Abstract
It is essential to prevent thromboembolic events in atrial fibrillation. The risks of thromboembolic and haemorrhagic events must be carefully assessed and weighed against one another, both in routine situations and in relation to invasive procedures. Vitamin K antagonists, until recently the first-line treatment for prophylaxis against thromboembolic events in patients with atrial fibrillation, have various drawbacks. Direct-acting oral anticoagulants overcome these limitations and are efficacious and safe. The recent developments of tests to monitor anticoagulant levels, and of target-specific reversal agents for these newer drugs, has facilitated their use in several situations, including emergencies. For these reasons, the European Society of Cardiology and other scientific societies now recommend direct-acting oral anticoagulants as first-line treatment for preventing thromboembolic events in atrial fibrillation.
Keywords: Atrial fibrillation, coagulation monitoring, direct-acting oral anticoagulants, periprocedural management, reversal agents, risk stratification
Atrial fibrillation (AF) is the most commonly encountered arrhythmia in clinical practice in Western countries. The prevalence of AF depends on the population studied[1] and especially on age.[2–9] It is affected by increasing longevity and is modulated by the prevalence of cardiovascular risk factors, especially arterial hypertension and related habits. In Spain, for example, the prevalence of AF among people >40 years of age is about 4.4 %,[9] rising to 8.5 % among those >60 years and reaching 16.5 % among those >85 years.[4] The prevalence of AF is expected to double in the next 50 years.[10,11]
AF is characterised by the anarchic (fast and disorganised) and unpredictable contraction of atrial muscle fibres. This arrhythmia is appears on an electrocardiogram as an absence of P-waves and irregular R-R intervals. It is usually associated with tachycardia. The resulting asynchrony leads to ineffective contraction, decreased ventricular ejection fraction and blood pooling, predisposing to coagulation inside the atrium and increasing the risk of thromboembolic events.
AF increases the risk of mortality and morbidity, resulting in high healthcare costs. It increases the probability of stroke by two- to sixfold and the probability of death by 1.5-fold to 2.2-fold.[12–18] Moreover, the risk of stroke recurrence is higher in patients with AF than in those without. AF has been also associated with cognitive dysfunction, diminished quality of life and diminished functional capacity.[19–22]
The prevention and treatment of AF is important for both patients and healthcare systems. The complexity of the mechanisms involved calls for a multidimensional approach. Since AF is potentially dangerous, efforts to correct and/or control it are required; however, for various reasons these efforts often fall short. The efficacy of antiarrhythmic drugs is unpredictable, depending mainly on the duration of AF and the patient’s underlying heart disease. Moreover, antiarrhythmic drug treatment can cause proarrhythmia. AF can also recur after catheter ablation. Given the uncertain success of attempts to directly treat AF, it is therefore important to manage the attendant increased risk of thromboembolic events; most patients with AF will eventually need anticoagulant therapy to prevent thromboembolism.[21,23,24]
During the past 50 years, vitamin K antagonists (VKAs) have become the first-line oral anticoagulant treatment of choice for preventing thromboembolic events.[25] Although VKAs improve prognosis by reducing thromboembolic events, they have diverse clinical limitations (see Figure 1).[1] VKAs significantly increase the risk of minor and major bleeding complications, of which intracranial haemorrhage is particularly harmful. They can interact with many drugs and foods, and their effects are also influenced by hepatic metabolism. Regular monitoring and dose adjustments are thus essential to keep patients within the narrow therapeutic range throughout VKA treatment.
Figure 1: Limitations of Treatment with Vitamin K Antagonists.
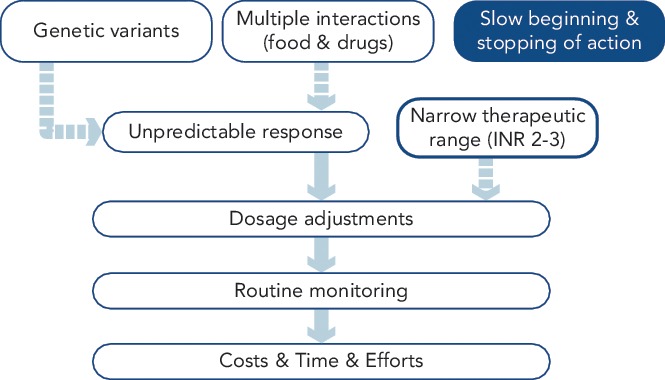
Drawbacks of vitamin K antagonists include the slow onset and offset of anticoagulation and a narrow therapeutic range. Genetic variants and multiple interactions with other drugs and food mean that patients’ responses are unpredictable, making routine monitoring and dose adjustments essential. Abbreviations: INR = international normalised ratio; VKA = vitamin K antagonists. Source: adapted from Martínez-Rubio et al.[1] With the permission from Spanish Society of Cardiology and Elsevier España S.L., © 2013, all rights reserved.
The novel direct-acting oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, apixaban and edoxaban have been developed to overcome the limitations of VKAs and are now considered a valid alternative.[21] Compared to VKAs, DOACs are at least as effective in reducing stroke and systemic embolism and are associated with a lower risk of haemorrhage.[26–29] Another benefit is their predictable, dose-related effects that do not require close monitoring.[26–30] The beneficial effects of DOACs over VKAs have been documented in several subsets of patients with AF including patients with diabetes mellitus, with heart failure and with previous stroke.[31]
Until recently, the main drawback of novel anticoagulants was the lack of an agent to reverse their effects. Now, however, various clinically-effective antidotes have been developed.[32–35] Another factor against the use of DOACs is their cost. Although they are more expensive than VKAs, comparisons of the overall costs of the two treatment strategies in various contexts have demonstrated that DOACs can be a cost-effective alternative to dose-adjusted warfarin for stroke prevention in AF in most patients.[36–41] For these reasons, the European Society of Cardiology and several other scientific societies now recommend using DOACs as first-line therapy.[23,42,43]
This review presents the current recommendations for the use of DOACs in patients with nonvalvular AF at high risk of bleeding.
Stratifying the Risk of Thromboembolism and Bleeding in AF
All anticoagulants increase the risk of bleeding. The benefits of decreasing the risk of thromboembolism must be weighed against the potential harm of increasing the risk of bleeding. Several scores have been proposed to assess these risks. Currently, the most widely recommended and used scores are the CHA2DS2-VASc (Congestive heart failure, Hypertension, Age >75 years, Diabetes, prior Stroke/transient ischemic attack, Vascular disease, Age 65–74 years, Sex category) for thromboembolism and the HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history of/predisposition, Labile international normalised ratio (INR), Elderly, Drug therapy/alcohol intake) for bleeding. These scores have been validated in very broad populations.[44–48]
The European Society of Cardiology has proposed an algorithm for managing thromboembolic and haemorrhagic risk in patients with AF.[23] Other strategies for reducing thromboembolic risk include aspirin and antiplatelet drugs; however, compared to anticoagulation alone, aspirin alone provokes similar indexes of intracranial bleeding and higher rates of other major bleeding events.[47] There is thus no argument for using aspirin instead of anticoagulation because of bleeding risk.[49] Aspirin plus antiplatelet therapy or, less effectively, aspirin alone may, however, be considered in patients who refuse oral anticoagulant treatment. Importantly, the HAS-BLED score should not be used to exclude patients from treatment; rather it should be used to correct potentially reversible risk factors and to determine whether selected patients with the highest risk of bleeding could benefit from low doses of DOACs. Chao et al. recently analysed the risk of stroke in 186,570 patients with AF not using antiplatelet or anticoagulant agents to determine whether patients with a single risk factor (apart from sex) should receive oral anticoagulation.[50] Analysing the impact of the components of the CHA2DS2-VASc score, they found that the weight of the components differed; the risk of stroke was highest for age, followed by the presence of diabetes mellitus. Thus, given the high risk of ischaemic stroke, oral anticoagulation is recommended in all patients with CHA2DS2-VASc scores greater than two and in most patients with CHA2DS2-VASc scores of one, unless the only risk factor is female sex.[49,50]
Results of Pivotal Clinical Trials Using DOACs
To date, four extensive randomised clinical trials comparing four DOACs (dabigatran, rivaroxaban, apixaban and edoxaban) with warfarin in different cohorts of patients with nonvalvular AF have been published.[26–29] Table 1 summarises the results of these trials, which have led to the authorisation of these DOACs for clinical use in AF in several countries. Since the publication of these trials, the results of two large observational studies that equalled or surpassed the clinical results obtained in the clinical trials have been published, further supporting the use of DOACs.[51,52] The efficacy and safety of DOACs can thus be considered as good as or possibly better than those of VKAs,[26–29,53] and DOACs have the additional advantage that their effects are dose-dependent and predictable. Furthermore, the advantages of DOACs over VKAs have been demonstrated in several specific groups of patients with AF (e.g. patients of both genders or with comorbidities such as heart failure, arterial hypertension, diabetes mellitus and previous stroke).[31]
Table 1: Percentage Relative Risk Reduction for Major Events Determined by the Pivotal Clinical Trials of Direct-acting Oral Anticoagulants versus Warfarin.
| Variable | Dabigatran 150 mg twice daily (%) | Dabigatran 110 mg twice daily (%) | Rivaroxaban 20 mg once daily (%) | Apixaban 5 mg twice daily (%) | Edoxaban 60 mg once daily (%) | Edoxaban 30 mg once daily (%) |
|---|---|---|---|---|---|---|
| Stroke and systemic embolism | 35 | n.s. | n.s. | 21 | n.s. | n.s. |
| Ischaemic stroke | 24 | n.s. | n.s. | n.s. | n.s. | ↑ 41 |
| Haemorrhagic stroke | 74 | 69 | 41 | 49 | 46 | 67 |
| Intracranial bleeding | 59 | 70 | 33 | 58 | 53 | 70 |
| Total mortality | n.s. | n.s. | n.s. | 11 | n.s. | 13 |
| Vascular mortality | 15 | n.s. | n.s. | n.s. | 14 | 15 |
Monitoring of DOACs
Although routine monitoring of coagulation levels is not necessary in patients on DOACs, simple and widely-available tests (see Table 2) help measure their anticoagulant effects if unexpected situations, such as urgent surgery, haemorrhagic events, overdose or acute renal failure, require it.
Table 2: Measurement of the Anticoagulant Effects of Direct Oral Anticoagulants using Specific and Non-specific Assays.
| Test | Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|---|
| Specific assay | Drug specific | Hemoclot and ecarin clotting-time | Anti-Xa | Anti-Xa |
| Non-specific assays | aPTT PT TT |
↑↑↑ ↑ ↑↑↑↑ |
↑ ↑↑ No effect |
↑ ↑↑ No effect |
Dabigatran prolongs the activated partial thromboplastin time (aPTT), but this effect is not linear and the sensitivity of aPTT reagents varies greatly. A trough aPTT (>12 hours after the most recent dose) >80 seconds or two- to three-times higher than the baseline value is associated with a higher risk of bleeding, whereas a normal aPTT indicates that dabigatran has no clinically-significant anticoagulant effect.[54] A normal thrombin time (TT) is an indicator of a drug concentration outside the clinically-relevant range.[55] The ecarin clotting time (ECT) measures dabigatran activity and the diluted thrombin time with an appropriate dabigatran calibrator (Hemoclot® thrombin inhibitor assay) measures dabigatran concentration. Dabigatran plasma concentration >200 ng/ml or an ECT three to four times the baseline value or >65 seconds at trough is associated with increased bleeding risk.[54] Prothrombin time (PT) and INR are not useful for measuring dabigatran’s effects.[56]
PT is of limited value for monitoring the anti-Xa anticoagulants rivaroxaban, apixaban and edoxaban. Rivaroxaban and apixaban may prolong PT, but PT is highly dependent on the reagent used in the assay.[55–57] A normal PT, however, indicates that these drugs are not having a clinically-significant effect. PT, aPTT and INR should not be used to measure edoxaban’s effects due to this lack of evidence, presumed insensitivity, the significant variation between reagents and lack of standardisation, which also effect the measurements of other direct anti-Xa inhibitors.[56] Anti-factor Xa assays using rivaroxaban, apixaban and edoxaban standards do, however, provide accurate information and seem the best approach to quantifying the anticoagulant effects of these drugs.[55,57]
Reversal of DOAC effects
To reverse the effects of a DOAC, it is essential to know the type of DOAC administered, the dosing regimen, the time since the last dose was administered and factors influencing plasma concentration, e.g. renal failure. Time reduces the effects of anticoagulants. Currently-available DOACs have short half-lives (about 12–15 hours), and their effects would be expected to completely disappear by four drug half-lives (after about 48–60 hours).[58] DOACs are absorbed and have an anticoagulant effect 1–4 hours after consumption, so early gastric lavage can be considered if little time has elapsed since the last dose. The administration of oral activated charcoal is useful within 2 hours of dabigatran intake and within 6 hours of apixaban intake.[58] The clearance of all DOACs depends to varying extents on renal function, so adequate hydration and diuresis are essential. Haemodialysis can be used for the emergency elimination of dabigatran; however, the risk of bleeding at puncture sites for dialysis needs to be carefully balanced against the risk of waiting. Nonspecific procoagulant agents (prothrombin complex concentrates and activated factor VIIa) have been used to treat serious bleeding, but the results are controversial.[58,59]
The recent advent of target-specific reversal agents that enable the effects of DOACs to be reversed within a few minutes (Table 3) represents a major safety advance in urgent situations.[32–35,60] One such agent, idarucizumab, is a humanised monoclonal antibody fragment with 350 times higher affinity for dabigatran than thrombin but lacks thrombin-like enzymatic activity and does not bind thrombin substrates.[60] It is easily administered intravenously (5 g as two 50-ml bolus infusions, no more than 15 minutes apart) and specifically and completely reverses the anticoagulant effects of dabigatran in few minutes. Ex vivo studies in rats have shown that steady-state dabigatran levels of 200 ng are completely reversed within 1 minute after the administration of an intravenous bolus of idarucizumab.[60] The safety and efficacy of idarucizumab have also been demonstrated in patients requiring urgent procedures or presenting with severe bleeding.[32] This drug is available for clinical use in some countries, obviating the need for dialysis in emergencies.[32,33]
Table 3: Agents that Reverse the Effects of Direct-acting Anticoagulants.
| Idaraucizumab | Andexanet alpha | Cirapantag | |
|---|---|---|---|
| Structure | Humanised Fab fragment | Human rXa variant | Synthetic water-soluble molecule |
| Target | Dabigatran | FXa inhibitors | Factor Xa and factor lla as well as heparin-based anticoagulants |
| Administration | Bolus | Bolus and infusion | Bolus |
| Clinical studies | Rapid, complete reversal | Rapid, complete reversal | Rapid, complete reversal |
Another target-specific reversal agent, andexanet alfa, has been designed specifically to reverse the anticoagulant effects of factor Xa inhibitors.[34,35,60] Andexanet alfa is a recombinant modified decoy of factor Xa. Its efficacy has been demonstrated in healthy volunteers treated with apixaban or rivaroxaban; it reverts anticoagulant activity within minutes after administration and for the duration of infusion.[35] In these healthy volunteers, transient increases in D-dimer and prothrombin fragments 1 and 2 without clinical thrombotic events have been observed. The dose of andexanet alfa depends on the DOAC. Whereas a 400-mg intravenous bolus followed by a continuous infusion of 4 mg/min for 120 minutes reverses the effects of 5 mg of apixaban twice daily, reversing the effects of 20 mg of rivaroxaban once daily requires 800 mg as an intravenous bolus (30 mg/min) followed by continuous infusion of 8 mg/min for 120 minutes.[35]
PER977, also called aripizine or ciraparantag, can also reverse the effects of factor Xa inhibitors. This small, synthetic, water-soluble molecule binds to direct inhibitors of factor Xa and factor IIa as well as to heparin-based anticoagulants. It antagonises the effects of all anticoagulants except VKAs and argatroban within 30 minutes after intravenous administration, and has a clearance half-life of about 1.5 hours.[60,61] To date, however, very few clinical data have been published[62] and the drug is not yet clinically and commercially available.
Periprocedural Management of Patients Treated with DOACs
One of the most important issues related to DOACs in daily clinical practice is appropriate periprocedural management to reduce the risk of bleeding events and the inherent risk of thromboembolic events. This challenge encompasses a wide range of clinical scenarios, including elective and urgent surgery as well as circumstances involving the risk of fatal haemorrhage, such as multiple traumas.
The first step in the periprocedural management of a patient on a DOAC is to determine the risks of thromboembolism with the CHADS-VASc score and bleeding with the HAS-BLED score.[23,24] Next, the inherent risk of bleeding associated with the invasive procedure to be undertaken must be determined and weighed against the benefit of remaining on anticoagulants on a case-by-case basis. Clinical guidelines detailing the risks involved in different invasive procedures and recommendations to minimise them[63,64] have proven very useful in clinical practice.[65]
The decision to continue or to pause anticoagulant treatment should be based on pharmacokinetic principles and the estimated thromboembolic and bleeding risks. Interestingly, accumulating evidence is leading to a consensus that bridging with heparin is unnecessary in patients treated with a DOAC[64–66] and that the availability of fast-acting reversal agents minimises anticoagulant-related bleeding during urgent or emergent interventions.
Conclusion
AF is very common and is associated with increased morbidity, mortality and healthcare costs. Appropriate clinical management, including the prevention of thromboembolic events, is thus crucial. Preventing thromboembolic events with VKAs has various clinical limitations; DOACs overcome these limitations and have proven efficacious and safe. The recent developments of tests that allow the monitoring of anticoagulant levels and of target-specific reversal agents for DOACs have facilitated the use of these drugs in several situations, including emergencies.
References
- 1.Martínez-Rubio A, Pujol Iglesias E, Bonastre Thió M et al. Epidemiologia de la fibrilación auricular en España. Rev Esp Cardiol. 2013;13:3–8. doi: 10.1016/j.recesp.2013.07.015. [DOI] [Google Scholar]
- 2.Masià R, Sala J, Marrugat J, Pena A. Prevalence of atrial fibrillation in the province of Girona, Spain: the REGICOR study. Rev Esp Cardiol. 2001;54:1240. doi: 10.1016/s0300-8932(01)76486-x. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Acuna JM, Gonzalez-Juanatey JR, Alegria Ezquerra E et al. Permanent atrial fibrillation in heart disease in Spain. The CARDIOTENS study 1999. Rev Esp Cardiol. 2002;55:943–52. [Article in Spanish] [PubMed] [Google Scholar]
- 4.Cea-Calvo L, Redón J, Lozano JV et al. Prevalence of atrial fibrillation in the Spanish population aged 60 years or more. The PREV-ICTUS study. Rev Esp Cardiol. 2007;60:616–24. doi: 10.1157/13107118. [Article in Spanish] [DOI] [PubMed] [Google Scholar]
- 5.Morillas P, Pallarés V, Llisterri JL et al. Prevalence of atrial fibrillation and use of antithrombotics in hypertensive patients aged >or=65 years. The FAPRES trial. Rev Esp Cardiol. 2010;63:943–50. doi: 10.1016/s1885-5857(10)70188-2. [DOI] [PubMed] [Google Scholar]
- 6.López Soto A, Formiga F, Bosch Xet al. en representación de los investigadores del estudio ESFINGE. Prevalence of atrial fibrillation and related factors in hospitalized old patients: ESFINGE study. Med Clinica 2012138231–7.[Article in Spanish] 10.1016/j.medcli.2011.05.023 [DOI] [PubMed] [Google Scholar]
- 7.Barrios V, Calderón A, Escobar C et al. Patients with atrial fibrillation in a primary care setting: Val-FAAP study. Rev Esp Cardiol. 2012;65:47–53. doi: 10.1016/j.recesp.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Clua-Espuny JL, Lechuga-Duran I, Bosch-Princep R et al. Prevalence of undiagnosed atrial fibrillation and of that not being treated with anticoagulant drugs: the AFABE study. Rev Esp Cardiol (Engl Ed) 2013;66:545–52. doi: 10.1016/j.rec.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Doblas JJ, Muñiz J, Martin JJA et al. OFRECE Study Collaborators. Prevalence of atrial fibrillation in Spain. OFRECE Study Results. Rev Esp Cardiol (Engl Ed) 2014;67:259–69. doi: 10.1016/j.rec.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Hylek EM, Phillips KA et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 11.Miyasaka Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 12.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–57. Anonymous. [PubMed] [Google Scholar]
- 13.Stewart S, Hart CL, Hole DJ et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–64. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 14.Flegel KM, Shipley MJ, Rose G. Risk of stroke in non-rheumatic atrial fibrillation. Lancet. 1987;1:526–9. doi: 10.1016/s0140-6736(87)90174-7. [DOI] [PubMed] [Google Scholar]
- 15.Krahn AD, Manfreda J, Tate RB et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Wolf PA, D’Agostino RB et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 17.Vidaillet H, Granada JF, Chyou Po et al. A population-based study of mortality among patients with atrial fibrillation or flutter. Am J Med. 2002;113:365–70. doi: 10.1016/s0002-9343(02)01253-6. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin EJ, Levy D, Vaziri SM et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 19.Fuster V, Ryden LE, Cannom DS et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Comm. J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Kelly-Hayes M, Beiser A, Kase CS et al. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–26. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 21.Camm AJ, Kirchhof P, Lip GYH et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–429. doi: 10.1093/eurheartj/ehq278. European Heart Rhythm Association, European Association for Cardio-THoracic Surgery. [DOI] [PubMed] [Google Scholar]
- 22.Knecht S, Oelschlager C, Duning T et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J. 2008;29:2125–32. doi: 10.1093/eurheartj/ehn341. [DOI] [PubMed] [Google Scholar]
- 23.John Camm A, Lip GYH, De Caterina R et al. ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 24.January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary. J Am Coll Cardiol. 2014;64:2246–80. doi: 10.1016/j.jacc.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 26.Connolly SJ, Ezekowitz MD, Yusuf S et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 27.Patel MR, Mahaffey KW, Garg J et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 28.Granger CB, Alexander JH, McMurray JJV et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 29.Giugliano RP, Ruff CT, Braunwald E et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Rubio A, Dan GA, Kaski JC. Rivaroxaban and stroke prevention in patients with atrial fibrillation: new evidence. Expert Rev Cardiovasc Ther. 2014;12:933–47. doi: 10.1586/14779072.2014.931223. [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Rubio A, Martínez-Torrecilla R. Current evidence for new oral anticoagulants in the treatment of nonvalvular atrial fibrillation: comparison of substudies. Rev Esp Cardiol (Engl Ed) 2015;68:185–9. doi: 10.1016/j.rec.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Pollack CV, Reilly PA, Eikelboom J et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–20. doi: 10.1056/NEJMoa. [DOI] [PubMed] [Google Scholar]
- 33.Eikelboom JW, Quinlan DJ, Van Ryn J et al. Idarucizumab: the antidote for reversal of dabigatran. Circulation. 2015;132:2412–22. doi: 10.1161/CIRCULATIONAHA.115.019628. [DOI] [PubMed] [Google Scholar]
- 34.Ansell JE. Universal, class-specific and drug-specific reversal agents for the new oral anticoagulants. J Thromb Thrombolysis. 2016;41:248–52. doi: 10.1007/s11239-015-1288-1. [DOI] [PubMed] [Google Scholar]
- 35.Siegal DM, Curnutte JT, Connolly SJ et al. Andexanetalfa for the reversal of Factor Xa inhibitor activity. N Engl J Med. 2015;373:2413–24. doi: 10.1586/17474086.2016.1135046. [DOI] [PubMed] [Google Scholar]
- 36.Sorensen S V, Kansal AR, Connolly S et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb Haemost. 2011;105:908–19. doi: 10.1160/TH11-02-0089. [DOI] [PubMed] [Google Scholar]
- 37.Pink J, Lane S, Pirmohamed M et al. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ. 2011;343:d6333. doi: 10.1136/bmj.d6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation. 2011;123:2562–70. doi: 10.1161/CIRCULATIONAHA.110. [DOI] [PubMed] [Google Scholar]
- 39.Freeman J V, Zhu RP, Owens DK et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1–11. doi: 10.7326/0003-4819-154-1-201101040-00289. [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Anglade MW, Pham D et al. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110:845–51. doi: 10.1016/j.amjcard.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 41.GonzÁlez-Juanatey JR, Álvarez-Sabin J, Lobos JMet al. Cost-effectiveness of dabigatran for stroke prevention in non-valvular atrial fibrillation in Spain. Rev Esp Cardiol (Engl Ed) 201265901–10. 10.1016/j.recesp.2012.06.006PMID: [DOI] [PubMed] [Google Scholar]
- 42.January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American college of Cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03. DOI: [DOI] [PubMed] [Google Scholar]
- 43.Verma A, Cairns JA, Mitchell LB et al. CCS Atrial fibrillation guidelines committee. Can J Cardiol. 2014;30:1114–30. doi: 10.1016/j.cjca.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Lip GYH, Nieuwlaat R, Pisters R et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 45.Pisters R, Lane DA, Nieuwlaat R et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 46.Lip GYH, Frison L, Halperin JL et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731–8. doi: 10.1161/STROKEAHA.110.590257. [DOI] [PubMed] [Google Scholar]
- 47.Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–10. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 48.Olesen JB, Lip GYH, Hansen ML et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lip GYH, Skjøth F, Rasmussen LH et al. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol. 2015;65:1385–94. doi: 10.1016/j.jacc.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 50.Chao TF, Liu CJ, Wang KL et al. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol. 2015;65:635–42. doi: 10.1016/j.jacc.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 51.Tamayo S, Frank Peacock W, Patel M et al. Characterizing major bleeding in patients with nonvalvular atrial fibrillation: a pharmacovigilance study of 27 467 patients taking rivaroxaban. Clin Cardiol. 2015;38:63–8. doi: 10.1002/clc.22373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graham DJ, Reichman ME, Wernecke M et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–64. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 53.Ruff CT, Giugliano RP, Braunwald E et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 54.Huisman MV, Lip GYH, Diener HC et al. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost. 2012;107(5):838–47. doi: 10.1160/TH11-10-0718. [DOI] [PubMed] [Google Scholar]
- 55.Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64:1128–39. doi: 10.1160/TH11-10-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nutescu EA, Burnett A, Fanikos J et al. Pharmacology of anticoagulants used in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:15–31. doi: 10.1007/s11239-015-1314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagakari K, Emmi M, Iba T. Prothrombin time tests for the monitoring of direct oral anticoagulants and their evaluation as indicators of the reversal effect. Clin Appl Thromb Hemost. 2016. epub ahead of press. [DOI] [PubMed]
- 58.Sartori MT, Prandoni P. How to effectively manage the event of bleeding complications when using anticoagulants. Expert Rev Hematol. 2016;9:37–50. doi: 10.1586/17474086.2016.1112733. [DOI] [PubMed] [Google Scholar]
- 59.Franchini M, Bonfanti C, Mannucci PM. Management of bleeding associated with new oral anticoagulants. Semin Thromb Hemost. 2015;41:788–801. doi: 10.1055/s-0035-1556046. [DOI] [PubMed] [Google Scholar]
- 60.Das A, Liu D. Novel antidotes for target specific oral anticoagulants. Exp Hematol Oncol. 2015;4:25. doi: 10.1186/s40164-015-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gomez-Outes A, Suarez-Gea ML, Lecumberri R et al. Specific antidotes in development for reversal of novel anticoagulants: a review. Recent Pat Cardiovasc Drug Discov. 2014;9:2–10. doi: 10.2174/1574890109666141205132531. [DOI] [PubMed] [Google Scholar]
- 62.Ansell JE, Bakhru SH, Laulicht BE et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. 2014;371:2141–42. doi: 10.1056/NEJMc1411800. [DOI] [PubMed] [Google Scholar]
- 63.Fleisher LA, Fleischmann KE, Auerbach AD et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130:e278–333. doi: 10.1161/CIR.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 64.Kristensen SD, Knuuti J. New ESC/ESA guidelines on non-cardiac surgery: Cardiovascular assessment and management. Eur Heart J. 2014;35:2344–5. doi: 10.1093/eurheartj/ehu285. [DOI] [PubMed] [Google Scholar]
- 65.Schulman S, Carrier M, Lee AYY et al. Perioperative management of dabigatran: a prospective cohort study. Circulation. 2015;132:167–73. doi: 10.1161/CIRCULATIONAHA.115.015688. [DOI] [PubMed] [Google Scholar]
- 66.Healey JS, Eikelboom J, Douketis J et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126:343–8. doi: 10.1161/CIRCULATIONAHA.111.090464. [DOI] [PubMed] [Google Scholar]


