ABSTRACT
Introduction
The SMART (Study for Monitoring Antimicrobial Resistance Trends) surveillance study monitors antimicrobial susceptibility and extended spectrum β-lactamases (ESBLs) in Gram-negative bacilli recovered from intra-abdominal infections (IAI).
Material and methods
Antimicrobial susceptibility of 5,343 isolates from IAI recovered in 11 centres during the 2011-2015 SMART-Spain program was analysed by standard microdilution (EUCAST criteria) and compared with that from 2002-2010. ESBLs were phenotypically detected.
Results
Escherichia coli, the most common isolate, significantly decreased in community acquired IAI (60.9% 2002-2010 vs. 56.1% 2011-2015, P=0.0003). It was followed in prevalence by Klebsiella pneumoniae that increased both in the community (8.9% vs. 10.8%, P=0.016) and nosocomial (9.2% vs. 10.8%, P=0.029) IAI and P. aeruginosa, which significantly increased in community acquired IAI (5.6% vs. 8.0%, P=0.0003). ESBLs were more prevalent in K. pneumoniae (16.3%) than in E. coli (9.5%) of nosocomial origin and were more frequently isolated from elderly patients (>60 years). Considering all Enterobacteriaceae, ertapenem (92.3-100%) and amikacin (95.5%-100%) were the most active antimicrobials. Ertapenem activity, unlike amoxicillin-clavulanate or piperacillin-tazobactam, remained virtually unchanged in ESBL (100%) and non-ESBL (98.8%) E. coli producers. Its activity decreased in ESBL-K. pneumoniae (74.7%) but was higher than that of amoxicillin-clavulanate (14.0%) and piperacillin-tazobactam (24.0%). Interestingly, ertapenem susceptibility was maintained in >60% of ESBL isolates that were resistant to amoxicillin-clavulanate, piperacillin-tazobactam or fluoroquinolones.
Conclusions
SMART-Spain results support current guidelines which include ertapenem as empiric treatment in mild-moderate community-acquired IAI, particularly with ESBL producers. These recommendations will need to be updated with the recently introduction of new antimicrobials.
Keywords: surveillance study, intra-abdominal infections, carbapenems, extended spectrum β-lactamases
RESUMEN
Introducción
El estudio SMART (Study for Monitoring Antimicrobial Resistance Trends) monitoriza la sensibilidad antimicrobiana y las β-lactamasas de espectro extendido (BLEE) en bacilos gramnegativos obtenidos de infecciones intraabdominales (IIA).
Material y Métodos
Se ha analizado la sensibilidad antimicrobiana (microdilución estándar, criterios EUCAST) y las BLEE (detección fenotípica) de 5.343 aislados de IIA en 11 centros del programa SMART-España durante 2011-2015 en comparación con 2002-2010.
Resultados
Escherichia coli, el microorganismo más prevalente, disminuyó significativamente en las IIA de origen comunitario (60,9% 2002-2010 vs. 56,1% 2011-2015, P=0,0003). Fue seguido en prevalencia por Klebsiella pneumoniae que aumentó tanto en IIA comunitaria (8,9% vs. 10,8%, P=0,016) como nosocomial (9,2% vs. 10,8%, P=0,029) y por P. aeruginosa que aumentó en la IIA comunitaria (5,6% vs. 8,0%, P=0,0003). Las BLEE fueron más prevalentes en la IIA nosocomial por K. pneumoniae (16,3%) que por E. coli (9,5%), siendo más frecuentes en pacientes de mayor edad (>60 años). Considerando todas las Enterobacteriaceae, ertapenem (92,3-100%) y amikacina (95,5%-100%) fueron los antimicrobianos más activos. La sensibilidad a ertapenem, al contrario que a amoxicilina-clavulánico o piperacilina-tazobactam, se mantuvo sin cambios en E. coli con (98,8%) y sin BLEE (100%). Su sensibilidad disminuyó en BLEE-K. pneumoniae (74,7%) pero fue mayor que la de amoxicilina-clavulánico (14,0%) o piperacilina-tazobactam (24,0%). Es de resaltar que esta actividad se mantuvo >60% en los aislados con BLEE resistentes a amoxicilina-clavulánico, piperacilina-tazobactam o fluoroquinolonas.
Conclusiones
El estudio SMART-España sustenta las guías actuales que incluyen al ertapenem como tratamiento empírico en la IIA leve-moderada comunitaria, en particular con BLEE. Estas recomendaciones precisaran actualizarse con la reciente introducción de nuevos antimicrobianos.
Keywords: estudio de vigilancia epidemiológica, infección intraabdominal, carbapenems, β-lactamasas de espectro extendido
INTRODUCTION
Intra-abdominal infections (IAI) are recognized as one of the most common adverse events in the healthcare settings and range in severity from appendicitis to serious peritonitis [1,4]. Antimicrobial treatment failure and increased morbidity and mortality of these infections are frequently recognized due to the absence of both an early diagnosis and increasing prevalence of antimicrobial resistance, particularly in high-risk patients [3,4,6]. These infections are often polymicrobial, including a wide variety of Gram-negative aerobic and facultative bacilli, such as Escherichia coli, Klebsiella spp., Proteus spp., Enterobacter spp., and, to a lesser extent, Pseudomonas aeruginosa and other non-fermenting Gram-negative bacilli [2,3,7,8]. As a result, empiric treatment with broad-spectrum antibiotics is recommended [4,6,8-10]. The antimicrobial agents currently used include the carbapenems and combinations of penicillins with β-lactamase inhibitors depending on the origin of the infection, and extended-spectrum cephalosporins and fluoroquinolones, usually in combination with metronidazole. Recently, new cephalosporin-β-lactamase inhibitor combinations, including ceftolozane-tazobactam and ceftazidime-avibactam have been approved for the IAI indication [4,11]. These new agents circumvent antimicrobial resistance mechanisms commonly present in organisms associated with IAIs which include extended-spectrum β-lactamases (ESBLs), plasmidic AmpC, AmpC hyperproduction and/or carbapenemases in Enterobacteriaceae and/or P. aeruginosa and are candidates to spare carbapenems, particularly those of class II (imipenem, meropenem and doripenem) [4,12].
On the other hand, and to address the problem of antimicrobial resistance in IAIs in line with antimicrobial stewardship programs, the implementation of epidemiological surveillance programs and the use of data collected in the design of therapeutic guidelines have been also proposed as first steps in the management of IAIs [8]. The SMART study (Study for Monitoring Antimicrobial Resistance Trends) is an ongoing global surveillance program started in 2002 involving over 180 hospitals from all over the world. It monitors the in vitro susceptibility to antimicrobials of aerobic and facultative anaerobic Gram-negative bacilli isolated from IAI, focusing on those producing ESBL. In addition, since 2009 it has enlarged its focus in urinary tract infection [13].
In this article we perform a sub-analysis of the SMART study and evaluate the susceptibility patterns of antimicrobials against aerobic and facultative anaerobic Gram-negative pathogens isolated from IAI in the period 2011-2015 in 11 Spanish hospitals, with particular focus on EBSL producers. These data are also compared with those previously published covering the 2002-2010 period [14].
MATERIAL AND METHODS
Microorganisms and participating sites
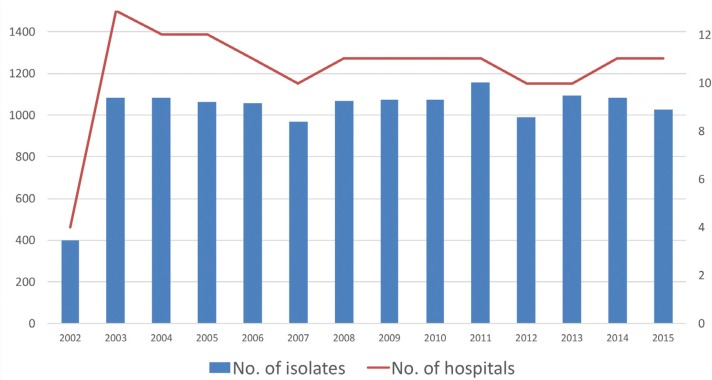
Isolates were recovered from abdominal samples from patients with diagnosis of an IAI. Each participating centre collected up to 100 non-selected consecutive isolates of aerobic and facultative anaerobic Gram-negative pathogens. To avoid duplicates, one strain per species and patient was included. During the 5 years of the study (2011 to 2015) a total of 11 hospitals participated (H. Basurto, Bilbao; H. Universitario Marqués de Valdecilla, Santander; H. Universitario Bellvitge, Hospitalet de Llobregat, Barcelona; H. Son Espases, Mallorca; H. Clínico Universitario Lozano Blesa, Zaragoza; H. Universitario y Politécnico La Fe, Valencia; H. Universitario Ramón y Cajal, Madrid; H. Universitario Gregorio Marañón, Madrid; H. Clínico San Carlos, Madrid; H. Universitario Virgen del Rocío, Sevilla; H. Universitario Virgen Macarena, Sevilla). Figure 1 shows the distribution of participating centres per year.
Figure 1.
Number of hospitals participating and microorganisms recovered per year in the SMART study in Spain from 2002 to 2015
A total of 5,343 isolates were collected from the Spanish centres and the most frequent intra-abdominal sample was peritoneal fluid (39%), followed by intra-abdominal abscesses (32%) and gall bladder (16%), and, to a lesser extent and in decreasing order, specimens from the liver, small bowel, appendix, pancreas, stomach, colon, rectum, and other minor sources. Most of the isolates were obtained during surgery procedures and others from paracentesis and percutaneous aspiration of intra-abdominal abscesses. Isolates from blood, urine, abdominal drainages, superficial wounds, and perirectal abscesses were excluded. The isolates were identified by species at each hospital and sent to a central laboratory (International Health Management Associates, Schaumburg, IL, US) to confirm identification and establish the antimicrobial susceptibility to different antimicrobials of choice in IAIs. The source of the sample, patient age and the results were incorporated in a centralized database. Following the conventional criteria of the Centers for Disease Control and Prevention (CDC) the organisms were rated as community-acquired when they were obtained in samples within 48 hours after hospitalization and as nosocomial-acquired when obtained in samples recovered after 48 hours of hospital stay [15].
Antimicrobial susceptibility
Antimicrobial susceptibility testing results were obtained at the central laboratory using the standard ISO broth microdilution method [16]. Dried MicroScan (Beckman, West Sacramento, CA, US) microdilution panels were used. The antimicrobials analyzed in this study were: piperacillin-tazobactam, ceftriaxone, ceftazidime, cefepime, imipenem, meropenem, ertapenem, amikacin, ciprofloxacin and levofloxacin. In addition, susceptibility to amoxicillin-clavulanate was measured with a gradient test (Etest®, bioMérieux, Lyon, France). For interpreting antibiotic susceptibility, the breakpoints proposed by the EUCAST in the year 2016 were used [17]. For amoxicillin-clavulanate, the amoxicillin value from this combination was used as a reference for the application of CLSI breakpoints [18].
The quality controls strains used were E. coli ATCC 25922, E. coli ATCC 35218, Klebsiella pneumoniae ATCC 700603 (positive ESBL control) and P. aeruginosa ATCC 27853. E. coli, Klebsiella spp. and Proteus mirabilis isolates were classified as ESBL producers if there was at least an 8-fold reduction of the MICs for ceftazidime and/or cefotaxime tested in combination with clavulanate compared with their MICs when tested alone, according to the CLSI and EUCAST specifications [18,19].
Statistical analysis
The frequency comparison (incidence between hospital and community isolates) was performed using the chi-squared test (χ2) taking P <0.05 as statistically significant.
RESULTS
The number of isolates and centres participating by year and compared with our previous study are presented in figure 1. Overall, 5,295 isolates were finally included in the study as 48 isolates (0.9%) were not available for further studies or information was not complete in the SMART database. The analysis of all microorganisms and the entire follow-up period showed that enterobacterial isolates (4,844) accounted for 91.4% of the isolates, with E. coli as the most common species (58.9%), followed by Klebsiella spp. (17.6%) and E. cloacae (6.0%). The most common non-fermenting Gram-negative bacilli were P. aeruginosa (6.6% of the total isolates). When the origin of the isolates was considered, 53.7% were of nosocomial origin and 46.3% community-acquired. In few isolates (0.9%) this information was not specified in the case report forms.
Table 1 shows the distribution of the 11 most commonly isolated microorganisms differentiating between community and nosocomial origin. There was a significant difference (P<0.01) in the percentage of E. coli isolates causing community (56.1%) and nosocomial (52.1%) IAIs but not in K. pneumoniae (10.8% in both cases). Contrary to what was expected, P. aeruginosa represented 8.0% and 5.5% of isolates from community and nosocomial origin, respectively. Unlike this finding, Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii, Morganella morganii and Serratia marcescens (all of them with chromosomal inducible AmpC β-lactamases) were also more common in IAIs of hospital origin (table 1).
Table 1.
Distribution of the most common Gram-negative organisms collected in intra-abdominal infections in Spain in the SMART study (2011-2015).
| Community acquired |
Nosocomial acquired |
||||
|---|---|---|---|---|---|
| Organisms | No. of isolates | No. | % | No. | % |
| Escherichia coli | 2,857 | 1,375 | 56.1 | 1,482 | 52.1 |
| Klebsiella pneumoniae | 570 | 264 | 10.8 | 306 | 10.8 |
| Klebsiella oxytoca | 283 | 138 | 5.6 | 145 | 5.1 |
| Proteus mirabilis | 222 | 79 | 3.2 | 143 | 5.0 |
| Proteus vulgaris | 32 | 13 | 0.5 | 19 | 0.7 |
| Enterobacter cloacae | 293 | 109 | 4.5 | 184 | 6.5 |
| Enterobacter aerogenes | 85 | 37 | 1.5 | 48 | 1.7 |
| Citrobacter freundii | 108 | 41 | 1.7 | 67 | 2.4 |
| Morganella morganii | 133 | 36 | 1.5 | 97 | 3.4 |
| Serratia marcescens | 59 | 27 | 1.1 | 32 | 1.1 |
| Other Enterobacteriaceae | 202 | 103 | 4.2 | 99 | 3.5 |
| Pseudomonas aeruginosa | 353 | 197 | 8,0 | 156 | 5,5 |
| Other Gram-negative bacilli | 98 | 30 | 1.2 | 68 | 2.4 |
| TOTAL | 5,295 | 2,449 | 46.3 | 2,846 | 53.7 |
When comparing this data (2011-2015) with that previously reported (2002-2010) [14], E. coli was also the most prevalent isolate but significantly decreased (P=0.0003) in community acquired IAI (60.9% 2002-2010 vs. 56.1% 2011-2015). However, P. aeruginosa significantly increased (P=0.0003) in community acquired IAI (5.6% 2002-2010 vs. 8.0% 2011-2015). For K. pneumoniae, figures revealed an increased prevalence both in the community (8.9% 2002-2010 vs. 10.8% 2011-2015, P=0.016) and nosocomial (9.2% 2002-2010 vs. 10.8% 2011-2015, P=0.029) IAI.
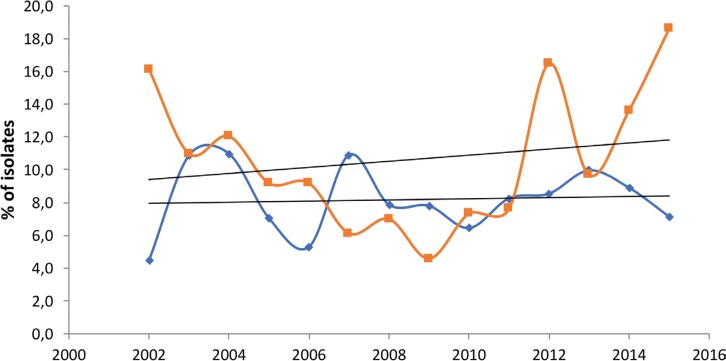
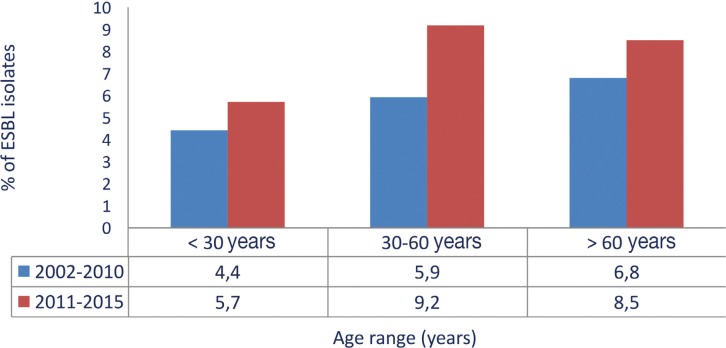
Considering all the enterobacterial isolates tested for ESBL (n=3,932; 2,857 E. coli, 853 Klebsiella spp. and 222 Proteus mirabilis), 338 (8.6%) were producers of these enzymes in the studied period (2011-2015). ESBLs were more prevalent in K. pneumoniae (16.3%) and E. coli (9.5%) isolates of nosocomial origin, followed by K. pneumoniae (9.5%) community-acquired isolates. With the exception of E. coli of nosocomial origin, all these figures increased when compared with the previous study (2002-2011) [14] (table 2). The incidence over time of ESBL-producing E coli and K. pneumoniae isolates is shown in figure 2, indicating a relative stability in E. coli and an overall increase in K. pneumoniae. In addition, an age-associated increase was observed in ESBL-producing isolates, reaching a frequency of more than 8% in patients over 60 years of age (figure 3). The increase of ESBL prevalence was observed in all patients irrespective of age when compared with the 2002-2010 period [14].
Table 2.
Frequency of Enterobacteriaceae with extended-spectrum β-lactamases (ESBLs) by origin of acquisition of infection in the SMART study in Spain comparing 2002-2010 and 2011-2015 periods.
| Acquisition of infection |
Escherichia coli |
Klebsiella pneumoniae |
Klesiella oxytoca |
Proteus mirabilis |
||||
|---|---|---|---|---|---|---|---|---|
| 2002-2010 | 2011-2015 | 2002-2010 | 2011-2015 | 2002-2010 | 2011-2015 | 2002-2010 | 2011-2015 | |
| <48 h | 6.2 | 8.0 | 5.3 | 9.5 | 1.3 | 1.4 | 0.7 | 1.3 |
| >48 h | 10.0 | 9.5 | 10.3 | 16.3 | 6.5 | 4.1 | 2.2 | 2.1 |
Figure 2.
Percentage of Escherichia coli and Klebsiella pneumoniae isolates with extended spectrum β-lactamases recovered per year in the SMART study in Spain from 2002 to 2015
Figure 3.
Frequency of Enterobacteriaceae (Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca and Proteus mirabilis) with extended spectrum β-lactamases according to age of the patients in the SMART study in Spain comparing 2002-2010 and 2011-2015 periods
The antibiotic susceptibility comparative profile of the most common organisms in IAIs isolated in 2011-2015 is shown in table 3. The compounds most active against Enterobacteriaceae were amikacin (susceptibility rates between 95.5 and 100%), ertapenem (92.3-100%) and imipenem (59.7-100%). Those which performed worst were the fluoroquinolones with, in general, lower rates of susceptibility. In the case of E. coli nearly 30% of the isolates were resistant to ciprofloxacin and levofloxacin. Enterobacterial isolates producing chromosomal inducible AmpC β-lactamases (E. cloacae, E. aerogenes, C. freundii, M. morganii and S. marcescens), known to be intrinsically resistant to the amoxicillin-clavulanate combination [20], were excluded from the analysis of resistance to this antibiotic. This ranged between 72.8% in E. coli and 92.0% in Proteus vulgaris. Piperacillin-tazobactam susceptibility of all the Enterobacteriaceae ranged from 72.9% in E. aerogenes to 98.7% in P. mirabilis. Piperacillin-tazobactam, ceftazidime, cefepime, impenem and amikacin maintained their activity against P. aeruginosa in a range from 81.6 to 89.3% of the isolates.
Table 3.
Activity of different antimicrobials used in intra-abdominal infections against the most common microorganisms collected in Spain in the SMART study (2011-2015).
| Microorganism | Percentage of susceptible isolatesa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUGa | P/T | CTX | CAZ | CPE | IMI | ETP | AK | CIP | LVX | |
| Escherichia coli | 72.8 | 90.0 | 90.5 | 90.4 | 92.2 | 99.8 | 99.9 | 98.1 | 71.7 | 73.4 |
| Klebsiella pneumoniae | 73.2 | 81.3 | 86.2 | 84.1 | 85.2 | 97.7 | 95.1 | 97.9 | 80.6 | 85.7 |
| Klebsiella oxytoca | 87.1 | 93.0 | 92.0 | 97.6 | 97.2 | 100.0 | 99.7 | 99.7 | 97.2 | 98.3 |
| Proteus mirabilis | 90.1 | 98.7 | 95.6 | 94.7 | 96.9 | 72.9 | 100.0 | 98.7 | 70.7 | 82.2 |
| Proteus vulgaris | 92.0 | 100.0 | 57.6 | 78.8 | 100.0 | 81.8 | 100.0 | 97.0 | 100.0 | 97.0 |
| Enterobacter cloacae | -b | 82.2 | 71.1 | 73.8 | 85.9 | 98.7 | 92.3 | 99.3 | 95.6 | 96.3 |
| Enterobacter aerogenes | -b | 72.9 | 61.2 | 60.0 | 92.9 | 98.8 | 96.5 | 100.0 | 89.4 | 92.9 |
| Citrobacter freundii | -b | 79.1 | 70.0 | 68.2 | 85.5 | 98.2 | 98.2 | 98.2 | 90.0 | 92.7 |
| Morganella morganii | -b | 96.3 | 75.4 | 64.2 | 97.8 | 59.7 | 100.0 | 95.5 | 71.6 | 85.8 |
| Serratia marcescens | -b | 96.6 | 94.9 | 96.6 | 96.6 | 96.6 | 100.0 | 100.0 | 89.8 | 94.9 |
| Other Enterobacteriaceae | 21.1 | 91.5 | 70.8 | 78.3 | 99.1 | 79.3 | 93.4 | 100.0 | 93.4 | 95.3 |
| Pseudomonas aeruginosa | -b | 84.2 | -b | 86.4 | 84.8 | 81.6 | -b | 89.3 | 77.7 | 74.0 |
EUCAST criteria except AUG in which CLSI criteria were considered;
This antimicrobial is not considered adequate against the microorganism tested.
AUG: amoxicillinclavulanate; P/T: piperacillin-tazobactam; CTX: cefotaxime; CAZ: ceftazidime; CPE: cefepime; IMI; imipenem; ETP: ertapenem; AK: amikacin; CP: ciprofloxacin; LVX: levofloxacin.
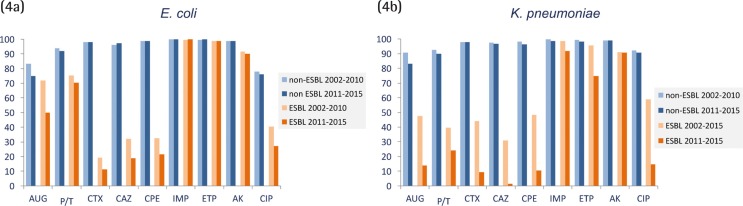
When only ESBL-producing E. coli and K. pneumoniae were taken into consideration and compared to non-ESBL-producers over time, imipenem and ertapenem activity remained virtually unchanged in E. coli irrespective of the ESBL production status (figure 4a) and was slightly affected in K. pneumoniae isolates (figure 4b). As expected, other β-lactam antibiotics were highly affected by the ESBL production and the reduction of susceptibility rates was higher in the 2011-2015 period. The decrease of susceptibility to all these antibiotics was higher in K. pneumoniae than in E. coli. It must be noted that ESBL-producing E. coli susceptibility rates for amoxicillin-clavulanate were 50%, and even lower in ESBL-producing K. pneumoniae in which susceptibility values of 14% were not achieved. The corresponding values for piperacillin-tazobactam were 70.5% for E. coli and 24.0% for K. pneumoniae.
Figure 4.
Activity of antimicrobials used in intra-abdominal infections against ESBL producing and non-producing Escherichia coli (4a) and Klebsiella pneumoniae (4b) isolates in the SMART study in Spain comparing 2002-2010 and 2011-2015 periods
When ertapenem activity was specifically analyzed, it was scarcely modified in ESBL and non-ESBL E. coli producers that were resistant to amoxicillin-clavulanic acid, piperacillin-tazobactam and levofloxacin. In all cases, susceptibility values were above 99% (table 4). In K. pneumoniae, ertapenem susceptibility was maintained in a range of 75.0% to 86.4% in non-ESBL producers and 59.6% to 72.5% in ESBL producers.
Table 4.
Activity of ertapenem in ESBL and non-ESBL producing Escherichia coli and Klebsiella pneumoniae isolates resistant to amoxicillin/clavulanate, piperacillin/tazobactam, and levofloxacin collected in the SMART study (2011-2015).
| Microorganisms | ESBL | Antimicrobial | % of resistant isolates | Ertapenem | ||
|---|---|---|---|---|---|---|
| Susceptible No. (%) | Intermediate No. (%) | Resistant No. (%) | ||||
| Escherichia coli | Negative | AMC | 11.4 | 297 (99.7) | 1 (0.3) | |
| Positive | 20.3 | 50 (100) | ||||
| Negative | P/T | 6.5 | 169 (99.4) | 1 (0.6) | ||
| Positive | 22.0 | 51 (99.4) | 1 (1.9) | 2 (3.7) | ||
| Negative | LVX | 21.8 | 571 (99.8) | 1 (0.2) | ||
| Positive | 69.5 | 170 (99.4) | 1 (0.6) | |||
| Klebsiella pneumoniae | Negative | AUG | 8.8 | 38 (86.4) | 2 (4.5) | 4 (9.1) |
| BLEE | 49.3 | 24 (64.9) | 2 (5.4) | 11 (29.7) | ||
| Negative | P/T | 7.2 | 27 (75.0) | 6 (16.7) | 3 (8.3) | |
| BLEE | 62.7 | 28 (59.6) | 2 (4.3) | 17 (36.2) | ||
| Negative | LVX | 4.8 | 19 (79.2) | 1 (4.2) | 4 (16.7) | |
| BLEE | 6.8 | 37 (72.5) | 1 (2.0) | 13 (25.5) | ||
ETP: ertapenem; AUG: amoxicillinclavulanate; P/T: piperacillin-tazobactam; LVX: levofloxacin.
DISCUSSION
Surveillance of antimicrobial resistance trends has been highlighted as a tool for fighting antimicrobial resistance and is an important tool in antimicrobial stewardship programs [21]. Local and international efforts in surveillance are being conducted and different governmental studies such as those promoted by the European Centre for Diseases Prevention and Control (ECDC) [22], the Centre for Diseases Control and Prevention (CDC) [23] and the World Health Organization [24] monitor susceptibility and resistance over time. These programs are complemented with those sponsored by pharmaceutical companies following specific microorganisms and antimicrobials (normally those marketed by the sponsor) and are focused on certain infections or resistance problems. Data from these sponsored surveillance studies are also useful when confronting antimicrobial use for monitoring their potential ecological impact on the development of resistance and for the implementation of antimicrobial guidelines in accordance with local epidemiology [4,25,26]. With the new antimicrobials and beyond the spectrum of activity, surveillance studies are used to define wild type populations, to understand the potential impact of resistance mechanisms on MIC distributions, and to perform PK/PD analysis [27]. All of them are essential in the procedure of setting clinical breakpoints, a role recognized by both the European Medicines Agency (EMA) and Food and Drugs Administration (FDA) not only in the process of commercialization of antimicrobials but also in the post-marketing period [28,29].
The SMART study, an ongoing surveillance program that collects antimicrobial susceptibility testing data among hospitalized patients on a global scale since 2002, was initially designed to monitor ertapenem susceptibility in IAI and ESBL producing Enterobacteriaceae [13]. Their objectives have now been enlarged to also monitor respiratory and urinary tract infections and, more recently, data on acquired carbapenemase-producing isolates are also being analysed [30]. In a previous publication [14], we analysed data from IAI obtained from the SMART study in Spain since its implementation in 2002 until 2010 with special focus on ertapenem susceptibility and also ESBLs. In the current study and with similar objectives we have enlarged this information in the 2011-2015 period. The SMART study provides a worldwide and local representative analysis of antimicrobial susceptibility profiles of microorganisms involved in IAI [13].
In line with recent publications [31], E. coli and K. pneumoniae are the most prevalent microorganisms in IAI in Spain, followed by Enterobacter spp. and P. aeruginosa. However, when comparing the current analysed period (2011-2015) with the previous one (2002-2010) [14], we found some differences when classifying the isolates as of nosocomial or community origin. The most important results were an increase of K. pneumoniae isolates both in the community and in nosocomial isolates and also in P. aeruginosa isolates among community isolates. These results might reflect a changing epidemiology due to a potential increase of patients with sociosanitary conditions or with previous antimicrobial therapy and/or hospital admission, all risk factors associated with these pathogens [11,32]. However, we cannot demonstrate this hypothesis with the patient’s data recorded in the SMART database.
As previously noted, surveillance studies also help to support empiric therapeutic recommendations in local guidelines. In Spain, the last IAI guidelines were published in 2009 [9] and in the USA [26] and at international level during 2017 [4]. These guidelines recommend carbapenems for empiric antimicrobial treatment of IAI; ertapenem or other carbapenems in patients at risk for infection with community-acquired ESBL-producing Enterobacteriaceae and meropenem, imipenem or doripenem for in-patients, including the critically ill, with healthcare-associated infections and these at higher risk for infection with multi-drug resistant microorganisms. On the other hand, they do not recommend (or questioned) the use of amoxicillin-clavulanate or ampicillin-sulbactam. Piperacillin-tazobactam, as meropenem, imipenem or doripenem, is recommended only for higher-risk patients. In Spain, and waiting for new recommendations with the approval of new antimicrobials with IAI indications, such as ceftazidime-avibactam and ceftolozane-tazobactam, these recommendations can also be supported with the SMART susceptibility testing results and data of ESBL producing Enterobacteriaceae.
Carbapenems, as well as amikacin, present the highest susceptibility values with percentages higher than 98% and 95% in E. coli and K. pneumoniae respectively. This situation was similar to that recently published for IAI microorganisms included in the SMART database in the USA [31]. Moreover, the overall prevalence of ESBL producers, specifically monitored in the SMART study, demonstrated higher values in K. pneumoniae (13.1%) than in E. coli (8.6%) isolates with a clear trend to increase over time in the former species but not in the latter (8.4% and 8.7%, respectively, in 2002-2011 period) [14]. This is consistent with the figures published in the SMART reports from the USA and Asia [13,31] and in other surveillance studies, such as EARS-net, TEST or SENTRY, that depict an overall increase of ESBLs irrespective of the origin of K. pneumoniae isolates [22,33,34]. They show that ESBL-producing isolates are more prevalent in the Mediterranean countries than in Northern Europe and North America, but lower than in Asian countries. Local epidemiology of ESBL producers is also highlighted to influence IAI empiric therapy [4,26]. Moreover, as in the analysis of SMART data from 2002-2010 [14], we demonstrated in the 2011-2015 study period a higher frequency of ESBL producers in older patients and in those with longer hospitalizations. Both situations have been repeatedly highlighted as risk factors for acquisition and infection due to ESBL-producing Enterobacteriaceae [11,32,35].
On the other hand, in our study we could also define co-resistances in ESBL producing isolates, a relevant issue for the design of treatment protocols and for the selection of antimicrobials [36]. The activity of penicillins plus β-lactamase inhibitors (amoxicillin-clavulanate and piperacillin-tazobactam), and to a higher degree extended spectrum cephalosporins (cefotaxime, ceftazidime and cefepime) and fluoroquinolones (levofloxacin and ciprofloxacin) was importantly affected in ESBL producers. Resistance values for ciprofloxacin were extremely high in ESBL-positive E. coli (72.9%) and K. pneumoniae (85.3%) isolates, even higher than those found previously (59.5% and 41.2%, respectively) [14]. This trend is also observed in other parts of the world [13,31].
Interestingly, further analysis of MIC values showed that carbapenems, including ertapenem, maintained a good activity in ESBL-producing isolates that were resistant to amoxicillin-clavulanate, piperacillin-tazobactam or fluoroquinolones. This effect was higher in E. coli than in K. pneumoniae (Table 4). This difference, more pronounced in the 2011-2015 than in the 2002-2010 period, might denote coproduction of carbapenemases in ESBL producers, a fact demonstrated when molecular characterization of β-lactamases was performed [30,37]. In Spain, coproduction of OXA-48-like or KPC carbapenemases with an ESBL is not an infrequent event and is linked to dissemination of specific clones [38,39].
The microbiological data of the SMART study in Spain support the current therapeutic guidelines in IAI which advocate ertapenem, a class I carbapenem remarkable for its long half-life and lower impact than class II carbapenems (imipenem, meropenem and doripenem) in the selection of resistant isolates [40], as the empiric treatment of choice for mild-moderate community-acquired infections, particularly for patients with risk factors for ESBL-producing or AmpC-hyperproducing Enterobacteriaceae and in patients not at risk of infection by P. aeruginosa [4,9,26]. In addition, the absence of a collateral effect or ecological impact on organisms with natural low susceptibility to ertapenem such as P. aeruginosa reinforces recommendation of class I carbapenems [40]. The selection of other carbapenems should depend on the type of patient, the possible origin of the infection and if P. aeruginosa infection is suspected. Nevertheless, the recent irruption of cephalosporins and β-lactamase inhibitors combinations, such as ceftazidime-avibactam and ceftolozane-tazobactam, in the therapeutic armamentarium and the need of sparing carbapenems, specifically of class II, due to the increasing prevalence of carbapenemases reinforces the need to update existing IAI guidelines [41].
ACKNOWLEDGEMENTS
We thank Merck Sharp & Dohme (MSD), España, S.A. (Madrid, Spain) and IHMA (International Health Management Associates, S.A., Schaumburg, Illinois, U.S.) for providing access to the database of the SMART epidemiological surveillance study. The manuscript has been prepared with an educational grant provided by MSD.
The SMART-Spain working group is represented by the following investigators who have participated in the study: R. Cisterna and O. Herrero (Hospital Basurto, Bilbao); J. Calvo and L. Martínez-Martínez (Hospital Universitario Marqués de Valdecilla, Santander); F. Tubau and M.A. Domínguez (Hospital Universitari Bellvitge-IDIBELL, Hospitalet de Llobregat. Barcelona); J.L. Pérez Sáenz and R. Barrón-Adúriz (Hospital Universitario Son Espases, Mallorca); F.J. Castillo and C. Seral (Hospital Clínico Universitario Lozano Blesa, Zaragoza); J.L. López-Hontangas (Hospital Universitario y Politécnico La Fe, Valencia); R. Cantón, M. García-Castillo, E. Loza, (Hospital Universitario Ramón y Cajal-IRYCIS, Madrid); E. Cercenado (Hospital Universitario Gregorio Marañón, Madrid); F. González Romo, José Prieto (Hospital Clínico San Carlos, Madrid); J. Aznar and V. González-Galán (Hospital Universitario Virgen del Rocío, Sevilla); A. Pascual and A.I. Suárez-Barrenechea (Hospital Universitario Virgen Macarena, Sevilla).
CONFLICTS OF INTEREST
Rafael Cantón has collaborated in educational meetings sponsored by MSD, Pfizer and AstraZeneca. He has also had research grants from MSD and AstraZeneca. F. Javier Castillo has collaborated in educational meetings sponsored by MSD.
All other authors declare that they have no conflicts of interest regarding this publication.
REFERENCES
- 1.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013; 34:1–14. PMID: . [DOI] [PubMed] [Google Scholar]
- 2.García-Sánchez JE, García-García MI, García-Garrote F, Sánchez-Romero I. Microbiological diagnosis of intra-abdominal infections. Enferm Infecc Microbiol Clin. 2013; 31:230-9PMID:. [DOI] [PubMed] [Google Scholar]
- 3.Shirah GR, O’Neill PJ. Intra-abdominal infections. Surg Clin North Am. 2014; 94:1319-33. PMID: . [DOI] [PubMed] [Google Scholar]
- 4.Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi AK, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017; 12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skrupky LP, Tellor BR, Mazuski JE. Current strategies for the treatment of complicated intraabdominal infections. Expert Opin Pharmacother. 2013; 14:1933-47. PMID: . [DOI] [PubMed] [Google Scholar]
- 6.Sartelli M, Catena F, Coccolini F, Pinna AD. Antimicrobial management of intra-abdominal infections: literature’s guidelines. World J Gastroenterol. 2012; 18:865-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Huang W. Epidemiological study of community- and hospital-acquired intra-abdominal infections. Chin J Traumatol. 2015; 18:84-9. PMID: . [DOI] [PubMed] [Google Scholar]
- 8.Guirao X, Arias J, Badía JM, García-Rodríguez JA, Mensa J, Alvarez-Lerma F et al. Recommendations in the empiric anti-infective agents of intra-abdominal infection. Rev Esp Quimioter 2009; 22: 151-72. PMID: . [PubMed] [Google Scholar]
- 9.Hoffmann C, Zak M, Avery L, Brown J. Treatment modalities and antimicrobial stewardship initiatives in the management of intra-abdominal infections. Antibiotics (Basel). 2016; 5(1). pii: E11. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:133-64 PMID: . [DOI] [PubMed] [Google Scholar]
- 11.Kaye KS, Pogue JM. Infections caused by resistant Gram-negative bacteria: epidemiology and management. Pharmacotherapy. 2015; 35:949-62 PMID: . [DOI] [PubMed] [Google Scholar]
- 12.Goodlet KJ, Nicolau DP, Nailor MD. Ceftolozane-tazobactam and ceftazidime-avibactam for the treatment of complicated intra-abdominal infections. Ther Clin Risk Manag 2016; 12:1811-26. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A Review of ten years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel). 2013; 6:1335-46PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantón R, Loza L, Aznar J, Calvo J, Cercenado E, Cisterna R, et al. Antimicrobial susceptibility of Gram-negative organisms from intra-abdominal infections and evolution of isolates with extended spectrum β-lactamases in the SMART study in Spain (2002-2010). Rev Esp Quimioter. 2011; 24: 223-32 PMID: . [PubMed] [Google Scholar]
- 15.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36:309-32. [DOI] [PubMed] [Google Scholar]
- 16.ISO.. Clinical laboratory testing and in vitro diagnostic test systems. Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. Part 1: Reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved. In: Infectious Diseases International Standard 20776-1. Geneva, Switzerland: ISO; 2006. [Google Scholar]
- 17.European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters Version 6.0, January 2016. (http://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/, last access January 8th, 2018).
- 18.Clinical and Laboratory Standards Institute.. Performance standards for antimicrobial susceptibility testing. Document M100-S26. Wayne, PA: CLSI, 2016. [Google Scholar]
- 19.Giske CG, Martinez-Martinez L, Cantón R, Stefani S, Skov R, Glupczynski Y, et al. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. European Committee on Antimicrobial Susceptibility Testing. 2013. http://www.eucast.org/resistance_mechanisms/ [Google Scholar]
- 20.European Committee of Antimicrobial Susceptibility Testing.. EUCAST Expert Rules Version 3.1. Intrinsic Resistance and Exceptional Phenotypes Tables. Available at http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Expert_Rules/Expert_rules_intrinsic_exceptional_V3.1.pdf, last access January 8th, 2018).
- 21.Tacconelli E, Sifakis F, Harbarth S, Schrijver R, van Mourik M, Voss A, et al. Surveillance for control of antimicrobial resistance. Lancet Infect Dis. 2017 pii: S1473-3099(17)30485-1. [DOI] [PubMed] [Google Scholar]
- 22.European Centre for Diseases Prevention and Control (ECDC).. European Antimicrobial Resistance Surveillance Network (EARS-Net) (http://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/ears-net, last access January 8th, 2018). [Google Scholar]
- 23.CDC.. Antibiotic/Antimicrobial resistance (http://www.cdc.gov/drugresistance/cdc_role.html, last access January 8th, 2018). [Google Scholar]
- 24.WHO . Global Antimicrobial Resistance Surveillance System (GLASS). (http://www.who.int/glass/en/http://www.who.int/glass/en/, last access January 8th, 2018). [Google Scholar]
- 25.Grau S, Bou G, Fondevilla E, Nicolás J, Rodríguez-Maresca M, Martínez-Martínez L. How to measure and monitor antimicrobial consumption and resistance. Enferm Infecc Microbiol Clin. 2013. September;31 Suppl 4:16-24PMID:. [DOI] [PubMed] [Google Scholar]
- 26.Mazuski JE, Tessier JM, May AK, Sawyer RG, Nadler EP, Rosengart MR, et al. The Surgical Infection Society Revised Guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt). 2017; 18:1-76. PMID: . [DOI] [PubMed] [Google Scholar]
- 27.Mouton JW, Brown DF, Apfalter P, Cantón R, Giske CG, Ivanova M, et al. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect. 2012. March; 18(3):E37-45. PMID: . [DOI] [PubMed] [Google Scholar]
- 28.Committee for Medicinal Products for Human Use (CHMP).. European Medicines Agency, Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. Document CPMP/EWP/558/95 rev 2. 15 December 2011. (http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003417.pdf, last access January 8th, 2018). [Google Scholar]
- 29.Food and Drug Administration.. The National Antimicrobial Resistance Monitoring System (NARMS) (https://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/default.htm, last access January 8th, 2018). [Google Scholar]
- 30.Karlowsky JA, Lob SH, Kazmierczak KM, Badal RE, Young K, Motyl MR, et al. In vitro activity of imipenem against carbapenemase-positive Enterobacteriaceae isolates collected by the SMART Global Surveillance Program from 2008 to 2014. J Clin Microbiol. 2017; 55:1638-49PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zalacain M, Biedenbach DJ, Badal RE, Young K, Motyl M, Sahm DF. Pathogen prevalence and antimicrobial susceptibility among Enterobacteriaceae causing hospital-associated intra-abdominal infections in adults in the United States (2012-2013). Clin Ther. 2016; 38:1510-21. PMID: . [DOI] [PubMed] [Google Scholar]
- 32.Cardoso T, Almeida M, Carratalà J, Aragão I, Costa-Pereira A, Sarmento AE, et al. Microbiology of healthcare-associated infections and the definition accuracy to predict infection by potentially drug resistant pathogens: a systematic review. BMC Infect Dis. 2015; 15:565. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kehl SC, Dowzicky MJ. Global assessment of antimicrobial susceptibility among Gram-negative organisms collected from pediatric patients between 2004 and 2012: results from the Tigecycline Evaluation and Surveillance Trial. J Clin Microbiol 2015; 53:1286-93. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sader HS, Farrell DJ, Flamm RK, Jones RN. . Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY Antimicrobial Surveillance Program, 2009-2012. Int J Antimicrob Agents. 2014; 43:328-34. PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, et al. European Society of Clinical Microbiology. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014. January;20 Suppl 1:1-55. PMID: . [DOI] [PubMed] [Google Scholar]
- 36.Cantón R, Ruiz-Garbajosa P. Co-resistance: an opportunity for the bacteria and resistance genes. Curr Opin Pharmacol. 2011; 11:477-85. PMID: . [DOI] [PubMed] [Google Scholar]
- 37.Hoban DJ, Badal R, Bouchillon S, Hackel M, Kazmierczak K, Lascols C, et al. In vitro susceptibility and distribution of beta-lactamases in Enterobacteriaceae causing intra-abdominal infections in North America 2010-2011 . Diagn Microbiol Infect Dis. 2014; 79:367-72. PMID: . [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Vázquez M, Oteo J, García-Cobos S, Aracil B, Harris SR, Ortega A, et al. Phylogeny, resistome and mobile genetic elements of emergent OXA-48 and OXA-245 Klebsiella pneumoniae clones circulating in Spain . J Antimicrob Chemother. 2016; 71:887-96. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oteo J, Pérez-Vázquez M, Bautista V, Ortega A, Zamarrón P, Saez D, et al. The spread of KPC-producing Enterobacteriaceae in SpaIn: WGS analysis of the emerging high-risk clones of Klebsiella pneumoniae ST11/KPC-2, ST101/KPC-2 and ST512/KPC-3. J Antimicrob Chemother. 2016; 71:3392-3399. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sousa D, Castelo-Corral L, Gutiérrez-Urbón JM, Molina F, López-Calviño B, Bou G, Llinares P. Impact of ertapenem use on Pseudomonas aeruginosa and Acinetobacter baumannii imipenem susceptibility rates: collateral damage or positive effect on hospital ecology? J Antimicrob Chemother. 2013; 68:1917-25. PMID: . [DOI] [PubMed] [Google Scholar]
- 41.Wilson APR. Sparing carbapenem usage. J Antimicrob Chemother. 2017; 72:2410-2417. PMID: . [DOI] [PubMed] [Google Scholar]