Abstract
There has been a great evolution in the development of coronary stents in order to avoid both restenosis and thrombosis. Improvements have led to improvements in the design and conformation of metallic or resorbable structures, with an adequate balance between trackability and radial force, the development of antiproliferative drugs and the polymers to control release and allow adequate endothelialisation and an optimal duration of the antiplatelet regimen. Some suggestions are provided about the ideal characteristics of future coronary stents.
Keywords: Drug-eluting stent, -limus drugs, biodegradable polymers, bioresorbable stent, stent thrombosis, double antiplatelet therapy
When Andreas Grüntzig introduced balloon coronary angioplasty in 1977 it represented the first alternative to coronary artery bypass graft surgery. However, balloon dilatation had inherent limitations – including elastic recoil and vessel closure in the acute phase, as well as negative remodelling and restenosis in the late phase – which limited its applicability and further expansion. In the 1980s, bare metal stents (BMS) rapidly demonstrated superiority over balloon angioplasty, improving angiographic results and clinical outcomes. Despite these improvements, neointimal hyperplasia and restenosis continued to be major limitations of BMS technology. Drug-eluting stents (DES) were designed to minimise neointimal hyperplasia and reduce repeat revascularisation, but an increased risk of late stent thrombosis (ST) was observed with the first generation of devices. New DES have been developed to ensure good acute and long-term results while minimising stent thrombosis rate. Continuous innovation and research to improve all aspects of DES technology, such as platform material and structure, polymers, coating distribution, additional coating and antiproliferative drugs, have led to newer, improved generations of DES (Figure 1).[1] In this article, we review the diverse features of current and future developments in DES.
Figure 1: Drug-eluting Stent Evolution.
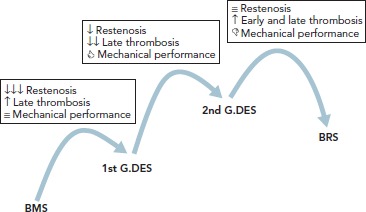
BMS = bare-metal stent; BRS = bioresorbable stent; G.DES = generation drug-eluting stent.
Metallic Drug-eluting Stents
Platform
The first DES were made of stainless steel and were coarse (up to 140 μm strut). New-generation DES are made of different kinds of alloys, such as cobalt chromium or platinum chromium, that are thinner (up to 60 μm strut), have high radial strength and radiopacity, and enhance biocompatibility as well as corrosion resistance.[2]
Thicker struts delay full neointimal coverage and increase the risk of subacute thrombosis. Research has shown that the thinner the strut, the better the endothelialisation.[3] Stents with thinner struts are more flexible, which enhances their trackability and crossability. In addition, stent strut thickness has been identified as an independent predictor of in-stent restenosis.[4]
Current stent designs are based on a sequential-ring construction method consisting of a series of expandable Z-shaped structural elements (known as struts) joined by connecting elements (known as bridges, hinges or nodes). In closed cell designs, the adjacent ring segments are connected at every possible junction. This provides greater radial force and scaffolding uniformity but reduces flexibility and conformability – even with flexible bridge connectors – compared to an open-cell design where some of the internal inflection points are joined by bridging connectors.[5,6] An open-cell configuration provides greater flexibility, adaptability and access to side-branches and has a higher resistance to fracture.
All of the currently available stents are made by laser-cutting metallic tubes. Continuous sinusoid technology is a manufacturing method that folds a single strand of cobalt alloy wire into a sinusoidal wave, enabling greater deliverability and conformability to the vessel wall. It remains to be seen whether nanotechnology will make the design of stents with ultrathin struts feasible in future.
Drug
First-generation DES used paclitaxel and sirolimus. Paclitaxel interferes with microtubule dynamics during mitosis by binding to the beta-tubulin subunit of the microtubules. The drug is cytostatic at the low doses used for coronary stents. It has a very high level of lipophilicity, which means that it can be linked to the stent without the use of a polymer. Sirolimus is a sophisticated natural antibiotic that was developed for its powerful immunosuppressive activity. It blocks protein synthesis, cell cycle progression and migration by inhibiting mammalian target of rapamycin.[7] Its better kinetics and wider therapeutic index are the reasons why the antirestenotic efficacy of sirolimus-eluting stents are higher than paclitaxel-eluting stents.[8,9] The antirestenotic efficacy of these drugs is the reason new-generation DES use the -limus family of drugs, which also includes everolimus, zotarolimus, umirolimus, novolimus and amphilimus. These drugs differ in terms of structure, molecular weight, potency and lipophilicity. Zotarolimus is a highly lipophilic analogue of sirolimus. It was designed to have a shorter in vivo half-life than sirolimus but the same high-affinity binding to the immunophilin FKBP12 along with comparable inhibition of t-cell proliferation in vitro.[10] Everolimus has a much higher interaction with mechanistic target of rapamycin complex 2, higher bioavailability and shorter half-life than sirolimus. Everolimus also reduces vascular inflammation.[11] The everolimus-eluting stent has shown more rapid endothelialisation.[12] Umirolimus has been specifically developed for local delivery to the coronary arteries. It is the most lipophilic of the common -limus drugs (around 10 times greater than sirolimus), which is why a low dose is used in free-polymer stents.[13] Novolimus is an active metabolite of sirolimus and has been shown to be a potent inhibitor of smooth muscle cells in in vitro studies.[14]
In the near future, the use of different drugs or combinations of drugs with different actions may address not only intimal proliferation but also thrombosis and, in the long term, in-stent neoatherosclerosis (Figure 2).
Figure 2: Optical Coherence Tomography Images of New-generation Drug-eluting Stents.
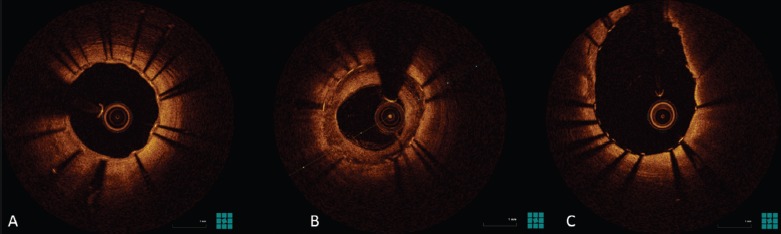
A: Well endothelialised struts. B: Restenosis. C: Uncovered struts.
Coating and Polymers
Great advances have been made in the field of coating and polymers. Drug release and availability are determined not only by the properties of the drug but also by the characteristics and architecture of the polymer that contains it. Depending on its composition, the polymer can cause undesirable inflammatory phenomena. The characteristics of an ideal polymer for use in a stent are given in Box 1.[17]
Box 1: Minimum Characteristics of an Ideal Polymer
Biocompatible and inert as well as compatible with the vessel. The polymer should not to produce inflammatory reactions or increase the risk of thrombosis but behave in a benign manner when implanted in a blood vessel.
Drug release from the polymer to the arterial wall should be predictable and capable of being modulated in time and dose in order to inhibit excess smooth muscle cell growth. Drug release should not affect the normal arterial endothelisation process.
Be highly elastic. The polymer particles should not be fractured, broken or detached on stent deployment.
Does not alter the activity of the drug or modify the structural and mechanical characteristics of the stent.
The durable polymers used in first-generation DES led to persistent arterial wall inflammation and delayed vascular healing, which contributed to stent thrombosis and delayed in-stent restenosis.[15] Although the durable polymers currently used in DES are less thrombogenic than those used in first-generation DES, doubts remain about their safety in the long term. This is why a huge effort has been made to develop both biodegradable-polymer and no-polymer stents.
Polymer coating can be conformal, inhibiting smooth cell proliferation over the entire surface of the stent, or abluminal, i.e. the release of the drug only has an effect on the surface in contact with the vessel wall. Abluminal coating reduces drug dose and polymer exposure, so it could theoretically reduce thrombogenicity while having a minimal affect on endothelial cell strut coverage.[16]
Of the new generation of biocompatible durable polymer coatings, two stand out: vinylidene-fluoride hexafluoropropylene copolymer and C10–C19–polyvinylpyrrolidone polymer.[18] Fluorinated copolymers such as vinylidene-fluoride hexafluoropropylene copolymer reduce protein adsorption, platelet adhesion and thrombus formation.[19] The blend of three different polymers – C10, C19 and polyvinylpyrrolidone – acts as an amphiphilic molecule. Its hydrophilic components face the stent surface, which is in contact with the cells, so it does not induce activated monocyte adhesion. This improves the biocompatibility of the polymer blend.
Biodegradable Polymers
It was hypothesised that durable polymers used in the first generation DES would trigger the inflammatory process and induce stent thrombosis.[16,20] Biodegradable polymer coatings, which are composed of lactic or glycolic acids, facilitate drug delivery to the vessel wall and are fully resorbed by hydrolysis after drug release without causing any long-term sequelae.[21]
Polylactic (PLLA, PDLLA), polyglycolic (PGA) and polylactic-co-glycolic (PLGA) copolymers are widely used for drug delivery. They differ not only in how they release the drug but also how long they take to degrade. A copolymer’s total degradation period may vary from 3 to 15 months. Future research is needed to optimise the composition and pharmacokinetics of copolymers.
Although the use of biodegradable polymers in newer-generation DES platforms looks promising, there are some issues that need to be resolved before their widespread clinical application.[22] Biodegradable polymers have a lower risk of late thrombosis than first-generation DES but not compared to the new generation of durable polymer DES.[23] Whether stents with biodegradable polymers require shorter double antiplatelet therapy (DAPT) than stents with durable polymers has yet to be properly assessed in randomised studies.[24]
Polymer-free Drug Eluting Stent
Inflammatory issues caused by the polymers used in stents can be avoided by eliminating the polymer coating completely and releasing the antiproliferative drug directly from the stent surface. Without a polymer coating, it would be expected that the elution rate would increase, which might affect the stent’s therapeutic efficacy.[25] To address this issue, stent manufacturers have adopted different approaches to decrease the elution rate. These approaches can be roughly separated into five categories: smooth surface, macroporous, microporous, nanoporous and drug-filled stents.[26] Perhaps the simplest polymer-free design is where the drug is coated directly onto the relatively unmodified smooth surface of the metal stent. With no polymer or pores to control drug release, the release rate is determined solely by the solubility and diffusion coefficient of the drug in the release medium and by the thickness of the coating. In macro-, micro- and nanoporous techniques, the surface of the stent is roughened. The drug is put into holes or slits in the body of macroporous stents. Pits and holes (in the order of microns) are made on the surface of microporous stents by a sandblasting or microabrasion process. The rough surface is then coated with the drug, resulting in the micropores being filled and a nominal layer covering the stent surface. The micropores act as a reservoir for the drug and aid adhesion to the stent surface. The nanopores of nanoporous stents are created by electrochemical treatments or sputter-coating techniques. These stents allow for a higher drug-loading capacity. Finally, drug-filled stents are a new and promising technology that allows the drug to be eluted from inside the stent through holes laser-drilled on the abluminal side.[27]
Additional Coating Technologies
PROBIO® is a passive amorphous silicon carbide coating that was developed to reduce the thrombogenic properties of metal stents. By providing a barrier against ion release, the silicon carbide coating creates a surface that reduces the deposition of fibrin, platelets and leucocytes, as well as enhancing the growth of endothelial cells. When a platelet comes into contact with the PROBIO coating, it remains in a resting state.[28]
Carbofilm™ is a high-density, ultra-thin (≤0.5 µm) turbostratic carbon film. It has a structure similar to diamond, giving it exceptional bio- and haemocompatibility and allowing extremely fast endothelialisation.[29]
Stents can also be coated with biological agents. CD34 antibodies are immobilised on the luminal surface of the stent from where they capture the circulating endothelial progenitor cells. The sheer stress triggered by the circulating blood and other cell signals leads the endothelial progenitor cells to differentiate and mature into endothelial cells, enhancing the vessel healing.[30]
Titanium nitride oxide can be used to coat all of the surfaces of a stent, resulting in the presence of nitric oxide particles. This nitric oxide coating is specifically active against restenosis and thrombosis, as well as being an accelerator of endothelialisation.[31] This, and other nanotextured ceramic coatings, could be advantageous but the benefits remain to be proved.[32] Some coronary stents characteristics are summed up in Table 1.
Table 1: Characteristics of Some Coronary Stents.
| Stent | Stent Material | Strut Thickness (µm) | Polymer | Polymer Type | Coating Distribution | Polymer Thickness (µm) | Absorption Time | Drug | Additional Coating | Drug Elution Time |
|---|---|---|---|---|---|---|---|---|---|---|
| Cypher | Stainless steel | 140 | PEVA/PBMA | Durable | Conformal | 13 | Permanent | Sirolimus | No | 90 days |
| Taxus Express | Stainless steel | 132 | SIBS | Durable | Conformal | 22 | Permanent | Paclitaxel | No | Lineal >180 days |
| Xience Alpine | CoCr | 81 | PVDF-HFP | Durable | Conformal | 7–8 | Permanent | Everolimus | No | 120 days |
| Xience Sierra | L-605 CoCr | 81 | PVDF-HFP | Durable | Conformal | 7–8 | Permanent | Everolimus | No | 120 days |
| Resolute Integrity | CoNi | 91 | BioLinx | Durable | Conformal | 6 | Permanent | Zotarolimus | No | 180 days |
| Resolute Onyx | CoNi with Pt-Ir | 81–91 | BioLinx | Durable | Conformal | 4.8 | Permanent | Zotarolimus | No | 180 days |
| Orsiro | CoCr | 60–80 | PLLA | Biodegradable | Conformal | 7 | 15 months | Sirolimus | Silicon carbide; conformal | 100–120 days |
| Ultimaster | CoCr | 80 | PDLLA-PCL | Biodegradable | Abluminal | 15 | 3–4 months | Sirolimus | No | 3–4 months |
| Synergy | PtCr | 79–81 | PLGA | Biodegradable | Abluminal | 4 | 3–4 months | Everolimus | No | 3 months |
| BioMatrix Nobori | Stainless steel | 120 | PDLLA | Biodegradable | Abluminal | 10 | 6–9 months | Biolimus | No | 6–9 months |
| Combo | Stainless steel | 100 | PDLLA-PLGA | Biodegradable | Abluminal | 5 | 90 days | Sirolimus | Anti CD-34 antibodies; comformal | 30–45 days |
| BioMatrix Flex | 316L Stainless steel | 112 | PDLLA | Biodegradable | Abluminal | 16.6 | 6–9 months | Biolimus | No | 180 days |
| BioFredoom | 316L Stainless steel | 119 | Polymer free | Polymer free | Abluminal | Polymer free | Polymer free | Biolimus | No | 30 days |
| Drug-filled stents | CoNi with Tantalum | 81 | Polymer free | Polymer free | Abluminal | Polymer free | Polymer free | Sirolimus | No | 180 days |
CoCr = cobalt–chromium; CoNi = cobalt–nickel; PBMA = poly n-butyl methacrylate; PCL = poly(e-caprolactone); PDLLA = poly-D, L-lactic acid; PEVA = poly-ethylene-co-vinyl acetate; PLGA = poly-lactic co-glycolic acid; PLLA = poly-L-lactic acid; PtCr = platinum–chromium; Pt-Ir = platinum–iridium; PVDF-HFP = co-polymer of vinylidene fluoride and hexafluoropropylene; SIBS = poly(styrene-b-isobutylene-b-styrene)
Bioresorbable Stent Technology
Bioresorbable stent (BRS) technology has been called the fourth revolution in interventional cardiology due to its potential advantages.[33] Apart from preventing acute vessel closure or recoil by transiently scaffolding the vessel, these fully-biodegradable scaffolds elute antiproliferative drugs that inhibit constrictive remodelling and neointimal hyperplasia. Complete resorption of the scaffold liberates the vessel from its cage and potentially restores vessel anatomy as well as vasomotor response, pulsatility, cyclical strain, physiological shear stress and mechanotransduction. In-stent restenosis secondary to low-grade inflammatory response to the polymer or device is mitigated. The risk of late or very late thrombosis is eliminated as the foreign material (platform plus coating) is replaced by connective tissue and the scaffolded segment healed with matured endothelium. The use of BRS could shorten the DAPT administration period and reduce complications due to secondary bleeding.
Currently, there are many materials used as the backbone for scaffolding (Table 2). Magnesium alloys, PLLA and tyrosine polycarbonate are the most common. The bioresorbable vascular scaffold system (based on polylactic polymers) was the first to become commercially available and is by far the most widely used and tested (Figure 3).
Table 2: Characteristics of Some Bioresorbable Scaffolds.
| Bioresorbable Scaffold | Strut material | Strut Thickness (µm) | Polymer Type | Coating Distribution | Resorption Time (months) | Eluting Polymer | Drug | Drug Elution Time (months) |
|---|---|---|---|---|---|---|---|---|
| Absorb | PLLA, Pt markers | 157 | Bioresorbable | Conformal | >24 | PDLLA | Everolimus | 3 |
| Absorb-GT1 | PLLA, Pt markers | 157 | Bioresorbable | Conformal | >24 | PDLLA | Everolimus | 3 |
| DESolve 100 | PLLA, Pt-Ir markers | 100 | Bioresorbable | Conformal | 24 | PLLA | Novolimus | 3 |
| MeRes | PLLA, Pt-Ir markers | 100 | Bioresorbable | Conformal | 24–36 | PDLLA | Sirolimus | 3 |
| Magmaris | Mg backbone, Ta markers | 150 | Bioresorbable | Conformal | 12 | PLLA | Sirolimus | 3 |
Ir = Iridium; Mg = magnesium; PDLLA = poly-D, L-lactic acid; PLLA = poly-L-lactic acid. Pt = platinum; Ta = tantalum;
Figure 3: Optical Coherence Tomography Images of Bioresorbable Scaffolds.
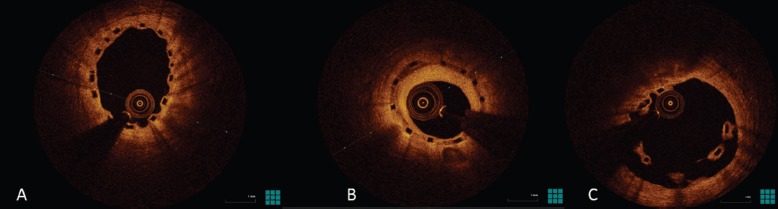
A: Well endothelialised struts. B: Restenosis. C: Covered but malappositioned struts.
There are still many issues that lead the interventional cardiologist to be cautious about BRS. There are practical concerns about strut thickness (up to 170 μm), which may lead to vessel injury, rheological disturbances, platelet deposition and poor deliverability. Thus, mechanical considerations are more challenging, especially when calcification or tortuosity are present. To address this issue, new bioresorbable designs with thinner struts (about 100 µm) will become available soon.[34] Regardless of lesion anatomy, due to lack of radial strength and poor deliverability in BRS, pre-dilatation is mandatory to facilitate lesion crossing and attain adequate expansion. Post-dilatation is also mandatory to ensure correct expansion and apposition. Defective healing and late adverse reactions may therefore not be completely avoided with the use of BRS. These technical particularities mean that the total cost and duration of percutaneous coronary intervention with BRS may be higher than with conventional DES.
There are other drawbacks to BRS. The most challenging issue is the process of resorption and scaffold disintegration in human coronary arteries with atherosclerosis. There is an increased scaffold fracture risk with over-dilatation of BRS; thus, significant upsizing is impossible. Recently, scaffold collapse has been described in subacute and late coronary thrombosis.[35,36] In addition to this, greater shear stress from the thick struts of current BRS may cause platelet activation. Observational findings from cases with very late thrombosis show the presence of largely dismantled scaffold remnants 2–3 years after implantation. These concerns have been confirmed clinically in trials. For example, there is a significantly higher risk of subacute and very late thrombosis with BRS compared to metallic everolimus-eluting stents.[37] Although resorption is achieved after a relatively short period of time in some devices, others take over 24 months (Table 2), and so the optimal duration of DAPT in conjunction with BRS application is unclear. Having highlighted these issues, we think this technology is worth pursuing and hope that research will overcome most, if not all, of its limitations.
Looking to the Future
Although considerable advances have been made, the ideal DES system has yet to be developed. The occurrence of stent thrombosis has accelerated technological evolution in interventional cardiology and the eradication of this fatal outcome should be the focus of new DES.
Since the advent of DES, restenosis figures have dropped to a single digit, even for the most complex lesions. The most recent generation of DES are associated with a greater reduction in the risk of early and late thrombosis than BMS. However, target lesion-related events are still observed years after implantation due to neoatherosclerosis. Future DES designs will have to address this issue. The ideal DES should incorporate a number of newer and improved materials and delivery systems to enhance safety, efficacy and cost-efficiency. The ideal system should include:[38]
A very low-profile stent delivery system
High flexibility and conformability due to a hybrid open-cell design
Thinner struts
Adequate radiopacity and radial strength
A high-pressure balloon that is suitable for direct stenting
Minimal late loss (≤0.2 mm)
A -limus drug.
Drug-elution for 60–90 days, followed by complete absence of drug release
Stimulation of early re-endothelialisation
A thrombus-resistant luminal surface
A very thin surface of durable or biodegradable polymer coating
A minimal duration of DAPT
The duration of DAPT for new DES could safely be shortened by up to 3 months in stable patients and 6 months in acute coronary syndrome patients who have a higher bleeding risk.[39] For specific models, such as BioFreedom, the duration of DAPT could be shortened to 1 month with better results than a BMS.[40] Other new DES are being evaluated in trials of 1 month of DAPT in patients with high bleeding risk.
The safety and efficacy of contemporary stents are supported by evidence from clinical trials and registries; however, larger trials and longer follow-up are necessary to assess the effectiveness of novel devices. The risk of late thrombosis with first-generation metallic DES and the risk of early and very late thrombosis with BRS were not properly identified in the preclinical stages of research. Animal models’ ability to reveal the significant long-term limitations of devices implanted in diseased coronary arteries of humans is limited. The more complex the interplay between a device and arterial wall-plaque, the harder it is to predict the long-term effects in humans. From BMS to DES, and particularly to BRS, stents are increasing in complexity. Computational models based on finite element analysis could complement the animal data but new advances in animal models will be crucial.
In summary, a validated and standardised set of preclinical studies is warranted before clinical studies of new stent models are conducted. Once a device is approved for use in humans, a well-designed programme of clinical studies is warranted before it is widely introduced on the market.
References
- 1.Stefanini GG, Byrne RA, Windecker S, Kastrati A. State of the art: coronary artery stents – past, present and future. EuroIntervention. 2017;13:706–16. doi: 10.4244/EIJ-D-17-00557. [DOI] [PubMed] [Google Scholar]
- 2.Karjalainen PP, Nammas W, Airaksinen J. Optimal stent design: past, present and future. Interv Cardiol. 2014;6:29–44. [Google Scholar]
- 3.Simon C, Palmaz JC, Sprague EA. Influence of topography on endothelization of stents: clues for new designs. J Long Term Eff Med Implant. 2000;10:143–51. doi: 10.1615/.v10.i12.120. [DOI] [PubMed] [Google Scholar]
- 4.Kastrati A, Mehilli J, Dirschinger J et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816–21. doi: 10.1161/01.CIR.103.23.2816. [DOI] [PubMed] [Google Scholar]
- 5.Stoeckel D, Bonsignore C, Duda S. A survey of stent designs. Min Invas Ther Allied Technol. 2002;11:137–47. doi: 10.1080/136457002760273340. [DOI] [PubMed] [Google Scholar]
- 6.Wholey M, Finol E. Designing the ideal stent. Endovascular Today. 2007;6:25–34. [Google Scholar]
- 7.Marx SO, Marks AR. Bench to bedside: the development of rapamycin and its application in stent restenosis. Circulation. 2001;104:852–5. doi: 10.1161/01.CIR.104.8.852. [DOI] [PubMed] [Google Scholar]
- 8.Stettler C, Wandel S, Allemann S et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–48. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 9.Schomig A, Dibra A, Windecker S et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J Am Coll Cardiol. 2007;50:1373–80. doi: 10.1016/j.jacc.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 10.Xue L, Sharma R, Cochran K. Effects of rapamycin derivative ABT-578 on canine smooth muscle cells and endothelial cell proliferation. Preclinica. 2004;2:451–5. [Google Scholar]
- 11.Klawitter J, Nashan B, Christians U. Everolimus and sirolimus in transplantation – related but different. Expert Opin Drug Saf. 2015;14:1055–70. doi: 10.1517/14740338.2015.1040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grube E, Buellesfeld L. Everolimus for stent-based intracoronary applications. Rev Cardiovasc Med. 2004;5:S3–8. [PubMed] [Google Scholar]
- 13.Costa R, Lansky A, Abizaid A. Angiographic results of the first human experience with the biolimus A9 drug-eluting stent for de novo coronary lesions. Am J Cardiol. 2006;98:443–6. doi: 10.1016/j.amjcard.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 14.Abizaid A, Costa JR, Jr, Feres F. TCT-429: Single center, first-in-man study of the elixir novolimus eluting coronary stent system with durable polymer 24-month clinical safety and efficacy results. Am J Cardiol. 2009;104:158D. doi: 10.1016/j.amjcard.2009.08.454. [DOI] [Google Scholar]
- 15.Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Puranik AS, Dawson ER, Peppas NA. Recent advances in drug eluting stents. Int J Pharm. 2013;441:665–79. doi: 10.1016/j.ijpharm.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serra A, Miranda F, Venegas R. Análisis y características de los stents farmacoactivos disponibles en la actualidad. Futuros desarrollos. Rev Esp Cardiol Suplementos. 2007;7:8E–28. doi: 10.1016/S1131-3587(07)75778-3. [in Spanish]. [DOI] [Google Scholar]
- 18.Mori H, Gupta A, Torii S et al. Clinical implications of blood-material interaction and drug eluting stent polymers in review. Expert Rev Med Devices. 2017;14:707–16. doi: 10.1080/17434440.2017.1363646. [DOI] [PubMed] [Google Scholar]
- 19.Szott LM, Irvin CA, Trollsas M et al. Blood compatibility assessment of polymers used in drug eluting stent coatings. Biointerphases. 2016;11:029806. doi: 10.1116/1.4944586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raungaard B, Christiansen EH, Bøtker HE et al. Comparison of durable-polymer zotarolimus-eluting and biodegradable-polymer biolimus-eluting coronary stents in patients with coronary artery disease: 3-year clinical 0utcomes in the randomized SORT OUT VI trial. JACC Cardiovasc Interv. 2017;10:255–64. doi: 10.1016/j.jcin.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Byrne RA, Iijima R, Mehilli J et al. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc Interv. 2009;2:291–9. doi: 10.1016/j.jcin.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Muramatsu T, Onuma Y, Zhang YJ et al. Progress in treatment by percutaneous coronary intervention: the stent of the future. Rev Esp Cardiol (Engl Ed) 2013;66:483–96. doi: 10.1016/j.recesp.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Kalra A, Rehman H, Khera S et al. New-generation coronary stents: current data and future directions. Curr Atheroscler Rep. 2017;19:14. doi: 10.1007/s11883-017-0654-1. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, Hai O, Garg A et al. Duration of dual antiplatelet therapy following drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. Curr Probl Cardiol. 2017;42:404–17. doi: 10.1016/j.cpcardiol.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Costa RA, Abizaid A, Mehran R et al. Polymer-free biolimus A9-coated stents in the treatment of de novo coronary lesions: 4- and 12-month angiographic follow-up and final 5-year clinical outcomes of the prospective, multicenter BioFreedom FIM clinical trial. JACC Cardiovasc Interv. 2016;9:51–64. doi: 10.1016/j.jcin.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 26.McGinty S, Vo TT, Meere M et al. Some design considerations for polymer-free drug-eluting stents: a mathematical approach. Acta Biomater. 2015;18:213–25. doi: 10.1016/j.actbio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Worthley SG, Abizaid A, Kirtane AJ et al. First-in-human evaluation of a novel polymer-free drug-filled stent: angiographic, IVUS, OCT, and clinical outcomes from the RevElution study. JACC Cardiovasc Interv. 2017;10:147–56. doi: 10.1016/j.jcin.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Dahm JB1, Willems T, Wolpers HG et al. Clinical investigation into the observation that silicon carbide coating on cobalt chromium stents leads to early differentiating functional endothelial layer, increased safety and DES-like recurrent stenosis rates: results of the PRO-Heal Registry (PRO-Kinetic enhancing rapid in-stent endothelialisation). EuroIntervention. 2009;4:502–8. doi: 10.4244/EIJV4I4A85. [DOI] [PubMed] [Google Scholar]
- 29.Visconti G, Focaccio A, Tavano D et al. The CID Chrono cobalt-chromium alloy carbofilm-coated coronary stent system. Int J Cardiol. 2011;149:199–204. doi: 10.1016/j.ijcard.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Nakazawa G, Granada JF, Alviar CL et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc Interv. 2010;3:68–75. doi: 10.1016/j.jcin.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Karjalainen PP, Nammas W. Titanium-nitride-oxide-coated coronary stents: insights from the available evidence. Ann Med. 2017;49:299–309. doi: 10.1080/07853890.2016.1244353. [DOI] [PubMed] [Google Scholar]
- 32.Karimi M, Zare H, Bakhshian Nik A et al. Nanotechnology in diagnosis and treatment of coronary artery disease. Nanomedicine (Lond) 2016;11:513–30. doi: 10.2217/nnm.16.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wykrzykowska JJ, Onuma Y, Serruys PW. Vascular restoration therapy: the fourth revolution in interventional cardiology and the ultimate “rosy” prophecy. EuroIntervention. 2009;5:F7–8. doi: 10.4244/EIJV5IFA1. [DOI] [PubMed] [Google Scholar]
- 34.Boeder NF, Dörr O, Bauer T et al. Impact of strut thickness on acute mechanical performance: A comparison study using optical coherence tomography between DESolve 150 and DESolve 100. Int J Cardiol. 2017;246:74–9. doi: 10.1016/j.ijcard.2017.05.087. [DOI] [PubMed] [Google Scholar]
- 35.Braun D, Baquet M, Massberg S et al. Collapse of a bioresorbable novolimus-eluting coronary scaffold. JACC Cardiovasc Interv. 2016;9:13–4. doi: 10.1016/j.jcin.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Salmerón RJ, Pereira S, de Araujo D. Bioresorbable vascular scaffold collapse causes subacute thrombosis. J Invasive Cardiol. 2014;26:98–9. [PubMed] [Google Scholar]
- 37.Sorrentino S, Giustino G, Mehran R et al. Everolimus-eluting bioresorbable scaffolds versus everolimus-eluting metallic stents. J Am Coll Cardiol. 2017;69:3055–66. doi: 10.1016/j.jacc.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Thakkar AS, Dave BA. Revolution of drug-eluting coronary stents: an analysis of market leaders. Eur Med J. 2016;4:114–25. [Google Scholar]
- 39.Valgimigli M, Bueno H, Byrne RA et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–60. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 40.Garot P, Morice MC, Tresukosol D et al. 2-year outcomes of high bleeding risk patients after polymer-free drug-coated stents. J Am Coll Cardiol. 2017;69:162–71. doi: 10.1016/j.jacc.2016.10.009. [DOI] [PubMed] [Google Scholar]


