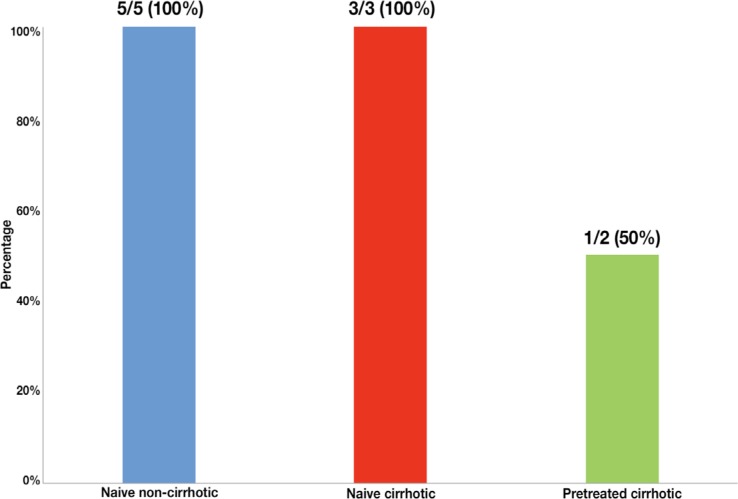
All naive non-cirrhotic patients (n=5) and naive cirrhotic patients (n=3) achieved SVR12. However, respect to pre-treated cirrhotic patients only 50% achieved SVR12 (figure 3).
Figure 3.
Percentage of different subgroups of patients (n = 10) who have achieved SVR12 with sofosbuvir/ledipasvir + rivabirin (SOF/LDV + RBV).
c) Sofosbuvir plus daclatasvir (n=20).
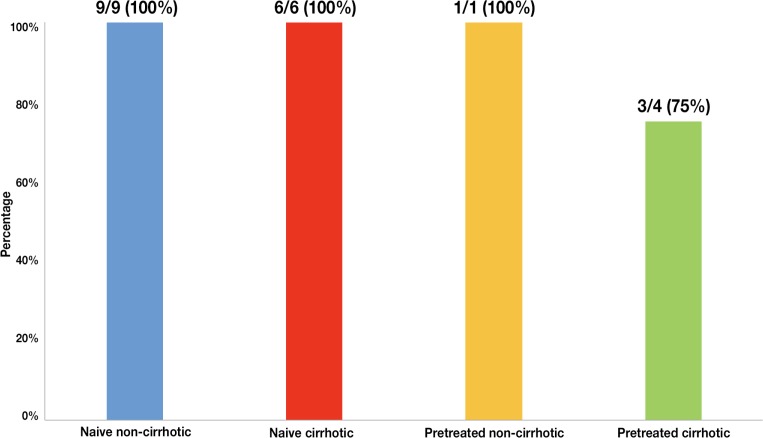
All patients: non-cirrhotic naive (n=9), naive cirrhotic (n=6) and pre-treated non-cirrhotic patients (n=1) achieved SVR12. Only 75% (n=3) pre-treated cirrhotic patients achieved SVR12 (figure 4).
Figure 4.
Percentage of different groups of patients (n = 20) who have achieved SVR12 with sofosbuvir plus daclatasvir (SOF + DCV).
Therefore, the most effective therapies to treat HCV geno-type 3 infection seem to be SOF + DCV and LDV/SOF + RBV. The sub-group of patients who responded better regardless of the therapy was the naive-non cirrhotic patients (85% achieved SVR12) and the worst group was pre-treated cirrhotic patients (57.14% achieved SVR12). Also it is remarkable that all patients who achieved SVR12 also achieved SVR24, regardless of the regimen received.
DISCUSSION
In this study, we have investigated the real-world effectiveness of various regimens of interferon-free treatments: LDV/SOF, LDV/SOF + RBV and SOF + DCV administered during 12 weeks in patients infected with HCV genotype 3 who met inclusion criteria, explained previously in materials and methods.
Our starting population was 586, whose genotypic distribution was similar to that one published by Ramos et al [11], concretely, the percentage of geno-type 1 of our patients was 73.54% vs. 78.4%, genotype 2 of our patients was 1.7% vs. 2.4%, genotype 3 was 12.11% vs. 9.7% and genotype 4 was 12.62% vs. 9.5%.
Patients with HCV genotype 3 are at a higher risk of liver disease progression and hepatocellular carcinoma development [12,13]. However, compared with other HCV genotypes, DAAs combinations have lower efficacy against genotype 3 in patients with liver cirrhosis. In our study, the global SVR12 in patients with HCV genotype 3 infection was 76.08% (35/46). In Ramos et al [11], the global SVR12 in patients with HCV genotype 3 infection was 93.3% (42/45). This difference may be due in part to the fact that in the Ramos et al study, 82.2% of the patients were treated with SOF + DCV, 6.7% with LDV/SOF and 11.1% with SOF unlike our study where 43.47% of patients were treated with SOF + DCV, 34.78% of them with LDV/SOF and 21.73% of total with LDV/SOF + RBV. It should be noted that all patients had 12 weeks of treatments in our study, however it is not known the percentage of patients treated during 12 or 24 weeks in the study made by Ramos et al [11].
In our cohort, 43.47% (20/46) of patients with this genotype were treated with SOF + DCV. In patients with liver cirrhosis we obtained a global SVR12 rate of 90% and 100% without. These results are in line with those obtained by Ramos et al: 82.2% (37/45) of patients with genotype 3 treated with SOF + DCV, with a global SRV12 rate of 90.3%-91.9% in patients with liver cirrhosis and 100% without, although our percentage of patients treated with SOF+DCV was lower than Ramos et al [11].
In others studies, in real-world settings, a global SVR12 of 60%-70% was achieved in genotype 3 infection with SOF + RBV [14,15]. All these studies had a remarkably low rate, which was likely related to the use of combinations that are currently not recommended because of their low efficacy [11]. Unlike our study where 43.47% of patients were treated with SOF + DCV, therapy of choice for non cirrhotic patients and alternative to SOF/velpatasvir (VEL) for cirrhotic patients [16].
On the other hand, if we analyzed every regimen used to treat HCV genotype 3 infection, it is essential to remark that 95% of the patients treated with SOF+DCV achieved SVR12, 90% of the patients treated with LDV/SOF + RBV achieved SVR12 and 43.75% of the patients treated with LDV/SOF achieved SVR12.
In the case of SOF+DCV regimen used to treat HCV geno-type 3 infection, it is important to point out that ALLY-3 clinical trial [7] supports the use of SOF + DCV during 12 weeks
in patients infected with genotype 3. Patients were either treatment naive (n=101) or treatment experienced (n=51). SVR12 rates were 90% (91 of 101) and 86% (44 of 51) in treatment-naive and treatment-experienced patients, respectively. Concretely, in patients without cirrhosis SOF + DCV for 12 weeks achieved SVR12 rates of 97% (73/75) in treatment-naive patients and 94% (32/34) in treatment-experienced patients with genotype 3 infection, while in our study we got SVR12 rates of 100% in both cases, but our sample (n=10) was smaller than in the ALLY-3 study. However, lower rates were obtained for cirrhotic patients in the ALLY-3 study, exactly 63% (20/32) achieved SVR12, while in our cohort 90% (9/10).
ELECTRON-2 study [8], evaluated LDV/SOF for genotype 3. Of the 51 naive patients included, 100% (26/26) achieved SVR12 in the treatment arm with LDV/SOF + RVB 12 weeks. These results are in line with those obtained in our study: 100% (8/8). In the treatment arm with LDV/SOF 12 weeks, only 64% (16/25) of them achieved SVR12 vs. 41.66% (5/12) in our cohort.
Moreover, in ELECTRON-2 study [8], SVR12 data from 50 more patients were reported, in this case pre-treated and all of them were treated with LDV/SOF + RBV for 12 weeks, and were divided into cirrhotic (n= 22) and non-cirrhotic (n=28). Overall SVR12 in pre-treated patients was 82% (41/50), 89% in non-cirrhotic (25/28) and 73% in cirrhotic (16/22). In our study only 2 pre-treated and cirrhotic patients were included with LDV/SOF + RBV for 12 weeks and 50% of them achieved SVR12.
In addition, if we disaggregate our patients in cirrhotic or non-cirrhotic patients, those treated with LDV/SOF + RBV 12 weeks (21.73%) achieved a global SVR12 rate of 80% in patients with liver cirrhosis and 100% without. Those treated with LDV/SOF 12 weeks (34.78%) achieved a global SVR12 rate of 28.57% in patients with liver cirrhosis and 55.55% without.
These results are aligned with the treatment regimens as valuable options for genotype 3 recommended by European Association for the Study of the Liver (EASL) (guideline 2016) [17]. EASL establishes that in patients infected with HCV genotype 3, the combination of LDV/SOF is not recommended because LDV is considerably less potent against genotype 3 than VEL or DCV.
This difference between LDV and DCV may be due to DCV´s higher potency in replicons containing HCV genotype 3a which is 0.003-1.25 nM [EC50] (50% effective concentration). Less clear is the benefit LDV adds to the efficacy of SOF with or without RBV. LDV´s lower potency in replicons containing HCV genotype 3a ([EC50] = 168 nM) as compared with those containing genotype 1a (EC50 = 0.031 nM) or 1b (EC50 = 0.004 nM) suggest that it would have minimal activity against HCV genotype 3 [8].
In addition to EC50, another important factor that we should keep in mind is the resistance-associated substitution (RAS). The clinical relevance of resistance testing has been limited to RASs in the NS5A gene. Two RASs in particular, Y93H and A30K, have emerged as the most clinically relevant polymorphisms in HCV-3 with the currently approved regimens [5]. It is important to highlight that Y93H RAS in a genotype 1a virus results in an EC50 of approximately 6 nM for LDV, therefore its activity is reduced clinically significant. Hence, one might expect that even at baseline the genotype 3 virus is effectively resistant to LDV [4]. However, in the ELECTRON-2 study, 6 patients with compensated cirrhosis treated with LDV/SOF + RBV achieved SVR12. These results clearly show that RBV is important but also suggest that LDV is more active against HCV genotype 3 than predicted based on the replicon data alone. It is also possible that RBV and/or SOF increase the sensitivity of HCV genotype 3 to LDV [4].
This study has the usual limitations related to its observational and retrospective design, electronic data collection and the small number of patients included in each arm of treatment. Resistance testing was not performed; thus, we were unable to assess the impact of this factor. The lack of randomization limited the ability to directly compare treatment groups, which is further compounded by the small number of patients in certain subgroups.
Subsequent to the period of inclusion of our study, other active DDAs against HCV genotype 3 have appeared more effective: SOF/VEL with SVR12 rates of 98% and 93% in naive patients and 91% y 89% in experienced patients with and without cirrhosis (ASTRAL-3) [18]. It would be desirable to confirm these results in patients with HCV in the real world.
In conclusion, in our cohort of patients, the combination of SOF + DCV followed by LDV/SOF + RBV 12 weeks were the most effective in patients with HCV genotype 3 and with cirrhosis (SVR12 90% and 80%, respectively) and in those without cirrhosis (SVR12 100% in both combinations). All patients who achieved SVR12 also achieved SVR24, regardless of the regimen received.
FUNDING
None to declare
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest
REFERENCES
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013; 57(4):1333–42. DOI: 10.1002/hep.26141 [DOI] [PubMed] [Google Scholar]
- 2.Lam BP, Jeffers T, Younoszai Z, Fazel Y, Younossi ZM. The changing landscape of hepatitis C virus therapy: focus on interferon-free treatment. Therap Adv Gastroenterol. 2015; 8(5):298–312. DOI: 10.1177/1756283X15587481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015; 61(1):77–87. DOI: 10.1002/hep.27259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feld JJ, Ramji A, Shafran SD, Willems B, Marotta P, Huchet E, et al. Ledipasvir-Sofosbuvir Plus Ribavirin in Treatment-Naive Patients With Hepatitis C Virus Genotype 3 Infection: An Open-Label Study. Clin Infect Dis. 2017. 65(1): 13-19. DOI: 10.1093/cid/cix289 [DOI] [PubMed] [Google Scholar]
- 5.Chan A, Patel K, Naggie S. Genotype 3 Infection: The Last Stand of Hepatitis C Virus. Drugs. 2017;77(2):131-144. DOI: 10.1007/s40265-016-0685-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministerio de Sanidad, Sercivios Sociales e Igualdad Estrategia terapéutica para la hepatitis crónica causada por el virus de hepatitis C en el Sistema Nacional de Salud. Recomendaciones generales y pautas actuales de tratamiento. 2015 [Cited 2017 July 10]. Available at: http://www.plataformadeafectadosporhepatitisc.org/sites/default/files/plan_estrategico_nacional_definitivo.pdf.
- 7.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015; 61(4):1127–35. DOI: 10.1002/hep.27726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gane EJ, Hyland RH, An D, Svarovskaia E, Pang PS, Brainard D, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology. 2015; 149(6):1454–1461.e1. DOI: 10.1053/j.gastro.2015.07.063 [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol 2015; 63(1): 199-236. DOI: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Bang CS, Kang HY, Choi GH, Kim SB, Lee W, Song IH. The Performance of Serum Biomarkers for Predicting Fibrosis in Patients with Chronic Viral Hepatitis. Korean J Gastroenterol. 2017;69(5):298–307. DOI: 10.4166/kjg.2017.69.5.298 [DOI] [PubMed] [Google Scholar]
- 11.Ramos H, Linares P, Badia E, Martin I, Gomez J, Almohalla C, et al. Interferon-free treatments in patients with hepatitis C genotype 1-4 infections in a real-world setting. World J Gastrointest Pharmacol Ther. 2017;8(2):137-146. DOI: 10.4292/wjgpt.v8.i2.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochud P-Y, Cai T, Overbeck K, Bochud M, Dufour J-F, Mullhaupt B, et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009; 51(4):655–66. DOI: 10.1016/j.jhep.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 13.Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirr-hosis. J Viral Hepat. 2011; 18(10):e516-22. DOI: 10.1111/j.1365-2893.2011.01441.x [DOI] [PubMed] [Google Scholar]
- 14.Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016; 151(3):457–471.e5. DOI: 10.1053/j.gastro.2016.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feld JJ, Maan R, Zeuzem S, Kuo A, Nelson DR, Di Bisceglie AM, et al. Effectiveness and Safety of Sofosbuvir-Based Regimens for Chronic HCV Genotype 3 Infection: Results of the HCV-TARGET Study. Clin Infect Dis. 2016; 63(6):776–83. DOI: 10.1093/cid/ciw387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guías AEEH / SEIMC de manejo de la Hepatitis C [Cited 2017 june 17] Available at: https//www.seimc.org/contenidos/documentoscientificos/guiasclinicas/seimc-clinicasclinicas-2016-Manejo_HepatitisC.pdf.
- 17.European Association for the Study of the Liver EASL Recommendations on Treatment of Hepatitis C 2016. J. Hepatol.2017;66(1):153-194. DOI: 10.1016/j.jhep.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 18.Graham R. Foster, Nezam Afdhal, Stuart K. Roberts, Norbert Bräu, Edward J. Gane et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015; 373:2608-2617. DOI: 10.1056/NEJMoa1512612 [DOI] [PubMed] [Google Scholar]