ABSTRACT
Pseudomonas aeruginosa is characterized by a notable intrinsic resistance to antibiotics, mainly mediated by the expression of inducible chromosomic β-lactamases and the production of constitutive or inducible efflux pumps. Apart from this intrinsic resistance, P. aeruginosa possess an extraordinary ability to develop resistance to nearly all available antimicrobials through selection of mutations. The progressive increase in resistance rates in P. aeruginosa has led to the emergence of strains which, based on their degree of resistance to common antibiotics, have been defined as multidrug resistant, extended-resistant and panresistant strains. These strains are increasingly disseminated worldwide, progressively complicating the treatment of P. aeruginosa infections. In this scenario, the objective of the present guidelines was to review and update published evidence for the treatment of patients with acute, invasive and severe infections caused by P. aeruginosa. To this end, mechanisms of intrinsic resistance, factors favoring development of resistance during antibiotic exposure, prevalence of resistance in Spain, classical and recently appeared new antibiotics active against P. aeruginosa, pharmacodynamic principles predicting efficacy, clinical experience with monotherapy and combination therapy, and principles for antibiotic treatment were reviewed to elaborate recommendations by the panel of experts for empirical and directed treatment of P. aeruginosa invasive infections.
Key words: Pseudomonas aeruginosa, treatment, guidelines
RESUMEN
Pseudomonas aeruginosa se caracteriza por una notable resistencia intrínseca a los antibióticos mediada fundamentalmente por la expresión de β-lactamasas cromosómicas inducibles y la producción constitutiva o inducible de bombas de expulsión. Además de esta resistencia intrínseca, P. aeruginosa posee una extraordinaria capacidad para desarrollar resistencia a prácticamente todos los antimicrobianos disponibles a través de la selección de mutaciones. El aumento progresivo de la resistencia en P. aeruginosa ha llevado a la aparición de cepas que, de acuerdo con el grado de resistencia frente a los antibióticos habituales, se han definido como multirresistentes, extensamente resistentes y panresistentes. Estas cepas se están diseminando mundialmente, complicando progresivamente el tratamiento de las infecciones por P. aeruginosa. En este escenario, el objetivo de las presentes recomendaciones es la revisión y puesta al día de la evidencia publicada para el tratamiento de pacientes con infección aguda, invasiva y grave por P. aeruginosa. Con este fin, se han revisado los mecanismos de resistencia intrínseca, factores que favorecen el desarrollo de resistencia durante la exposición a anti-bióticos, prevalencia de la resistencia en España, antibióticos clásicos así como los de reciente introducción activos frente a P. aeruginosa, principios farmacodinámicos predictores de eficacia, experiencia clínica con tratamientos en monoterapia o terapia combinada y principios del tratamiento antibiótico para elaborar por un panel de expertos recomendaciones para el tratamiento empírico o dirigido de infecciones invasivas por P. aeruginosa.
Palabras clave: Pseudomonas aeruginosa, tratamiento, recomendaciones
Pseudomonas aeruginosa is not part of normal microbiota in healthy humans [1]. Significant and/or prolonged colonization by P. aeruginosa occurs following loss of resistance to colonization due to changes in the composition of normal micro-biota as consequence of antibiotic treatment and/or pre-existence of severe disease. Clinical and experimental observations indicate that, in both cases, colonization occurs within the first 3-5 days of exposure to an environment with high exposure pressure, as in hospitals, mainly in Intensive Care Units (ICUs). In the 2016 ENVIN study, P. aeruginosa was the second most frequent isolated microorganism, just behind Escherichia coli, as cause of nosocomial infections in ICUs, and the third most frequent (after E. coli and Staphylococcus aureus) in community-acquired infections requiring ICU admission. Mortality of bacteremia by P. aeruginosa is 20-39% [2-11], values similar to or greater than those for bacteremia by S. aureus and candidemia episodes. In ventilator associated pneumonia (VAP), mortality is even higher, reaching 44% [12,13].
The progressive increase in resistance rates in P. aeruginosa has led to the emergence of strains which, based on their degree of resistance to common antibiotics, have been defined as multidrug resistant (MDR), extended-drug-resistant (XDR) and pan-drug-resistant (PDR) strains [14]. However, two new antibiotics active against P. aeruginosa have been introduced in the therapeutic armamentarium recently: 1) a new cephalosporin, ceftolozane, associated with tazobactam, active against most of the strains resistant to the remaining β-lactams [15,16], and 2) ceftazidime associated with a new β-lactamase inhibitor, avibactam, able to block AmpC β-lactamases, including those produced by P. aeruginosa [17,18]. Selection of the most appropriate antibiotic, dose, and route of administration, as well as potential association with other antibiotics, are critical decisions to obtain optimal clinical efficacy with the lowest risk of resistance increase and development of toxicity.
From the clinical point of view, infections caused by P. aeruginosa can be classified as: 1) acute superficial, noninvasive, infections in immunocompetent patients, 2) acute invasive infections in patients with significant comorbidities or immunodepression, and 3) chronic infections. The first group includes the following entities: external otitis (swimmer’s ear), perichondritis, queratitis associated with the use of contact lens, hydromassage-associated folliculitis, paronychia (green nail syndrome), palmoplantar hidradenitis, foot bones osteomyelitis (secondary to puncture wounds by objects penetrating sport shoes), and interdigital intertrigo. In all these cases, the infection that follows the exposure to a high P. aeruginosa inocula could be self-limited or respond to topical or oral ciprofloxacin treatment, and only exceptionally could pose problems in relation to the presence or development of resistance. The second group includes, among others, bacteremia, nosocomial pneumonia or VAP, endocarditis in parenteral drug users, pacemaker infections, necrotizing enterocolitis in the neutropenic patient, post-surgical meningitis, cerebrospinal fluid shunt infection, necrotizing fasciitis, gangrenous ecthyma, tertiary peritonitis or peritonitis associated with ambulatory peritoneal dialysis, malignant external otitis, central. venous catheter infection, burn wound infection, and urinary tract infection (pyelonephritis or prostatitis) in patients with vesical catheters. In all these circumstances, the severity of the infection and the risk of resistance in the infecting strain and of the empirical treatment resulting inadequate or generating higher degree of resistance, make important the knowledge of criteria guiding most appropriate treatment selection. The objective of the present guidelines is the treatment of this group of patients with acute, invasive, and usually severe infections by P. aeruginosa. In the third group, chronic infections are included. Usually, isolates of P. aeruginosa from patients with cystic fibrosis produce an extracellular polysaccharide, alginate, conferring mucoid-type colonies. The same pheno-type could be observed in bronchial infections in patients with bronchiectasis, advanced COPD (GOLD IV) or panbronchiolitis. These strains are usually less virulent and rarely produce bacteremia or extend beyond the lung. However, growth within biofilms makes difficult its eradication, and in advanced stages it is not possible with current treatments.
The present document does not address the treatment of chronic infections observed in patients with cystic fibrosis or bronchiectasis since it was subject of two recently published consensus [19,20]. We have reviewed the mechanisms of intrinsic and acquired resistance in P. aeruginosa, and their prevalence in Spain, to review afterwards the principles of treatment, basis for the further analysis of the main antibiotics with activity against P. aeruginosa. Lastly, recommendations for empirical and directed treatments are formulated.
MECHANISMS OF INTRINSIC RESISTANCE IN P. aeruginosa AND RESISTANCE DEVELOPMENT DURING TREATMENT
P. aeruginosa is characterized by its notable intrinsic resistance to antibiotics, mainly determined by the expression of inducible chromosomic AmpC β-lactamase and the production of constitutive (MexAΒ-OprM) or inducible (MexXY) efflux pumps [21]. The expression of inducible AmpC is determinant in the natural resistance of P. aeruginosa to most penicillins and cephalosporins [22]. Besides, the constitutive expression of MexAΒ-OprM contributes to the reduced susceptibility of P. aeruginosa to all β-lactams (except imipenem) and fluoroquinolones [23]. In addition, the inducible expression of MexXY has a major role in the lower basal activity and adaptive (inducible) resistance to aminoglycosides in P. aeruginosa [24]. Similarly, the inducible expression of operon arnBCADTEF, responsible for the addition of a 4-aminoarabinose residual to lipid A of the lipopolysaccharide, is critical for the development of inducible/adaptive resistance to polymyxins [25].
Apart from its notable intrinsic resistance, P. aeruginosa possess an extraordinary ability to develop resistance to nearly all available antimicrobials, through the selection of mutations in a complex network of genes implicated in resistance and their regulation [21,26]. This fact has major consequences for the efficacy of treatments for P. aeruginosa infections, mainly among critical patients at the ICU or those with chronic infections where the problem is magnified due to the high frequency of hypermutator strains, which present a spontaneous mutation rate up to 1000 times higher than normal [27]. The rate of spontaneous mutation for development of resistance usually ranges from 10-6 (1 mutant per million bacteria) to 10-8 (1 mutant per 100 millions) for most antibiotics. Therefore, in those infections linked to high bacterial load (as respiratory infections) the probability of resistance development is elevated for most classical antipseudomonal compounds, even for strains with normal rate of spontaneous mutation (non hypermutator strains). In fact, for most antipseudomonals mutant prevention concentrations (MPCs) [28] are frequently above concentrations achieved by systemic administration; colistin and ceftolozane/tazobactam being among the few exceptions [29]. Table 1 summarizes the characteristics of resistance development for the main antipseudomonals, including: a) main mechanisms of resistance developed through exposure to each antibiotic, b) the relatively frequency of spontaneous occurrence, c) the baseline minimal inhibitory concentrations (MICs) and MPCs, and d) development of cross-resistance to other antipseudomonals.
Table 1.
Activity and frequency of individual and cross-resistance resistance development for the different antipseudomonals, according to mechanisms implicated
| Antimicrobiala | PIP-TZ | CAZ | FEP | TOL-TZ | ATM | IMP | MER | FQ | AMG | COL | FOS | MIC(mg/L) | MPC(mg/L) | Primary R MEC | Secondary R MEC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIP/TZ | +++ | +++ | ++ | - | ++ | - | + | -/+ | - | - | - | 2 | >32 | ↑ AmpC | ↑ MexAB |
| CAZ | +++ | +++ | ++ | -/+ | ++ | - | + | -/+ | - | - | - | 1 | >32 | ↑ AmpC | ↑ MexAB |
| FEP | ++ | ++ | +++ | -/+ | +++ | - | ++ | + | + | - | - | 1 | >32 | ↑ MexAB/XY | ↑ AmpC |
| TOL/TZ | -/+ | + | + | + | -/+ | - | -/+ | - | - | - | - | 0.5 | 2 | ↑ AmpC+mut AmpC | PBP3 |
| ATM | ++ | ++ | +++ | -/+ | +++ | - | ++ | + | - | - | - | 4 | >32 | ↑ MexAB/XY | ↑ AmpC |
| IMP | -/+ | -/+ | -/+ | - | -/+ | +++ | ++ | -/+ | - | - | - | 1 | >32 | OprD | MexST (↑ MexEF ↓ OprD) |
| MER | + | + | + | - | + | ++ | ++ | + | - | - | - | 0.5 | 8 | OprD | ↑ MexAB, PBP3 |
| FQb | + | + | ++ | - | ++ | -/+ | + | +++ | + | - | - | 0.12 | 2 | QRDR | ↑ MexAB/XY/CD/EF |
| AMGc | - | - | + | - | - | - | - | + | ++ | - | - | 1 | 8 | ↑ MexXY | FusA |
| COL | - | - | -/+ | - | - | -/+ | -/+ | -/+ | + | - | 0.5 | 2 | pmrAB/phoPQ | parRS | |
| FOS | - | - | - | - | - | - | - | - | - | - | ++++ | 64 | >1,024 | GlpT |
PIP-TZ: piperacillin-tazobactam; CAZ: ceftazidime; FEP: cefepime; TOL-TZ: ceftolozane-tazobactam; ATM: aztreonam; IMP: imipenem; MER: meropenem; FQ: Fluoroquinolones; AMG: aminoglycosides; COL: colistin; FOS: fosfomycin. MIC: minimum inhibitory concentration. MPC: mutant prevention concentration (concentration preventing selection of resistant mutants). R MEC: resistance mechanism
Frequency of spontaneous development of clinical resistance (EUCAST resistance breakpoints) to antibiotics in columns by exposure to antibiotics in rows. (++++) Extremely elevated resistance development, (+++) Very elevated resistance development, (++) Elevated resistance development, (+) Moderate resistance development, (-/+) Low or improbable resistance development, (-) Non expected resistance development. Data shown in the Table refer to wild-type strains without acquired mechanisms of resistance, using as reference strain PAO1 (28;236; A. Oliver data non published).
FQ resistance development: levofloxacin > ciprofloxacin (pumps hyperexpression). Data shown in the Table refer to ciprofloxacin.
Resistance development aminoglycosides: gentamicin > amikacin > tobramycin. Data shown in the Table refer to tobramycin.
The main mechanism of development of resistance to penicillins (ticarcillin, piperacillin, piperacillin-tazobactam) and cephalosporins (ceftazidime and cefepime) active against P. aeruginosa, is the selection of mutants with constitutive hyperproduction (derepression) of inducible AmpC chromosomic cephalosporinase [30]. Although AmpC derepression also increases MIC of ceftolozane-tazobactam, clinical resistance to this new combination requires an additional structural modification of AmpC, thus explaining the lower development of resistance [31]. The new combination of ceftazidime with the β-lactamase inhibitor avibactam, equally preserves activity against AmpC hyperproducer strains [32]. Among the great number of mutational resistance mechanisms stand out the repression or inactivation of the OprD porine which, together with the inducible expression of AmpC, confers resistance to imipenem and reduced susceptibility to meropenem [22]. Frequently, inactivation of OprD also synergically acts with derepression of AmpC, confering resistance to all available β-lactams except ceftolozane/tazobactam [33] and ceftazidime/avibactam [32]. Finally, the hyperexpression of any of the multiple efflux pumps, mainly MexAΒ-OprM and MexXY-OprM and to a lesser entent Mex-EF-OprN and MexCD-OprJ, significantly contributes to the resistance phenotypes [23]. MexAΒ-OprM is the efflux pump presenting the larger substrate profile. Its constitutive expression plays an important role in intrinsic resistance and its hyperexpression by chromosomic mutations affects all classical β-lactams (except imipenem) and fluoroquinolones. Hyperexpression of MexAΒ-OprM plus OprD inactivation is one of the most frequent causes of clinical resistance to meropenem [34]. The expression of inducible MexXY plays an important role in the intrinsic resistance to aminoglycosides, and its mutational hyperexpression in the acquired resistance to cefepime. Hyperexpression of MexEF-OprN and MexCD-OprJ is less frequent and mainly affects quinolones. However, mutations (mexT/mexS) leading to hyperexpression of MexEF-OprN also confer decreased susceptibility to carbapenems through repression of oprD. Quinolone resistance is frequently produced by mutations in topoisomerases including ADN gyrase (GyrA/GyrB) and type IV topoisomerases (ParC/ParE). Lastly, development of resistance to polymyxins generally implies the modification of lipopolysaccharide mediated by mutations in the two-component systems involving PmrAB, PhoPQ or ParRS [35]. Interactions between all these mutations are complex, but it should be taken into account that in many cases the selection of a first mutation facilitates the subsequent selection of others, frequently resulting in MDR/XDR phenotypes close to panresistance.
PREVALENCE AND MECHANISMS OF PRIMARY RESISTANCE IN SPAIN
Although there are local important differences that should be analyzed and considered at each institution, table 2 shows the estimated prevalence and resistance mechanisms in P. aeruginosa that could be expected in Spanish hospitals. Overall, resistance rates are over 20% for most antipseudomonal antibiotics, including penicillins (piperacillin, piperacillin-tazobactam), cephalosporins (ceftazidime, cefepime), monobactams (aztreonam), carbapenems (imipenem, meropenem), fluoroquinolones (ciprofloxacin, levofloxacin) and aminoglycosides (gentamicin and tobramycin). Among the available antipseudomonal antibiotics, only colistin, amikacin and the recently introduced combination ceftolozane-tazobactam exhibit an activity close to 95%. The prevalence of MDR strains is already above 30% worldwide, including Spanish hospitals; approximately half of MDR strains would be also XDR [9]. The increasing prevalence of MDR/XDR phenotypes results from the combination of the extraordinary ability of P. aeruginosa to develop resistance against nearly all available antimicrobials through selection of chromosomal mutations, together with the increasing frequency of exogenous resistance determinants, generally localized in integrons codified in transferable genetic elements (plasmids or transposons) [21]. Among these determinants, due to its clinical importance, the genes of β-lactamases with higher hydrolytic profile, class B carbapenemases (metallo-β-lactamases, MBLs) and extended-spectrum β-lactamases (ESBLs), usually associated with determinants of aminoglycoside resistance should be highlighted [36]. No doubt that intra-hospital dissemination, originating epidemic/endemic situations of MDR/XDR strains, plays an important role in the increasing magnitude of this problem. Even more important if posible is the alarming evidence of epidemic MDR/XDR strains widely disseminated worldwide, the so-called high-risk clones, mainly ST111, ST175 and ST235 [37]. A recent Spanish multicenter study (2015) showed that the most prevalent clone was by far ST175, being responsible for 68% cases of XDR P. aeruginosa in our country [38]. This study also showed that 20% of XDR strains were carbapenemase-producers (mainly VIM-type MBLs), while in the remaining 80% β-lactam resistance was mediated by chromosomal mutations (OprD inactivation + AmpC hyperproduction). It should be highlighted that although all XDR strains were resistant to all classical antipseudomonal β-lactams, only those carbapenemase-producing strains were highly resistant (MIC > 8 mg/L) to ceftolozane-tazobactam. In fact, 68% of XDR strains were susceptible to this combination, although in many cases MICs were close to EUCAST and CLSI breakpoints (4 mg/L) [38].
Table 2.
Prevalence and primary resistance mechanisms expected in P. aeruginosa in Spain.
| Antimicrobiala | % I+R (R)a | In order of frequency implicated mechanisms of resistanceb |
|---|---|---|
| PIP-TZ | 20-30 | ↑AmpC (++), ↑MexAB (+), MBL (+), OXAs and other ESBL (+) |
| CAZ | 20-30 | ↑AmpC (++), ↑ MexAB (+), MBL (+), OXAs and other ESBL (+) |
| FEP | 20-30 | ↑MexAB/XY (++), ↑AmpC (++), MBL (+), OXAs and other ESBL (+) |
| TOL-TZ | 1-5 | MBL (+), OXAs and other ESBL (+) ↑AmpC+mut AmpC (-/+) |
| ATM | >50 (20-30) | ↑MexAB/XY (+++) ↑AmpC (++), OXAs and other ESBL (+) |
| IMP | 20-30 (20-30) | OprD (+++), MBL (+) |
| MER | 20-30 (5-20) | OprD (+++), ↑MexAB (++), MBL (+) |
| CIP | 30-50 | QRDR (+++), ↑MexAB/XY (++), ↑MexCD/EF (+) |
| TOB | 20-30 | Modified enzyme AMG (++) ↑MexXY (+) |
| AMK | 5-20 (1-5) | ↑MexXY (++),modified enzyme AMG (+) |
| COL | 1-2 | pmrAB/phoPQ/parRS (-/+) |
PIP-TZ: piperacillin-tazobactam; CAZ: ceftazidime; FEP: cefepime; TOL-TZ: ceftolozane-tazobactam; ATM: aztreonam; IMP: imipenem; MER: meropenem; CIP: ciprofloxacin; TOB: tobramycin; AMK: amikacin; COL: colistin
Prevalence of primary resistance expected in Spain, according to 2017 EUCAST breakpoints. When there is an intermediate susceptibility category, prevalence of non-susceptible strains (I+R) is shown and prevalence of resistant strains are in parenthesis. Data estimated using available information from EARS-Net (https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/ears-net), multicenter studies (29;33;101;237) and microbiology department in several Spanish hospitals (H. Son Espases, Palma de Mallorca; H. Clinic, Barcelona; H. A Coruña, A Coruña).
Relative frequency of resistance mechanisms: +++ (20-30%), ++ (5-20%), + (1-5%), -/+ (<1%).
PRINCIPLES FOR THE TREATMENT OF INFECTIONS CAUSED BY P. aeruginosa
Principles guiding election of antibiotic treatment, whether empirical or directed treatment, in case of suspected or confirmed P. aeruginosa infections, are those also applying to any severe infection, but with some peculiarities as follows:
1) MIC of main antibiotics active against P. aeruginosa. The breakpoint used to categorize P. aeruginosa as resistant to one β-lactam or aminoglycoside is from 2-times (piperacillin-tazobactam, imipenem, tobramycin, gentamicin) to 8-times (ceftazidime, cefepime) higher than the one used to consider resistant an enterobacteria. Against most clinical isolates of P. aeruginosa susceptible to β-lactams, the MIC of an antibiotic is usually at or close to its breakpoint value (2-8 mg/L). For this reason, high doses of β-lactams are recommended, even if the strain has been categorized as susceptible in in vitro susceptibility tests.
Clinical and/or bacteriological efficacy of β-lactams is related with the time of exposure of the microorganism to the antibiotic or the percentage of time that the free fraction of the antibiotic exceeds the MIC (% fT>CMI). In the treatment of infections by Gram-negative bacilli, including P. aeruginosa, ceftazidime and cefepime exhibits bactericidal effect (reduction of 2-3 log10 CFU) when serum concentrations exceed the MIC for more than 60% of the dosing interval [39]. Clinical cure, especially in severe infections, has been related with exposure to antibiotic concentrations 4-times higher than the MIC for 100% of the dosing interval [40,41]. In an in vitro P. aeruginosa growth model, the PK/PD index predicting efficacy for piperacillin-tazobactam was a maintained antibiotic concentration 5-times above the MIC [42]. In another similar study performed with a P. aeruginosa inocula of 108 CFU/mL, the consecution of a Cmin/MIC index ≥3.8 avoided emergence of resistance [43].
Elimination half-lives for most β-lactams are 1-2 hours. After 30-minutes administration of standard doses at 8-hour intervals, serum concentrations decrease below 4-8 mg/L before the 4th-6th hours from administration, especially in septic patients with a volume of distribution (Vd) and renal clearance presumably elevated. For the treatment of severe or high bacterial load infections, produced by microorganisms exhibiting MIC ≥4 mg/L of the β-lactam, only elevated doses administered by continuous or extended infusion reach free antibiotic concentrations exceeding 4-times the MIC [44-46]. Nevertheless, serum antibiotic concentrations in the first hours (until the steady state is reached) are noticeably lower after continuous infusion than with a 30-min dose administration. Consequences of the delay may be important for critically ill patients or patients with severe immunodepression or severe infection. In these circumstances it is necessary to start antibiotic treatment with an additional loading dose, by bolus infusion, followed by the total daily dose administered as continuous infusion. The initial dose by bolus infusion allows early achievement of an elevated Cmax, favoring diffusion of the free fraction of the antibiotic to the infectious foci, on one side, and on the other, to relatively compensate the increased Vd and renal clearance in early phases of sepsis.
Several studies performed in patients with P. aeruginosa infections analyzed the potential advantages of maintaining serum β-lactam concentrations over MIC values for the maximum possible time through iv continuous or extended infusion administration. In a retrospective study including 87 patients with bacteremia and/or pneumonia by P. aeruginosa, the extended infusion of cefepime (MIC50=4 mg/L) significantly decrease mortality and days in the ICU compared with the standard intermittent administration [47]. In other study, piperacillin-tazobactam was administered as intermittent doses or 4-hour extended infusion in the treatment of 194 patients with infections by P. aeruginosa [48]. For the extended infusion, doses were lower than those used for intermittent administration; however, both mortality was lower and mean hospital stay was shorter in the group of patients receiving extended infusion. The difference was significant only in the subgroup of more severe patients (APACHE II ≥17) [48]. In cystic fibrosis patients with acute exacerbations of P. aeruginosa infection, extended or continuous infusion of a β-lactam (generally ceftazidime) has shown to be better than intermittent dose administration, with respect to improvement of FEV1, forced vital capacity and extension of exacerbation-free intervals [49]. The potential greater efficacy of continuous infusion has also been shown in Montecarlo simulations with patients treated with meropenem [50] or piperacillin-tazobactam [51] and in one case of carbapenem-resistant P. aeruginosa infection well controlled by 12g of meropenem extended infusion [52]. In rabbit models of infectious endocarditis caused by P. aeruginosa, maintained ceftazidime concentrations 4-5 times over the MIC provided optimal clinical efficacy [53,54]. In vitro models of P. aeruginosa infection also indicate that continuous infusion is the most efficient administration for β-lactams [55-57].
In most clinical studies [58-66] but not in all [67-69], continuous or extended infusion of piperacillin-tazobactam, cefepime, ceftazidime or meropenem, for the treatment of infections by Gram-negative bacilli (including P. aeruginosa) was more efficacious than intermittent administration with respect to the one or more following parameters: clinical cure rate, microbiological eradication, days with fever, length of ICU or hospital stay and decrease in severity (measured by APACHE II) and/or mortality. Negative or non-conclusive results in some studies might be explained by one or more of these facts: 1) the infecting microorganism was highly susceptible to the antibiotic used (very low MIC) and the antibiotic administration as intermittent doses was enough to maintain a serum concentration over the MIC for most of the dosing interval [67], 2) patients were not critically ill and/or did not suffer a severe infection [69], 3) the dose used for intermittent administration was frequently higher than the dose used for continuous infusion [63,69], 4) a significant number of patients was treated with a concomitant antibiotic (aminoglyco-side or fluoroquinolone) [69], and 5) other factors that might have attenuated potential advantages of continuous infusion were absence of an initial loading dose, lack of consideration of the favorable effect of renal function impairment on the intermittent dosification and the recruitment of an insufficient number of patients to obtain significant differences [70]. The conclusion of three meta-analyses [71-73] including most of the above referred studies, was favorable to the use of extended or continuous infusion with respect to the risk of death. On the contrary, a third meta-analysis [74] including, among others, studies carried out in patients with COPD exacerbations, did not show differences in outcome in relation to ways of antibiotic administration.
A recent study [75] showed that in septic patients attended at the Emergency department, a first-to-second antibiotic dose delay of near 4h (for 6-hour dosing intervals) was seen in >50% patients. The delay in the second dose administration was associated with a significant increase in mortality. Antibiotic continuous infusion can preclude the risk of an eventual prolongation of the dosing interval.
The main determinant for clinical response to an aminoglycoside treatment is the Cmax/MIC value [76]. For the reasons exposed below, the greatest efficacy for a treatment is obtained when Cmax/MIC ≥10. For a MIC value for P. aeruginosa of 2-4 mg/L of tobramycin and gentamicin, the recommended Cmax is 30-40 mg/L and for amikacin MIC of 8 mg/L, Cmax should be between 60 and 80 mg/L [77]. As later commented, usually these values are not achieved with standard doses.
2) Importance of the bacterial load in the infectious foci. In P. aeruginosa infectious foci as pneumonia, purulent tracheobronchitis in the intubated patient, secondary peritonitis, neutropenic colitis and skin and soft tissue infections (gangrenous ecthyma, cellulitis in a diabetic foot wound or wound infection in severely burned patients), the bacterial load at antibiotic treatment initiation is usually high (≥107-108 CFU). This bacterial inocula is between 100 and 1000 higher than standard inocula used in in vitro susceptibility tests. The intrinsic activity of most antibiotics decreases when bacterial load is high. In the case of β-lactams, this effect could be due to a reduced growth rate and/or expression of different PBPs with reduced affinity to β-lactams in the stationary phase of growth or the increase of β-lactamase concentrations due to bacterial lysis. Piperacillin and piperacillin-tazobactam seems the most affected by the inoculum size followed by ceftazidime, with meropenem in the third place [78]. For β-lactams, time over MIC is the most important factor for low bacterial inocula or very susceptible microorganisms. However, when the microorganism is less susceptible or the inoculum is high, the β-lactam activity shows certain dependence of the antibiotic concentration [40].
The ability of granulocytes to eradicate microorganisms is saturable [79]. In rat models of pneumonia by P. aeruginosa, when the bacterial load was close to or higher than 2.5 x 106 CFU/g of tissue, the bacteriolytic ability of granulocytes was surpassed and bacterial growth occurred [80,81]. The authors of these studies suggested that in infections with high bacte-rial load, as VAP, an early and rapid ≥2 log10 CFU/mL decrease produced by the antibiotic treatment might decrease bacterial density below the cut-off level of granulocyte activity saturation, allowing an optimal contribution for microorganism eradication.
Another important consequence of the presence of a high bacterial load is the increased risk of selection of resistant mutants.
3) Mutation ability and development of resistance in P. aeruginosa. Frequency of emergence of resistant mutants within P. aeruginosa populations ranges from 10-6 to 10-8 depending on the antibiotic [82]. In the presence of agents damaging DNA (fluoroquinolones) and in biofilm-embedded bacterial growth, the basal rate of emergence of resistant mutants can be around 100 times increased. These are strains with mutations in genes involved in repair mechanisms of DNA replication errors. These hypermutants strains are usually seen in the mucoid phenotype present in patients with cystic fibrosis and other situations as chronic bronchial infections [83-87].
A bacterial density ≥ 107-108 CFU at treatment initiation involves high risk of selection and amplification of the resistant subpopulation under the selective antibiotic pressure. Measures to counter this risk include: a) reduction of the bacterial load through the control of the infectious foci (drainage, debridement, de-obstruction or removal of catheter or infected foreign body), b) initiation of treatment with associations of antibiotics not sharing the main resistance mechanism [88], and c) use of doses and/or routes of administration able to generate an antibiotic concentration higher than MIC for potential resistant mutants in the infectious foci.
If the P. aeruginosa infecting strain is susceptible to the antibiotics used and the dose and the administration schedule are appropriate, after 48-72 hours of treatment, the residual bacterial load in the infectious foci would presumably be lower to the one needed to generate a significant number of resistant mutants, i.e., lower than the inferior limit of the spontaneous mutation rate (10-6). From then, the risk of development of resistance in the infectious foci could be considered as negligible and, if there are no other reasons justifying the association (see below), treatment can be continued as monotherapy with the β-lactam chosen based on the antibiogram.
Antibiotics (aminoglycoside, ciprofloxacin or levofloxacin) associated with the β-lactam during the first 48-72 h, among other purposes to avoid selection of resistant mutants, should be administered at doses achieving concentrations over the corresponding MPCs. Although MPCs are unknown and could not be predicted from MIC values, generally for these antibiotics they are from 8 to 12 times higher than the MIC. In any case, the activity of these antibiotics is concentration-dependent and, higher the concentration in the infection foci, higher the bactericidal effect and lower the number of resistant mutants surviving antibiotic exposure. In vitro studies carried out with P. aeruginosa strains have shown that exposure to high tobramycin concentrations for 1-4 h [89] and to high ciprofloxacin concentrations along 1 and 10h [90] widely reduce bacterial population without selection/amplification of resistant mutants. However, in both experiments the addition of a second antibiotic was needed to prevent regrowth of the residual bacterial population that remained susceptible.
At the 2nd-3rd day of treatment, when deescalation to monotherapy is considered, most patients remain colonized by P. aeruginosa in mucosa and bronchial secretion (in case of pneumonia, tracheal intubation or previous bronchial pathology), especially if no inhaled antibiotic treatment with tobramycin, colistin or aztreonam had been administered. Persistence of bronchial colonization does not justify by itself prolongation of iv administration of the aminoglycoside more than 3-5 days. Despite reaching a Cmax in serum ≥10 times the MIC, there is a low probability that the concentration and the activity of the aminoglycoside in bronchial secretion exceeds the MPC, thus hardly precluding development of resistance at the expense of a higher risk of renal toxicity secondary to treatment prolongation. The same concept could be applied to colistin administered by systemic route, but not to ciprofloxacin and levofloxacin with better diffusion to bronchial secretion.
4) Importance of an appropriate empirical treatment. Studies performed in patients with VAP [13,91] or bacteremia [2-4,8,11,92-94] caused by P. aeruginosa showed high mortality rates if the initial empirical antibiotic treatment is not appropriate. Non appropriate antibiotics are those for which the microorganism shows resistance in in vitro susceptibility tests. Early administration of an appropriate antibiotic treatment has special relevance when the infection presents clinical or biological severity criteria, the patient suffers important immunodepression or comorbidities or has advanced age. These are particularly frequent clinical situations in patients with P. aeruginosa infections [3,95-97]. Given the current high prevalence of P. aeruginosa strains resistant to β-lactams, treatment initiation with a β-lactam associated with amikacin, ciprofloxacin or colistin (chosen based on local resistance rates) increases the probability of the appropriateness of the initial empirical schedule, that is, the P. aeruginosa strain is at least susceptible to one of the two antibiotics administered [91,93,94,98,99].
5) Value of antibiotic associations. Usually, the association of a β-lactam and an aminoglycoside shows in vitro synergistic activity. However, in clinical practice, the potential synergy of the association does not seem to turn into a tangible improvement of prognosis estimated as survival rate. Most studies carried out in patients with bacteremia [5,92,94,100-104] or VAP [91,105,106] by P. aeruginosa, as well as several meta-analyses [98,99,107], did not found significant differences in mortality rates between patients receiving β-lactam monotherapy and those receiving a β-lactam and aminoglycoside association. Nevertheless, there are several aspects raising doubts with respect to the strength of these results. Most studies were retrospective analyses, treatments were not ran-domized, the most severe patients tended to be treated with antibiotic associations [107] and analyses were not adjusted by possible confounding factors. In a significant number of patients, the origin of bacteremia was an urinary tract infection or venous catheter removal, thus, non-severe infections and low bacterial load. In addition, in the aminoglycoside arm nephrotoxicity masking the benefits of the association could not be ruled out since renal failure is an important prognostic factor in critically ill patients. On the other hand, in other studies, a favorable effect of the association versus monotherapy has been reported in the treatment of bacteremia caused by P. aeruginosa [2,108], particularly in neutropenic patients [109-111], in cystic fibrosis exacerbations [112] and in a meta-analysis of studies on bacteremia by Gram-negative bacilli [113]. However, these results are neither conclusive because in the monotherapy arm patients treated with aminoglycosides were often included [110,113]. The efficacy of aminoglycosides is lower than that of β-lactams [92,111] except in urinary tract infections [114].
The results of all these so far published studies on P. aeruginosa infections comparing monotherapy of a β-lactam with combinations of β-lactams and aminoglycosides, are at least questionable since the aminoglycoside concentration in serum was never optimized in the first 24-48 hours. This could be a critical issue explaining the apparent lack of in vivo synergy and other possible favorable effects of the combination, particularly in the case of P. aeruginosa infection for two reasons: the first one in relation to the mechanism of synergy and the second one related to the adaptive resistance phenomenon. At low or intermediate tobramycin concentrations (<4 mg/L) the main mechanism of bacterial lysis is the block of protein synthesis at the ribosome, while at more elevated concentrations (≥8 mg/L), the main lytic mechanism is the aminoglycoside interaction with divalent cations stabilizing lipopolysaccharide molecules of the outer membrane. Since aminoglycosides molecules are bigger than Ca2+ and Mg2+ ions, their substitution by the aminoglycoside causes the disruption of the external membrane, with the subsequent increase in permeability [115]. In Gram-negative bacilli, and specially in P. aeruginosa, the outer membrane constitutes the main barrier for penetration of many antibiotics. The achievement of a high aminoglycoside concentration in the infectious foci is, probably, an important target if synergistic activity is to be obtained.
The result of P. aeruginosa exposure to aminoglycosides is an early and rapid concentration-dependant bacterial lysis followed by a refractory phase characterized by a low and concentration-independant bacterial destruction known as adaptive resistance [24]. This phenotype of partial and transitory resistance is due to the fact that the aminoglycoside, even at subinhibitory concentrations, induces the expression of genes codifying the MexXY efflux pump [116]. A similar phenomenon is observed in anaerobic or hyperosmolar media, at acidic pH and in the presence of elevated concentrations of divalent ions (Ca2+ or Mg2+) [117]. This effect is more pronounced against P. aeruginosa. Several of these conditions are present in urine and bronchial secretion. Adaptative resistance justifies, among others, the administration of aminoglycosides as single daily doses. If after the first aminoglycoside dose, a Cmax approximately 10 times the MIC is not reached, the intrinsic bactericidal activity of the antibiotic is lower than optimal, not surpassing the MPC and reduces the possibility and/or the degree of β-lactam synergy. This decrease in efficacy precisely occurs during the first 24-48 hours of treatment, when there is a need for a rapid elimination of the high bacterial load and for countering selection of resistant mutants, this justifying the β-lactam and aminoglycoside association. Clinical experience supports the importance of optimizing the aminoglycoside PK/PD parameters from the beginning. A published study [118] analyzed outcome in 78 patients with pneumonia treated with antibiotic regimens including aminoglycosides with the aim of determining if optimization of PK/PD parameters result in more rapid therapeutic responses (defined as days until fever and leukocytosis resolution). The logistic regression analysis predicted 90% probability of fever and leukocytosis resolution after 7 days if during the first 48h treatment with the aminoglycoside a Cmax/MIC >10 ratio was reached [118]. In another study including 38 patients with bacteremia by P. aeruginosa, the probability of clinical cure was ≥90% when the Cmax/MIC ratio was at least 8 [119].
Until mid 90’s, aminoglycosides (gentamicin, netilmicin and tobramycin) were used at doses of 3 to 5 mg/kg/day with bid or tid schedules. These regimens reached a Cmax of approximately 5 mg/L from day 2-3 on [106,120,121]. The potential effects on outcome when aminoglycosides are administered at suboptimal doses are hardly valorable in infections by P. aeruginosa (tobramycin MIC are usually 2 mg/L). From 1990’s on, schedules progressively changed to single daily doses of 5-7 mg/kg/day (gentamicin and tobramycin) and of 15-20 mg/kg/day for amikacin [122]. Nevertheless, even with these doses, often Cmax continues to be suboptimal (especially for the treatment of P. aeruginosa infections) due to the elevated Vd and/or increase of renal clearance normally present in patients with severe sepsis or septic shock, mechanical ventilation, neutropenia, polytraumatism, severely burn, cystic fibrosis or morbid obesity (if doses are calculated for the lean body mass) [123-126]. In an ICU study, septic patients were treated with a mean gentamicin dose of 6.6 ± 2.3 mg/kg and only 1 out of 24 patients (4%) reached the desired Cmax ≥30 mg/L [127]. In another study carried out in patients with severe sepsis or septic shock treated with an amikacin initial dose of 25 mg/kg, the desired Cmax of at least 60 mg/L was not reached in up to 30% of cases [128]. Other authors have reported similar results [129-133]. In a 2013-14 French study, two years after the implementation of a guideline for aminoglycoside administration [77], 37% prescriptions were not in line with the recommendations [134]. With the aminoglycoside once daily administration the risk of renal toxicity is reduced through the reduction in the time that the proximal tubule is exposed to the antibiotic. Treatment duration for the aminoglycoside in the combined therapy with a β-lactam should be limited to the first 3-5 days [135].
In VAP patients, a low aminoglycoside Cmax might be primarily unfavorable due to its limited diffusion to the alveolar space and specially to the bronchial secretion [136-141] and the potential loss of activity in both sites. A reduction in the activity of tobramycin has been observed in the presence of pulmonary surfactant, particularly at low concentrations (0.25-1 x MIC) [142], probably due to its linkage to surfactant phospholipid proteins. In bronchial secretion, aminoglycosides are partially inactivated, mainly if the sputum is purulent, due to the electrostatic binding to mucin polysaccharides and to the DNA, to the presence of divalent cations and to pH ≤ 7 [143]. Concentrations up to 25 times higher the MIC of tobramycin are required to achieve bactericidal activity in sputum [144,145].
Even though clinical experience does not permit to firmly rule out the existence of a favorable result when associating a β-lactam and an aminoglycoside, if a benefit exists, it does not imply a significant improvement in the prognosis and it does not justify the risk of the aminoglycoside nephrotoxicity. In most clinical situations, the treatment of choice for a β-lactam susceptible P. aeruginosa infection is β-lactam monotherapy except in the following cases: 1) during the first 72 hours if the infection presents criteria of severe sepsis or septic shock, 2) in the neutropenic patient, and 3) in nervous central system (meningitis, abscess) or endovascular (endocarditis) infections. Use of associations including a β-lactam should be considered even for the treatment of infections caused by β-lactam resistant pathogens, especially if the resistance level is moderate (MIC 2-4 times higher than the breakpoint value). In this situation, the potential synergy with the second antibiotic could revert β-lactam non-susceptibility, if succeed in lowering the MIC below the resistance level.
6) Clinical efficacy of different antibiotics as mono-therapy. Clinical experience evidences that monotherapy with β-lactams shows higher efficacy and/or lower toxicity than monotherapy with aminoglycosides [92,111,114] or colistin [146-148] and similar to monotherapy with a fluoroquinolone (ciprofloxacin) [149-151] in the treatment of gramnegative infections, including those by P. aeruginosa. However, in some infection sites, as in external malignant otitis, prostatitis, or cystic fibrosis bronchial infections, the use of ciprofloxacin may have advantages over a β-lactam, based on the possibility of oral administration, better penetration in the infectious foci and the probable greater activity in biofilms.
7) Measures to increase antibiotic concentrations in the infectious foci. As mentioned in points 1 and 2, to optimize the PK/PD index and to avoid selection/amplification of resistant subpopulations, high (aminoglycosides, fluoroquinolones) and maintained (β-lactams) antibiotic concentrations are required in the infectious foci. Nevertheless, in certain infection sites (as in pneumonia in the intubated patient, ventriculitis, meningitis), even with the maximum tolerated dose, MPCs are not exceeded or the associated toxicity is unacceptably high. In these cases, the possibility of directly introducing the antibiotic into the infectious foci using the inhalatory, intrathecal or other routes (depending on the infection site) should be considered. Antibiotic administration by the inhalatory route allows concentrations in bronchial mucous and the epithelial lining fluid around 100 times higher than those obtained with the same dose by iv route. This result in a higher probability of bacteriological eradication, even for microorganisms considered as resistant in in vitro susceptibility tests together with a reduction in the risk of selection and growth of the resistant population.
The review of clinical experience on the treatment of P. aeruginosa respiratory infections using inhaled antibiotics surpasses the extension limit of the present document. In chronic respiratory infections by P. aeruginosa in cystic fibrosis patients, inhaled tobramycin, colistin or aztreonam are considered treatments of choice, from the first exacerbation by P. aeruginosa, even in case of strains susceptible to β-lactams [19,152]. Studies performed in VAP patients [153-164], and several meta-analyses on VAP [165-167] or bronchiectasis infections [168], indicate that the addition of inhaled antibiotics improves clinical success and bacteriological eradication, especially when causal microorganisms harbour resistance mechanisms. In a study on patients with VAP by P. aeruginosa, the administration of inhaled antibiotics was compared with the administration of the same compounds by iv route, randomly assigning patients to receive ceftazidime and amikacin as treatments [169]. In the inhaled treatment arm, several patients were infected by strains exhibiting intermediate resistance to the antibiotics used, while in the iv treatment arm, in case of intermediate resistance to amikacin, this drug was changed to ciprofloxacin. No statistically significant differences in clinical outcome were observed. Resistances only emerged in the iv treatment arm [169]. In the respiratory infection by P. aeruginosa, if the infection presents severity criteria, the radiologic image is extensive or shows cavitations, or the isolated strain is multidrug resistant, inhaled treatment administration of tobramycin, colistin or aztreonam through a vibrating-membrane nebulizer should be considered. Presence of severe hypoxia (PaO2/FiO2 < 200) might contraindicate the use of inhalatory route.
Table 3 shows main recommendations in relation to antibiotic treatment for acute invasive infections by P. aeruginosa.
Table 3.
Recommendations for antibiotic treatment of acute invasive infection produced by P. aeruginosa
|
ANTIBIOTICS ACTIVE AGAINST P. aeruginosa
β-lactams. Nowadays, in most Spanish hospitals resistance rates in P. aeruginosa to piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, imipenem or meropenem are ≥20% (table 2). Ceftolozane-tazobactam is active against nearly 95% isolates and the ceftazidime-avibactam association restores ceftazidime susceptibility in nearly 80% resistant strains. With the exception of imipenem, poorly stable at room temperature, all other β-lactams active against P. aeruginosa should be administered at high doses and using extended or continuous infusion after an initial loading dose. This recommendation is based on: their time-dependant bactericidal activity, the possible inoculum effect of a high bacterial load (present at treatment initiation), the need for optimization of the PK/PD parameter for the high MIC against P. aeruginosa, the increase in Vd and/or renal clearance [170] and the need to exceed the MPC. In relation to the latter, table 1 shows MPC values for different β-lactams against a P. aeruginosa strains not harboring additional resistance determinants. Several studies have reported for ceftazidime and meropenem values similar to those shown in table 1 [171-173]. With a MPC value >32 mg/L of ceftazidime, cefepime, aztreonam, piperacillin-tazobactam and imipenem, the probability that concentrations of these antibiotics in serum are within the mutant selection window is very high, even when administered at maximum doses by extended/continuous infusion. The risk is especially high if the infection involves a bacterial load equal to or higher than the spontaneous mutation rate (10-6-10-8 CFU). The risk is moderate for meropenem (MPC of 8 mg/L) administered at 6 g daily dose by extended infusion, and very low for ceftolozane-tazobactam (MPC of 2 mg/L) at 1.5-3 g dose by 3-4 hours infusion every 8 hours. In an in vitro study using one wild-type and one hypermutant P. aeruginosa strains exposed to ceftazidime, meropenem and ceftolozane-tazobactam, high-level resistance first to ceftazidime and after to meropenem was rapidly developed in both strains [31]. None of the selected mutants showed cross-resistance with ceftolozane-tazobactam. Development of resistance to ceftolozane-tazobactam was slower and only was of high-level in the hypermutant strain [31]. Other studies have confirmed the greater ability of ceftazidime versus meropenem in selecting P. aeruginosa resistant mutants both from wild-type and hyper-mutant strains [174].
In clinical practice, most isolates of P. aeruginosa harbors one or more resistance mechanisms and MPC values are higher than those for fully susceptible strains. In these cases, failure and/or resistance development may occur with meropenem and, eventually, with ceftolozane-tazobactam monotherapies, even at high doses.
The main side effect with the use of a β-lactam high dose is neurotoxicity produced by inhibition of GABA- GABAA receptors binding, characterized by a slow and progressive appearance of somnolence, confusion, disorientation, agitation, myoclonus, asterixis, seizures, non-convulsive epileptic status and coma. The electroencephalogram shows a diffuse slow wave activity with triphasic waves, suggestive of toxic encephalopathy. Neurotoxicity is more frequent with cefepime, followed by ceftazidime, cefazoline and the remaining β-lactams. Patients with pathologies involving the central nervous system, with renal impairment and advanced age are especially vulnerable [175,176]. Some authors consider that steady state concentrations should not exceed the 100 mg/L threshold to avoid neurological toxicity with piperacillin, aztreonam or ceftazidime [177,178].
Piperacillin-tazobactam has been identified as a factor responsible for the delay in renal function restoration in the critically ill patient [179].
The review of the resistance mechanisms to different β-lactams in table 1 shows that ceftazidime and piperacillin share the same primary resistance mechanism, as well as occurs for cefepime and aztreonam and for meropenem and imipenem. Resistance to any of these antibiotics makes probable (but not certain) the resistance to its couple [180].
High number of in vitro studies on the association of two β-lactams or one β-lactam with other antibiotics, mainly aminoglycosides and fluoroquinolones, has been published. Chromosomal cephalosporinases (AmpC) of the species Enterobacter, Citrobacter, Serratia and Pseudomonas hydrolyze aztreonam, but the half-life of this reaction is long enough to maintain the enzyme inactivated along several generations of bacterial growth. In this way aztreonam can protect ceftazidime, and specially cefepime, from hydrolysis by AmpC in P. aeruginosa strains resistant by derepressed production of the enzyme [181-185]. The benefit is higher in the case of cefepime due to its more rapid cross of the external bacterial membrane. However, clinical experience is limited to a study including 13 patients with infection by P. aeruginosa resistant to all β-lactams treated with the association of cefepime plus aztreonam. Outcome was favorable for 69% of cases. Nevertheless, 11 out of 13 patients additionally received an aminoglycoside and 5 inhaled colistin. Aztreonam is resistant to hydrolysis by MBLs. It could be associated with ceftazidime-avibactam for the treatment of infections caused by P. aeruginosa strains producing a MBL plus derepressed AmpC. In a Galleria mellonella larvae model of P. aeruginosa infection, several β-lactam associations (not including aztreonam) showed in vivo synergism, which was bad correlated with in vitro interaction results [186]. Another possible synergistic mechanism for β-lactam combinations is the complementarity of PBPs inhibition profiles. In a recently published study [187], the association of cefepime, piperacillin or meropenem with zidebactam, a non- β-lactam PBP2 specific inhibitor, was synergistic against MDR and MBL-producing P. aeruginosa strains. However, there are not clinical experience, not even in infection animal models, supporting the potential advantage of the combination of a potent PBP2 inhibitor (carbapenem) with a potent PBP3 inhibitor (ceftazidime, cefepime or aztreonam).
Aminoglycosides. Tobramycin is the aminoglycoside showing the highest intrinsic activity against P. aeruginosa, being two-times more active than gentamicin and from 3 to 4 times than amikacin. Nevertheless, amikacin is susceptible to inactivation by a lower number of enzymes, thus being active against a higher percentage of P. aeruginosa isolates (90-95%) compared to tobramycin (80%).
The concentration-dependent bactericidal activity of aminoglycosides reaches its optimal efficacy in the treatment of P. aeruginosa infection when a Cmax/CMI ≥10 ratio is obtained in the first 24-48 hours of treatment initiation [118,119]. Aminoglycosides, due its hydrophilic nature, are distributed in the interstitial space and renally eliminated. The increase in Vd and in renal clearance, observed in critically ill patients with an important systemic inflammatory response, significantly reduces the aminoglycoside concentration in serum after the first dose. The recommended dose in the first 48-72 h of treatment, in patients with normal renal function and severe P. aeruginosa infection, is up to 8 mg/kg for gentamicin or tobramycin and of 20-30 mg/kg for amikacin [77].
The combination of an aminoglycoside and a β-lactam might be in vitro synergistic against gramnegative bacilli by means of the increase in the permeability of the external membrane, as previously commented. Another mechanism that could contribute, at least in part, to the synergy is the one observed in AmpC-producing P. aeruginosa resistant to cefepime. The addition of tobramycin at 7 mg/kg/day doses suppress protein synthesis, and with that, β-lactamase expression, facilitating the cephalosporin activity [188].
Fluoroquinolones. The current resistance rate to ciprofloxacin and levofloxacin in P. aeruginosa, in most Spanish hospitals, exceeds 30% (table 2). Ciprofloxacin is intrinsically more active than levofloxacin (MIC 2-4 dilutions lower).
The concentration-dependent bactericidal activity of fluoroquinolones reaches an optimal efficacy with Cmax/MIC >8. Nevertheless, the bactericidal effect of fluoroquinolones is slower than that of aminoglycosides and lysis of resistant mutants requires longer exposures. Bacteriological eradication without resistance development has been related with AUC24h/MIC >100 [189,190]. The combination of both indexes minimizes resistance emergence [90]. MPC of ciprofloxacin and levofloxacin is 2 and 8 mg/L, respectively [191]. Diffusion of fluoroquinolones (especially levofloxacin) to cerebrospinal flu-id, lung parenchyma, bronchial secretion and prostate is superior to that of β-lactams, aminoglycosides and colistin.
In in vitro studies carried out with P. aeruginosa, the association of levofloxacin and imipenem precluded emergence of resistance, even when strains exhibiting intermediate resistance to one or both antibiotics due to loss of OprD or efflux pumps overexpression were used [192,193]. In several studies, the association of levofloxacin with meropenem had more rapid bactericidal effect and resulted in resistance suppression [194] or meropenem MPC decrease [195], even when the strain was resistant to levofloxacin [196]. Levofloxacin and meropenem are eliminated by MexAB and the overexpression of this pump should affect both. The authors suggest that the β-lactam access to the pump through the periplasmic space could saturate its ability to extract levofloxacin from the cytoplasm [194]. The association of ceftazidime or cefepime with a fluoroquinolone (ciprofloxacin, levofloxacin or moxifloxacin) at 0.5 x MIC concentrations was synergistic for more than 50% P. aeruginosa strains [197]. However, in another study, the association of ceftazidime with ciprofloxacin led to emergence of resistance due to overexpression of MexAB [198].
Clinical experience indicates that ciprofloxacin is similar to [151] or better than imipenem [149] in the treatment of severe nosocomial pneumonia. Ciprofloxacin associated to metronidazole was similar to imipenem in intraabdominal infections [199] and equivalent to the association of ceftazidime and amikacin in febrile episodes in neutropenic patients [150]. In a study including 740 patients with VAP, treatment with meropenem monotherapy (1 g every 8 hours) was compared with meropenem associated with ciprofloxacin (400 mg/12 hours), in both cases by iv route. Treatment allocation was randomized. No differences in mortality, days in the ICU or hospital, clinical or microbiological response or emergence of resistance were observed. Nevertheless, in the analysis of the subgroup of 56 patients who had infection due to P. aeruginosa, Acinetobacter spp., and multidrug-resistant Gram-negative bacilli, the combined initial treatment was appropriate in 84% patients (versus 18.8%; p< 0.001) and the response was favorable for the association in microbiological eradication (64% versus 29.4%; p = 0.05) and favorable but non-significant in 28-days clinical resolution rates, days in the ICU and days with mechanical ventilation [200]. In the analysis of a series of 235 episodes of bacteremia by P. aeruginosa, definitive treatments with associations including ciprofloxacin showed a significantly lower 30-days mortality if the strain was susceptible. On the contrary, the association with tobramycin did not modify the prognosis [2]. A similar result was reported in another study on patients with bacteremia by gramnegative bacilli and Pittsburgh score <4 [201].
The use of high fluoroquinolone doses, exceptionally might produce confusion, orofacial dyskinesias, myoclonus, psychosis and non-convulsive epileptic status [175] by GABAA inhibition or NMDA receptor activation.
Colistin. Around 98% P. aeruginosa strains are colistin susceptible with MICs of 0.5-1 mg/L (table 2). Colistin Cmax after standard doses does not exceed 2-3 mg/L. Although its bactericidal activity is concentration-dependent, the therapeutic margin is very narrow and the increase in serum concentrations is not possible due to the risk of renal toxicity. The activity decreases in the presence of high inocula [202,203]. In P. aeruginosa rat models of pneumonia, a fAUC0–24 /MIC of 40 predicted a bacterial reduction ≥ 2 log10 [204]. Some P. aeruginosa strains, apparently susceptible to colistin, presented heteroresistance [205] with the MIC of the resistant subpopulation far above the achievable maximum concentration in serum. Colistin should not be used as monotherapy, especially if the MIC is > 1 mg/L, the bacterial load is high or in the case of low accessible foci (lung, CNS). The association with a β-lactam (cefazidime or meropenem), a fluoroquinolone (ciprofloxacin or levofloxacin) or rifampicin can exert synergistic effects [206-211]. It is recommended to start treatment with a 6-9 MU iv loading dose to avoid the delay of 48-72 hours needed to reach the stationary state [212,213], followed by iv 4.5 MU/12 hours. Nevertheless, in a recent study [214] no relation between 28-days mortality and administration of a loading dose followed by high doses (9 MU/days) was observed when compared with the use of lower doses (4-6 MU/day) without loading dose. On the contrary, renal toxicity and appearance of seizures were significantly more frequent with the use of high doses. The most frequently isolated microorganisms were Acinetobacter baumannii and Klebsiella pneumoniae, and against both, colistin MIC was low (MIC90 0.5 mg/L). Thus, probably, an optimal exposure was achieved with both doses [215]. Until more experience in the treatment of infections caused by microorganisms exhibiting MIC ≥ 2 mg/L is available, the administration of a loading dose followed by high doses should be considered.
Diffusion of colistin in the alveolar space and the bronchial secretion is limited [216], and its activity significantly decreases in the presence of mucus [217]. As well, concentration in cerebrospinal fluid is only 5% of the serum concentration [218].
Fosfomycin. Against nearly 33% P. aeruginosa strains, MIC of fosfomycin is ≤64 mg/L. Its time-dependent bacteriostatic activity is highly influenced by the inoculum size [219]. Heteroresistance is frequent among susceptible strains, and for this reason monotherapy is not recommended. The association with tobramycin [220,221], amikacin [222,223], ciprofloxacin [224,225] and different β-lactams [226-229] is frequently synergistic and decreases emergence of resistance [220-222]. Clinical experience is limited to the treatment of MDR P. aeruginosa exacerbations of cystic fibrosis. In the largest published study, 30 exacerbations in 15 patients treated with iv 5 g/8h fosfomycin associated with tobramycin, colistin or a β-lactam were analyzed [230]. The authors considered that treatment outcome was favorable. In a literature review analyzing 6 studies, including 33 patients treated with fosfomycin (associated with other antibiotic in 25 cases), 91% patients had a favorable outcome [231]. Optimal efficacy against P. aeruginosa is obtained with 16-24 g/day continuous infusion [226]. The disodium salt for iv administration contains 13.5 mEq of sodium per gram; caution is needed when administered to patients with heart insufficiency or under hemodialysis. Rapid high doses administration may produce hypopotassemia.
ANTIBIOTICS OF CHOICE FOR THE TREATMENT OF INFECTIONS CAUSED BY P. aeruginosa
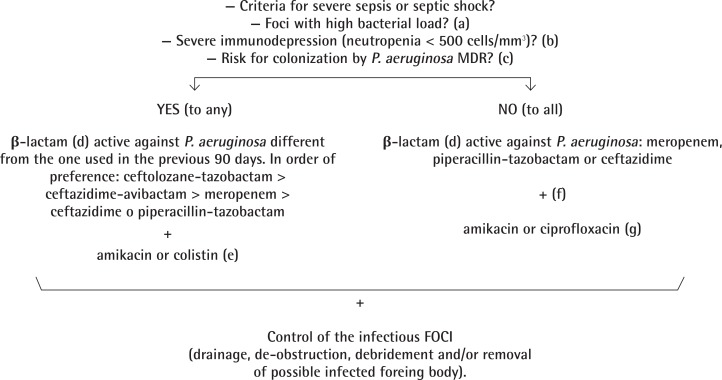
Empirical treatment (figure 1). Empirical selection of the most appropriate antibiotic treatment for a possible infection by P. aeruginosa is based on: a) presence of severity criteria and b) presence of risk factors for infection by a strains harboring resistance mechanisms. Severity criteria include criteria of severe sepsis or septic shock, severe immunodepression (especially neutropenia <500 cells/mm3), and infections involving high bacterial load, being not surgically controllable, as extensive pneumonia or pneumonia with cavitations/necrosis. The possibility of infection by a MDR strain should be considered in patients treated with a β-lactam active against P. aeruginosa within the previous 30-90 days, and in hospitalized patients admitted to units with a prevalence of MDR/XDR P. aeruginosa ≥10-20% for >3-5 days or which have history of previous colonization/infection by MDR/XDR P. aeruginosa. Among risk factors for selection of MDR/XDR strains we do not include exposure to β-lactams not active against P. aeruginosa, fluoroquinolones or aminoglycosides, since, under these conditions, the probability of colonization by strains resistant to antipseudomonal β-lactams is lower.
Figure 1.
Election of empirical antibiotic treatment active against P. aeruginosa
a) High bacterial load not surgically correctable (extensive pneumonia or pneumonia with necrosis/cavitation)
b) Includes neutropenia < 500 cells/mm3 and treatment with corticoid doses >20 mg/kg during >3 weeks
c)Treatment within the last 30-90 days with a β-lactam active against P. aeruginosa, admission during > 3-5 days in an hospitalization unit with a prevalence of MDR P. aeruginosa >10-20% or previous history of colonization/infection by MDR P. aeruginosa
d) Initial loading dose followed by high doses administered as continuous (or extended) infusion during the first 48-72 h
e) According to local epidemiology and susceptibility of possible previous isolates
f) Monotherapy in case of urinary tract infection or venous catheter infection. Association with amikacin or fluoroquinolone (levofloxacin or ciprofloxacin) in situations with high bacterial load (pneumonia)
g) Ciprofloxacin as treatment of choice for malignant external otitis, prostatitis and bronchial infection in patients with cystic fibrosis
If the patient fulfills any of the above criteria, treatment with a β-lactam different from the one received within the previous 90 days should be used. By order, preference should be given to 1.5-3 g/8 h iv ceftolozane-tazobactam, 2 g/8 h iv meropenem and 2 g/8 h iv ceftazidima or 4.5 g/6 h iv piperacillin-tazobactam. They should be administered as extended infusion (ceftolozane-tazobactam, meropenem) or continuous infusion with a loading dose (ceftazidime, piperacillin-tazobactam), together with a second antibiotic as 25 mg/kg/día iv ami-kacin as single daily dose or colistin (loading dose of 6-9 MU iv followed by 4.5 MU/12 h iv). For the election of the second antibiotic, it should be taken into account the epidemiology of the unit or hospital, and in the case of previous colonization/infection by P. aeruginosa, the susceptibility of the isolate.
If the patient does not fulfill severity criteria and has not risk factors for infection by a MDR/XDR P. aeruginosa strain, treatment could be initiated with a β-lactam (meropenem, ceftazidime or piperacillin-tazobactam) alone (urinary tract infection or venous catheter infection) or associated with amikacin or a fluoroquinolone (levofloxacin or ciprofloxacin) when bacterial load is higher (pneumonia).
In any of the previous situations, adequate surgical control of the infectious foci (drainage, de-obstruction, debridement) and/or removal of the infected foreign body (catheter or others) is critical.
Once culture results and antibiogram are available, treatment should be adjusted to the susceptibility of the isolated microorganism. If P. aeruginosa infection is confirmed and clinical evolution is favorable, from the 3rd day on treatment can be continued as monotherapy with a β-lactam chosen in accordance with the antibiogram. If all cultures are negative and clinical evolution is favorable, from the 3rd day on treatment can be continued as monotherapy with the initial β-lactam. If a rectal swab is available, and the patient is not colonized by P. aeruginosa, treatment continuation with a β-lactam not active against this microorganism can be considered.
Directed treatment. Election of antibiotic treatment when the susceptibility profile of the isolated P. aeruginosa strain is known, can be made according to the following recommendations:
a) Strain resistant to meropenem, ceftazidime and piperacillin-tazobactam, but susceptible to ceftolozane-tazobactam and ceftazidime-avibactam.
Against these strains, MIC of ceftolozane is often 2-4 mg/L. A possible treatment is 3 g/8 h iv ceftolozane-tazobactam. ESBL- or class A carbapenemase (GES o KPC)- producing P. aeruginosa strains can be resistant to ceftolozane-tazobactam, maintaining susceptibility to ceftazidime-avibactam that can be used at 2.5 g/8 h iv doses. If the strain produces a MBL-type carbapenemase, therapeutic options are limited to the use of associations of aztreonam with ceftazidime-avibactam with or without colistin.
b) Strain resistant to one of the β-lactams active against P. aeruginosa.
In case of resistance to ceftazidime and/or piperacillin-tazobactam, treatment can be ceftolozane-tazobactam, ceftazidime-avibactam or meropenem. The election depends on the risk of emergence of resistance, which in turn is related with the expected size of the bacterial load in the infectious foci. If the infection involves a high bacterial load (pneumonia), it is adviced to give priority to the antibiotic having the greatest probability to surpass the MPC, in this case, ceftolozane-tazobactam. Meropenem can be used for urinary tract infections, venous catheter infections or other infections with low bacterial load. In case of resistance to meropenem, treatment can be ceftolozane-tazobactam, ceftazidime or piperacillin-tazobactam. Again, the decision should be taken based on the bacterial inoculum size.
c) Strain susceptible to all β-lactams.
In this case, treatment options can be meropenem, ceftazidime or piperacillin-tazobactam. However, in VAP, severe pneumonia in COPD patients or in patients with bronchiectasis, and pneumonia with cavitation/necrosis, treatment with ceftolozane-tazobactam at 3 g/8 hours should be considered due to the high risk of resistance emergence.
In any of the three previous situations, in case of septic shock and in neutropenic patients, along the first 48-72 hours of directed treatment, an additional antibiotic (chosen according to the strain susceptibility) can be added: 400 mg/8 h ciprofloxacin or 8 mg/kg/day iv tobramycin (25-30 mg/kg/day amikacin in case of resistance to tobramycin). Occasionally, the resistance pattern makes necessary associations with colistin 4.5 MU/12 h or fosfomycin at 16-24 g/day iv dose administered as continuous infusion. Inhaled antibiotics (tobramycin, colistin or aztreonam) are reserved for cases of severe pneumonia or pneumonia caused by MDR P. aeruginosa strains. Nevertheless, their use should also be considered for infections caused by strains not harboring resistance mechanisms when the patient is intubated or suffers a relevant chronic bronchial pathology (GOLD-4 COPD, cystic fibrosis, bronchiectasis, bronchiolitis), circumstances in which the high bacterial load together with the limited antibiotic diffusion to bronchial secretions drives to an important risk for treatment failure and/or resistance emergence.
The treatment of CNS infections by P. aeruginosa poses two additional problems: antibiotic diffusion through the meninges and the risk of encephalopathy (seizures) associated with elevated doses of β-lactams (cefepime, ceftazidime or imipenem) and to lesser extent with fluoroquinolones. Treatment can be 2 g/8 h iv meropenem or ceftazidime [232], associated or not (according to the strain susceptibility) with 400 mg/8 h iv ciprofloxacin. Among other potentially efficacious antibiotics, if the strain is susceptible, they should be considered 16-24 g/day fosfomycin and intratecal or intraventricular administration [233,234] of 5-20 mg/day tobramycin, 30 mg/day amikacin or 10-20 mg/day colistin as colistimethate (1 mg of colistimethate = 12,500 UI) [235]. Up to now, no experience with the use of ceftolozane-tazobactam is available.
Table 4 shows initial doses of antibiotics active against P. aeruginosa for severe infections.
Table 4.
Initial posology of antibiotics with activity against P. aeruginosa for the treatment of severe infections
| Antibiotic | Posology |
|---|---|
| Ceftazidime | 1-2 g loading dose + 6 g/24 h CI |
| Ceftazidime-avibactam | 2/0.5 g/8 h EI |
| Piperacillin-tazobactam | 2/0.25 g loading dose + 16/2 g/24 h CI |
| Ceftolozane-tazobactam | 1/0.5 or 2/1 g/8 h EI |
| Aztreonam | 1-2 g loading dose + 6 g/24 h CI |
| Meropenem | 1-2 g loading dose + 2 g/8 h EI |
| Fosfomycin | 2-4 g loading dose + 16-24 g/24 h CI |
| Colistin | 6-9 MU loading dose + 4.5 MU/12 h |
| Ciprofloxacin | 400 mg/8 h in 30-60 minutes |
| Levofloxacin | 500 mg/12 h in 30-60 minutes |
| Tobramycin | 8 mg/kg/24 h in 60 minutes |
| Amikacin | 25 mg/kg/24 h in 60 minutes |
CI: continuous infusion; EI: extended infusion (3-4 h); MU: million units
REFERENCES
- 1.Segata N, Haake SK, Mannon P et al. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol 2012; 13 (6):R42 DOI: 10.1186/gb-2012-13-6-r42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulsson M, Granrot A, Ahl J et al. Antimicrobial combination treatment including ciprofloxacin decreased the mortality rate of Pseudomonas aeruginosa bacteraemia: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2017;36:1187-1196. DOI: 10.1007/s10096-017-2907-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheong HS, Kang CI, Wi YM et al. Clinical Significance and Predictors of Community-Onset Pseudomonas aeruginosa Bacteremia. Am J Med 2008; 121(8):709-714. DOI: 10.1016/j.amjmed.2008.03.034 [DOI] [PubMed] [Google Scholar]
- 4.Kang C, Kim S, Kim H et al. Pseudomonas aeruginosa Bacteremia: Risk Factors for Mortality and Influence of Delayed Receipt of Effective Antimicrobial Therapy on Clinical Outcome. Clin Infect Dis 2003; 37(6):745-751. DOI: 10.1086/377200 [DOI] [PubMed] [Google Scholar]
- 5.Siegman-Igra Y, Ravona R, Primerman H, Giladi M. Pseudomonas aeruginosa bacteremia: an analysis of 123 episodes, with particular emphasis on the effect of antibiotic therapy. Int J Infect Dis 1998; 2(4):211-215. [DOI] [PubMed] [Google Scholar]
- 6.Suarez C, Pena C, Gavalda L et al. Influence of carbapenem resistance on mortality and the dynamics of mortality in Pseudomonas aeruginosa bloodstream infection. Int J Infect Dis 2010; 14 Suppl 3:e73-e78. DOI: 10.1016/j.ijid.2009.11.019 [DOI] [PubMed] [Google Scholar]
- 7.Pena C, Suarez C, Gozalo M et al. Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob Agents Chemother 2012; 56(3):1265-1272. DOI: 10.1128/AAC.05991-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morata L, Cobos-Trigueros N, Martínez JA et al. Influence of Multi-drug Resistance and Appropriate Empirical Therapy on the 30-Day Mortality Rate of Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother 2012; 56(9):4833-4837. DOI: 10.1128/AAC.00750-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pena C, Cabot G, Gomez-Zorrilla S et al. Influence of virulence genotype and resistance profile in the mortality of Pseudomonas aeruginosa bloodstream infections. Clin Infect Dis 2015; 60(4):539-548. DOI: 10.1093/cid/ciu866 [DOI] [PubMed] [Google Scholar]
- 10.Thaden JT, Park LP, Maskarinec SA, Ruffin F, Fowler VG Jr., van DD. Increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa as compared to other bacteria: Results of a 13-year prospective cohort study. Antimicrob Agents Chemother 2017; 61(6). pii: e02671-16. DOI: 10.1128/AAC.02671-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumbarello M, Repetto E, Trecarichi EM et al. Multidrug-resistant Pseudomonas aeruginosa bloodstream infections: risk factors and mortality. Epidemiol Infect 2011; 139(11):1740-1749. DOI: 10.1017/S0950268810003055 [DOI] [PubMed] [Google Scholar]
- 12.Micek ST, Wunderink RG, Kollef MH et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care 2015; 19:219 DOI: 10.1186/s13054-015-0926-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tumbarello M, De Pascale G, Trecarichi EM et al. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med 2013; 39(4):682-692. DOI: 10.1007/s00134-013-2828-9 [DOI] [PubMed] [Google Scholar]
- 14.Magiorakos AP, Srinivasan A, Carey RB et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18(3):268-281. DOI: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 15.Juan C, Zamorano L, Perez JL, Ge Y, Oliver A. Activity of a new antipseudomonal cephalosporin, CXA-101 (FR264205), against carbapenem-resistant and multidrug-resistant Pseudomonas aeruginosa clinical strains. Antimicrob Agents Chemother 2010; 54(2):846-851. DOI: 10.1128/AAC.00834-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sader HS, Farrell DJ, Castanheira M, Flamm RK, Jones RN. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011-2012). J Antimicrob Chemother 2014; 69(10):2713-2722. DOI: 10.1093/jac/dku184 [DOI] [PubMed] [Google Scholar]
- 17.Nichols WW, de Jonge BL, Kazmierczak KM, Karlowsky JA, Sahm DF. In Vitro Susceptibility of Global Surveillance Isolates of Pseudomonas aeruginosa to Ceftazidime-Avibactam (INFORM 2012 to 2014). Antimicrob Agents Chemother 2016; 60(8):4743-4749. DOI: 10.1128/AAC.00220-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huband MD, Castanheira M, Flamm RK, Farrell DJ, Jones RN, Sader HS. In vitro activity of ceftazidime-avibactam against contemporary Pseudomonas aeruginosa isolates from United States medical centers by Census region (2014). Antimicrob Agents Chemother 2016; 60(4):2537-2541. DOI: 10.1128/AAC.03056-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canton R, Maiz L, Escribano A et al. Spanish consensus on the prevention and treatment of Pseudomonas aeruginosa bronchial infections in cystic fibrosis patients. Arch Bronconeumol 2015; 51(3):140-150. DOI: 10.1016/j.arbres.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 20.Polverino E, Goeminne PC, McDonnell MJ et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50(3). DOI: 10.1183/13993003.50017-2017 [DOI] [PubMed] [Google Scholar]
- 21.Lister PD, Wolter DJ, Hanson ND. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin Microbiol Rev 2009; 22(4):582-610. DOI: 10.1128/CMR.00040-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livermore DM. Interplay of impermeability and chromosomal beta-lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 1992; 36(9):2046-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XZ, Plésiat P, Nikaido H. The Challenge of Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Clin Microbiol Rev 2015; 28(2):337-418. DOI: 10.1128/CMR.00117-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hocquet D, Vogne C, El GF et al. MexXY-OprM efflux pump is necessary for a adaptive resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother 2003; 47(4):1371-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skiada A, Markogiannakis A, Plachouras D, Daikos GL. Adaptive resistance to cationic compounds in Pseudomonas aeruginosa. Int J Antimicrob Agents 2011; 37(3):187-193. DOI: 10.1016/j.ijantimicag.2010.11.019 [DOI] [PubMed] [Google Scholar]
- 26.Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol 2011; 2:65 DOI: 10.3389/fmicb.2011.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Causape C, Rojo-Molinero E, Macia MD, Oliver A. The problems of antibiotic resistance in cystic fibrosis and solutions. Expert Rev Respir Med 2015; 9(1):73-88. DOI: 10.1586/17476348.2015.995640 [DOI] [PubMed] [Google Scholar]
- 28.Zhao X, Drlica K. Restricting the selection of antibiotic-resistant mutant bacteria: measurement and potential use of the mutant selection window. J Infect Dis 2002; 185(4):561-565. DOI: 10.1086/338571 [DOI] [PubMed] [Google Scholar]
- 29.Riera E, Macia MD, Mena A et al. Anti-biofilm and resistance suppression activities of CXA-101 against chronic respiratory infection phenotypes of Pseudomonas aeruginosa strain PAO1. J Antimicrob Chemother 2010; 65(7):1399-1404. DOI: 10.1093/jac/dkq143 [DOI] [PubMed] [Google Scholar]
- 30.Cabot G, Ocampo-Sosa AA, Tubau F et al. Overexpression of AmpC and Efflux Pumps in Pseudomonas aeruginosa Isolates from Bloodstream Infections: Prevalence and Impact on Resistance in a Spanish Multicenter Study. Antimicrob Agents Chemother 2011; 55(5):1906-1911. DOI: 10.1128/AAC.01645-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabot G, Bruchmann S, Mulet X et al. Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC. Antimicrob Agents Chemother 2014; 58(6):3091-3099. DOI: 10.1128/AAC.02462-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torrens G, Cabot G, Ocampo-Sosa AA et al. Activity of Ceftazidime-Avibactam against Clinical and Isogenic Laboratory Pseudomonas aeruginosa Isolates Expressing Combinations of Most Relevant beta-Lactam Resistance Mechanisms. Antimicrob Agents Chemother 2016; 60(10):6407-6410. DOI: 10.1128/AAC.01282-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moya B, Zamorano L, Juan C, Perez JL, Ge Y, Oliver A. Activity of a new cephalosporin, CXA-101 (FR264205), against beta-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob Agents Chemother 2010; 54(3):1213-1217. DOI: 10.1128/AAC.01104-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riera E, Cabot G, Mulet X et al. Pseudomonas aeruginosa carbapenem resistance mechanisms in Spain: impact on the activity of imipenem, meropenem and doripenem. J Antimicrob Chemother 2011; 66(9):2022-2027. DOI: 10.1093/jac/dkr232 [DOI] [PubMed] [Google Scholar]
- 35.Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 2014; 5:643 DOI: 10.3389/fmicb.2014.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel G, Bonomo RA. Status report on carbapenemases: challenges and prospects. Expert Rev Anti Infect Ther 2011; 9(5):555-570. DOI: 10.1586/eri.11.28 [DOI] [PubMed] [Google Scholar]
- 37.Oliver A, Mulet X, Lopez-Causape C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat 2015; 21-22:41-59. DOI: 10.1016/j.drup.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 38.Del Barrio-Tofino E, Lopez-Causape C, Cabot G et al. Genomics and Susceptibility Profiles of Extensively Drug-Resistant (XDR) Pseudomonas aeruginosa from Spain. Antimicrob Agents Chemother 2017; 61(11). pii: e01589-17. DOI: 10.1128/AAC.01589-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacVane SH, Kuti JL, Nicolau DP. Clinical Pharmacodynamics of Antipseudomonal Cephalosporins in Patients with Ventilator-Associated Pneumonia. Antimicrob Agents Chemother 2014; 58(3):1359-1364. DOI: 10.1128/AAC.01463-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 2008; 31(4):345-351. DOI: 10.1016/j.ijantimicag.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 41.Tam VH, McKinnon PS, Akins RL, Rybak MJ, Drusano GL. Pharmaco-dynamics of cefepime in patients with Gram-negative infections. J Antimicrob Chemother 2002; 50(3):425-428. [DOI] [PubMed] [Google Scholar]
- 42.Bergen PJ, Bulitta JB, Kirkpatrick CM et al. Effect of different renal function on antibacterial effects of piperacillin against Pseudomonas aeruginosa evaluated via the hollow-fibre infection model and mechanism-based modelling. J Antimicrob Chemother 2016; 71(9):2509-2520. DOI: 10.1093/jac/dkw153 [DOI] [PubMed] [Google Scholar]
- 43.Tam VH, Chang KT, Zhou J et al. Determining beta-lactam exposure threshold to suppress resistance development in Gram-negative bacteria. J Antimicrob Chemother 2017; 72(5):1421-1428. DOI: 10.1093/jac/dkx001 [DOI] [PubMed] [Google Scholar]
- 44.Nicasio AM, Ariano RE, Zelenitsky SA et al. Population Pharmacokinetics of High-Dose, Prolonged-Infusion Cefepime in Adult Critically Ill Patients with Ventilator-Associated Pneumonia. Anti-microb Agents Chemother 2009; 53(4):1476-1481. DOI: 10.1128/AAC.01141-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buijk SL, Gyssens IC, Mouton JW, Van Vliet A, Verbrugh HA, Bruining HA. Pharmacokinetics of ceftazidime in serum and peritoneal exudate during continuous versus intermittent administration to patients with severe intra-abdominal infections. J Antimicrob Chemother 2002; 49(1):121-128. [DOI] [PubMed] [Google Scholar]
- 46.Boselli EM, Breilh DP, Rimmele TM et al. Alveolar concentrations of piperacillin/tazobactam administered in continuous infusion to patients with ventilator-associated pneumonia. Crit Care Med 2008; 36(5):1500-1506. DOI: 10.1097/CCM.0b013e318170ba21 [DOI] [PubMed] [Google Scholar]
- 47.Bauer KA, West JE, O’Brien JM, Goff DA. Extended-Infusion Cefepime Reduces Mortality in Patients with Pseudomonas aeruginosa Infections. Antimicrob Agents Chemother 2013; 57(7):2907-2912. DOI: 10.1128/AAC.02365-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lodise TP Jr Lomaestro B, Drusano GL. Piperacillin-Tazobactam for Pseudomonas aeruginosa Infection: Clinical Implications of an Extended-Infusion Dosing Strategy. Clin Infect Dis 2007; 44(3):357-363. DOI: 10.1086/510590 [DOI] [PubMed] [Google Scholar]
- 49.Prescott WA Jr Gentile AE, Nagel JL, Pettit RS. Continuous-infusion antipseudomonal Beta-lactam therapy in patients with cystic fibrosis. P & T 2011; 36(11):723-763. [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts JA, Kirkpatrick CMJ, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 2009; 64(1):142-150. DOI: 10.1093/jac/dkp139 [DOI] [PubMed] [Google Scholar]
- 51.Felton TW, Hope WW, Lomaestro BM et al. Population Pharmacokinetics of Extended-Infusion Piperacillin-Tazobactam in Hospitalized Patients with Nosocomial Infections. Antimicrob Agents Chemother 2012; 56(8):4087-4094. DOI: 10.1128/AAC.00521-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taccone FS, Cotton Fdr, Roisin S, Vincent JL, Jacobs Fdr. Optimal Meropenem Concentrations To Treat Multidrug-Resistant Pseudomonas aeruginosa Septic Shock. Antimicrob Agents Chemother 2012; 56(4):2129-2131. DOI: 10.1128/AAC.06389-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robaux MA, Dube L, Caillon J et al. In vivo efficacy of continuous infusion versus intermittent dosing of ceftazidime alone or in combination with amikacin relative to human kinetic profiles in a Pseudomonas aeruginosa rabbit endocarditis model. J Antimicrob Chemother 2001; 47(5):617-622. [DOI] [PubMed] [Google Scholar]
- 54.Navas D, Caillon J, Gras-Le Guen C et al. Comparison of in vivo intrinsic activity of cefepime and imipenem in a Pseudomonas aeruginosa rabbit endocarditis model: effect of combination with tobramycin simulating human serum pharmacokinetics. J Antimicrob Chemother 2004; 54(4):767-771. DOI: 10.1093/jac/dkh381 [DOI] [PubMed] [Google Scholar]
- 55.Alou L, Aguilar L, Sevillano D et al. Is there a pharmacodynamic need for the use of continuous versus intermittent infusion with ceftazidime against Pseudomonas aeruginosa? An in vitro pharmacodynamic model. J Antimicrob Chemother 2005; 55(2):209-213. DOI: 10.1093/jac/dkh536 [DOI] [PubMed] [Google Scholar]
- 56.Tam VH, Schilling AN, Neshat S, Poole K, Melnick DA, Coyle EA. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother 2005; 49(12):4920-4927. DOI: 10.1128/AAC.49.12.4920-4927.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tessier PR, Nicolau DP, Onyeji CO, Nightingale CH. Pharmacodynamics of intermittent- and continuous-infusion cefepime alone and in combination with once-daily tobramycin against Pseudomonas aeruginosa in an in vitro infection model. Chemotherapy 1999; 45(4):284-295. DOI: 10.1159/000007198 [DOI] [PubMed] [Google Scholar]
- 58.Dulhunty JM, Roberts JA, Davis JS et al. Continuous infusion of Beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis 2013; 56(2):236-244. DOI: 10.1093/cid/cis856 [DOI] [PubMed] [Google Scholar]
- 59.Chytra I, Stepan M, Benes J et al. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open-label controlled trial. Crit Care 2012; 16(3):R113 DOI: 10.1186/cc11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicasio AM, Eagye KJ, Nicolau DP et al. Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J Crit Care 2010; 25(1):69-77. DOI: 10.1016/j.jcrc.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 61.Paterson DL. Resistance in gram-negative bacteria: enterobacteriaceae. Am J Med 2006; 119(6 Suppl 1):S62-S70. DOI: 10.1016/j.amjmed.2006.03.013 [DOI] [PubMed] [Google Scholar]
- 62.Lorente L, Jiménez A, Martín MM, Iribarren JL, Jiménez JJ, Mora ML. Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int J Antimicrob Agents 2009; 33(5):464-468. DOI: 10.1016/j.ijantimicag.2008.10.025 [DOI] [PubMed] [Google Scholar]
- 63.Rafati MR, Rouini MR, Mojtahedzadeh M et al. Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int J Antimicrob Agents 2006; 28(2):122-127. DOI: 10.1016/j.ijantimicag.2006.02.020 [DOI] [PubMed] [Google Scholar]
- 64.Yost RJ, Cappelletty DM. The Retrospective Cohort of Extended-In-fusion Piperacillin-Tazobactam (RECEIPT) study: a multicenter study. Pharmacotherapy 2011; 31(8):767-775. DOI: 10.1592/phco.31.8.767 [DOI] [PubMed] [Google Scholar]
- 65.Grant EM, Kuti JL, Nicolau DP, Nightingale C, Quintiliani R. Clinical efficacy and pharmacoeconomics of a continuous-infusion piperacillin-tazobactam program in a large community teaching hospital. Pharmacotherapy 2002; 22(4):471-483. [DOI] [PubMed] [Google Scholar]
- 66.Lorente L, Lorenzo L, Martin MM, Jimenez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother 2006; 40(2):219-223. DOI: 10.1345/aph.1G467 [DOI] [PubMed] [Google Scholar]
- 67.Hanes SD, Wood GC, Herring V et al. Intermittent and continuous ceftazidime infusion for critically ill trauma patients. Am J Surg 2000; 179(6):436-440. [DOI] [PubMed] [Google Scholar]
- 68.Dulhunty JM, Roberts JA, Davis JS et al. A Multicenter Randomized Trial of Continuous versus Intermittent beta-Lactam Infusion in Severe Sepsis. Am J Respir Crit Care Med 2015; 192(11):1298-1305. DOI: 10.1164/rccm.201505-0857OC [DOI] [PubMed] [Google Scholar]
- 69.Patel GW, Patel N, Lat A et al. Outcomes of extended infusion piperacillin/tazobactam for documented Gram-negative infections. Diagn Microbiol Infect Dis 2009; 64(2):236-240. DOI: 10.1016/j.diagmicrobio.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 70.Abdul-Aziz MH, Dulhunty JM, Bellomo R, Lipman J, Roberts JA. Continuous beta-lactam infusion in critically ill patients: the clinical evidence. Ann Intensive Care 2012; 2(1):37 DOI: 10.1186/2110-5820-2-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. Clinical Outcomes With Extended or Continuous Versus Short-term Intravenous Infusion of Carbapenems and Piperacillin/Tazobactam: A Systematic Review and Meta-analysis. Clin Infect Dis 2013; 56(2):272-282. DOI: 10.1093/cid/cis857 [DOI] [PubMed] [Google Scholar]
- 72.Roberts JA, Abdul-Aziz MH, Davis JS et al. Continuous versus Intermittent beta-Lactam Infusion in Severe Sepsis. A Meta-analysis of Individual Patient Data from Randomized Trials. Am J Respir Crit Care Med 2016; 194(6):681-691. DOI: 10.1164/rccm.201601-0024OC [DOI] [PubMed] [Google Scholar]
- 73.Teo J, Liew Y, Lee W, Kwa AL. Prolonged infusion versus intermittent boluses of beta-lactam antibiotics for treatment of acute infections: a meta-analysis. Int J Antimicrob Agents 2014; 43(5):403-411. DOI: 10.1016/j.ijantimicag.2014.01.027 [DOI] [PubMed] [Google Scholar]
- 74.Tamma PD, Putcha N, Suh YD, Van Arendonk KJ, Rinke ML. Does prolonged beta-lactam infusions improve clinical outcomes compared to intermittent infusions? A meta-analysis and systematic review of randomized, controlled trials. BMC Infect Dis 2011; 11:181 DOI: 10.1186/1471-2334-11-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leisman D, Huang V, Zhou Q et al. Delayed Second Dose Antibiotics for Patients Admitted From the Emergency Department With Sepsis: Prevalence, Risk Factors, and Outcomes. Crit Care Med 2017; 45(6):956-965. DOI: 10.1097/CCM.0000000000002377 [DOI] [PubMed] [Google Scholar]
- 76.Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 1987; 155(1):93-99. [DOI] [PubMed] [Google Scholar]
- 77.Update on good use of injectable aminoglycosides, gentamycin, tobramycin, netilmycin, amikacin. Pharmacological properties, indications, dosage, and mode of administration, treatment monitoring. Med Mal Infect 2012; 42(7):301-308. DOI: 10.1016/j.medmal.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 78.Tam VH, Schilling AN, Melnick DA, Coyle EA. Comparison of β-lactams in counter-selecting resistance of Pseudomonas aeruginosa. Diag Microbiol Infect Dis 2005; 52(2):145-151. DOI: 10.1016/j.diagmicrobio.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 79.Drusano GL, Fregeau C, Liu W, Brown DL, Louie A. Impact of Burden on Granulocyte Clearance of Bacteria in a Mouse Thigh Infection Model. Antimicrob Agents Chemother 2010; 54(10):4368-4372. DOI: 10.1128/AAC.00133-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drusano GL, VanScoy B, Liu W, Fikes S, Brown D, Louie A. Saturability of Granulocyte Kill of Pseudomonas aeruginosa in a Murine Model of Pneumonia. Antimicrob Agents Chemother 2011; 55(6):2693-2695. DOI: 10.1128/AAC.01687-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drusano GL, Liu W, Fikes S et al. Interaction of drug- and granulocyte-mediated killing of Pseudomonas aeruginosa in a murine pneumonia model. J Infect Dis 2014; 210(8):1319-1324. DOI: 10.1093/infdis/jiu237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Breidenstein EB, de lF-N, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol 2011; 19(8):419-426. DOI: 10.1016/j.tim.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 83.Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 2000; 288(5469):1251-1254. [DOI] [PubMed] [Google Scholar]
- 84.Hogardt M, Hoboth C, Schmoldt S, Henke C, Bader L, Heesemann J. Stage-Specific Adaptation of Hypermutable Pseudomonas aeruginosa Isolates during Chronic Pulmonary Infection in Patients with Cystic Fibrosis. J Infect Dis 2007; 195(1):70-80. DOI: 10.1086/509821 [DOI] [PubMed] [Google Scholar]
- 85.Oliver A. Clinical relevance of Pseudomonas aeruginosa hypermu-tation in cystic fibrosis chronic respiratory infection. J Cyst Fibros 2015; 14(4):e1-e2. DOI: 10.1016/j.jcf.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 86.Cabot G, Zamorano L, Moyà B et al. Evolution of Pseudomonas aeruginosa Antimicrobial Resistance and Fitness under Low and High Mutation Rates. Antimicrob Agents Chemother 2016; 60(3):1767-1778. DOI: 10.1128/AAC.02676-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waine DJ, Honeybourne D, Smith EG, Whitehouse JL, Dowson CG. Association between Hypermutator Phenotype, Clinical Variables, Mucoid Phenotype, and Antimicrobial Resistance in Pseudomonas aeruginosa. J Clin Microbiol 2008; 46(10):3491-3493. DOI: 10.1128/JCM.00357-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mouton JW. Combination therapy as a tool to prevent emergence of bacterial resistance. Infection 1999; 27 Suppl 2:S24-S28. [DOI] [PubMed] [Google Scholar]
- 89.Rees VE, Bulitta JB, Oliver A et al. Resistance suppression by high-intensity, short-duration aminoglycoside exposure against hypermutable and non-hypermutable Pseudomonas aeruginosa. J Antimicrob Chemother 2016; 71(11):3157-3167. DOI: 10.1093/jac/dkw297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rees VE, Bulitta JB, Nation RL, Tsuji BT, Sörgel F, Landersdorfer CB. Shape does matter: short high-concentration exposure minimizes resistance emergence for fluoroquinolones in Pseudomonas aeruginosa. J Antimicrob Chemother 2015; 70(3):818-826. DOI: 10.1093/jac/dku437 [DOI] [PubMed] [Google Scholar]
- 91.Garnacho-Montero J, Sa-Borges M, Sole-Violan J et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med 2007; 35(8):1888-1895. DOI: 10.1097/01.CCM.0000275389.31974.22 [DOI] [PubMed] [Google Scholar]
- 92.Bodey GP, Jadeja L, Elting L. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med 1985; 145(9):1621-1629. PMID: 3927867 [DOI] [PubMed] [Google Scholar]
- 93.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 2005; 49(4):1306-1311. DOI: 10.1128/AAC.49.4.1306-1311.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoon YK, Kim HA, Ryu SY et al. Tree-structured survival analysis of patients with Pseudomonas aeruginosa bacteremia: A multi-center observational cohort study. Diagn Microbiol Infect Dis 2017; 87(2):180-187. DOI: 10.1016/j.diagmicrobio.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 95.Al Hasan MN, Wilson JW, Lahr BD, Eckel-Passow JE, Baddour LM. Incidence of Pseudomonas aeruginosa Bacteremia: A Population-Based Study. Am J Med 2008; 121(8):702-708. DOI: 10.1016/j.amjmed.2008.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schechner V, Nobre V, Kaye K et al. Gram-Negative Bacteremia upon Hospital Admission: When Should Pseudomonas aeruginosa Be Suspected? Clin Infect Dis 2009; 48(5):580-586. DOI: 10.1086/596709 [DOI] [PubMed] [Google Scholar]
- 97.Vidal F, Mensa J, Almela M et al. Bacteraemia in adults due to glucose non-fermentative Gram-negative bacilli other than P. aeruginosa. QJM 2003; 96(3):227-234. [DOI] [PubMed] [Google Scholar]
- 98.Paul M, Leibovici L. Editorial Commentary: Combination Therapy for Pseudomonas aeruginosa Bacteremia: Where Do We Stand? Clin Infect Dis 2013; 57(2):217-220. DOI: 10.1093/cid/cit220 [DOI] [PubMed] [Google Scholar]
- 99.Vardakas KZ, Tansarli GS, Bliziotis IA, Falagas ME. beta-Lactam plus aminoglycoside or fluoroquinolone combination versus beta-lactam monotherapy for Pseudomonas aeruginosa infections: a meta-analysis. Int J Antimicrob Agents 2013; 41(4):301-310. DOI: 10.1016/j.ijantimicag.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 100.Vidal F, Mensa J, Almela M et al. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch Intern Med 1996; 156(18):2121-2126. [PubMed] [Google Scholar]
- 101.Chatzinikolaou I, Abi-Said D, Bodey GP, Rolston KV, Tarrand JJ, Samonis G. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: Retrospective analysis of 245 episodes. Arch Intern Med 2000; 160(4):501-509. [DOI] [PubMed] [Google Scholar]
- 102.Bliziotis IA, Petrosillo N, Michalopoulos A, Samonis G, Falagas ME. Impact of definitive therapy with beta-lactam monotherapy or combination with an aminoglycoside or a quinolone for Pseudomonas aeruginosa bacteremia. PLoS ONE 2011; 6(10):e26470 DOI: 10.1016/j.msard.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pena C, Suarez C, Ocampo-Sosa A et al. Effect of Adequate Single-Drug vs Combination Antimicrobial Therapy on Mortality in Pseudomonas aeruginosa Bloodstream Infections: A Post Hoc Analysis of a Prospective Cohort. Clin Infect Dis 2013; 57(2):208-216. DOI: 10.1093/cid/cit223 [DOI] [PubMed] [Google Scholar]
- 104.Bowers DR, Liew YX, Lye DC, Kwa AL, Hsu LY, Tam VH. Outcomes of Appropriate Empiric Combination versus Monotherapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother 2013; 57(3):1270-1274. DOI: 10.1128/AAC.02235-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Planquette B, Timsit JF, Misset BY et al. Pseudomonas aeruginosa ventilator-associated pneumonia. predictive factors of treatment failure. Am J Respir Crit Care Med 2013; 188(1):69-76. DOI: 10.1164/rccm.201210-1897OC [DOI] [PubMed] [Google Scholar]
- 106.Cometta A, Baumgartner JD, Lew D et al. Prospective randomized comparison of imipenem monotherapy with imipenem plus netilmicin for treatment of severe infections in nonneutropenic patients. Antimicrob Agents Chemother 1994; 38(6):1309-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu Y, Li L, Li W et al. Combination antibiotic therapy versus mono-therapy for Pseudomonas aeruginosa bacteraemia: A meta-analysis of retrospective and prospective studies. Int J Antimicrob Agents 2013; 42(6):492-496. DOI: 10.1016/j.ijantimicag.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 108.Park SY, Park HJ, Moon SM et al. Impact of adequate empirical combination therapy on mortality from bacteremic Pseudomonas aeruginosa pneumonia. BMC Infect Dis 2012; 12:308 DOI: 10.1186/1471-2334-12-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim YJ, Jun YH, Kim YR et al. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia; retrospective study of impact of combination antimicrobial therapy. BMC Infect Dis 2014; 14:161 DOI: 10.1186/1471-2334-14-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome cor-relations in a prospective study of 200 patients. Am J Med 1989; 87(5):540-546. [DOI] [PubMed] [Google Scholar]
- 111.Leibovici L, Paul M, Poznanski O et al. Monotherapy versus beta-lactam-aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob Agents Chemother 1997; 41(5):1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith AL, Doershuk C, Goldmann D et al. Comparison of a beta-lactam alone versus beta-lactam and an aminoglycoside for pulmonary exacerbation in cystic fibrosis. J Pediatr 1999; 134(4):413-421. [DOI] [PubMed] [Google Scholar]
- 113.Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis 2004; 4(8):519-527. DOI: 10.1016/S1473-3099(04)01108-9 [DOI] [PubMed] [Google Scholar]
- 114.Vidal L, Gafter-Gvili A, Borok S, Fraser A, Leibovici L, Paul M. Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chem-other 2007; 60(2):247-257. DOI: 10.1093/jac/dkm193 [DOI] [PubMed] [Google Scholar]
- 115.Bulitta JB, Ly NS, Landersdorfer CB et al. Two mechanisms of killing of Pseudomonas aeruginosa by tobramycin assessed at multiple inocula via mechanism-based modeling. Antimicrob Agents Chem-other 2015; 59(4):2315-2327. DOI: 10.1128/AAC.04099-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fernández La, Hancock REW. Adaptive and Mutational Resistance: Role of Porins and Efflux Pumps in Drug Resistance. Clin Microbiol Rev 2012; 25(4):661-681. DOI: 10.1128/CMR.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fernandez L, Breidenstein EB, Hancock RE. Creeping baselines and adaptive resistance to antibiotics. Drug Resist Updat 2011; 14(1):1-21. DOI: 10.1016/j.drup.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 118.Kashuba AD, Nafziger AN, Drusano GL, Bertino JS Jr. Optimizing Aminoglycoside Therapy for Nosocomial Pneumonia Caused by Gram-Negative Bacteria. Antimicrob Agents Chemother 1999; 43(3):623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zelenitsky SA, Harding GK, Sun S, Ubhi K, Ariano RE. Treatment and outcome of Pseudomonas aeruginosa bacteraemia: an antibiotic pharmacodynamic analysis. J Antimicrob Chemother 2003; 52(4):668-674. DOI: 10.1093/jac/dkg403 [DOI] [PubMed] [Google Scholar]
- 120.Triginer C, Izquierdo I, Fernandez R et al. Gentamicin volume of distribution in critically ill septic patients. Intensive Care Med 1990; 16(5):303-306. [DOI] [PubMed] [Google Scholar]
- 121.Triginer C, Izquierdo I, Fernandez R et al. Changes in gentamicin pharmacokinetic profiles induced by mechanical ventilation. Eur J Clin Pharmacol 1991; 40(3):297-302. [DOI] [PubMed] [Google Scholar]
- 122.Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 1995; 39(3):650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Udy AA, Varghese JM, Altukroni M et al. Sub-therapeutic initial beta-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest 2012; 142(1):30-39. DOI: 10.1378/chest.11-1671 [DOI] [PubMed] [Google Scholar]
- 124.Udy AA, Roberts JA, Lipman J. Implications of augmented renal clearance in critically ill patients. Nat Rev Nephrol 2011; 7(9):539-543. DOI: 10.1038/nrneph.2011.92 [DOI] [PubMed] [Google Scholar]
- 125.Taccone FS, Laterre PF, Dugernier T et al. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care 2010; 14(4):R126 DOI: 10.1186/cc9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roberts JA, Ulldemolins M, Roberts MS et al. Therapeutic drug monitoring of [beta]-lactams in critically ill patients: proof of concept. Int J Antimicrobial Agents 2010; 36(4):332-339. DOI: 10.1016/j.ijantimicag.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 127.Roger C, Nucci B, Molinari N et al. Standard dosing of amikacin and gentamicin in critically ill patients results in variable and subtherapeutic concentrations. Int J Antimicrob Agents 2015; 46(1):21-27. DOI: 10.1016/j.ijantimicag.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 128.Taccone FS, Laterre PF, Spapen H et al. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care 2010; 14(2):R53 DOI: 10.1186/cc8945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Blackburn LM, Tverdek FP, Hernandez M, Bruno JJ. First-dose pharmacokinetics of aminoglycosides in critically ill haematological malignancy patients. Int J Antimicrob Agents 2015; 45(1):46-53. DOI: 10.1016/j.ijantimicag.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 130.Zeitany RG, El Saghir NS, Santhosh-Kumar CR, Sigmon MA. Increased aminoglycoside dosage requirements in hematologic malignancy. Antimicrob Agents Chemother 1990; 34(5):702-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Montmollin E, Bouadma L, Gault N et al. Predictors of insufficient amikacin peak concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med 2014; 40(7):998-1005. DOI: 10.1007/s00134-014-3276-x [DOI] [PubMed] [Google Scholar]
- 132.Hodiamont CJ, Juffermans NP, Bouman CS, de J, Mathot RA, van Hest RM. Determinants of gentamicin concentrations in critically ill patients: a population pharmacokinetic analysis. Int J Antimicrob Agents 2017; 49(2):204-211. DOI: 10.1016/j.ijantimicag.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 133.Bracco D, Landry C, Dubois MJ, Eggimann P. Pharmacokinetic variability of extended interval tobramycin in burn patients. Burns 2008; 34(6):791-796. DOI: 10.1016/j.burns.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 134.Robert J, Pean Y, Alfandari S et al. Application of guidelines for aminoglycosides use in French hospitals in 2013-2014. Eur J Clin Microbiol Infect Dis 2017;36:1083-1090. DOI: 10.1007/s10096-016-2892-5 [DOI] [PubMed] [Google Scholar]
- 135.Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. Back to the Future: Using Aminoglycosides Again and How to Dose Them Optimally. Clin Infect Dis 2007; 45(6):753-760. DOI: 10.1086/520991 [DOI] [PubMed] [Google Scholar]
- 136.Mombelli G, Coppens L, Thys JP, Klastersky J. Anti-Pseudomonas activity in bronchial secretions of patients receiving amikacin or tobramycin as a continuous infusion. Antimicrob Agents Chemother 1981; 19(1):72-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rodvold KA, George JM, Yoo L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 2011; 50(10):637-664. DOI: 10.2165/11594090-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 138.Boselli E, Breilh D, Djabarouti S et al. Reliability of mini-broncho-alveolar lavage for the measurement of epithelial lining fluid concentrations of tobramycin in critically ill patients. Intensive Care Med 2007; 33(9):1519-1523. DOI: 10.1007/s00134-007-0688-x [DOI] [PubMed] [Google Scholar]
- 139.Carcas AJ, Garcia-Satue JL, Zapater P, Frias-Iniesta J. Tobramycin penetration into epithelial lining fluid of patients with pneumonia. Clin Pharmacol Ther 1999; 65(3):245-250. DOI: 10.1016/S0009-9236(99)70103-7 [DOI] [PubMed] [Google Scholar]
- 140.Panidis D, Markantonis SL, Boutzouka E, Karatzas S, Baltopoulos G. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest 2005; 128(2):545-552. DOI: 10.1378/chest.128.2.545 [DOI] [PubMed] [Google Scholar]
- 141.Sulaiman H, Abdul-Aziz MH, Roberts JA. Pharmacokinetic/Pharmacodynamics-Optimized Antimicrobial Therapy in Patients with Hospital-Acquired Pneumonia/Ventilator-Associated Pneumonia. Semin Respir Crit Care Med 2017; 38(3):271-286. DOI: 10.1055/s-0037-1602716 [DOI] [PubMed] [Google Scholar]
- 142.van ‘t Veen A, Mouton JW, Gommers D, Kluytmans JA, Dekkers P, Lachmann B. Influence of pulmonary surfactant on in vitro bactericidal activities of amoxicillin, ceftazidime, and tobramycin. Anti-microb Agents Chemother 1995; 39(2):329-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Konig C, Simmen HP, Blaser J. Effect of pathological changes of pH, pO2 and pCO2 on the activity of antimicrobial agents in vitro. Eur J Clin Microbiol Infect Dis 1993; 12(7):519-526. [DOI] [PubMed] [Google Scholar]
- 144.Mendelman PM, Smith AL, Levy J, Weber A, Ramsey B, Davis RL. Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am Rev Respir Dis 1985; 132(4):761-765. DOI: 10.1164/arrd.1985.132.4.761 [DOI] [PubMed] [Google Scholar]
- 145.Levy J, Smith AL, Kenny MA, Ramsey B, Schoenknecht FD. Bioactivity of gentamicin in purulent sputum from patients with cystic fibrosis or bronchiectasis: comparison with activity in serum. J Infect Dis 1983; 148(6):1069-1076. [DOI] [PubMed] [Google Scholar]
- 146.de Oliveira MS, de Assis DB, Freire MP et al. Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect 2015; 21(2):179.e1-7. DOI: 10.1016/j.cmi.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 147.Kvitko CH, Rigatto MH, Moro AL, Zavascki AP. Polymyxin B versus other antimicrobials for the treatment of Pseudomonas aeruginosa bacteraemia. J Antimicrob Chemother 2011; 66(1):175-179. DOI: 10.1093/jac/dkq390 [DOI] [PubMed] [Google Scholar]
- 148.Paul M, Bishara J, Levcovich A et al. Effectiveness and safety of colistin: prospective comparative cohort study. J Antimicrob Chemother 2010; 65(5):1019-1027. DOI: 10.1093/jac/dkq069 [DOI] [PubMed] [Google Scholar]
- 149.Fink MP, Snydman DR, Niederman MS et al. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double-blind trial comparing intravenous ciprofloxacin with imipenem-cilastatin. The Severe Pneumonia Study Group. Antimicrob Agents Chemother 1994; 38(3):547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Giamarellou H, Bassaris HP, Petrikkos G et al. Monotherapy with intravenous followed by oral high-dose ciprofloxacin versus combination therapy with ceftazidime plus amikacin as initial empiric therapy for granulocytopenic patients with fever. Antimicrob Agents Chemother 2000; 44(12):3264-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Torres A, Bauer TT, Leon-Gil C et al. Treatment of severe nosocomial pneumonia: a prospective randomised comparison of intravenous ciprofloxacin with imipenem/cilastatin. Thorax 2000; 55(12):1033-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mogayzel PJ Jr., Naureckas ET, Robinson KA et al. Cystic Fibrosis Foundation pulmonary guideline. pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann Am Thorac Soc 2014; 11(10):1640-1650. DOI: 10.1513/AnnalsATS.201404-166OC [DOI] [PubMed] [Google Scholar]
- 153.Arnold HM, Sawyer AM, Kollef MH. Use of adjunctive aerosolized antimicrobial therapy in the treatment of Pseudomonas aeruginosa and Acinetobacter baumannii ventilator-associated pneumonia. Respir Care 2012; 57(8):1226-1233. DOI: 10.4187/respcare.01556 [DOI] [PubMed] [Google Scholar]
- 154.Rattanaumpawan P, Lorsutthitham J, Ungprasert P, Angkasekwinai N, Thamlikitkul V. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J Antimicrob Chemother 2010; 65(12):2645-2649. DOI: 10.1093/jac/dkq360 [DOI] [PubMed] [Google Scholar]
- 155.Doshi NM, Cook CH, Mount KL et al. Adjunctive aerosolized colistin for multi-drug resistant gram-negative pneumonia in the critically ill: a retrospective study. BMC Anesthesiol 2013; 13(1):45 DOI: 10.1186/1471-2253-13-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hallal A, Cohn SM, Namias N et al. Aerosolized tobramycin in the treatment of ventilator-associated pneumonia: a pilot study. Surg Infect (Larchmt ) 2007; 8(1):73-82. DOI: 10.1089/sur.2006.051 [DOI] [PubMed] [Google Scholar]
- 157.Ghannam DE, Rodriguez GH, Raad II, Safdar A. Inhaled aminoglyco-sides in cancer patients with ventilator-associated Gram-negative bacterial pneumonia: safety and feasibility in the era of escalating drug resistance. Eur J Clin Microbiol Infect Dis 2009; 28(3):253-259. DOI: 10.1007/s10096-008-0620-5 [DOI] [PubMed] [Google Scholar]
- 158.Tumbarello MM, De Pascale GMP, Trecarichi EMM et al. Effect of Aerosolized Colistin as Adjunctive Treatment on the Outcomes of Microbiologically Documented Ventilator-Associated Pneumonia Caused by Colistin-Only Susceptible Gram-Negative Bacteria. Chest 2013; 144(6):1768-1775. DOI: 10.1378/chest.13-1018 [DOI] [PubMed] [Google Scholar]
- 159.Kofteridis D, Alexopoulou C, Valachis A et al. Aerosolized plus Intravenous Colistin versus Intravenous Colistin Alone for the Treatment of Ventilator-Associated Pneumonia: A Matched Case-Control Study. Clin Infect Dis 2010; 51(11):1238-1244. DOI: 10.1086/657242 [DOI] [PubMed] [Google Scholar]
- 160.Horianopoulou M, Kanellopoulou M, Paraskevopoulos I, Kyriakidis A, Legakis NJ, Lambropoulos S. Use of inhaled ampicillin-sulbactam against multiresistant Acinetobacter baumannii in bronchial secretions of intensive care unit patients. Clin Microbiol Infect 2004; 10(1):85-86. [DOI] [PubMed] [Google Scholar]
- 161.Naesens R, Vlieghe E, Verbrugghe W, Jorens P, Ieven M. A retrospective observational study on the efficacy of colistin by inhalation as compared to parenteral administration for the treatment of nosocomial pneumonia associated with multidrug-resistant Pseudomonas aeruginosa. BMC Infect Dis 2011; 11:317 DOI: 10.1186/1471-2334-11-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Korbila IP, Michalopoulos A, Rafailidis PI, Nikita D, Samonis G, Falagas ME. Inhaled colistin as adjunctive therapy to intravenous colistin for the treatment of microbiologically documented ventilator-associated pneumonia: a comparative cohort study. Clin Microbiol Infect 2010; 16(8):1230-1236. DOI: 10.1111/j.1469-0691.2009.03040.x [DOI] [PubMed] [Google Scholar]
- 163.Abdellatif S, Trifi A, Daly F, Mahjoub K, Nasri R, Ben LS. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann Intensive Care 2016; 6(1):26 DOI: 10.1186/s13613-016-0127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brown RB, Kruse JA, Counts GW, Russell JA, Christou NV, Sands ML. Double-blind study of endotracheal tobramycin in the treatment of gram-negative bacterial pneumonia. The Endotracheal Tobramycin Study Group. Antimicrob Agents Chemother 1990; 34(2):269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ioannidou E, Siempos II, Falagas ME. Administration of antimicrobials via the respiratory tract for the treatment of patients with nosocomial pneumonia: a meta-analysis. J Antimicrob Chemother 2007; 60(6):1216-1226. DOI: 10.1093/jac/dkm385 [DOI] [PubMed] [Google Scholar]
- 166.Valachis A, Samonis G, Kofteridis DP. The Role of Aerosolized Colistin in the Treatment of Ventilator-Associated Pneumonia: A Systematic Review and Metaanalysis. Crit Care Med 2015; 43(3):527-533. DOI: 10.1097/CCM.0000000000000771 [DOI] [PubMed] [Google Scholar]
- 167.Sole-Lleonart C, Rouby JJ, Blot S et al. Nebulization of Antiinfective Agents in Invasively Mechanically Ventilated Adults: A Systematic Review and Meta-analysis. Anesthesiology 2017; 126(5):890-908. DOI: 10.1097/ALN.0000000000001570 [DOI] [PubMed] [Google Scholar]
- 168.Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non-cystic fibrosis bronchiectasis: a systematic review. Eur Respir J 2014; 44(2):382-393. DOI: 10.1183/09031936.00018414 [DOI] [PubMed] [Google Scholar]
- 169.Lu Q, Yang J, Liu Z et al. Nebulized Ceftazidime and Amikacin in Ventilator-associated Pneumonia caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med 2011; 184(1):106-115. DOI: 10.1164/rccm.201011-1894OC [DOI] [PubMed] [Google Scholar]
- 170.Goncalves-Pereira J, Povoa P. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of beta-lactams. Crit Care 2011; 15(5):R206 DOI: 10.1186/cc10441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Feng Y, Hodiamont CJ, van Hest RM, Brul S, Schultsz C, Ter Kuile BH. Development of Antibiotic Resistance during Simulated Treatment of Pseudomonas aeruginosa in Chemostats. PLoS ONE 2016; 11(2):e0149310 DOI: 10.1371/journal.pone.0149310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dahdouh E, Shoucair SH, Salem SE, Daoud Z. Mutant prevention concentrations of imipenem and meropenem against Pseudomonas aeruginosa and Acinetobacter baumannii. Scientific World Journal 2014; 2014:979648 DOI: 10.1155/2014/979648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Credito K, Kosowska-Shick K, Appelbaum PC. Mutant prevention concentrations of four carbapenems against gram-negative rods. Antimicrob Agents Chemother 2010; 54(6):2692-2695. DOI: 10.1128/AAC.00033-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Henrichfreise B, Wiegand I, Luhmer-Becker I, Wiedemann B. Development of Resistance in Wild-Type and Hypermutable Pseudomonas aeruginosa Strains Exposed to Clinical Pharmacokinetic Profiles of Meropenem and Ceftazidime Simulated In Vitro. Anti-microb Agents Chemother 2007; 51(10):3642-3649. DOI: 10.1128/AAC.00160-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381-393. DOI: 10.1111/j.1365-2125.2011.03991.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chow KM, Hui AC, Szeto CC. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis 2005; 24(10):649-653. DOI: 10.1007/s10096-005-0021-y [DOI] [PubMed] [Google Scholar]
- 177.Moriyama B, Henning SA, Childs R et al. High-dose continuous infusion beta-lactam antibiotics for the treatment of resistant Pseudomonas aeruginosa infections in immunocompromised patients. Ann Pharmacother 2010; 44(5):929-935. DOI: 10.1345/aph.1M717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moriyama B, Henning SA, Neuhauser MM, Danner RL, Walsh TJ. Continuous-infusion beta-lactam antibiotics during continuous venovenous hemofiltration for the treatment of resistant gram-negative bacteria. Ann Pharmacother 2009; 43(7):1324-1337. DOI: 10.1345/aph.1L638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jensen JU, Hein L, Lundgren B et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open 2012; 2(2):e000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mustafa MH, Chalhoub H, Denis O et al. Antimicrobial Susceptibility of Pseudomonas aeruginosa Isolated from Cystic Fibrosis Patients in Northern Europe. Antimicrob Agents Chemother 2016; 60(11):6735-6741. DOI: 10.1128/AAC.01046-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sader HS, Rhomberg PR, Jones RN. In vitro activity of beta-lactam antimicrobial agents in combination with aztreonam tested against metallo-beta-lactamase-producing Pseudomonas aeruginosa and Acinetobacter baumannii. J Chemother 2005; 17(6):622-627. DOI: 10.1179/joc.2005.17.6.622 [DOI] [PubMed] [Google Scholar]
- 182.Bosso JA, Saxon BA, Matsen JM. In vitro activities of combinations of aztreonam, ciprofloxacin, and ceftazidime against clinical isolates of Pseudomonas aeruginosa and Pseudomonas cepacia from patients with cystic fibrosis. Antimicrob Agents Chemother 1990; 34(3):487-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sader HS, Huynh HK, Jones RN. Contemporary in vitro synergy rates for aztreonam combined with newer fluoroquinolones and beta-lactams tested against gram-negative bacilli. Diagn Microbiol Infect Dis 2003; 47(3):547-550. [DOI] [PubMed] [Google Scholar]
- 184.Sader HS, Jones RN. Comprehensive in vitro evaluation of cefepime combined with aztreonam or ampicillin/sulbactam against multi-drug resistant Pseudomonas aeruginosa and Acinetobacter spp. Int J Antimicrob Agents 2005; 25(5):380-384. DOI: 10.1016/j.ijantimicag.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 185.Lister PD, Sanders WE Jr., Sanders CC. Cefepime-Aztreonam: a Unique Double beta -Lactam Combination for Pseudomonas aeruginosa. Antimicrob Agents Chemother 1998; 42(7):1610-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Krezdorn J, Adams S, Coote PJ. A Galleria mellonella infection model reveals double and triple antibiotic combination therapies with enhanced efficacy versus a multidrug-resistant strain of Pseudomonas aeruginosa. J Med Microbiol 2014; 63(Pt 7):945-955. DOI: 10.1099/jmm.0.074245-0 [DOI] [PubMed] [Google Scholar]
- 187.Moya B, Barcelo IM, Bhagwat S et al. WCK 5107 (Zidebactam) and WCK 5153 Are Novel Inhibitors of PBP2 Showing Potent “beta-Lactam Enhancer” Activity against Pseudomonas aeruginosa, Including Multidrug-Resistant Metallo-beta-Lactamase-Producing High-Risk Clones. Antimicrob Agents Chemother 2017; 61(6):e02529-16. DOI: 10.1128/AAC.02529-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Drusano GL, Bonomo RA, Bahniuk N et al. Resistance emergence mechanism and mechanism of resistance suppression by tobramycin for cefepime for Pseudomonas aeruginosa. Antimicrob Agents Chemother 2012; 56(1):231-242. DOI: 10.1128/AAC.05252-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thomas JK, Forrest A, Bhavnani SM et al. Pharmacodynamic Evaluation of Factors Associated with the Development of Bacterial Resistance in Acutely Ill Patients during Therapy. Antimicrob Agents Chemother 1998; 42(3):521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Drusano GL, Preston SL, Fowler C, Corrado M, Weisinger B, Kahn J. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J Infect Dis 2004; 189(9):1590-1597. DOI: 10.1086/383320 [DOI] [PubMed] [Google Scholar]
- 191.Hansen GT, Zhao X, Drlica K, Blondeau JM. Mutant prevention concentration for ciprofloxacin and levofloxacin with Pseudomonas aeruginosa. Int J Antimicrob Agents 2006; 27(2):120-124. DOI: 10.1016/j.ijantimicag.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 192.Lister PD, Wolter DJ, Wickman PA, Reisbig MD. Levofloxacin/imipenem prevents the emergence of high-level resistance among Pseudomonas aeruginosa strains already lacking susceptibility to one or both drugs. J Antimicrob Chemother 2006; 57(5):999-1003. DOI: 10.1093/jac/dkl063 [DOI] [PubMed] [Google Scholar]
- 193.Lister PD, Wolter DJ. Levofloxacin-imipenem combination prevents the emergence of resistance among clinical isolates of Pseudomonas aeruginosa. Clin Infect Dis 2005; 40 Suppl 2:S105-S114. DOI: 10.1086/426190 [DOI] [PubMed] [Google Scholar]
- 194.Louie A, Bied A, Fregeau C et al. Impact of different carbapenems and regimens of administration on resistance emergence for three isogenic Pseudomonas aeruginosa strains with differing mechanisms of resistance. Antimicrob Agents Chemother 2010; 54(6):2638-2645. DOI: 10.1128/AAC.01721-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhanel GG, Mayer M, Laing N, Adam HJ. Mutant Prevention Concentrations of Levofloxacin Alone and in Combination with Azithromycin, Ceftazidime, Colistin (Polymyxin E), Meropenem, Piperacillin-Tazobactam, and Tobramycin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2006; 50(6):2228-2230. DOI: 10.1128/AAC.01620-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhanel GG, Vashisht V, Tam ED, Hoban DJ, Karlowsky JA. Mutant prevention concentrations of doripenem and meropenem alone and in combination with colistin (polimixin E), levofloxacin and tobramycin inPseudomonas aeruginosa. Can J Infect Dis Med Microbiol 2009;20(suppl-A): 67A-71A. [Google Scholar]
- 197.Fish DN, Choi MK, Jung R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J Antimicrob Chemother 2002; 50(6):1045-1049. [DOI] [PubMed] [Google Scholar]
- 198.Vestergaard M, Paulander W, Marvig RL et al. Antibiotic combination therapy can select for broad-spectrum multidrug resistance in Pseudomonas aeruginosa. Int J Antimicrobi Agents 2016; 47(1):48-55. DOI: 10.1016/j.ijantimicag.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 199.Solomkin JS, Reinhart HH, Dellinger EP et al. Results of a rand-omized trial comparing sequential intravenous/oral treatment with ciprofloxacin plus metronidazole to imipenem/cilastatin for intra-abdominal infections. The Intra-Abdominal Infection Study Group. Ann Surg 1996; 223(3):303-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Heyland DK, Dodek P, Muscedere J, Day A, Cook D. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med 2008; 36(3):737-744. DOI: 10.1097/01.CCM.0B013E31816203D6 [DOI] [PubMed] [Google Scholar]
- 201.Al-Hasan MN, Wilson JW, Lahr BD et al. Beta-lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by gram-negative bacilli. Antimicrob Agents Chemother 2009; 53(4):1386-1394. DOI: 10.1128/AAC.01231-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bulitta JB, Yang JC, Yohonn L et al. Attenuation of Colistin Bactericidal Activity by High Inoculum of Pseudomonas aeruginosa Characterized by a New Mechanism-Based Population Pharmaco-dynamic Model. Antimicrob Agents Chemother 2010; 54(5):2051-2062. DOI: 10.1128/AAC.00881-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tam VH, Schilling AN, Vo G et al. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2005; 49(9):3624-3630. DOI: 10.1128/AAC.49.9.3624-3630.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dudhani RV, Turnidge JD, Coulthard K et al. Elucidation of the Pharmacokinetic/Pharmacodynamic Determinant of Colistin Activity against Pseudomonas aeruginosa in Murine Thigh and Lung Infection Models. Antimicrob Agents Chemother 2010; 54(3):1117-1124. DOI: 10.1128/AAC.01114-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ly NS, Bulitta JB, Rao GG et al. Colistin and doripenem combinations against Pseudomonas aeruginosa: profiling the time course of synergistic killing and prevention of resistance. J Antimicrob Chemother 2015; 70(5):1434-1442. DOI: 10.1093/jac/dku567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Aoki N, Tateda K, Kikuchi Y et al. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J Antimicrob Chemother 2009; 63(3):534-542. DOI: 10.1093/jac/dkn530 [DOI] [PubMed] [Google Scholar]
- 207.Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2003; 47(3):905-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rynn C, Wootton M, Bowker KE, Alan HH, Reeves DS. In vitro assessment of colistin’s antipseudomonal antimicrobial interactions with other antibiotics. Clin Microbiol Infect 1999; 5(1):32-36. [DOI] [PubMed] [Google Scholar]
- 209.Giamarellos-Bourboulis EJ, Sambatakou H, Galani I, Giamarellou H. In vitro interaction of colistin and rifampin on multidrug-resistant Pseudomonas aeruginosa. J Chemother 2003; 15(3):235-238. DOI: 10.1179/joc.2003.15.3.235 [DOI] [PubMed] [Google Scholar]
- 210.Zusman O, Avni T, Leibovici L et al. Systematic Review and Meta-Analysis of In Vitro Synergy of Polymyxins and Carbapenems. Antimicrob Agents Chemother 2013; 57(10):5104-5111. DOI: 10.1128/AAC.01230-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lenhard JR, Nation RL, Tsuji BT. Synergistic combinations of polymyxins. Int J Antimicrob Agents 2016; 48(6):607-613. DOI: 10.1016/j.ijantimicag.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sandri AM, Landersdorfer CB, Jacob J et al. Population Pharmacokinetics of Intravenous Polymyxin B in Critically Ill Patients: Implications for Selection of Dosage Regimens. Clin Infect Dis 2013; 57(4):524-531. DOI: 10.1093/cid/cit334 [DOI] [PubMed] [Google Scholar]
- 213.Garonzik SM, Li J, Thamlikitkul V et al. Population Pharmacokinetics of Colistin Methanesulfonate and Formed Colistin in Critically Ill Patients from a Multicenter Study Provide Dosing Suggestions for Various Categories of Patients. Antimicrob Agents Chemother 2011; 55(7):3284-3294. DOI: 10.1128/AAC.01733-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Benattar YD, Omar M, Zusman O et al. The Effectiveness and Safety of High-Dose Colistin: Prospective Cohort Study. Clin Infect Dis 2016; 63(12):1605-1612. DOI: 10.1093/cid/ciw684 [DOI] [PubMed] [Google Scholar]
- 215.Pogue JM, Ortwine JK, Kaye KS. Editorial Commentary: Colistin Dosing: Does the Fun Ever Start? Clin Infect Dis 2016; 63(12):1613-1614. DOI: 10.1093/cid/ciw685 [DOI] [PubMed] [Google Scholar]
- 216.Imberti RM, Cusato MP, Villani PB et al. Steady-State Pharmacokinetics and BAL Concentration of Colistin in Critically Ill Patients After IV Colistin Methanesulfonate Administration. Chest 2010; 138(6):1333-1339. DOI: 10.1378/chest.10-0463 [DOI] [PubMed] [Google Scholar]
- 217.Huang JX, Blaskovich MAT, Pelingon R et al. Mucin Binding Reduces Colistin Antimicrobial Activity. Antimicrob Agents Chemother 2015; 59(10):5925-5931. DOI: 10.1128/AAC.00808-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Markantonis SL, Markou N, Fousteri M et al. Penetration of Colistin into Cerebrospinal Fluid. Antimicrob Agents Chemother 2009; 53(11):4907-4910. DOI: 10.1128/AAC.00345-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Walsh CC, McIntosh MP, Peleg AY, Kirkpatrick CM, Bergen PJ. In vitro pharmacodynamics of fosfomycin against clinical isolates of Pseudomonas aeruginosa. J Antimicrob Chemother 2015; 70(11):3042-3050. DOI: 10.1093/jac/dkv221 [DOI] [PubMed] [Google Scholar]
- 220.Rodriguez-Rojas A, Couce A, Blazquez J. Frequency of spontaneous resistance to fosfomycin combined with different antibiotics in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2010; 54(11):4948-4949. DOI: 10.1128/AAC.00415-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Díez-Aguilar M, Morosini MA, Tedim AP, Rodríguez I, Aktas, Cantón R. Antimicrobial Activity of Fosfomycin-Tobramycin Combination against Pseudomonas aeruginosa Isolates Assessed by Time-Kill Assays and Mutant Prevention Concentrations. Antimicrob Agents Chemother 2015; 59(10):6039-6045. DOI: 10.1128/AAC.00822-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Montgomery AB, Rhomberg PR, Abuan T, Walters KA, Flamm RK. Amikacin-Fosfomycin at a Five-to-Two Ratio: Characterization of Mutation Rates in Microbial Strains Causing Ventilator-Associated Pneumonia and Interactions with Commonly Used Antibiotics. Antimicrob Agents Chemother 2014; 58(7):3708-3713. DOI: 10.1128/AAC.02779-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Montgomery AB, Rhomberg PR, Abuan T, Walters KA, Flamm RK. Potentiation Effects of Amikacin and Fosfomycin against Selected Amikacin-Nonsusceptible Gram-Negative Respiratory Tract Pathogens. Antimicrob Agents Chemother 2014; 58(7):3714-3719. DOI: 10.1128/AAC.02780-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gómez-Garcés JL, Gil-Romero Y, Sanz-Rodríguez N, Muñoz-Paraíso C, Regodón-Domínguez M. Actividad in-vitro de fosfomicina, sola o en combinaciones, frente a aislamientos clínicos de Pseudomonas aeruginosa resistentes a carbapenémicos. Enf Infecc Mi-crobiol Clín 2016; 34(4):228-231. DOI: 10.1016/j.eimc.2015.06.016 [DOI] [PubMed] [Google Scholar]
- 225.Yamada S, Hyo Y, Ohmori S, Ohuchi M. Role of ciprofloxacin in its synergistic effect with fosfomycin on drug-resistant strains of Pseudomonas aeruginosa. Chemotherapy 2007; 53(3):202-209. DOI: 10.1159/000100811 [DOI] [PubMed] [Google Scholar]
- 226.Asuphon O, Montakantikul P, Houngsaitong J, Kiratisin P, Sonthisombat P. Optimizing intravenous fosfomycin dosing in combination with carbapenems for treatment of Pseudomonas aeruginosa infections in critically ill patients based on pharmacokinetic/pharmacodynamic (PK/PD) simulation. Int J Infect Dis 2016; 50:23-29. DOI: 10.1016/j.ijid.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 227.Chin NX, Neu NM, Neu HC. Synergy of fosfomycin with beta-lactam antibiotics against staphylococci and aerobic gram-negative bacilli. Drugs Exp Clin Res 1986; 12(12):943-947. [PubMed] [Google Scholar]
- 228.Zeitlinger MA, Marsik C, Georgopoulos A, M³ller M, Heinz G, Joukhadar C. Target site bacterial killing of cefpirome and fosfomycin in critically ill patients. Int J Antimicrob Agents 2003; 21(6):562-567. [DOI] [PubMed] [Google Scholar]
- 229.Okazaki M, Suzuki K, Asano N et al. Effectiveness of fosfomycin combined with other antimicrobial agents against multidrug-resistant Pseudomonas aeruginosa isolates using the efficacy time index assay. J Infect Chemother 2002; 8(1):37-42. DOI: 10.1007/s101560200004 [DOI] [PubMed] [Google Scholar]
- 230.Mirakhur A, Gallagher MJ, Ledson MJ, Hart CA, Walshaw MJ. Fosfomycin therapy for multiresistant Pseudomonas aeruginosa in cystic fibrosis. J Cyst Fibros 2003; 2(1):19-24. DOI: 10.1016/S1569-1993(02)00143-1 [DOI] [PubMed] [Google Scholar]
- 231.Falagas ME, Kastoris AC, Karageorgopoulos DE, Rafailidis PI. Fosfomycin for the treatment of infections caused by multidrug-resistant non-fermenting Gram-negative bacilli: a systematic review of microbiological, animal and clinical studies. Int J Antimicrob Agents 2009; 34(2):111-120. DOI: 10.1016/j.ijantimicag.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 232.Tunkel AR, Hasbun R, Bhimraj A et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis 2017;64:e34-e65. DOI: 10.1093/cid/ciw861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Remes F, Tomas R, Jindrak V, Vanis V, Setlik M. Intraventricular and lumbar intrathecal administration of antibiotics in post-neurosurgical patients with meningitis and/or ventriculitis in a serious clinical state. J Neurosurg 2013; 119(6):1596-1602. DOI: 10.3171/2013.6.JNS122126 [DOI] [PubMed] [Google Scholar]
- 234.Pai S, Bedford L, Ruramayi R et al. Pseudomonas aeruginosa meningitis/ventriculitis in a UK tertiary referral hospital. QJM 2016; 109(2):85-89. DOI: 10.1093/qjmed/hcv094 [DOI] [PubMed] [Google Scholar]
- 235.Gilbert B, Morrison C. Evaluation of intraventricular colistin utilization: A case series. J Crit Care 2017; 40:161-163. DOI: 10.1016/j.jcrc.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 236.Plasencia V, Borrell N, Macia MD, Moya B, Perez JL, Oliver A. Influence of High Mutation Rates on the Mechanisms and Dynamics of In Vitro and In Vivo Resistance Development to Single or Combined Antipseudomonal Agents. Antimicrob Agents Chemother 2007; 51(7):2574-2581. DOI: 10.1128/AAC.00174-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tato M, Garcia-Castillo M, Bofarull AM, Canton R. In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa and Enterobacteriaceae recovered in Spanish medical centres: Results of the CENIT study. Int J Antimicrob Agents 2015;46:502-510. DOI: 10.1016/j.ijantimicag.2015.07.004 [DOI] [PubMed] [Google Scholar]