Abstract
The Brugada syndrome (BrS) is a hereditary arrhythmic syndrome manifesting as syncope or sudden cardiac death (SCD) in individuals without overt structural heart disease. Currently, its diagnosis is mainly based on the presence of a spontaneous or Na+-channel blocker induced so-called “type 1” Brugada electrocardiographic (ECG) pattern typically seen in leads V1 and V2 recorded from the 4th to 2nd intercostal spaces. Presently the main unresolved clinical problem in the BrS is the identification of patients at high risk of SCD who need implantable cardioverter-defibrillator (ICD). Current guidelines recommend ICD implantation only in patients with spontaneous type 1 ECG pattern and either history of aborted cardiac arrest or documented sustained ventricular tachycardia (class I) or syncope of arrhythmic origin (class IIa) because they are at high risk of recurrent arrhythmias. However, the majority of BrS patients are asymptomatic when diagnosed and have generally low risk (0.5 % annually or lower) and therefore are not indicated for ICD. Most of SCD victims in the BrS have had no symptoms prior to the fatal event and therefore were not protected with an ICD. Currently there are no reliable methods to identify these potential victims of SCD. Although some ECG markers such as QRS fragmentation and infero-lateral early repolarisation have been demonstrated to signify increased arrhythmic risk their value still needs to be confirmed in large prospective studies. Novel risk assessment strategies need to be developed based on computerised quantitative ECG analysis of large digital ECG databases in patients with BrS and their relatives, and combined assessment of the most important factors of ventricular arrhythmogenesis.
Keywords: Brugada syndrome, electrocardiogram, sudden cardiac death, risk stratification, heredtary arrhythmic syndromes
Introduction
The Brugada syndrome (BrS) is a hereditary arrhythmia syndrome manifesting as recurrent syncope or sudden cardiac death (SCD) due to polymorphic ventricular (VT) or fibrillation (VF) in the absence of overt structural heart disease or myocardial ischaemia.[1,2] The prevalence of the syndrome is estimated at around 15 per 10,000 in South East Asia including Japan and around 2 per 10,000 in the Western countries.[3,4] The BrS may be responsible for up to 4 % of all sudden cardiac deaths (SCD) and at least 20 % of SCDs in patients with structurally normal hearts.[5] It is 8–10 times more prevalent in males than in females[6] and males are at considerably higher risk of dying suddenly.[7] In South-East Asia, the BrS is the leading cause of non-traumatic death in men younger than 40 years.[8] This review will briefly summarise current knowledge about the BrS with emphasis on the methods for diagnosis and risk assessment.
Cellular Mechanisms of the BrS
BrS is considered a heritable autosomal dominant disease[9] and more than 390 mutations have been identified in the SCN5A gene encoding the α-subunit of the cardiac INa-channel.[10] However, presently SCN5A mutations are found only in 11–37 % of the genotyped patients.[7,11] Recent data has suggested that heritability may be related to common genetic variation instead of being strictly monogenic.[12] Many patients with the BrS have no family history presumably due to under-diagnosis in the other family members, low penetrance or sporadic disease.[13]
The cellular basis of the BrS is still not fully understood.[14] According to the “repolarisation theory”, reduction of the inward Na+ current leads to unopposed transient outward (Ito) current in some epicardial regions of the right ventricular outflow tract (RVOT), which causes either delayed expression of the action potential (AP) dome and epicardial AP prolongation or loss of the dome and AP shortening. The net effect is a potentially arrhythmogenic magnification of repolarisation dispersion between the RVOT endo- and epicardium, and between different RVOT epicardial regions. The repolarisation theory was initially promoted on the basis of experimental studies[15,16,17] and was later supported by clinical data such as “spike and dome” configuration with deep notching of monophasic action potentials (MAP) from the RVOT epicardium but not endocardium,[18] paradoxical shortening of the RVOT epicardial activation-recovery intervals (ARI) during augmentation of Brugada-type ST segment elevation,[19] steep AP duration restitution (slope >1) in the RVOT[20,21,22] (both clinically and experimentally), longer ARI in the RVOT epicardium recorded from the conus branch of the right coronary artery than in the endocardium of patients with BrS and type 1 ECG pattern but not in controls[23] and others.
There is also mounting evidence from experimental,[22] histopathological,[24] computational,[25] clinical electrophysiological[23,25] and imaging[26] studies for the presence of conduction abnormalities in the RVOT and their importance for the genesis of ventricular arrhythmias in BrS[22,23] (“depolarisation theory”). Delay of the RVOT activation relative to the rest of the RV has also been proposed as a mechanism of the Brugada type ECG changes on the surface ECG.[27] The presence of late potentials and prolonged filtered QRS duration on signal-averaged ECG (SAECG) as well as increased notching and fragmentation of the QRS on the standard ECG are linked to increased arrhythmic risk in BrS.[28,29,30,31] The reported cases of patients presenting with both arrhythmogenic right ventricular cardiomyopathy (ARVC) and BrS with SCN5A mutations,[32] further attest to the likely role of conduction abnormalities in the BrS. A third hypothesis unifying the above two explains the BrS with abnormal expression of the neural crest cells during the embryological development of the RVOT. This defect in the embryogenesis of the RVOT leads to both abnormally augmented electrical gradients during repolarisation as well as to delayed activation of the RVOT.[33]
From electrocardiographic point of view, the characteristic elevation of the J point and ST segment of the type 1 Brugada ECG pattern (see below) results from early relative (intracellular) positivity of the unaffected zone (RVOT endocardium according to the “repolarisation theory” or normally activated myocardium outside the RVOT according to the “depolarisation theory”), whereas the negative T wave is an expression of late epicardial relative (intracellular) positivity in the affected RVOT zone due to either prolongation of the epicardial APs or its delayed activation.
Clinical Manifestations of the BrS
The symptoms associated with the BrS are due to re-entry ventricular arrhythmias typically arising in the affected zone of the RV. If they last briefly (seconds) and terminate spontaneously they can be asymptomatic or cause palpitations; longer arrhythmias lead to syncope or nocturnal agonal respiration, or can degenerate into VF and cardiac arrest. The duration of the arrhythmia is unpredictable with currently available methods and every arrhythmic episode can be fatal. Therefore, the assessment of the degree of arrhythmic risk and the need for prophylactic treatment is by far the most important aspect of the management of these patients.
The Electrocardiogram – a Key to the Diagnosis of the Brugada Syndrome
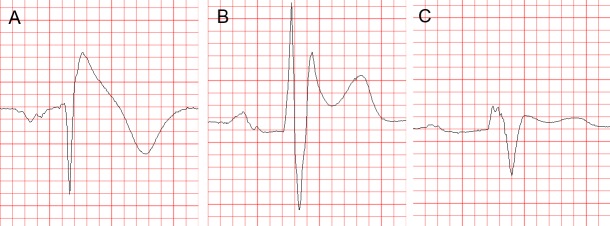
The standard 12-lead ECG (with some additional modifications, as explained below) is crucial for the diagnosis and likely also for determining the prognosis (i.e. the level of arrhythmic risk) in the BrS. The diagnostic hallmark of syndrome is the so-called “coved” or “type 1 Brugada ECG pattern” characterised by J-point elevation with slowly descending or concave ST segment elevation merging into a negative or reaching the isoelectric line symmetric T wave7,[34] (see Figure 1A). The type 1 Brugada ECG pattern is observed most frequently in leads V1 and V2, much less frequently in lead V3.[35] When type 1 Brugada ECG pattern is observed in lead V3 it is always accompanied by the same ECG pattern in at least one more right precordial lead (unpublished observations).
Figure 1: Brugada Type ECG Patterns.
Brugada Type 1 (A), Type 2 (B) and Type 3 (C) ECG Pattern On this figure and all subsequent figures, the ECGs are presented at 25 mm/s, 1 cm/mV. See the text for details.
In patients investigated for the BrS, leads V1 and V2 should always be recorded both from the 4th intercostal (i.c.) space as well as from the 3rd and 2nd i.c. space, because the “high” positions increase the sensitivity of leads V1 and V2 for detecting type 1 pattern without loss of specificity[36] (see Figure 2). This has been noted already in 1960,[37] long before the discovery of the Brugada syndrome. Since the exact anatomic relation between RV and the thoracic cage is individually specific and any of the three i.c. spaces could be closest to the RVOT, it is best to record simultaneously leads V1 and V2 in the 4th, 3rd and 2nd i.c. space. One small study with cardiovascular magnetic resonance imaging found that the maximum RVOT area was most frequently in the 3rd followed by the 4th and 2nd i.c. space.[38] In our experience, when type 1 Brugada pattern is observed in the 4th i.c. space it is always also observed in the 3rd or 2nd i.c. space whereas the converse is not the case (unpublished observations). Positioning lead V3 one or two i.c. spaces higher also increases its sensitivity to detect type 1 pattern (unpublished data) (see Figure 2). Bipolar precordial leads between the V2 electrode (positive pole) and V4 or V5 electrodes (negative pole) which can be computed from the standard unipolar leads V2, V4 and V5 seem to be more sensitive and equally specific compared to the unipolar lead V2 for detecting the diagnostic type 1 Brugada ECG pattern.[39] Type 1 Brugada ECG pattern sometimes also can be observed in the inferior[40,41,42,43] or lateral[44,45] leads (the so-called “atypical” BrS).
Figure 2: Diagnostic Value of Higher Intercostal Positions.
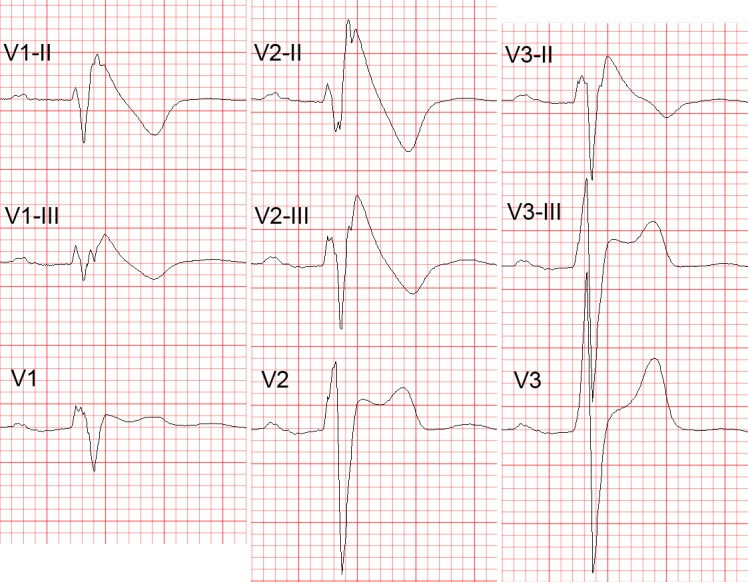
Recording Leads V1 to V3 from One or Two Intercostal (i.c.) spaces higher than their standard positions (i.e. 3rd or 2nd i.c. space for leads V1 and V2) increases their sensitivity for detecting the diagnostic type 1 Brugada ECG pattern. Note the transition of the ECG complexes in lead V3 from a normal morphology (in the standard position) to type 2 (one i.c. space higher) and type 1 Brugada ECG pattern (two i.c. spaces higher). V1-III, V2-III, V1-II, V2-II = leads V1 and V2 from the 3rd and 2nd i.c. spaces, respectively; V3-III, V3-II = lead V3 recorded one and two spaces higher, respectively. ECG = electrocardiogram.
Another ECG pattern of J point and ST segment elevation with a positive T wave in the right precordial leads, the so-called “saddle-back” pattern, is considered suspicious but not diagnostic of BrS, unless converted into type 1 pattern following administration of Na+ channel blocking agents. Traditionally the “saddle back” patterns are further divided into “type 2” and “type 3” Brugada ECG pattern depending on the level of J point and ST segment elevation2 (see Figure 1B,C). A recently published expert Consensus Report on the ECG characteristics of the BrS proposed “type 2” and “type 3” patterns to be unified into one “saddle-back” Brugada pattern because, according to the authors’ opinion, the small morphological differences between the two patterns had no diagnostic or prognostic significance.[34] In the latest HRS/EHRA/APHRS Expert Consensus Statement on the Diagnosis and Management of Patients with Inherited Primary Arrhythmia Syndromes published in December 2013,[7] however, type 2 and type 3 Brugada ECG patterns are still mentioned separately.
Up to 40 % of patients with the BrS present with normal or non-diagnostic resting ECG.[46] In these patients, the diagnostic “coved” ECG pattern can be elicited by i.v. administration of sodium channel blocker (ajmaline, procainamide, flecainide).[47,48] Currently the BrS is definitely diagnosed when type 1 pattern is observed in at least one of leads V1 and V2 recorded from the 4th, 3rd or 2nd i.c. space either spontaneously or following administration of Na+channel.[7] The presence of gene mutations is not considered essential for the diagnosis.[2,7]
It is important to distinguish between type 2 Brugada ECG pattern and the r’or R’-pattern (incomplete right bundle branch block (iRBBB) pattern) in leads V1 and V2 (especially when recorded from the 3rd or 2nd i.c. space) which can be observed in healthy subjects (frequently in athletes[49]). It has been reported that a broader angle between the ascending and descending limb of the r’/R’-wave[50] or a broader base of the triangle formed by the two limbs of the r’/R’-wave measured at 5 mm from the highest point[51,52] can reliably distinguish type 2 Brugada ECG pattern from IRBBB pattern. The classical diagnostic type 1 Brugada ECG pattern needs to be distinguished from similar “Brugada-like” patterns caused by RBBB, septal hypertrophy, arrhythmogenic right ventricular cardiomyopathy (ARVC), pectus excavatum and other conditions, and also from the transient appearance of typical Brugada pattern in the cause of various acute processes such as acute ischaemia, Prinsmetal angina, pulmonary embolism, pericarditis, metabolic disorders, various medications and others (the so-called “Brugada phenocopies”,[53] see www.brugadaphenocopy.com). The ECG characteristics of the BrS and the methods to distinguish them from other conditions presenting with similar ECG changes are reviewed in great detail in the above mentioned ECG Consensus Report.[34]
ECG acquisition with inappropriate high-pass filtering (e.g. non-linear phase high-pass filter of 0.5 Hz instead of the recommended 0.05 Hz)[54] can cause considerable ST segment distortion and even mimic type 1 or 2 Brugada pattern.[34,55,56]
The ECG in BrS characteristically shows considerable dynamic variability; it can be completely normal at one time and demonstrate diagnostic type 1 pattern at another. Vagal influences (slow heart rate, post-prandial state, nighttime) tend to augment the J point and ST segment elevation and the type 1 pattern,[57] whereas exercise and catecholamine infusion tend to have the opposite effect (however, in some BrS patients the ST segment elevation might become more prominent during exercise).[58] Autonomic influences play important role also in the genesis of malignant arrhythmias because most of the arrhythmic events in BrS occur at night, two long RR intervals often precede episodes of VT/VF,[59] whereas catecholamine infusion is used as a first line treatment of such episodes.[60] Patients with BrS have increased incidence (10–53 %) of atrial fibrillation (AF).[61,62]
Additional ECG findings which support the diagnosis of the BrS in asymptomatic patients with spontaneous or induced by Na-channel blockers type 1 pattern include the presence of atrial fibrillation, atrio-ventricular or intraventricular conduction abnormalities (first degree A-V block, fragmented and/or prolonged QRS, abnormal signal-averaged ECG (SAECG), left axis deviation of the QRS complex, prolonged HV interval), ventricular ectopic beats with left bundle branch block (LBBB) morphology and short (<200 ms) ventricular effective refractory period.[7]
Assessment of the Arrhythmic Risk – the Most Important Clinical Problem in the Brugada Syndrome
The identification of BrS patients with high arrhythmic risk especially among those without previous history of arrhythmia-related symptoms is currently the most important and yet unresolved clinical problem in the BrS. In some aspects, this problem is similar to one of the main (also still not fully resolved) problems of modern cardiology – the identification of patients with ischaemic heart disease (IHD) at high risk of dying suddenly who need prophylactic implantable cardioverter-defibrillator (ICD).
In BrS patients with a previous history of arrhythmic syncope or aborted cardiac arrest the annual event rate of sustained VT or VF is relatively high – between 1.9 %[63] and 8.8 %[64] and between 7.7 %[62] and 13.8 %[63], respectively. It is universally accepted that those with aborted cardiac arrest or documented spontaneous sustained VT (with or without syncope) should receive an ICD which is the single therapy with proven efficacy (Class I indication, “…is recommended”).[6] There is also evidence that patients with spontaneous type 1 pattern and syncope judged to be of arrhythmic origin (“intermittent risk” group) also are indicated for ICD implantation (currently class IIa indication, “…can be useful.”), whereas asymptomatic patients with spontaneous type 1 pattern are currently considered to represent a “low risk” group (class IIb indication, “…may be considered” depending on the presence or absence of other, not yet fully established risk factors, see below).[6] A very recently published Japanese multicentre study confirmed the difference in the level of risk between the latter two patient groups (2.2 % vs 0.5 % during a mean follow-up of 62 months).[65]
However, the decision to offer an ICD even to a BrS patient with a syncope of presumably arrhythmic origin often is difficult because unlike IHD patients, most of them are relatively young, apparently healthy and without any previous awareness of cardiac problems. Most importantly, it is often very difficult to exclude non-arrhythmic cause of the syncope. In addition, the rate of ICD-related complications (20–30 % annually including inappropriate shocks) is higher than the rate of appropriate activation of the device (2.6–8 % annually).[66,67,68] This suggests that novel, better methods of risk-stratification could benefit even some symptomatic BrS patients (i.e. those with the current class IIa indications).[6]
The majority of BrS patients (64 % in the largest reported series of 1029 BrS patients, the France, Italy, Netherlands, Germany (FINGER) study[62] and 63 % in the report of the Brugada syndrome investigators in Japan[69]) have no symptoms at the time of establishment of the diagnosis. The annual rate of SCD or sustained VT in these patients is low – between 0 %[70,71] and 0.8[72] (0.5 % in the FINGER study[62] and the Japanese multicentre study,[66] 0.4–1 % in several Japanese studies[73,74,75]) and cannot justify ICD implantation in all of them. On the other hand, the majority of the patients in this heterogeneous group have generally structurally normal hearts and are young or middle aged when diagnosed (median age 45 years in the FINGER study), and therefore the low annual risk can translate into a considerable cumulative arrhythmic risk for the next several decades of their life expectancy. In fact, the majority of victims of SCD in BrS come from this “low risk” (according to current standards) population. One study showed that among patients with the BrS who have died suddenly 68 % had no previous history of arrhythmia-related symptoms and therefore had not been protected by ICD.[13] Currently, there are no firmly established reliable methods for the identification of these patients. Similarly, the largest absolute number of patients with IHD who die suddenly also comes from a large patient population considered to have generally low risk (i.e. post-myocardial infarction patients with relatively preserved left ventricular ejection fraction).[76]
While some studies[77,78,79] reported increased occurrence of arrhythmic events in BrS patients with SCN5A mutations these findings have not been confirmed by other studies.[80] The genetic analysis is expensive, time-consuming and available only in specialised centres. The role of programmed ventricular stimulation (PVS) during EPS for induction of VT has been an object of controversy and debate since the 1990s. While some early studies supported its value for risk stratification mainly due to its high negative predictive value,[81,82] most recent studies failed to confirm its independent predictive value.[66,83,84] In the FINGER study,[62] inducibility during EPS also did not predict arrhythmic events in multivariate analysis whereas in the multicentre PRELUDE study which tested uniform protocol of PVS during EPS in all 308 patients, the rate arrhythmic events during an average follow-up of three years was not significantly different between the 126 inducible (3.9 %) and the 182 non-inducible patients (4.9 %).[85] Currently its role of EPS for risk stratification is accepted only as a Class IIb (“may be considered”) indication.[7,86] The method is inherently limited by its invasive character and probably also by the labile nature of the underlying electrophysiologic substrate.[87]
ECG-derived Parameters for Risk Assessment
Similarly to other diseases with intraventricular conduction abnormalities such as IHD and cardiomyopathies, the presence of notched or fragmented QRS,[31,83] (see Figure 3) has been consistently demonstrated to indicate increased arrhythmic risk independently of other clinical and ECG variables (HR 4.9, 95 % CI 1.6-15.4 in the PRogrammed ELectrical stimUlation preDictive valuE (PERLUDE) study).[85] However, currently the presence of notching/fractionation is assessed only visually using arbitrary descriptive criteria (e.g. number of QRS peaks). The presence of late potentials on the signal-averaged ECG (SAECG) is another marker of intraventricular conduction disturbances which also indicates increased arrhythmic risk in BrS30 independently of QRS fractionation. The standard time-domain SAECG, however, cannot detect conduction abnormalities within the QRS complex, has uncertain value in patients with bundle branch block, and uses only a single-lead ECG complex which is derived from the XYZ orthogonal leads and does not contain any regional information.
Figure 3: QRS Notching in the Brugada Syndrome.
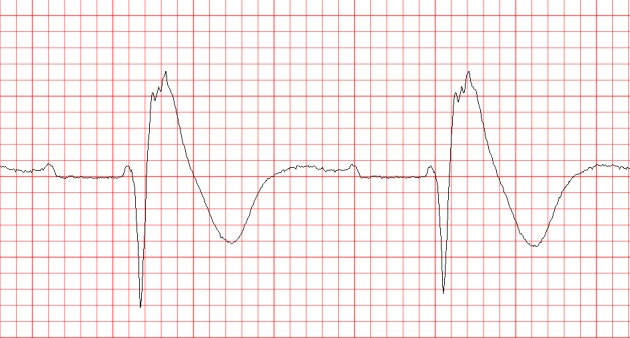
Marked fractionation of the QRS complex in lead V2(2nd i.c. space) during positive diagnostic ajmaline test in a 20-year old man with aborted cardiac arrest and a non-diagnostic resting ECG (not shown).
Among asymptomatic BrS patients, those with Brugada ECG pattern in the infero-lateral leads in addition to the right precordial leads,[88] (increased “spatial Brugada burden”) were recently demonstrated to have several times higher risk of VF compared to those with Brugada type 1 changes only in the right precordial leads.[89] On the other hand, the number of right precordial leads displaying type 1 pattern and the degree of J-point or ST-segment elevation do not seem to correlate with the arrhythmic risk.[35,36] The “high” (3rd or 2nd i.c. space) positions of leads V1 and V2 are diagnostically more sensitive than their standard positions in the 4th i.c. space[90,91,92] but prognostically their value is the same.[93]
Infero-lateral early repolarisation (ER) (see Figure 4) is not only more common (up to 15 %[94]) in BrS patients,[70,94,95] but also carries up to 4-fold increased risk of spontaneous VF[67,70] and even higher risk if the ER has “malignant” morphology,[96] i.e. is associated with horizontal/descending (as opposed to rapidly ascending) ST segment after the J point.[68,75] The combination of infero-lateral ER and fractionated QRS complex seems to indicate indicate a particularly high arrhythmic risk.[97]
Figure 4: Early Repolarisation in the Brugada Syndrome.
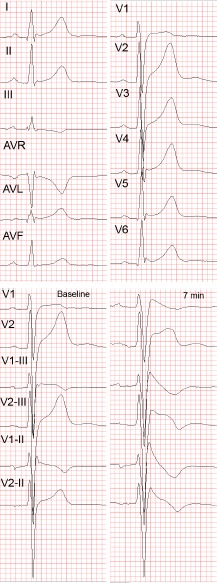
Lateral early repolarisation (ER) in a 34-year-old man with aborted cardiac arrest and Brugada syndrome. A: Standard 12-lead ECG. Note the ER changes in leads V4-V6 as well as, less pronounced, in leads I and AVL. B: Leads V1 and V2 recorded in the standard positions, in the 3rd and 2nd i.c. space at baseline (left panel) and 7 minutes after the start of ajmaline administration (right panel). Note the appearance of diagnostic type 1 Brugada ECG pattern in leads V1(3rd and 2nd i.c. space) and lead V2 (2nd i.c. space) after ajmaline administration. There were no Brugada-like changes in the peripheral leads following ajmaline administration (not shown).The abbreviations are the same as in Figure 2.
Currently there are only limited data suggesting that other ECG parameters may indicate increased arrhythmic risk. These parameters include changes in repolarisation dynamics (QT/RR and Tpeak-Tend/RR intervals relations),[98] deep negative T wave in lead V1,[99] QTc interval more than 460 ms in lead V2 and prolonged Tpeak-Tend interval,[100] dynamic alterations in the amplitude of the ST elevation,[101] prolonged PR-interval,[93] presence of atrial arrhythmias,[60,61,102] and augmentation of the ST segment elevation during the early recovery phase of exercise test.[103]
Summary – Risk Stratification in the Brugada Syndrome – Current Status and Future Diretions
Whereas the diagnosis of the BrS is relatively straightforward with currently available ECG-based methods, the identification of high risk patients who need prophylactic ICD implantation is still an unresolved issue. Currently the only class I indications for ICD implantation in patients diagnosed with the BrS endorsed by the 2013 HRS/EHRA/APHRS Expert Consensus Statement[7] is history of aborted cardiac arrest or documented spontaneous sustained VT, whereas syncope judged to be likely of arrhythmic origin is only a Class IIa indication which mainly reflects the difficulty of excluding a non-cardiac origin of syncope. The guidelines of the Japanese Cardiac Society of 2011 accept practically the same Class I indications whereas for Class IIa indication they require the presence of at least two of the following risk factors: history of syncope, family history of sudden cardiac death and inducible VF during EPS.[104] Obviously, these guidelines do not offer solution to the problem of identifying the high risk asymptomatic patients with the BrS. Strict adherence to the HRS/EHRA/APHRS guidelines means that each year in the UK alone, approximately 40 asymptomatic BrS patients are likely to experience their first and potentially lethal arrhythmic event without ICD protection (assuming 0.5 % annual rate in asymptomatic patients [approximately 2/3 of all BrS patients] × estimated 12,600 (2 per 10,000) BrS patients in the UK). Clearly, there is a pressing need to develop novel, easily applicable (e.g. ECG based) risk stratifiers (or combinations thereof) and to confirm prospectively the value of the most promising available ones (e.g. QRS fractionation, infero-lateral ER, possibly others).
Obvious obstacles along the path to this goal are the low rate of arrhythmic events (i.e. end-point events in prospective studies), the small number of patients in the individual centres (since the prevalence of the disease outside South-East Asia is generally low), difficult organisation of big multicentre prospective studies and, possibly, inherent differences between various patient populations (e.g. Western vs Japanese).[66] Less appreciated obstacle is the fact that currently ECG research studies (as well as everyday clinical practice) still use mainly 12-lead paper ECGs (or digital ECG images) which are amenable only to visual assessment and simple manual measurement. Computerised mathematical methods for quantitative assessment of QRS and ST-T wave abnormalities have been developed and successfully tested in various cardiac diseases[105,106] but they require the availability of digital ECGs (digital files containing the raw ECG signal and not just digital image files). Finally, the development of sustained VT/VF in the BrS is likely a complex event resulting from interaction between the arrhythmic substrate (repolarisation and depolarisation abnormalities) and various triggering and modifying factors (e.g. ventricular ectopic beats, atrial arrhythmias, autonomic modulations such as vagal surge, fever, etc.).[14,107,108] Therefore a successful ECG-based risk stratification in BrS should likely involve the combined quantitative assessment of several most important elements of arrhythmogenesis.
References
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. J Am Coll Cardiol. 1992;20:1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Antzelevitch C, Brugada P, Borggrefe M et al. Brugada Syndrome. Report of the Second Consensus Conference. Circulation. 2005;111:659–70. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 3.Kamakura S. Epidemiology of Brugada syndrome in Japan and rest of the world. Journal of Arrhythmia. 2013;29:52–55. [Google Scholar]
- 4.Juang JM, Huang SK. Brugada syndrome—an under-recognized electrical disease 843 in patients with sudden cardiac death. Cardiology. 2004;101:157–69. doi: 10.1159/000076693. [DOI] [PubMed] [Google Scholar]
- 5.Derval N, Simpson CS, Birnie DH et al. Prevalence and characteristics of early repolarization in the CASPER registry: cardiac arrest survivors with preserved ejection fraction registry. J Am Coll Cardiol. 2011;58:722–8. doi: 10.1016/j.jacc.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 6.HRS/EHRA/APHRS Expert Consensus Statement on the Diagnosis and Management of Patients with Inherited Primary Arrhythmia Syndromes. Heart Rhythm. 2013;10:1932–63. doi: 10.1016/j.hrthm.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Kapplinger JD, Tester DJ, Alders M et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nademanee K, Veerakul G, Nimmannit S et al. Arrhythmogenic marker for the sudden unexplained death syndrome in Thai men. Circulation. 1997;96:2595–600. doi: 10.1161/01.cir.96.8.2595. [DOI] [PubMed] [Google Scholar]
- 9.Horie M, Ohno S. Genetic basis of Brugada syndrome. J Arrhythmia. 2013;29:71–6. [Google Scholar]
- 10.The Gene Connection for the Heart. Genetic mutations and inherited arrhythmias. http://www.fsm.it/cardmoc/ http://www.fsm.it/cardmoc/ Accessed on 24/03/2014.
- 11.Gehi AK, Duong TD, Metz LD et al. Risk Stratification of Individuals with the Brugada Electrocardiogram: A Meta-Analysis. J Cardiovasc Electrophysiol. 2006;17:577–83. doi: 10.1111/j.1540-8167.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 12.Bezzina CR, Barc J, Mizusawa Y et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet. 2013;45:1044–9. doi: 10.1038/ng.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raju H, Papadakis M, Govindan M et al. Low Prevalence of Risk Markers in Cases of Sudden Death Due to Brugada Syndrome. Relevance to Risk Stratification in Brugada Syndrome. J Am Coll Cardiol. 2011;57:2340–5. doi: 10.1016/j.jacc.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 14.Morita M, Zipes DP. Brugada syndrome: Insights of ST elevation, arrhythmogenicity, and risk stratification from experimental observations. Heart Rhythm. 2009;6:S34–43. doi: 10.1016/j.hrthm.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Yan G-X, Antzelevitch C. Cellular Basis for the Brugada Syndrome and Other Mechanisms of Arrhythmogenesis Associated With ST-Segment Elevation. Circulation. 1999;100:1660–6. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 16.Yan G-X, Shimizu W, Antzelevitch C. Characteristics and Distribution of M Cells in Arterially Perfused Canine Left Ventricular Wedge Preparations. Circulation. 1998;98:1921–7. doi: 10.1161/01.cir.98.18.1921. [DOI] [PubMed] [Google Scholar]
- 17.Antzelevitch C. The Brugada syndrome: ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12:268–72. doi: 10.1046/j.1540-8167.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 18.Kurita T, Shimizu W, Inagaki M et al. The Electrophysiologic Mechanism of ST-Segment Elevation in Brugada Syndrome. J Am Coll Cardiol. 2002;40:330–4. doi: 10.1016/s0735-1097(02)01964-2. [DOI] [PubMed] [Google Scholar]
- 19.Simizu W, Aiba T, Kurita T, Kamakura S. Paradoxic Abbreviation of Repolarization in Epicardium of the Right Ventricular Outflow Tract During Augmentation of Brugada-Type ST Segment Elevation. J Cardiovasc Electrophysiol. 2001;12:1418–21. doi: 10.1046/j.1540-8167.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- 20.Narayan SM, Kim J, Tate C, Berman BJ. Steep restitution of ventricular action potential duration and conduction slowing in human Brugada syndrome. Heart Rhythm. 2007;4:1087–9. doi: 10.1016/j.hrthm.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Aiba T, Shimizu W, Hidaka I et al. Cellular Basis for Trigger and Maintenance of Ventricular Fibrillation in the Brugada Syndrome Model High-Resolution Optical Mapping Study. J Am Coll Cardiol. 2006;47:2074–85. doi: 10.1016/j.jacc.2005.12.064. [DOI] [PubMed] [Google Scholar]
- 22.Lambiase PD, Ahmed AK, Ciaccio EJ et al. High-Density Substrate Maping in Brugada Syndrome: Combined Role of Conduction and Repolarization Heterogeneities in Arrhythmogenesis. Circulation. 2009;120:106–17. doi: 10.1161/CIRCULATIONAHA.108.771401. [DOI] [PubMed] [Google Scholar]
- 23.Nagase S, Kusano KF, Morita H et al. Longer Repolarization in the Epicardium at the Right Ventricular Outflow Tract Causes Type 1 Electrocardiogram in Patients With Brugada Syndrome. J Am Coll Cardiol. 2008;51:1154–61. doi: 10.1016/j.jacc.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 24.Coronel R, Casini S, Koopmann TT et al. Right Ventricular Fibrosis and Conduction Delay in a Patient With Clinical Signs of Brugada Syndrome. A Combined Electrophysiological, Genetic, Histopathologic, and Computational Study. Circulation. 2005;112:2769–77. doi: 10.1161/CIRCULATIONAHA.105.532614. [DOI] [PubMed] [Google Scholar]
- 25.Nagase S, Kusano KF, Morita H et al. Epicardial Electrogram of the Right Ventricular Outflow Tract in Patients With the Brugada Syndrome. Using the Epicardial Lead. J Am Coll Cardiol. 2002;39:1992–5. doi: 10.1016/s0735-1097(02)01888-0. [DOI] [PubMed] [Google Scholar]
- 26.Tukkie R, Sogaard P, Vleugels J et al. Delay in Right Ventricular Activation Contributes to Brugada Syndrome. Circulation. 2004;109:1272–7. doi: 10.1161/01.CIR.0000118467.53182.D1. [DOI] [PubMed] [Google Scholar]
- 27.Meregalli PG, Wilde AAM, Tan HL. Pathophysiological mechanisms of Brugada syndrome: Depolarization disorder, repolarization disorder, or more? Cardiovasc Res. 2005;67:367–78. doi: 10.1016/j.cardiores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda T, Sakurada H, Sakabe K et al. Assessment of Noninvasive Markers in Identifying Patients at Risk in the Brugada Syndrome: Insight Into Risk Stratification. J Am Coll Cardiol. 2001;37:1628–34. doi: 10.1016/s0735-1097(01)01197-4. [DOI] [PubMed] [Google Scholar]
- 29.Furushima H, Chinushi M, Hirono T et al. Relationship Between Dominant Prolongation of the Filtered QRS Duration in the Right Precordial Leads and Clinical Characteristics in Brugada Syndrome. J Cardiovasc Electrophysiol. 2005;16:1311–7. doi: 10.1111/j.1540-8167.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z, Patel C, MD et al. Role of signal-averaged electrocardiograms in arrhythmic risk stratification of patients with Brugada syndrome: A prospective study. Heart Rhythm. 2009;6:1156–62. doi: 10.1016/j.hrthm.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Morita H, Kusano KF, Miura D et al. Fragmented QRS as a Marker of Conduction Abnormality and a Predictor of Prognosis of Brugada Syndrome. Circulation. 2008;118:1697–704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 32.Peters S. A second case with arrhythmogenic cardiomyopathy, provocable Brugada ECG and SCN5A mutation. Int J Cardiol. 2014;171:e117–8. doi: 10.1016/j.ijcard.2013.12.038. [DOI] [PubMed] [Google Scholar]
- 33.Elizari MV, Levi R, Acunzo RS et al. Abnormal expression of cardiac neural crest cells in heart development: A different hypothesis for the etiopathogenesis of Brugada syndrome. Heart Rhythm. 2007;4:359–65. doi: 10.1016/j.hrthm.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Bayés de Luna A, Brugada J, Baranchuk A, Borggrefe M, Breithardt G, Goldwasser D, Lambiase P et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433–42. doi: 10.1016/j.jelectrocard.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Richter S, Sarkozy A, Paparella G et al. Number of electrocardiogram leads displaying the diagnostic coved-type pattern in Brugada syndrome: a diagnostic consensus criterion to be revised. Eur Heart J. 2010;31:1357–64. doi: 10.1093/eurheartj/ehq049. [DOI] [PubMed] [Google Scholar]
- 36.Govindan M, Batchvarov VN, Raju H et al. Utility of high and standard right precordial leads during ajmaline testing for the diagnosis of Brugada Syndrome. Heart. 2010;96:1904–8. doi: 10.1136/hrt.2010.201244. [DOI] [PubMed] [Google Scholar]
- 37.Roesler H. An electrocardiographic study of high take-off of the R(R’)-T segment in right precordial leads. Am J Cardiol. 1960;6:920–8. doi: 10.1016/0002-9149(60)90292-7. [DOI] [PubMed] [Google Scholar]
- 38.Veltmann CT, Papavassiliu T, Konrad T. Insights into the location of type I ECG in patients with Brugada syndrome: Correlation of ECG and cardiovascular magnetic resonance imaging. Heart Rhythm. 2012;9:414–21. doi: 10.1016/j.hrthm.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 39.Batchvarov VN, Govindan M, Macfarlane PF et al. Diagnostic Utility of Bipolar Precordial Leads During Ajmaline Testing for Suspected Brugada Syndrome. Heart Rhythm. 2010;7:208–15. doi: 10.1016/j.hrthm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Takagi M, Aihara N, Takaki H et al. Clinical Characteristics of Patients With Spontaneous or Inducible Ventricular Fibrillation Without Apparent Heart Disease Presenting with J Wave and ST Segment Elevation in Inferior Leads. J Cardiovasc Electrophysiol. 2000;11:844–8. doi: 10.1111/j.1540-8167.2000.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 41.Chinushi M, Izumi D, Furushima H et al. Multiple Premature Beats Triggered Ventricular Arrhythmias During Pilsicainide Infusion in a Patient with Inferior ST-Segment Elevation. Pacing Clin Electrophysiol. 2006;29:1445–8. doi: 10.1111/j.1540-8159.2006.00562.x. [DOI] [PubMed] [Google Scholar]
- 42.Kalla H, Yan G-X, Marinchak R. Ventricular Fibrillation in a Patient with Prominent J (Osborn) Waves and ST Segment Elevation in the Inferior Electrocardiographic Leads: A Brugada Syndrome Variant? J Cardiovasc Electrophysiol. 2000;11:95–8. doi: 10.1111/j.1540-8167.2000.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 43.Batchvarov VN, Govindan M, Camm AJ, Behr ER. Brugada-Like Changes in the Peripheral Leads During Diagnostic Ajmaline Test in Patients With Suspected Brugada Syndrome. Pacing Clin Electrophysiol. 2009;32:695–703. doi: 10.1111/j.1540-8159.2009.02353.x. [DOI] [PubMed] [Google Scholar]
- 44.Van den Berg MP, Wiesfeld ACP. Brugada Syndrome with ST-Segment Elevation in the Lateral Leads. J Cardiovasc Electrophysiol. 2006;17:1. doi: 10.1111/j.1540-8167.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 45.Bonakdar H, Haghjoo M, Sadr-Ameli MA. Brugada Syndrome Manifested by the Typical Electrocardiographic Pattern both in the Right Precordial and the High Lateral Leads. Ind Pacing Electrophysiol J. 2008;8:137–40. [PMC free article] [PubMed] [Google Scholar]
- 46.Rivero-Ayerza M, Brugada R, Brugada J . Dynamic Electrocardiography. Futura/Blackwell Publishing; NY: 2004. Electrocardiogram of Brugada Syndrome and its Dynamic Patterns. In: Malik M, Camm AJ (editors) pp. 417–24. [Google Scholar]
- 47.Brugada R, Brugada J, Antzelevitch C et al. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510–5. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 48.Rolf S, Bruns H-J, Wichter T et al. The Ajmaline challenge in Brugada syndrome: Diagnostic impact, safety, and recommended protocol. Eur Heart J. 2003;24:1104–12. doi: 10.1016/s0195-668x(03)00195-7. [DOI] [PubMed] [Google Scholar]
- 49.Chung EH, McNeely DE, Gehi AK et al. Brugada-type patterns are easily observed in high precordial lead ECGs in collegiate athletes. J Electrocardiol. 2014;47:1–6. doi: 10.1016/j.jelectrocard.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Chevallier S, Forclaz A, Tenkorang J et al. New Electrocardiographic Criteria for Discriminating Between Brugada Types 2 and 3 Patterns and Incomplete Right Bundle Branch Block. J Am Coll Cardiol. 2011;58:2290–8. doi: 10.1016/j.jacc.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 51.Serra G, Baranchuk A, Bayes de Luna A et al. New electrocardiographic criteria to differentiate the Type-2 Brugada pattern from electrocardiogramof healthy athletes with r′-wave in leads V1/V2. Europace. 2014;16:1639–45. doi: 10.1093/europace/euu025. [DOI] [PubMed] [Google Scholar]
- 52.García-Niebla J, Baranchuck A, Bayés de Luna A. True Brugada pattern or only high V1-V2 electrode placement? J Electrocardiol. 2014;47:756–8. doi: 10.1016/j.jelectrocard.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 53.Baranchuk A, Nguyen T, Ryu MH et al. Brugada Phenocopy: New Terminology and Proposed Classification. Ann Noninvas Electrocardiol. 2012;17:299–314. doi: 10.1111/j.1542-474X.2012.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kligfield P, Gettes LS, Bailey JJ et al. AHA/ACC/HRS Ssinetific Statements. Recommendations for the Standardization and Interpretation of the Electrocardiogram Part I: The Electrocardiogram and Its Technology. J Am Coll Cardiol. 2007;49:1109–27. doi: 10.1016/j.jacc.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 55.García-Niebla J, Serra-Autonell G, Bayés de Luna A. Brugada syndrome electrocardiographic pattern as a result of improper application of a high pass filter. Am J Cardiol. 2012;110:318–20. doi: 10.1016/j.amjcard.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 56.García-Niebla J, Serra-Autonell G, Fiol M, Bayés de Luna A. Brugada electrocardiographic pattern: Reality or fiction? J Electrocardiol. 2014;47:362–3. doi: 10.1016/j.jelectrocard.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Mizumaki K, Fujiki A, Nishida K et al. Bradycardia-dependent ECG changes in Brugada syndrome. Circ J. 2006;70:896–901. doi: 10.1253/circj.70.896. [DOI] [PubMed] [Google Scholar]
- 58.Amin AS, de Groot EA, Ruijter JM et al. Exercise-induced ECG changes in Brugada syndrome. Circ Arrhythm Electrophysiol. 2009;2:531–9. doi: 10.1161/CIRCEP.109.862441. [DOI] [PubMed] [Google Scholar]
- 59.Matsuo K, Shimizu W, Kurita T et al. Dynamic changes of 12-lead electrocardiograms in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 1998;9:508–12. doi: 10.1111/j.1540-8167.1998.tb01843.x. [DOI] [PubMed] [Google Scholar]
- 60.Veerakul G, Nademanee K. Treatment of electrical storms in Brugada syndrome. J Arrhythmia. 2013;29:117–24. [Google Scholar]
- 61.Morita H, Kusano-Fukushima Nagase S et al. Atrial fibrillation and atrial vulnerability in patients with Brugada syndrome. J Am Coll Cardiol. 2002;40:1437–44. doi: 10.1016/s0735-1097(02)02167-8. [DOI] [PubMed] [Google Scholar]
- 62.Kusano KF, Taniyama MK, Nakamura K et al. Atrial fibrillation in patients with Brugada syndrome relationships of gene mutation, electrophysiology, and clinical backgrounds. J Am Coll Cardiol. 2008;51:1169–75. doi: 10.1016/j.jacc.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 63.Probst V, Veltmann C, Eckardt L et al. Long-Term Prognosis of Patients Diagnosed With Brugada Syndrome: Results from the FINGER Brugada syndrome Registry. Circulation. 2010;121:635–43. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 64.Brugada J, Brugada R, Antzelevitch C et al. Long-term follow-up of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Circulation. 2002;105:73–8. doi: 10.1161/hc0102.101354. [DOI] [PubMed] [Google Scholar]
- 65.Takagi M, Sekiguchi Y, Yokoyama Y et al. Long-term prognos is inpatients with Brugada syndrome based on Class II indication for implantable cardioverter-defibrillator in the HRS/EHRA/APHRS Expert Consensus Statement: Multicenter study in Japan. Heart Rhythm. 2014;11:1716–20. doi: 10.1016/j.hrthm.2014.06.033. for the Japan Idiopathic Ventricular Fibrillation Study (J-IVFS )Investigators. [DOI] [PubMed] [Google Scholar]
- 66.Sacher F, Probst V, Iesaka Y et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study. Circulation. 2006;114:2317–24. doi: 10.1161/CIRCULATIONAHA.106.628537. [DOI] [PubMed] [Google Scholar]
- 67.Sarkozy A, Boussy T, Kourgiannides G et al. Long-term follow-up of primary prophylactic implantable cardioverter-defibrillator therapy in Brugada syndrome. Eur Heart J. 2007;28:334–44. doi: 10.1093/eurheartj/ehl450. [DOI] [PubMed] [Google Scholar]
- 68.Rosso R, Glick A, Glikson M et al. Outcome after implantation of cardioverter defibrillator [corrected] in patients with Brugada syndrome: a multicenter Israeli study (ISRABRU). Isr Med Assoc J. 2008;10:435–9. [PubMed] [Google Scholar]
- 69.Kamakura S, Ohe T, Nakazawa K et al. Long-term prognosis of probands with Brugada-pattern ST-elevation in leads V1– V3. Circulat Arrhythmia Electro-Physiol. 2009;2:495–503. doi: 10.1161/CIRCEP.108.816892. [DOI] [PubMed] [Google Scholar]
- 70.Letsas KP, Weber R, Efremidis M et al. Long-term prognosis of asymptomatic individuals with spontaneous or drug-induced type 1 electrocardiographic phenotype of Brugada syndrome. J Electrocardiol. 2011;44:346–9. doi: 10.1016/j.jelectrocard.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Champagne J, Philippon F, Gilbert M et al. The Brugada syndrome in Canada: a unique French-Canadian experience. Can J Cardiol. 2007;23(Suppl B):71B. doi: 10.1016/s0828-282x(07)71014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eckardt L, Probst V, Smits JP et al. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation. 2005;111:257. doi: 10.1161/01.CIR.0000153267.21278.8D. [DOI] [PubMed] [Google Scholar]
- 73.Atarashi H, Ogawa S. Idiopathic Ventricular Fibrillation Investigators. New ECG criteria for high-risk Brugada syndrome. Circ J. 2003;67:8. doi: 10.1253/circj.67.8. [DOI] [PubMed] [Google Scholar]
- 74.Takagi M, Aonuma K, Sekiguchi Y et al. The Japan Idiopathic Ventricular Fibrillation Study. The prognostic value of early repolarization (J wave) and ST-segment morphology after J wave in Brugada syndrome: Multicenter study in Japan (J-IVFS) Investigators. Heart Rhythm. 2013;10:533–9. doi: 10.1016/j.hrthm.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 75.Yokokawa M, Noda T, Okamura H et al. Comparison of long-term follow-up of electrocardiographic features in Brugada syndrome between the SCN5A-positive probands and the SCN5A-negative probands. Am J Cardiol. 2007;100:649. doi: 10.1016/j.amjcard.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 76.Huikuri HV, Castellanos A, Myerburg RJ. Sudden Death Due to Cardiac Arrhythmias. N Engl J Med. 2001;345:1473–82. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 77.Sommariva E, Pappone C, Martinelli Boneschi F et al. Genetics can contribute to the prognosis of Brugada syndrome: a pilot model for risk stratification. Eur J Human Genetics. 2013;21:911–7. doi: 10.1038/ejhg.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishii N, Ogawa M, Morita H et al. SCN5A mutation is associated with early and frequent recurrence of ventricular fibrillation in patients with Brugada syndrome. Circ J. 2010;74:2572–8. doi: 10.1253/circj.cj-10-0445. [DOI] [PubMed] [Google Scholar]
- 79.Meregalli PG, Tan HL, Probst V et al. Type of SCN5A mutation determines clinical severity and degree of conduction slowing in loss-of-function sodium channelopathies. Heart Rhythm. 2009;6:341–8. doi: 10.1016/j.hrthm.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Priori SG, Napolitano C, Gasparini M et al. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105:1342–7. doi: 10.1161/hc1102.105288. [DOI] [PubMed] [Google Scholar]
- 81.Brugada J, Brugada R, Brugada P. Electrophysiologic testing predicts events in Brugada syndrome Patients. Heart Rhythm. 2011;8:1595–7. doi: 10.1016/j.hrthm.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 82.Sarkozy A, Paparella G, Boussy T et al. The usefulness of the consensus clinical diagnostic criteria in Brugada syndrome. Int J Cardiol. 2013;167:2700–4. doi: 10.1016/j.ijcard.2012.06.115. [DOI] [PubMed] [Google Scholar]
- 83.Gasparini M, Priori SG, Mantica M et al. Provocative tests in the Brugada syndrome: do we have the right tools? Circulation. 2000;102(suppl2):677. [Google Scholar]
- 84.Eckardt L, Kirchhof P, Schulze-Bahr E et al. Electrophysiologic investigation in Brugada syndrome. Yield of programmed ventricular stimulation at two ventricular sites with up to three premature beats. European Heart Journal. 2002;23:1394–401. doi: 10.1053/euhj.2002.3256. [DOI] [PubMed] [Google Scholar]
- 85.Priori SG, Gasparini M, Napolitano C et al. Risk stratification in Brugada syndrome results of the PRELUDE (Programmed Electrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59:37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 86.Zipes DP, Camm AJ, Borggrefe M et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. Circulation. 2006;114:e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 87.Wichter T, Matheja P, Eckardt L et al. Cardiac Autonomic Dysfunction in Brugada Syndrome. Circulation. 2002;105:702–6. doi: 10.1161/hc0602.103677. [DOI] [PubMed] [Google Scholar]
- 88.Rollin A, Sacher F, Gourraud JB et al. Prevalence, characteristics, and prognosis role of type 1 ST elevation in the peripheral ECG leads in patients with Brugada syndrome. Heart Rhythm. 2013;10:1012–8. doi: 10.1016/j.hrthm.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 89.Viskin S, Adler A, Rosso R. Brugada burden in Brugadasyndrome: The way to go in risk stratification? Heart Rhythm. 2013;7:1019–20. doi: 10.1016/j.hrthm.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 90.Shimizu W, Matsuo K, Takagi M, Tanabe Y, Aiba T, Taguchi A, Suyama K, Kurita T, Aihara N, Kamakura S. Body surface distribution and response to drugs of ST segment elevation in Brugada syndrome: clinical implication of eighty-seven-lead body surface potential mapping and its application to twelve-lead electrocardiograms. J Cardiovasc Electrophysiol. 2000;11:396–404. doi: 10.1111/j.1540-8167.2000.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 91.Sangwatanaroj S, Prechawat S, Sunsaneewitayakul B, Sitthisook S et al. New electrocardiographic leads and the procainamide test for the detection of the Brugada sign in sudden unexplained death syndrome survivors and their relatives. Eur Heart J. 2001;22:2290–6. doi: 10.1053/euhj.2001.2691. [DOI] [PubMed] [Google Scholar]
- 92.Teijeiro R, Garro HA, Acunzo RS et al. Recording of High V1-V3 Precordial Leads May Be Essential to the Diagnosis of Brugada Syndrome During the Ajmaline Test. J Cardiovasc Pharmacol and Therapeutics. 2006;11:153–5. doi: 10.1177/1074248406288760. [DOI] [PubMed] [Google Scholar]
- 93.Miyamoto K, Yokokawa M, Tanaka K et al. Diagnostic and prognostic value of a type 1 Brugada electrocardiogram at higher (third or second) V1 to V2 recording in men with Brugada syndrome. Am J Cardiol. 2007;99:53–7. doi: 10.1016/j.amjcard.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 94.Sarkozy A, Chierchia GB, Paparella G et al. Inferior and lateral electrocardio-graphic repolarization abnormalities in Brugada syndrome. Circulat Arrhythmia Electrophysiol. 2009;2:154–61. doi: 10.1161/CIRCEP.108.795153. [DOI] [PubMed] [Google Scholar]
- 95.Bastiaenen R, Raju H, Sharma S et al. Characterization of early repolarisation during ajmaline provocation and exercise tolerance testing. Heart Rhythm. 2013;10:247–54. doi: 10.1016/j.hrthm.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 96.Rosso R, MD, Glikson E, Belhassen B et al. Distinguishing “benign” from “malignant early repolarization”: The value of the ST-segment morphology. Heart Rhythm. 2012;9:225–9. doi: 10.1016/j.hrthm.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 97.Tokioka K, Kusano KF, Morita H et al. Electrocardiographic parameters and fatal arrhythmic events in patients with Brugada syndrome: combination of depolarization and repolarization abnormalities. J Am Coll Cardiol. 2014;63:2131–8. doi: 10.1016/j.jacc.2014.01.072. [DOI] [PubMed] [Google Scholar]
- 98.Sangawa M, Morita H, Nakatsu T et al. Abnormal transmural repolarization process in patients with Brugada syndrome. Heart Rhythm. 2009;6:1163–9. doi: 10.1016/j.hrthm.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 99.Miyamoto A, Hayashi H, Makiyama T et al. Risk determinants in individuals with a spontaneous type 1 Brugada ECG. Circ J. 2011;75:844–51. doi: 10.1253/circj.cj-10-0903. [DOI] [PubMed] [Google Scholar]
- 100.Castro Hevia J, Antzelevitch C, Tornes Barzaga F et al. Tpeak–Tend and Tpeak–Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828–34. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Take Y, Morita H, Wu J et al. Spontaneous electrocardiogram alterations predict ventricular fibrillation in Brugada syndrome. HeartRhythm. 2011;8:1014–21. doi: 10.1016/j.hrthm.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 102.Bordachar P, Reuter S, Garrigue S et al. Incidence, clinical implications and prognosis of atrial arrhythmias in Brugada syndrome. Eur Heart J. 2004;25:879–84. doi: 10.1016/j.ehj.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Makimoto H, Nakagawa E, Takak I H et al. Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J Am Coll Cardiol. 2010;56:1576–84. doi: 10.1016/j.jacc.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 104.Shimizu A. Indication of ICD in Brugada syndrome. J Arrhythmia. 2013;29:110–6. [Google Scholar]
- 105.Okin PM, Devereux RB, Fabsitz RR et al. Principal Component Analysis of the T Wave and Prediction of Cardiovascular Mortality in American Indians. The Strong Heart Study. Circulation. 2002;105:714–9. doi: 10.1161/hc0602.103585. [DOI] [PubMed] [Google Scholar]
- 106.Addison PS. Wavelet transforms and the ECG: a review. Physiol Meas. 2005;26:R155–99. doi: 10.1088/0967-3334/26/5/R01. [DOI] [PubMed] [Google Scholar]
- 107.Cellular basis for trigger and maintenance of ventricular fibrillation in the Brugada syndrome model: high-resolution optical mapping study. J Am Coll Cardiol. 2006;47:2074–85. doi: 10.1016/j.jacc.2005.12.064. [DOI] [PubMed] [Google Scholar]
- 108.Morita H, Zipes DP, Lopshire J et al. T wave alternans in an invitro canine tissue model of Brugada syndrome. Am J Physiol Heart Circ Physiol. 2006;291:H421–8. doi: 10.1152/ajpheart.01259.2005. [DOI] [PubMed] [Google Scholar]