Abstract
Hypertension is the most common preventable cause of cardiovascular disease. Home blood pressure monitoring (HBPM) is a self-monitoring tool that can be incorporated into the care for patients with hypertension and is recommended by major guidelines. A growing body of evidence supports the benefits of patient HBPM compared with office-based monitoring: these include improved control of BP, diagnosis of white-coat hypertension and prediction of cardiovascular risk. Furthermore, HBPM is cheaper and easier to perform than 24-hour ambulatory BP monitoring (ABPM). All HBPM devices require validation, however, as inaccurate readings have been found in a high proportion of monitors. New technology features a longer inflatable area within the cuff that wraps all the way round the arm, increasing the ‘acceptable range’ of placement and thus reducing the impact of cuff placement on reading accuracy, thereby overcoming the limitations of current devices.
Keywords: Home blood pressure monitoring, hypertension
Hypertension increases the risk of heart attack, stroke, kidney disease, and heart failure[1] and is the leading preventable risk factor for global cardiovascular (CV) disease burden worldwide.[2] At ages 40–69 years, each increase of 20 mmHg in systolic blood pressure (BP) is associated with more than a doubling of the baseline mortality rate from cardiovascular disease (CVD).[3] However, despite the fact that the impact of BP on CV risk is supported by one of the greatest bodies of clinical trial data in medicine, few clinical studies have been devoted to the issue of BP measurement and its validity. Studies also lack consistency in the reporting of BP measurements and some do not even provide details on how BP monitoring was performed.[4] This article aims to discuss the advantages and disadvantages of home BP monitoring (HBPM) and examines new technology aimed at improving its accuracy.
The Use of Home Blood Pressure Monitoring
Office BP measurement is associated with several disadvantages. Large variability in office BP readings have been reported, both in clinical trials[5] and in the primary care setting.[6] A study in which repeated BP measurements were made over a 2-week period under research study conditions found variations of as much as 30 mmHg with no treatment changes.[7] A recent observational study required primary care physicians (PCPs) to measure BP on 10 volunteers. Two trained research assistants repeated the measures immediately after the PCPs. The PCPs were then randomised to receive detailed training documentation on standardised BP measurement (group 1) or information about high BP (group 2). The BP measurements were repeated a few weeks later and the PCPs’ measurements compared with the average value of four measurements by the research assistants (gold standard). At baseline, the mean BP differences between PCPs and the gold standard were 23.0 mmHg for systolic and 15.3 mmHg for diastolic BP. Following PCP training, the mean difference remained high (group 1: 22.3 mmHg and 14.4 mmHg; group 2: 25.3 mmHg and 17.0 mmHg). As a result of the inaccuracy of the BP measurement, 24–32 % of volunteers were misdiagnosed as having systolic hypertension and 15–21 % as having diastolic hypertension.[6]
Two alternative technologies are available for measuring out-of-office BP. Ambulatory BP monitoring (ABPM) devices are worn by patients over a 24-hour period with multiple measurements and are considered the gold standard for BP measurement.[8] The average of multiple measurements at home tends to be lower compared with the measurements in a surgery[5] and is more reproducible than clinic measurements.[5] It also has the advantage of measuring nocturnal BP and therefore allowing the detection of an attenuated dip during the night. However, ABPM monitors are expensive and, while cost-effective for the diagnosis of hypertension, are not practical for the long-term monitoring of BP.
In the past decade, HBPM has emerged as an effective and convenient means of screening for hypertension,[4,9] as well as being cost-effective.[10] Methods for non-invasive BP measurement include auscultatory, oscillometric, tonometry and pulse wave record and analysis. HBPM uses the same technology as ABPM monitors, but allows patients to monitor BP as often as they wish. The advantages and disadvantages of HBPM are summarised in Table 1. While ABPM provides BP information at many timepoints on a particular day during unrestricted routine daily activities, HBPM provides BP information obtained under fixed times and conditions over a long period; thus, HBPM gives stable readings with high reproducibility and has been shown to be as reliable as ABPM.[11–13]
Table 1: Advantages and Limitations of Home Blood Pressure Monitoring.
| Advantages | Limitations |
|---|---|
|
|
BP = blood pressure; CV = cardiovascular. Adapted from Parati, 2010.[15]
Recommendations for the Use of Home Blood Pressure Monitoring
National Institute for Clinical Excellence (NICE) guidelines[14] for HBPM recommend that when using HBPM to confirm a diagnosis of hypertension it is necessary to ensure that:
for each BP recording, two consecutive measurements are taken, at least 1 minute apart with the person seated;
BP is recorded twice daily, ideally in the morning and evening; and
BP recording continues for at least 4 days, ideally for 7 days.
Measurements taken on the first day should be discarded and the average value of the remaining days after day one is discarded be used.
Except for special cases (for example, patients with arrhythmias trained in auscultatory BP measurement), the use of auscultatory devices (mercury, aneroid or other) is not recommended for HBPM.[15] Monitors that use the oscillometric method are accurate, reliable, easy to use and relatively inexpensive.[16] Recommendations are in place to ensure the accuracy of monitoring devices:[17–19] in the UK and Ireland the Dabl Educational Trust[20] and the British Hypertension Society have produced lists of validated devices.[21] The European Society of Hypertension Working Group on Blood Pressure Monitoring has produced a detailed consensus document on guidelines for HBPM.[15] It recommends semi-automated (manual cuff inflation) or automated electronic devices that measure BP at the upper arm as the preferred option for HBPM. Such devices are easier to use and avoid observer bias. Monitors equipped with an automated memory should prevent patients from misreporting their BP measurements. Finger and wrist devices are less accurate and are not recommended, unless brachial measurements are difficult or impossible to obtain (for example, in subjects with very large arm circumference or extreme obesity).
Advantages of Home Blood Pressure Monitoring
Reproducibility and Accuracy
It has been found that HBPM readings are often lower than readings taken in the office and closer to the average BP recorded during 24-hour ABPM.[22] HBPM allows increased numbers of readings, achieves more reproducible readings than office readings and provides improved correlations with measures of target organ damage.[16,23–26]
A randomised controlled trial (n=555) compared manual BP measurement and automated measurement in an office setting and concluded that the quality and accuracy of automated office BP measurement was significantly higher compared with manual office BP measurement.[27] A retrospective analysis of a clinical trial (n=163) compared the within-patient variability of the different methods of BP measurement for at least 6 weeks and found coefficients of variation of 8.6 %, 5.5 %; and 4.2 % for office BPM, ABPM and HPBM, respectively. The study concluded that a week of self-monitoring was the most accurate method of measuring BP.[28] Another study (n=133) found that HBP has superior reproducibility compared with both office BPM and ABPM.[29]
White-coat Effect and Masked Hypertension
White-coat effect, defined as elevated office and low ambulatory or home BP, can manifest with very high clinic readings. The use of automated BP measurements have significantly reduced this effect in primary care settings.[27] The reverse phenomenon – i.e. normal clinic BP and elevated out-of-clinic BP – is termed masked hypertension and is associated with increased CV risk.[30,31] Some studies have found that HBPM is as effective as and more convenient than ABPM in the diagnosis of this phenomenon,[32,33] but others suggest that ABPM has greater sensitivity.[34] A recent meta-analysis found that HPBM is particularly useful in risk stratification in masked hypertension.[31] Both phenomena are relatively common, occurring in 10–15 % of patients with hypertension, but diagnosis requires physicians to be alert to the possibility, particularly with regard to masked hypertension.[30,35] The European Society of Hypertension has recommended that both white-coat and masked hypertension can be diagnosed using ambulatory or home BP measurements.[17]
Prediction of Cardiovascular and Stroke Morbidity and Mortality
Many,[24,26,36–39] but not all,[40,41] prospective studies have found that HBPM predicts CV and stroke[42] morbidity and mortality more accurately than office BP; the major studies are summarised in Table 2. A meta-analysis included 5,008 people who had home and conventional BP measurements and were not being treated with antihypertensive medication that would influence the prognostic outcome. Participants were stratified into five categories of BP: optimal, 120/80 mmHg; normal, 120–129/80–84 mmHg; high–normal, 130–139/85–89 mmHg; mild hypertension, 140–159/90–99 mmHg; and severe hypertension, ≥160/≥100 mmHg. At every level of BP below severe hypertension, the additional measurements obtained from HBPM improved risk stratification, supporting the use of HBPM in routine assessment of risk.[31] This finding could refine risk stratification in people with optimal, normal or high-normal BP, who are not conventionally treated. A recent systematic review (19 studies) compared HBPM with ABPM in terms of outcomes including heart attack, stroke, kidney failure and/or all-cause mortality and concluded that HBPM encourages patient-centred care and improves BP control and patient outcomes.[43]
Table 2: Prospective Studies Comparing HBPM and Office BPM in Terms of Stroke and Cardiovascular Risk.
| Study Type | Study Population | Findings | Reference |
|---|---|---|---|
| Prospective cohort study, n=1,769, mean 6.6 years | Age ≥40 years | Average of multiple (taken more than three times) home SBP but not screening values was significantly and strongly related to the CV mortality risk according to Cox model | Ohkubo et al., 1998[38] |
| Longitudinal study, n=209 | Age 31–86 | Correlation between LVMI, HBP and OBP was closer for HPB | Tsuonda, 2002[26] |
| Prospective cohort study, n=4,939, mean 3.2 years | Age 70 ± 6.5 years | For BP self-measurement at home, each 10 mmHg increase in SBP increased the risk of a cardiovascular event by 17.2 % (95 % CI 11.0 %–23.8 %); each 5 mmHg increase in diastolic BP increased that risk by 11.7 % (95 % CI 5.7 %-18.1 %). The same increase in BP observed using office measurement was not associated with a significant increase in the risk of a cardiovascular event | Bobrie et al., 2004[36] |
| Prospective cohort study, n=1,702, mean 11 years | Age ≥40 years | The JNC-7 classification had a stronger predictive power of stroke using HBP-based classification compared with CBP-based classification | Asayama et al., 2004[42] |
| Prospective cohort study, n=2,051, mean 131 months | Age 25–74 years | Office, home and ambulatory BP values showed a significant exponential direct relationship with risk of CV or all-cause death, greater for systolic than for diastolic BP and for night than for day BP, but not better for home or ambulatory than for office BP. The slope of the relationship, however, was progressively greater from office to home and ambulatory BP | Sega, 2005[40] |
| Prospective cohort study, n=391, median 10.9 years | Age 71 ± 9 years; | Incidence of major CV events (cardiovascular death, MI and stroke) was related to the BPs by use of multivariate Cox regression analysis. Prognostic value of home BP was better than that of office BP | Fagard et al., 2005[37] |
| Prospective cohort study, n=163, compared OBPM, ABPM and HPBM | Age 53.9 ± 14.5 years | In a multivariate regression analysis in which age, sex, body mass index, OBP, awake ABP and HBPM were included, only age, sex and HBP were significant predictors of LVMI | Shimbo et al., 2007[24] |
| Cross-sectional study, n=662, compared OBPM and HPBM | Mean age 54.1 ± 17.6 years | HBPM and office BPM were both significant predictors of cardiovascular risk but there was no significant prognostic superiority of HBPM over office BPM | Stergiou et al., 2007[41] |
| Prospective cohort study, n=2,081, median 6.8 years | Age 45–74 years | HBPM (HR 1.22/1.15, 95 % CI 1.09 to 1.37/1.05 to 1.26), but not office BP (HR 1.01/1.06, 95 % CI 0.92 to 1.12/0.97 to 1.16), was predictive of cardiovascular events. Systolic home BP was the sole predictor of total mortality (HR 1.11; 95 % CI 1.01/1.23) | Niiranen et al., 2010[39] |
| Meta-analysis, n=5,008, median 8.3 years | Mean age 57.1 years | HBPM substantially refines risk stratification at office BPM levels assumed to carry no or only mildly increased risk, in particular in the presence of masked hypertension | Asayama et al., 2014[31] |
| RCT, n=778 Intervention = usual care (control), HBPM monitoring + website training (group 1), or HBPM + website training plus pharmacist care management delivered through website (group 2). | Aged 25–75 years with uncontrolled hypertension | Group 1: non-significant increase in controlled BP 36 % [95 % CI 58, 30 %–42 %] versus 31 % (95 % CI 25 %–37 %); 0 = 0.21). Group 2: controlled BP in 56 %; 95 % CI 49 %–62% (p0<0.001) SBP decreased stepwise from control to group 1 to group 2. DBP decreased only group 2 |
Green et al., 2008[57] |
| 2 × 2 RCT, n=636 intervention = usual care, behavioural intervention (group 1), HBPM (group 2) or HPPM + behavioural intervention (group 3), 24 months | Mean age was 61 years, 49 % were African American, and 19 % reported having inadequate incomes | Improvements in BP control in 4.3 % (95 % CI -4.5 % to 12.9 %) of group 1, 7.6 % (CI -1.9 % to 17.0 %) of group 2, and 11.0 % (CI 1.9 %, 19.8 %) in group 3. Change in SBP was -0.6 mmHg (CI -2.2 to 3.4 mmHg) in group 1, -0.6 mmHg (CI -3.6 to 2.3 mmHg) in group 2, and -3.9 mmHg (CI -6.9 to -0.9 mmHg) in group 3; patterns were similar for DBP | Bosworth et al., 2009[58] |
| Cochrane review of interventions to control BP in hypertension, 72 RCT | Mixed | HBPM was associated with reduction in SBP (-2.5 mmHg, 95 % CI -3.7 to -1.3 mmHg) and DBP (-1.8 mmHg, 95 % CI: -2.4 to -1.2 mmHg) | Glynn et al., 2010[51] |
| RCT, n=527, 12 month Intervention = HBPM + telemonitoring | Aged 35–85 years, BP >140/90 mmHg despite antihypertensive treatment | Mean SBP decreased by 12.9 mmHg (95 % CI 10.4–15.5) at 6 month in self-management group and by 9.2 mmHg (6.7–11.8) in control group (p=0.013). At 12 months, SBP decreased by 17.6 mmHg (14.9-20.3) in self-management group and by 12.2 mmHg (9.5-14.9) in control group (p=0.0004) | McManus et al., 2010[61] |
| Meta analysis, 25 RCTs, | Mixed | HBPM was associated with reduction of SBP of -3.82 mmHg (95% confidence interval -5.61 to -2.03), and DBP -1.45 mmHg (-1.95 to -0.94). Self-monitoring increased the chance of meeting office BP targets RR = 1.09 (1.02 to 1.16)). Significant heterogeneity was observed between studies | Bray et al., 2010[49] |
| Meta analysis, 37 RCTs, n=9449 | Mixed | HPBM was associated with reductions in SBP (-2.63 mmHg; 95% CI -4.24, -1.02), DBP (-1.68 mmHg; 95% CI -2.58, -0.79 reductions in antihypertensive medication (RR 2.02 [95% CI 1.32 to 3.11]) and less therapeutic inertia defined as unchanged medication despite elevated BP (RR for unchanged medication, 0.82 [95% CI 0.68 to 0.99]) | Agarwal et al., 2011[50] |
| Cluster randomised trial, n=450, 12 month intervention and 6-month post-intervention follow up. Intervention = HBPM + telemonitoring | Uncontrolled BP, mean age 61.1±14 years | BP was controlled at 6 and 12 month in 57.2 % (95 % CI 44.8 % to 68.7 %) of intervention group versus 30.0 % (95 % CI 23.2 % to 37.8 %) of usual care (p = 0.001). At 18 mo, BP was controlled in 71.8 % (95 % CI 65.0 % to 77.8 %) of intervention group versus 57.1 % (95 % CI 51.5% to 62.6%) of usual care group (p = 0.003). SBP decreased more in intervention group at 6 months (-10.7 mmHg [95 % CI -14.3 to -7.3 mmHg]; p<.001), at 12 months (-9.7 mmHg [95 % CI -13.4 to -6.0 mmHg]; p<0.001), and at 18 months (-6.6 mmHg [95% CI -10.7 to -2.5 mmHg]; p=0.004). DBP decreased at 6 months (-6.0 mmHg [95 % CI -8.6 to -3.4 mmHg]; p<0.001), at 12 months (-5.1 mmHg [95 % CI -7.4 to -2.8 mmHg]; p<0.001), and at 18 months (-3.0 mmHg [95 % CI -6.3 to 0.3 mmHg]; p =0 .07) | Margolis et al., 2013[62] |
| Prospective cohort study. 9 months | Predominantly black and Hispanic adults with uncontrolled hypertentsion from clinics in low-income, medically underserved communities | 53 % of the patients had controlled hypertension at follow-up. Systolic and DBP decreased by 18.7 mmHg and 8.5 mmHg, respectively, at follow-up | Angell et al., 2013[52] |
| Randomised controlled trial, n=552, 12 months | History of stroke, coronary heart disease, diabetes, or CKD and with baseline blood pressure of at least 130/80 mmHg | Mean SBP decreased by 9.2 mmHg (95 % CI 5.7-12.7) in systolic and diastolic by 3.4 mmHg (95 % CI 1.8-5.0) | McManus et al., 2014[54] |
| Randomised controlled trial, n=900, 9 months | Predominantly black and Hispanic adults with uncontrolled hypertension from clinics in low-income, medically underserved communities | SBP decreased (intervention, 14.7 mmHg; control, 14.1 mmHg; p=0.70). Control was achieved in 38.9 % of intervention and 39.1% of control participants at the end of follow-up. No significant difference between groups | Yi et al., 2015[53] |
CI = confidence interval; CKD = chronic kidney disease; DBP = diastolic blood pressure; HBPM = home blood pressure monitoring; HR = hazard ratio; JNC-7 = Joint National Committee 7; LVMI = left ventricular mass index; RCT = randomised controlled trial; RR = relative risk; SBP = systolic blood pressure.
Large-scale studies are now investigating the optimum use of HBPM in prevention of CV outcomes. The multicentre Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED-BP; 2001–2010) trial (n=3,518) proved the feasibility of adjusting antihypertensive drug treatment via a computer algorithm that automatically generated treatment recommendations based on HBP and suggested that a systolic HBP level of 130 mmHg should be an achievable and safe target.[44] The Home Blood Pressure Measurement With Olmesartan Naive Patients to Establish Standard Target Blood Pressure (HONEST) study, a prospective observational study (n=21,591), found that morning HBP should be controlled to <145 mmHg.[45]
Other Advantages
Different methods of measuring BP response might clinically influence treatment decisions. A meta-analysis of more than 6,000 patients found that antihypertensive response to therapy measured by HBPM was 20 % less than office measurements.[46] In addition, a clinical study found that HBPM was similar to 24-hour ABPM in assessing BP response to the antihypertensive agents atenolol and hydrochlorothiazide.[47] Office BP measurements overestimated BP response compared with HBPM, with an average 4.6 mmHg greater reduction in SBP (p<0.0001) and 2.1 mmHg greater reduction in diastolic BP (p<0.0001) across all therapies.[47] These findings indicate that HBPM can influence management decisions in hypertension, particularly given the relative ease of incorporating HBPM into daily activities.
Compliance and Improvement of Blood Pressure Control
Incorporation of HBPM into routine management of patients with uncontrolled hypertension may improve BP control. Several meta-analyses have shown that compared with usual care, the use of HBPM is associated with significant reductions in systolic and diastolic BP,[48–51] as well as reductions in antihypertensive medication and therapeutic inertia, defined as unchanged medication despite elevated BP.[50] While most studies have focused on white populations, some studies have looked at ethnically diverse adults with uncontrolled BP[52,53] and high-risk patients (i.e. history of stroke, coronary heart disease, diabetes or chronic kidney disease (CKD) and with baseline BP of at least 130/80 mmHg) from clinics in low-income, medically underserved communities.[54] Studies are also ongoing in stroke and at-risk groups.[55]
HBPM is most effective when accompanied by input from a healthcare professional, e.g. telemonitoring, whereby readings made at home are instantly relayed to a primary healthcare professional who can guide treatment along a predetermined algorithm in such a way that treatment is effected by readings obtained in a more direct manner.[56–60] Numerous studies support the use of HBPM and telemonitoring,[61–63] and other studies are planned.[64] The use of telemonitoring avoids travel for the patient and saves time for the healthcare team. It has also been hypothesised that if patients can understand their own BP measurements and appreciate the impact of treatment, then they may be more likely to comply with medical therapy in the longer term, even if the treatment does not appear to be making them feel better.[4] There is a need for clinical trial data to confirm this hypothesis.
Some studies suggest that HBPM may contribute towards medication adherence in hypertensive patients,[65,66] although others have not reached this conclusion.[67] NICE guidelines still recommend ABPM where possible.
Cost-effectiveness of Home Blood Pressure Monitoring
A recent cost-benefit analysis found that HBPM is more effective than conventional clinic BP monitoring in the diagnosis and management of hypertension, is easier to implement and requires less labour and capital investment than ABPM.[10,68]
As a result of these findings, the American Heart Association, the American Society of Hypertension and the Preventive Cardiovascular Nurses’ Association have released a statement suggesting that HBPM be incorporated into usual care.[16] European guidelines also support the use of HBPM as an adjunct to conventional office management.[15,69]
Limitations of Home Blood Pressure Monitoring
Patient Groups
Further research is required in the clinical application of HBPM in certain patient groups; this includes children and adolescents. A systematic review of 27 studies found that HBPM has similar diagnostic value in children as in adults and appears to be a reliable alternative to ABPM monitoring in the detection of white-coat hypertension. However, systolic daytime BP readings in children was found to be lower when measured with than daytime ABPM, whereas no such difference exists in adults.[70] In patients with CKD, preliminary data suggest that HBPM outperforms office BP monitoring in predicting progression to end-stage renal disease or death.[71] When combined with additional support such as telemonitoring, medication titration or behavioural therapy, HBPM results in a sustained improvement in BP control. However, HBPM does not provide nocturnal recordings and therefore cannot give information on diurnal patterns in BP, which are more prevalent in the CKD population and are important CV risk factors.[71] Finally, caution should be exercised in the use of HBPM in the elderly. In a comparison of Korotkoff (K-BP, the traditional means of BP measurement employed in HBPM and office methods) and Strain-Gauge-Finger-Plethysmography (SG-BP) methods, K-BP underestimated BP in 46 % of subjects with SG-BP ≥140 mmHg at age 81.[72]
Accuracy of Home Blood Pressure Monitors
Accuracy of devices remains a limiting factor associated with HBPM. In a 2009 study to determine the accuracy of 554 automated HBPM devices, only 30 % of the devices were found to have acceptable validation, while 72 % of the automated monitors were inaccurate. The frequency of accuracy was higher among validated devices compared with non-validated devices.[73] In a 2011 study, only 30 % of the 382 devices studied had been acceptably validated and 24 % of the devices were inaccurate. Upper arm devices were more accurate than wrist devices. The categorisation of upper arm devices into validated and ‘others’ showed that the validated devices were more accurate than the ‘others’.[74] A recent retrospective review analysed ‘real use’ data from 210 patients attending hypertension clinics and found that 30 % of HBPM readings were >5 mmHg different and 8 % were >10 mmHg different from mercury systolic BP measurement taken in the clinic (see Figure 1). For diastolic BP, the proportions were 32 % and 9 %, respectively.[75] In addition, a 2005 analysis of 30 studies found that the accuracy of most devices tends to decrease at higher BP levels.[76] However, the study’s author suggested that the reported decrease in accuracy might be explained by the fact that BP is more variable at higher levels and by the use of sequential measurements.
Figure 1: Accuracy of Home Blood Pressure Monitors in the Measurement of Systolic Blood Pressure.
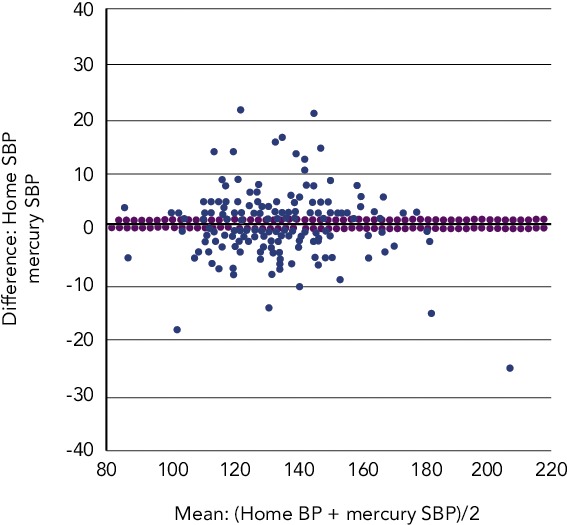
BP = blood pressure; SBP = systolic blood pressure. Source: Hiremath, 2014.[75]
The match between upper arm circumference and cuff size is essential to the accuracy of HBPM monitors; inappropriate cuff size has been associated with inaccuracy; studies have suggested that different cuffs should be used for BP measurement in child, adult and obese patients.[77,78] The inflatable bladder of the cuff should cover 80–100 % of the individual’s arm circumference.[15] The use of too small a cuff for the size of the arm can result in overestimation of BP, whereas a too large one results in an underestimation. Although standard cuffs are appropriate for most patients, in those with small (<24 cm) or large (>32 cm) arm circumference only the devices equipped with appropriate sized cuffs should be used.[15] A study of six cuffs of various lengths and widths concluded a single long bladder cuff can measure BP with comparable accuracy both in subjects with large arms and in subjects with normal sized arms.[79]
New Technology to Improve Reading Accuracy of Home Blood Pressure Monitoring
In order to measure BP accurately, the cuff must be wrapped correctly and proper posture of the patient during the measurement is essential. Users can find it difficult to wrap the cuff in the correct position, especially if they are inexperienced. If the cuff is wrapped incorrectly then the result is less accurate. In addition, irregular pulses due to arrhythmias lead to inaccurate BP readings. Home monitoring devices equipped with automated cuff wrapping and a display indicating correct positioning have been introduced.[80] These devices can detect irregular pulses that cause inaccurate BP readings. Moreover, they detect noises and wave pulses within the cuff that are caused by arm movements. These device functions may help provide more accurate BP readings.[80]
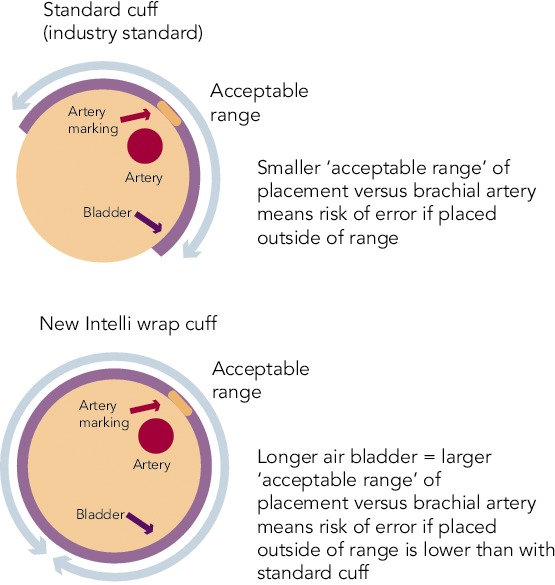
The latest development in HPBM technology is Intelli wrap cuff technology. This features a longer inflatable area within the cuff that wraps all the way round the arm. This reduces pressure loss on the brachial artery, increasing the ‘acceptable range’ of placement and thus reducing the impact of cuff placement on accuracy (see Figure 2). It is also pre-formed, enabling it to be easily fitted with one hand. An ongoing study evaluated a validated oscillometric device (Omron M6-Comfort; HEM-7321-E) coupled with the Intelli wrap cuff in subjects (planned n=50) aged 50.7 ± 16.0 years with arm circumference 33.3 ± 4.4cm, body mass index 32.8 ± 7.9 kg/m2 and in stable clinical condition. Interim data from this study has recently been presented and shows that incorrect positioning of a conventional cuff significantly affects BP measurement results, with the greatest overestimation of BP when the bladder centre is displaced by 90° laterally or by 180° compared with the correct position. When the Intelli Wrap cuff was used, there was no significant effect resulting from cuff position. BP values obtained with the oscillometric device tended to be lower than those obtained by reference method because of relative undercuffing with the mercury device equipped with standard size cuff in subjects with a large arm circumference.[81]
Figure 2: Intelli Wrap Cuff Technology.
Summary and Conclusion
The growing burden of hypertension has seen an increase in the use and availability of HBPM devices. HBPM provides extensive BP information obtained under fixed timeframes and conditions over a long period; thus, the mean values of HBP are stable and the reproducibility are high. The use of HBPM devices is cost-effective and has stronger prognostic value in terms of CV risk when compared with clinic BP measurement. HBPM is easy to incorporate into normal daily routines, can accurately assess response to hypertensive therapy and enables remote consultations by use of telemonitoring. HBPM also appears to be valuable in assessing patients at risk who would not usually be considered as potentially benefitting from treatment. However, recent data suggest that around a third of HBPM devices are inaccurate.[75] The Intelli Wrap cuff technology may reduce the impact of cuff placement and arm movements on accuracy, although further studies are required to confirm this.
Acknowledgments
Medical Media Communications (Scientific) Ltd provided medical writing and editing support to the authors, funded by Omron Healthcare.
References
- 1.Chobanian AV, Bakris GL, Black HR et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report, JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010, Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies, Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 4.Padfield PL. The case for home monitoring in hypertension, BMC Med. 2010;8:55. doi: 10.1186/1741-7015-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benediktsson R, Padfield PL. Maximising the benefit of treatment in mild hypertension:three simple steps to improve diagnostic accuracy, QJM. 2004;97:15–20. doi: 10.1093/qjmed/hch005. [DOI] [PubMed] [Google Scholar]
- 6.Sebo P, Pechere-Bertschi A, Herrmann FR et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens, 2014;32:509–17. doi: 10.1097/HJH.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 7.Padfield PL. Self-monitored blood pressure: a role in clinical practice?, Blood Press Monit. 2002;7:41–4. doi: 10.1097/00126097-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Little P, Barnett J, Barnsley L et al. Comparison of agreement between different measures of blood pressure in primary care and daytime ambulatory blood pressure. BMJ. 2002;325:254. doi: 10.1136/bmj.325.7358.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai Y, Obara T, Asamaya K et al. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res. 2013;36:661–72. doi: 10.1038/hr.2013.38. [DOI] [PubMed] [Google Scholar]
- 10.Arrieta A, Woods JR, Qiao N et al. Cost-benefit analysis of home blood pressure monitoring in hypertension diagnosis and treatment: an insurer perspective, Hypertension. 2014;64:891–6. doi: 10.1161/HYPERTENSIONAHA.114.03780. [DOI] [PubMed] [Google Scholar]
- 11.Appel LJ, Stason WB. Ambulatory blood pressure monitoring and blood pressure self-measurement in the diagnosis and management of hypertension, Ann Intern Med. 1993;118:867–82. doi: 10.7326/0003-4819-118-11-199306010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Jula A, Puukka P, Karanko H. Multiple clinic and home blood pressure measurements versus ambulatory blood pressure monitoring. Hypertension. 1999;34:261–6. doi: 10.1161/01.hyp.34.2.261. [DOI] [PubMed] [Google Scholar]
- 13.Ntineri A, Nasothimiou E, Kollias A et al. 3c.05: Diagnostic agreement of the European Society of Hypertension Home Blood Monitoring Schedule with ambulatory blood pressure monitoring in untreated and treated subjects. J Hypertens. 2015;33(Suppl 1):e38. [Google Scholar]
- 14.https://www.nice.org.uk/guidance/cg127. https://www.nice.org.uk/guidance/cg127 NICE. Hypertension: Clinical management of primary hypertension in adults. Available at: (accessed 29 June 2015).
- 15.Parati G, Stergiou GS, Asmar R et al. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779–85. doi: 10.1038/jhh.2010.54. [DOI] [PubMed] [Google Scholar]
- 16.Pickering TG, Miller NH, Ogedegbe G et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1–9. doi: 10.1161/HYPERTENSIONAHA.107.189011. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien E, Waeber B, Parati G et al. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–6. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien E, Petrie J, Littler W et al. An outline of the revised British Hypertension Society protocol for the evaluation of blood pressure measuring devices, J Hypertens. 1993;11:677–9. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Vol. 40 Arlington, VA: AAMI; 1993. ANSI/AAMI SP 10 1992. Association for the Advancement of Medical Instrumentation. American National Standard. Electronic or automated sphygmomanometers. [Google Scholar]
- 20.Sphygmomanometers for Self-measurement of Blood Pressure (SBPM). http://www.dableducational.org/sphygmomanometers/recommended_cat.html. http://www.dableducational.org/sphygmomanometers/recommended_cat.html dabl Educational Trust. Available at: (accessed 28 April 2015).
- 21.Blood pressure monitors validated for home use. www.bhsoc.org//index.php?cID=246. www.bhsoc.org//index.php?cID=246 British Hypertension Society. (accessed 28 April 2015).
- 22.McGowan N, Padfield PL. Self blood pressure monitoring: a worthy substitute for ambulatory blood pressure? J Hum Hypertens. 2010;24:801–6. doi: 10.1038/jhh.2010.15. [DOI] [PubMed] [Google Scholar]
- 23.Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919–27. doi: 10.1097/HJH.0b013e32830c4368. [DOI] [PubMed] [Google Scholar]
- 24.Shimbo D, Pickering TG, Spruill TM et al. Relative utility of home, ambulatory, and office blood pressures in the prediction of end-organ damage, Am J Hypertens. 2007;20:476–82. doi: 10.1016/j.amjhyper.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mule G, Caimi G, Cottone S et al. Value of home blood pressures as predictor of target organ damage in mild arterial hypertension. J Cardiovasc Risk. 2002;9:123–9. doi: 10.1177/174182670200900208. [DOI] [PubMed] [Google Scholar]
- 26.Tsunoda S, Kawano Y, Horio T et al. Relationship between home blood pressure and longitudinal changes in target organ damage in treated hypertensive patients. Hypertens Res. 2002;25:167–73. doi: 10.1291/hypres.25.167. [DOI] [PubMed] [Google Scholar]
- 27.Myers MG, Godwin M, Dawes M et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial, BMJ. 2011;342:d286. doi: 10.1136/bmj.d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren RE, Marshall T, Padfield PL et al. Variability of office, 24-hour ambulatory, and self-monitored blood pressure measurements. Br J Gen Pract. 2010;60:675–80. doi: 10.3399/bjgp10X515403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stergiou GS, Baibas NM, Gantzarou AP et al. Reproducibility of home, ambulatory, and clinic blood pressure: implications for the design of trials for the assessment of antihypertensive drug efficacy. Am J Hypertens. 2002;15:101–4. doi: 10.1016/s0895-7061(01)02324-x. [DOI] [PubMed] [Google Scholar]
- 30.Stergiou GS, Asayama K, Thijs L et al. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675–82. doi: 10.1161/HYPERTENSIONAHA.113.02741. [DOI] [PubMed] [Google Scholar]
- 31.Asayama K, Thijs L, Brguljan-Hitij J et al. Risk stratification by self-measured home blood pressure across categories of conventional blood pressure: a participant-level meta-analysis. PLoS Med. 2014;11:e1001591. doi: 10.1371/journal.pmed.1001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stergiou GS, Salgami EV, Tzamouranis DG et al. Masked hypertension assessed by ambulatory blood pressure versus home blood pressure monitoring: is it the same phenomenon? Am J Hypertens. 2005;18:772–8. doi: 10.1016/j.amjhyper.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Mancia G, Bombelli M, Brambilla G et al. Long-term prognostic value of white-coat hypertension: an insight from diagnostic use of both ambulatory and home blood pressure measurements. Hypertension. 2013;62:168–74. doi: 10.1161/HYPERTENSIONAHA.111.00690. [DOI] [PubMed] [Google Scholar]
- 34.Kang YY, Li Y, Huang QF et al. Accuracy of home versus ambulatory blood pressure monitoring in the diagnosis of white-coat and masked hypertension. J Hypertens. 2015;33:1580–7. doi: 10.1097/HJH.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 35.Hansen TW, Kikuya M, Thijs L et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25:1554–64. doi: 10.1097/HJH.0b013e3281c49da5. [DOI] [PubMed] [Google Scholar]
- 36.Bobrie G, Chatellier G, Genes N et al. Cardiovascular prognosis of “masked hypertension” detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA. 2004;291:1342–9. doi: 10.1001/jama.291.11.1342. [DOI] [PubMed] [Google Scholar]
- 37.Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens. 2005;19:801–7. doi: 10.1038/sj.jhh.1001903. [DOI] [PubMed] [Google Scholar]
- 38.Ohkubo T, Imai Y, Tsuji I et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16:971–5. doi: 10.1097/00004872-199816070-00010. [DOI] [PubMed] [Google Scholar]
- 39.Niiranen TJ, Hanninen MR, Johansson J et al. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension. 2010;55:1346–51. doi: 10.1161/HYPERTENSIONAHA.109.149336. [DOI] [PubMed] [Google Scholar]
- 40.Sega R, Facchetti R, Bombelli M et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–83. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 41.Stergiou GS, Baibas NM, Kalogeropoulos PG. Cardiovascular risk prediction based on home blood pressure measurement: the Didima study. J Hypertens. 2007;25:1590–6. doi: 10.1097/HJH.0b013e3281ab6c69. [DOI] [PubMed] [Google Scholar]
- 42.Asayama K, Ohkubo T, Kikuya M et al. Prediction of stroke by self-measurement of blood pressure at home versus casual screening blood pressure measurement in relation to the Joint National Committee 7 classification: the Ohasama study. Stroke. 2004;35:2356–61. doi: 10.1161/01.STR.0000141679.42349.9f. [DOI] [PubMed] [Google Scholar]
- 43.Breaux-Shropshire TL, Judd E, Vucovich LA et al. Does home blood pressure monitoring improve patient outcomes? A systematic review comparing home and ambulatory blood pressure monitoring on blood pressure control and patient outcomes. Integr Blood Press Control. 2015;8:43–9. doi: 10.2147/IBPC.S49205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asayama K, Ohkubo T, Metoki H et al. Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertens Res. 2012;35:1102–10. doi: 10.1038/hr.2012.125. [DOI] [PubMed] [Google Scholar]
- 45.Kario K, Saito I, Kushiro T et al. Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy: primary results of HONEST, a large-scale prospective, real-world observational study. Hypertension. 2014;64:989–96. doi: 10.1161/HYPERTENSIONAHA.114.04262. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa J, Carroll DJ, Kuruvilla S et al. Changes in home versus clinic blood pressure with antihypertensive treatments: a meta-analysis. Hypertension. 2008;52:856–64. doi: 10.1161/HYPERTENSIONAHA.108.115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beitelshees AL, Gong Y, Bailey KR et al. Comparison of office, ambulatory, and home blood pressure antihypertensive response to atenolol and hydrochlorthiazide. J Clin Hypertens (Greenwich), 2010;12:14–21. doi: 10.1111/j.1751-7176.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cappuccio FP, Kerry SM, Forbes L et al. Blood pressure control by home monitoring: meta-analysis of randomised trials. BMJ. 2004;329:145. doi: 10.1136/bmj.38121.684410.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bray EP, Holder R, Mant J et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371–86. doi: 10.3109/07853890.2010.489567. [DOI] [PubMed] [Google Scholar]
- 50.Agarwal R, Bills JE, Hecht TJ et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29–38. doi: 10.1161/HYPERTENSIONAHA.110.160911. [DOI] [PubMed] [Google Scholar]
- 51.Glynn LG, Murphy AW, Smith SM et al. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;CD005182 doi: 10.1002/14651858.CD005182.pub4. [DOI] [PubMed] [Google Scholar]
- 52.Angell S, Guthartz S, Dalal M et al. Integrating self-blood pressure monitoring into the routine management of uncontrolled hypertension: translating evidence to practice. J Clin Hypertens (Greenwich), 2013;15:180–5. doi: 10.1111/jch.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi SS, Tabaei BP, Angell SY et al. Self-blood pressure monitoring in an urban, ethnically diverse population: a randomized clinical trial utilizing the electronic health record. Circ Cardiovasc Qual Outcomes. 2015;8:138–45. doi: 10.1161/CIRCOUTCOMES.114.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McManus RJ, Mant J, Haque MS et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799–808. doi: 10.1001/jama.2014.10057. [DOI] [PubMed] [Google Scholar]
- 55.O’Brien C, Bray EP, Bryan S et al. Targets and self-management for the control of blood pressure in stroke and at-risk groups (TASMIN-SR): protocol for a randomised controlled trial. BMC Cardiovasc Disord. 2013;13:21. doi: 10.1186/1471-2261-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rogers MA, Small D, Buchan DA et al. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med. 2001;134:1024–32. doi: 10.7326/0003-4819-134-11-200106050-00008. [DOI] [PubMed] [Google Scholar]
- 57.Green BB, Cook AJ, Ralston JD et al. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299:2857–67. doi: 10.1001/jama.299.24.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bosworth HB, Olsen MK, Grubber JM et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med. 2009;151:687–95. doi: 10.1059/0003-4819-151-10-200911170-00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang V, Smith VA, Bosworth HB et al. Economic evaluation of telephone self-management interventions for blood pressure control. Am Heart J. 2012;163:980–6. doi: 10.1016/j.ahj.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Magid DJ, Ho PM, Olson KL et al. A multimodal blood pressure control intervention in 3 healthcare systems. Am J Manag Care. 2011;17:e96–103. [PubMed] [Google Scholar]
- 61.McManus RJ, Mant J, Bray EP et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163–72. doi: 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
- 62.Margolis KL, Asche SE, Bergdall AR et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46–56. doi: 10.1001/jama.2013.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mengden T, Ewald S, Kaufmann S et al. Telemonitoring of blood pressure self measurement in the OLMETEL study. Blood Press Monit. 2004;9:321–5. doi: 10.1097/00126097-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Spruill TM, Williams O, Teresi JA et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97. doi: 10.1186/s13063-015-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fletcher BR, Hartmann-Boyce J, Hinton L, McManus RJ. The effect of self-monitoring of blood pressure on medication adherence and lifestyle factors: a systematic review and meta-analysis. Am J Hypertens. 2015;28:1209–21. doi: 10.1093/ajh/hpv008. [DOI] [PubMed] [Google Scholar]
- 66.Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens (Greenwich) 2006;8:174–80. doi: 10.1111/j.1524-6175.2006.04872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hosseininasab M, Jahangard-Rafsanjani Z, Mohagheghi A et al. Self-monitoring of blood pressure for improving adherence to antihypertensive medicines and blood pressure control: a randomized controlled trial. Am J Hypertens. 2014;27:1339–45. doi: 10.1093/ajh/hpu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lovibond K, Jowett S, Barton P et al. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet. 2011;378:1219–30. doi: 10.1016/S0140-6736(11)61184-7. [DOI] [PubMed] [Google Scholar]
- 69.Parati G, Stergiou GS, Asmar R et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–26. doi: 10.1097/HJH.0b013e328308da66. [DOI] [PubMed] [Google Scholar]
- 70.Stergiou GS, Karpettas N, Kapoyiannis A et al. Home blood pressure monitoring in children and adolescents: a systematic review. J Hypertens. 2009;27:1941–7. doi: 10.1097/HJH.0b013e32832ea93e. [DOI] [PubMed] [Google Scholar]
- 71.Sanghavi S, Vassalotti JA. Practical use of home blood pressure monitoring in chronic kidney disease. Cardiorenal Med. 2014;4:113–22. doi: 10.1159/000363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siennicki-Lantz A, Elmstahl S. Phenomenon of declining blood pressure in elderly-high systolic levels are undervalued with Korotkoff method. BMC Geriatr. 2011;11:57. doi: 10.1186/1471-2318-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akpolat T, Dilek M, Aydogdu T et al. Home sphygmomanometers: validation versus accuracy. Blood Press Monit. 2009;14:26–31. doi: 10.1097/MBP.0b013e3283262f31. [DOI] [PubMed] [Google Scholar]
- 74.Akpolat T, Aydogdu T, Erdem E et al. Inaccuracy of home sphygmomanometers: a perspective from clinical practice. Blood Press Monit. 2011;16:168–71. doi: 10.1097/mbp.0b013e328348ca52. [DOI] [PubMed] [Google Scholar]
- 75.the American Society of Nephrology Kidney Week. Philadelphie, PA: Nov 11–16, 2014. Are Home Blood Pressure Monitors Accurate Compared to Validated Devices? Hiremath Sea, Presented at. Abstract no SA-PO187, 2014. [Google Scholar]
- 76.Braam RL, Thien T. Is the accuracy of blood pressure measuring devices underestimated at increasing blood pressure levels? Blood Press Monit. 2005;10:283–9. doi: 10.1097/01.mbp.0000180671.76279.c7. [DOI] [PubMed] [Google Scholar]
- 77.Bur A, Herkner H, Vlcek M et al. Factors influencing the accuracy of oscillometric blood pressure measurement in critically ill patients, Crit Care Med. 2003;31:793–9. doi: 10.1097/01.CCM.0000053650.12025.1A. [DOI] [PubMed] [Google Scholar]
- 78.Pickering TG, Hall JE, Appel LJ et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 79.van Montfrans GA, van der Hoeven GM, Karemaker JM et al. Accuracy of auscultatory blood pressure measurement with a long cuff. Br Med J (Clin Res Ed) 1987;295:354–5. doi: 10.1136/bmj.295.6594.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi H, Yoshika M, Yokoi T. Validation of two automatic devices for the self-measurement of blood pressure according to the ANSI/AAMI/ISO81060–2:2009 guidelines: the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z). Vasc Health Risk Manag. 2015;11:49–53. doi: 10.2147/VHRM.S72438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bilo G, Sala O, Perego C Incorrect positioning of cuff for blood pressure measurement – clinical relevance and usefulness of novel cuff design, Presented at the 25th European Meeting on Hypertension and Cardiovascular Protection, Milan. Jun, 2015. pp. 12–15.


