Abstract
The use of diuretics is common in patients with heart failure (HF), to relieve the congestive symptoms of HF. Although they are widely used, there are limited data on their ability to modulate HF-related morbidity and mortality. Diuretic efficacy may be limited by adverse neurohormonal activation and by ‘congestion-like’ symptoms. Diuretics are an extremely useful and varied class of agent for the management of hypervolaemic states. This review summarises the basic features of diuretics, including their mechanism of action, indications and adverse effects in heart failure.
Keywords: Heart failure, diuretic therapy, diuretic resistance, loop diuretics, thiazide diuretics, potassium-sparing diuretics
Heart failure (HF) is a syndrome defined by the failure of the heart to deliver oxygen at a rate commensurate with the requirements of the metabolising tissues, despite normal filling pressures (or only at the expense of increased filling pressures),[1] secondary to an abnormality of the cardiac structure or function.
HF is the most common cause of hospitalisation in patients over the age of 65.[2] The main manifestations of the syndrome are symptoms resulting from vascular congestion, such as shortness of breath, abdominal distension, oedema formation and symptoms resulting from low systemic perfusion. HF syndrome is of relevant economic importance and in the ADHERE study signs and symptoms of congestion were the most frequent cause of hospital admission.1 Congestion often develops gradually before admission and many patients may have elevated left ventricular (LV) filling pressures even when congestion (dyspnoea, jugular venous distension or oedema)[3] is absent. Diuretic therapy, and especially loop diuretic therapy, are the usual way of managing congestion, especially in volume-overloaded patients.[4] The most commonly used diuretics in HF are loop diuretics, thiazides and potassium-sparing diuretics.
This review focuses on the classes of diuretics, their role in cases of HF with volume overload and current approaches when treating this complex subset of patients.
Class of Diuretics
Loop Diuretics
Loop diuretics, reversibly, inhibit the Na+⁄2Cl-⁄K+ co-transporter of the thick ascending loop of Henle where one-third of filtered sodium is reabsorbed. This causes decreased sodium and chloride reabsorption and increased diuresis.[5]
Loop diuretics also enhance the synthesis of prostaglandins, which cause renal and venous dilatation. This explains some of the cardiac effects, such as reduction in pulmonary wedge pressure.[6] However, it is important to recognise that the diuretic actions of loop diuretics may be decreased by the concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs), possibly because this inhibits renal prostaglandin synthesis. Loop diuretics include furosemide, bumetanide, torsemide and ethacrynic acid.
While the bioavailability of oral furosemide ranges from 40 to 80 %, the bioavailability of torasemide and bumetanide exceeds 80 %; so these two molecules may be more effective in treating patients suffering from HF.[7]
A well-known consequence of loop diuretic therapy is depletion of other electrolytes, such as potassium, magnesium, calcium and chloride (see Table 1).
Table 1: Summary of Diuretic Drugs used in Heart Failure.
| Drug | Site of Action | Duration of Action | Common Starting Dosage | Maximum Dosage | Common Side Effects |
|---|---|---|---|---|---|
| Loop diuretics | Inhibition of Na-K-CI co-transporter in the thick ascending loop of Henle | Hypokalaemia, hypomagnesaemia, hyperuricaemia, hypocalcaemia, hyponatraemia, otoxicity | |||
| Furosemide | 7 h | 20 to 40 mg once or twice | 600 mg | ||
| Bumetanide | 4 to 6 h | 0.5 to 1.0 mg once or twice | 10 mg | ||
| Torasemide | 12 to 16 h | 10 to 20 mg once | 200 mg | ||
| Ethacrynic acid | 6 h | 25–50 mg once or twice | 200 mg | ||
| Thiazide-like diuretics | Inhibition of Na-Cl transporter at distal nephron | Hypokalaemia, hypomagnesaemia, hypercalcaemia, hyponatraemia, hyperuricaemia | |||
| Chlorothiazide | 6 to 12 h | 250 to 500 mg | Once or twice | 1,000 mg | |
| Chlorthalidone | 24 to 72 h | 12.5 to 25 mg once | 100 mg | ||
| Indapamide | 36 h | 2.5 mg once | 20 mg | ||
| Potassium-sparing diuretics | Inhibition of mineralcarticoid receptor or its effectors at distal nephron | Hyperkalaemia | |||
| Amiloride | 24 h | 5 mg once | 20 mg | ||
| Triamterene | 7 to 9 h | 50 to 75 mg twice | 200 mg | ||
| Spironolactone | 1 to 3 h | 12.5 to 25.0 mg once | 50 mg | Gynecomastia |
Thiazide Diuretics and Metolazone
Benzothiazide diuretics inhibit the sodium–chloride transporter at the distal portion of the ascending limb and the first part of the distal tubule. They prevent maximal dilution of urine, thus increasing free water clearance and excretion of sodium and chloride through the renal tubular epithelium. The increased delivery of sodium to the collecting ducts enhances the exchange of sodium with potassium and, as a result, potassium depletion.
They are less effective in patients with reduced glomerular filtration, because they exert their diuretic effects from the luminal side of the nephron. Although they are less potent than loop diuretics, they may work in synergy with them when a sequential segmental nephron blockade is achieved.
Thiazides also decrease peripheral vascular resistance by a mechanism which is, at present, not well understood, resulting in a decrease of blood pressure.[8]
Metolazone is not a thiazide but acts in a similar way. Metolazone is more potent than hydrochlorothiazide and retains its effectiveness even when there is severe glomerular filtration rate (GFR) reduction.
Potassium-sparing Diuretics
The potassium-sparing diuretics used for treating HF are the aldosterone receptor antagonists spironolactone and eplerenone. They act at the cortical collecting duct, in particular by reducing the absorption of sodium and water and increasing the excretion of hydrogen ions and potassium, and their action is mediated by the antagonism of the actions of mineral corticoids. Only 3 % of filtered sodium is reabsorbed at the collecting duct, so this class of drugs does not have an appreciable diuretic effect. However they are often used in association with other more effective diuretics to correct or prevent potassium deficiency. They are also significantly efficacious in reducing the deleterious effects of aldosterone on the cardiovascular system. Spironolactone is a non-selective aldosterone receptor antagonist, and thus endocrine-related adverse effects (such as gynecomastia) are relatively common when it is used. Eplerone has greater selectivity on the mineral corticoid receptor, and has fewer side effects.[9]
Diuretics in Chronic Heart Failure
Diuretics are used to achieve and maintain euvolaemia (the patient’s ‘dry weight’) with the lowest possible dose. This means that the dose must be adjusted, particularly after restoration of the dry body weight, to avoid the risk of dehydration, which leads to hypotension and renal dysfunction.[10] It is important that treatment with diuretics is always coupled with neuro-hormonal system blocking, in order to slow down the progress of the disease.
In general, due to their greater effectiveness, loop diuretics, such as furosemide, are the mainstay of diuretic therapy in HF. Indeed loop diuretics produce more intense and shorter diuresis than thiazides, which results in more gentle and prolonged diuresis. They are, however, less effective in patients with reduced kidney function.[10] As a general rule, doses of loop diuretics should be as low as possible, in order to maintain a euvolaemic state. Restricting the amount of sodium and water, daily weight monitoring and avoidance of NSAIDs are critical in preventing salt and water retention.
The commonly used loop diuretics only act for a short time, so common therapy schemes require twice-daily administration, in order to avoid post-diuretic rebound sodium retention.
Furosemide is by far the most common oral loop diuretic, but patients with resistance to oral furosemide therapy may benefit from trials with second-generation oral loop diuretics (bumetanide and torasemide). These may be more efficacious, due to their increased oral bioavailability and potency. The longer half-life of torasemide may limit the previously described rebound phenomenon.[11] In the prospective TORasemide In Chronic heart failure (TORIC) study, the use of torasemide was associated with lower mortality than furosemide in patients with HF. Furthermore, torasemide has been reported to attenuate LV remodelling in patients with congestive HF (CHF) to a greater extent than furosemide.[12] Torasemide has also been reported to attenuate LV remodelling in patients with HF to a greater extent than furosemide.[13] Although international guidelines do not define which diuretic should be preferred, there is not enough strong evidence to recommend torasemide and bumetanide over furosemide in HF.
Careful monitoring and supplementation of electrolytes, particularly potassium and magnesium, are a crucial aspect of loop diuretic therapy.
Randomised clinical trials have shown that potassium-sparing diuretics are able to reduce both hospitalisations and mortality in patients with chronic HF, although they are less useful than loop diuretics in cases of acute decompensate HF.[14] Aldosterone levels are elevated in patients with acute decompensated heart failure (ADHF) despite the use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and beta-blockers. In this setting, aldosterone elevation may contribute to cardiorenal dysfunction, increasing the risk of death and ventricular arrhythmias.[15,16]
Studies have shown benefits using aldosterone antagonists in HF using non-diuretic doses of mineralcorticoid receptor antagonists. The objective was to completely inhibit the angiotensin–aldosteron axis. In the Emphasis-HF study, a double-blinded trial enrolling patients with chronic HF and low ejection fraction (EF), the aldosterone antagonist eplerenone compared with placebo showed a significant reduction in deaths from all causes, hospitalisation for HF and of the primary outcome (cardiovascular death or hospitalisation for HF).[17]
For these reasons, their use is strongly recommended in patients with HF. Their greater usefulness, as has already been mentioned, is not their diuretic properties, but their ability to antagonise the many harmful effects of hyperaldosteronism on the cardiovascular system. There are few studies in the literature describing the usefulness of high diuretic doses of aldosterone antagonists in ADHF in order to overcome congestion. In a exploratory study in ADHF patients, high doses of mineralcorticoid receptor antagonists (in more detail, about 100 mg spironolactone) were safe and were also associated with an earlier resolution of the congestive signs and with a more pronounced N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) reduction.[18]
Potassium-sparing diuretics have the disadvantage that their use results in a greater incidence of hyperkalaemia. However, when combined with loop diuretics, as happens frequently in clinical practice, this side effect is greatly reduced.
After overcoming the acute phase of HF, in selected subgroups it will be possible to make an attempt to withdraw diuretics. A history of hypertension, baseline furosemide dose of >40 mg/day, and a low LVEF (<27 %) were independent predictors of diuretic restarting.[19]
Diuretic Resistance
Diuretic resistance is a common problem in HF patients. Removal of excessive fluid is usually achieved by a combination of salt restriction and loop diuretics, but in some cases congestion persists despite adequate diuretic therapy. This has been termed diuretic resistance. The prevalence of diuretic resistance in the HF population is unknown due to the heterogeneity of the populations studied, the frequent comorbidity, the different treatment regimens, as well as to the different definitions used in various clinical trials. In a retrospective analysis of 1,153 patients with advanced HF, 402 patients had diuretic resistance (defined in this study as requirement of furosemide >80 mg or bumetanide >2 mg daily).[20] Diuretic resistance was independently associated with total mortality, sudden death and pump failure death. Loop diuretics are ‘threshold drugs’. HF shifts the dose-response curve for loop diuretics downward and to the right. Thus a higher starting dose of loop diuretics is needed in order to achieve the same level of sodium excretion.[21]
The shift of the dose–response curve in HF implicates insufficient dosing as a common cause of a lack of diuretic response (see Figure 1).[21,22] The magnitude of natriuresis following a defined dose of diuretics declines over time, even in normal subjects. This is the so-called ‘braking phenomenon’ and it is the result of both haemodynamic changes at the glomerulus as well as adaptive changes in the distal nephron. In a seminal study on rats by Kaissling, furosemide treatment was associated with cell hypertrophy at the distal convoluted tubule, the connecting tubule and the cortical collecting duct.[23] These structural changes after furosemide treatment suggest an increase in active transcellular transport capacity of this segment.[24] A partial explanation of these anatomical modifications may be the increased stimulation mediated by the renin-angiotensin and sympathetic nervous systems.[23] An abrupt increase in diuretic resistance in HF patients may be due to concomitant NSAID use or to an excessive intake of sodium. This may result in renal function deteriorating and development of cardiorenal syndrome.[25]
Figure 1: Schematic of a Dose-response Curve of Loop Diuretics in Heart Failure Patients Compared with Controls.
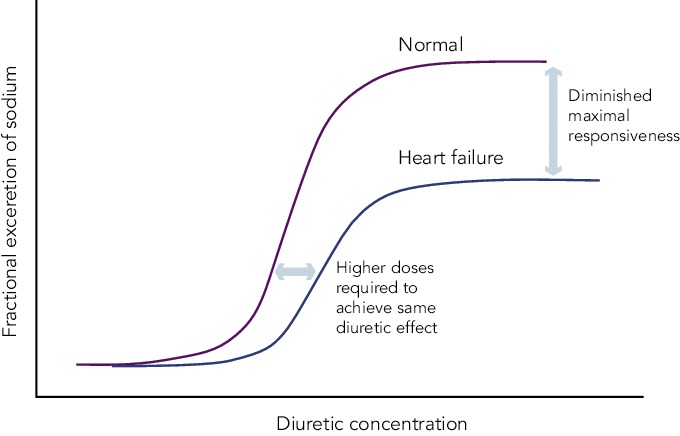
In heart failure patients, higher doses are required to achieve a given diuretic effect and the maximal effect is blunted. Adapted, with permission, from Ellison[21] and reprinted, with permission, from Felker Reproduced with permission from Felker.[22]
A response reduction to diuretic therapy is a common problem in patients with HF and while many studies have tried to give an exact clinical definition of diuretic resistance, others have tried to find a solution to the clinical problems that this causes. Probably the single most used and reproducible marker of cardiovascular congestion is body weight. As a result, HF guidelines advocate daily body weight monitoring in order to detect the pre-symptomatic phase in patients at risk to develop acute decompensated HF.[10] An interesting attempt to create a quantitative index of response to diuretic therapy was undertaken by Valente el al.[26] This index was obtained by comparing the administered dose of diuretic with the reduction of body weight and was intended to measure its effectiveness. It showed a significant correlation with relevant clinical variables and also highlighted a correlation with adverse events.
In another study, Testani et al. tested a metrical index of diuretic efficiency, which was defined as the net fluid lost per milligram of loop diuretic, thus demonstrating that low diuretic efficiency during decongestive therapy portends poorer long-term outcomes in patients hospitalised with decompensated HF.[27]
Once correctable variables and blockage of the neuroendocrine system have been excluded, a possible way of overcoming diuretic resistance is to use infusion therapy to avoid the limitations of oral bioavailability. For patients refractory to escalating doses of intravenous diuretics, options include use of continuous infusion rather than intermittent boluses. This strategy was tested in the DOSE study,[28] but no significant difference was noted between the two treatment groups.
Another approach is to administer two classes of diuretics together, a loop diuretic combined with a thiazide-like diuretic, thus performing a sequential nephron blockade.[29] Various mechanisms explain the success of this combination strategy: the longer half-life of thiazide diuretics helps to counteract the rebound post-diuretic effect (see Figure 2).[30] Thiazide-type diuretics inhibit sodium reabsorption in the distal nephron and primarily benefit patients who have distal nephron hypertrophy and hyperfunction due to chronic treatment with loop diuretics. Indeed, inhibiting NaCl transport along the distal tubule counteracts the reabsorption due to hyper-functioning cells in the distal tubule. In addition, they markedly increase the fractional sodium excretion, which is needed to achieve a neutral or negative sodium balance if the GFR is depressed.[31]
Figure 2: Mechanism of Diuretic Resistance.
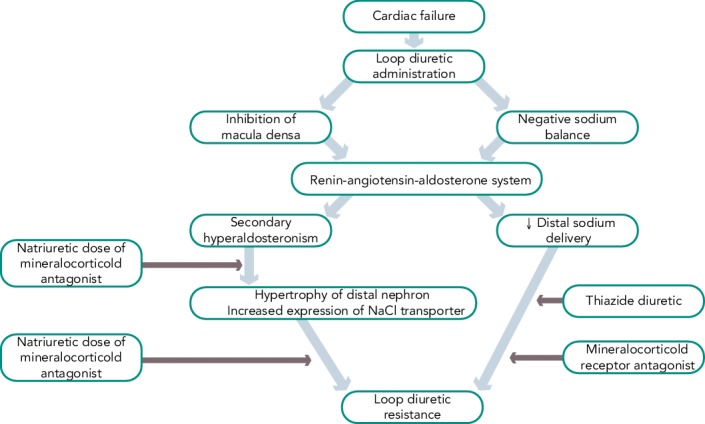
Reproduced with permission from Schrier et al.[30]
Numerous thiazide-like diuretics have been evaluated in combination with loop diuretics with similar results overall and there is no clear evidence that any single thiazide-like diuretic is superior to another, suggesting a class effect. It has been suggested that metolazone is superior to other thiazide-like diuretics in patients with advanced kidney disease, but other thiazide-like diuretics also increased the response to loop diuretics, even in patients with advanced renal failure. More recently, a small, retrospective, single-centre cohort study compared two of the most commonly used thiazide-like diuretics (oral metolazone and intravenous chlorothiazide) as add-on therapy to loop diuretics and no statistically significant differences in efficacy or safety were found.[32] In some European countries, metolazone and chlorothiazide are not available and the most commonly used thiazide-like diuretics for ADHF are hydrochlorothiazide and chlorthalidone. Chorthalidone’s half-life (48–72 hours) is longer than that of hydrochlorothiazide (6–12 hours), which might increase risk of adverse events in patients hospitalised for ADHF. Moreover, head-to-head studies comparing these for treating hypertension described an increased risk of hyponatraemia with chorthalidone.[33]
For these reasons, hydrochlorothiazide or metalazone could be the diuretic of choice for treating ADHF. The main problem when using sequential nephron blockage is the excessive depletion of water and electrolytes. Chronic thiazide diuretics use is a predictor of worsening renal function in chronic HF and this is of concern, given the adverse prognosis associated with worsening renal function in these patients. Impaired renal function with diuretic therapy can result from direct alterations in glomerular haemodynamics due to neurohormonal and intrarenal feedback mechanisms or from overt volume depletion. To address these common concerns we need to await results of ongoing clinical trials (between these, the ‘Safety and efficacy of the combination of loop with thiazide type diuretics in patients with decompensated HF’, will compare the strategy of sequential block through add-on hydrochlorothiazide versus therapy with loop diuretics alone). As a result of the above considerations, nowadays it is not easy to apply sequential nephron blockage to outpatient settings.[34]
Diuretic Therapy in Acute Decompensated Heart Failure
Fluid overload is a major pathophysiological mechanism underlying both acute decompensation episodes of HF and the progress of the syndrome. Loop diuretics remain a cornerstone in the pharmacological treatment of ADHF and are administered in about 90 % of patients hospitalised for HF.[1] These drugs are routinely used as initial therapy in ADHF due to their ability to greatly improve the symptoms. Conversely, because of their lower natriuretic effect, thiazide diuretics are used infrequently and are limited to cases where there is diuretic resistance. The same is true for potassium-sparing diuretics, which are only used in cases of refractory oedema or concomitant hypokalaemia.
One of the major concerns of clinicians is the effect of excessive diuretic therapy on the intra-arterial volume and, consequently, on the possible deleterious effects on renal function. Several studies have, indeed, demonstrated that there is a correlation between doses of diuretics and the worsening of the prognosis in patients with acute decompensated HF.[35] However, no definite causal relationship has been established between diuretic therapy, its dosage, and cardiovascular mortality. It is, indeed, virtually impossible to distinguish between the multiple confounding factors, because sicker patients present often with greater congestion and therefore receive higher doses of diuretics. The pathophysiological basis of many of these concerns is that these drugs, which cause intravascular volume depletion, could increase the hyperactivation of the neuroendocrine system with resulting detrimental consequences.[36,37]
Nowadays, despite many studies in ADHF on diuretic therapy, the only certainty is that such therapies can relieve the patient’s symptoms and reduce vascular congestion. It remains unclear what the preferred loop diuretic should be, what should be the appropriate combination, what is the optimal dosage and what should be the clinical goal. Current guidelines from the American College of Cardiology and the American Heart Association suggest that ‘Diuretics should be administered at doses sufficient to achieve optimal volume status and relieve congestion without inducing an excessively rapid reduction in intravascular volume.’[38]
New Approaches
Although in the majority of patients congestion symptoms are controlled by loop diuretic therapy, in a minority of cases other adjunctive therapies are needed. This is because of the progression of the disease or the worsening of the renal function.
Other solutions have been tested in addition to the aforementioned combination therapy (sequential nephron blockade). Some trials demonstrated the positive effects of incorporating hypertonic saline solution (HSS) with standard loop diuretic therapy.[39] In a large study of 1,771 patients, the SMAC-HF study, in-hospital HSS administration, combined with moderate sodium restriction, reduces hospitalisation time and increased diuresis. However, a long-term follow-up found that moderate salt restriction was associated with a better prognosis than a low sodium diet.[40] The potential benefits of this therapy are the faster recovery of intra-arterial volume. This reduces the neuro-endocrine stimulation and improves glomerular perfusion, thus counteracting the common mechanisms that underlie fluid overload in various clinical scenarios.[36] Regardless, this was an unblinded study and use of HSS is not recommended in current guidelines. Larger prospective and blinded studies need to be undertaken before this approach can be recommended for clinical use.
HF with concomitant severe hyponatraemia is of particular clinical relevance, due to its particular prognostic and therapeutic implications.[41] Such patients may benefit from treatment with arginin vasopressin antagonist (vaptans). This class of drugs can be useful in several cases of resistance to diuretics because of their specific action mechanisms.[42] Despite this and other anecdotal reports, after the results of the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan trial (Everest), tolvaptan is today approved by the US Food and Drug Administration only for the treatment of clinically significant hypervolaemic and euvolaemic hyponatraemia (serum sodium less than 125 meq/lL). This includes patients with HF and the syndrome of inappropriate antidiuretic hormone secretion.
Indeed in the EVEREST trial, an international, multicentre, randomised, double-blind, placebo-controlled trial in a population of hospitalised chronic HF patients, there was no difference in the global clinical status of the two groups, although the tolvaptan group had significantly decreased dyspnoea on day 1, and decreased weight and oedema after 7 days. It is noteworthy that patients in the tolvaptan group had significantly decreased loop diuretic use compared with the placebo group. Despite these initial results, the long-term primary outcome trial showed no significant difference in overall mortality.[43] In the future It would be interesting to design a specific clinical trial on use of vaptans in patients who developed diuretic resistance.
Another option to be used in most complex patients is the use of diuretics in association with ultrafiltration (UF) therapy. UF moves water and small to medium weight solutes across a semi-permeable membrane to reduce volume overload.
The first interesting, but controversial, data comes from the Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated HF (UNLOAD) trial. In this study treatment with UF resulted in significantly fewer hospital readmissions due to HF during a 90-day follow-up.[44] Unfortunately, the study was harshly criticised because of the low doses of diuretics used and the consequent reduced clinical reproducibility. In the recent Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF), a study designed to compare the effect of UF with that of stepped pharmacological therapy on renal function and weight loss in patients with HF who have worsening renal function and persistent congestion, UF patients in the UF group had a significantly greater increase in serum creatinine and more adverse events, including bleeding and vascular complications, as well as progressive renal dysfunction. Moreover, there was no significant difference in the outcome, including mortality and rehospitalisation, at 60 days.[45] However the latest American guidelines suggest that UF may be considered for use after all diuretic strategies have failed.[38] Further studies will be needed to assess what should be the exact role of UF in the management of patients with ADHF.
Conclusions
HF remains the most common cause of hospitalisation in patients over the age of 65 and the main symptoms are vascular congestion. Fluid overload is a major pathophysiological mechanism underlying both acute decompensation in HF and the progression of the syndrome. Although there has been a lot of controversy on the possible negative effects of diuretic therapy, due to the reduced intra-arterial volume with neuro-endocrine hyperactivation, no definite causal relationship has been established between diuretic therapy, its dosage and cardiovascular mortality.
Although there are three main classes of diuretics (loop diuretics, thiazide diuretics with metolazone and potassium-sparing diuretics), loop diuretics are most commonly used, because they have the most potent natriuretic action. Conversely, despite having a weak diuretic effect, potassium sparing diuretics have been shown to be significantly efficacious in improving the long-term prognosis in symptomatic HF patients.
Nowadays, the primary role of thiazide-like diuretics in CHF is to attempt to overcome diuretic resistance, thus performing a sequential nephron blockade when administered in association with loop diuretics.
Despite various attempts, due to the many confounding factors and the extreme heterogeneity of studied population, randomised trials failed to find any significant differences on optimal dosages and modality of administration of loop diuretics in acute HF.
More data will be needed before using arginine vasopressin antagonist clinically, since the results of randomised trials failed to show the expected benefits. The same is true for UF – until stronger clinical data are available, its use will be limited to selected cases in accordance with current guidelines.
Research of new physiology-based approaches designed to offset the primary determinants of water retention could improve the management of patients affected by CHF. Until then, diuretic therapy will remain the cornerstone in CHF.
Acknowledgments
The authors want to thank Dr Paola Berne for helpful discussions during the writing of this paper.
References
- 1.Adams KF Jr, Fonarow GC, Emerman CL et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119:S3–S10. doi: 10.1016/j.amjmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Goldsmith SR, Brandimarte F, Gheorghiade M. Congestion as a therapeutic target in acute heart failure syndromes. Prog Cardiovasc Dis. 2010;52:383–92. doi: 10.1016/j.pcad.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Brater DC. Diuretic therapy. N Engl J Med. 1998;339:387–95. doi: 10.1056/NEJM199808063390607. [DOI] [PubMed] [Google Scholar]
- 6.Raftery EB. Haemodynamic effects of diuretics in heart failure. Br Heart J. 1994;72(Suppl.):S44–S47. doi: 10.1136/hrt.72.2_suppl.s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray MD, Deer MM, Ferguson JA et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513–20. doi: 10.1016/s0002-9343(01)00903-2. [DOI] [PubMed] [Google Scholar]
- 8.Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther. 2014;19:5–13. doi: 10.1177/1074248413497257. [DOI] [PubMed] [Google Scholar]
- 9.Struthers A, Krum H, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clinical Cardiology. 2008;31:153–8. doi: 10.1002/clc.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMurray JJ, Adamopoulos S, Anker SD et al. ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 11.Masuyama T, Tsujino T, Origasa H et al. Superiority of long-acting to short-acting loop diuretics in the treatment of congestive heart failure. Circ J. 2012;76:833–42. doi: 10.1253/circj.cj-11-1500. [DOI] [PubMed] [Google Scholar]
- 12.Cosin J, Diez J. TORIC Investigators. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507–13. doi: 10.1016/s1388-9842(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 13.Lopez B, Querejeta R, Gonzalez A et al. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol. 2004;43:2028–35. doi: 10.1016/j.jacc.2003.12.052. [DOI] [PubMed] [Google Scholar]
- 14.Pitt B, Remme W, Zannad F et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 15.Aronson D, Burger AJ. Neurohormonal prediction of mortality following admission for decompensated heart failure. Am J Cardiol. 2003;91:245–8. doi: 10.1016/s0002-9149(02)03119-3. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt BM, Sammer U, Fleischmann I et al. Rapid nongenomic effects of aldosterone on the renal vasculature in humans. Hypertension. 2006;47:650–5. doi: 10.1161/01.HYP.0000205224.58715.cc. [DOI] [PubMed] [Google Scholar]
- 17.Zannad F, McMurray JJ, Krum H et al. Eplerenone in patients with systolic heart failure and mild symptoms. New Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira JP, Santos M, Almeida S et al. Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med. 2014;25:67–72. doi: 10.1016/j.ejim.2013.08.711. [DOI] [PubMed] [Google Scholar]
- 19.Grinstead WC, Francis MJ, Marks GF et al. Discontinuation of chronic diuretic therapy in stable congestive heart failure secondary to coronary artery disease or to idiopathic dilated cardiomyopathy. Am J Cardiol. 1994;73:881–6. doi: 10.1016/0002-9149(94)90815-x. [DOI] [PubMed] [Google Scholar]
- 20.Neuberg GW, Miller AB, O’Connor CM et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144:31–8. doi: 10.1067/mhj.2002.123144. [DOI] [PubMed] [Google Scholar]
- 21.Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology. 2001;96(3–4):132–43. doi: 10.1159/000047397. [DOI] [PubMed] [Google Scholar]
- 22.Felker MG. Diuretic management in heart failure. Congest Heart Fail. 2010;16(Suppl. 1):S68–S72. doi: 10.1111/j.1751-7133.2010.00172.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaissling B, Stanton BA. Adaptation of distal tubule and collecting duct to increased sodium delivery. I. Ultrastructure. Am J Physiol. 1988;255(6 Pt 2):F1256–68. doi: 10.1152/ajprenal.1988.255.6.F1256. [DOI] [PubMed] [Google Scholar]
- 24.Kaissling B, Bachmann S, Kriz W. Structural adaptation of the distal convoluted tubule to prolonged furosemide treatment. Am J Physiol. 1985;248(3 Pt 2):F374–81. doi: 10.1152/ajprenal.1985.248.3.F374. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson LW, Nohria A, Mielniczuk L. Torrent or torment from the tubule? Challenge of the cardiorenal connections. J Am Coll Card. 2005;45:2004–7. doi: 10.1016/j.jacc.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Valente MA, Voors AA, Damman K et al. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35:1284–93. doi: 10.1093/eurheartj/ehu065. [DOI] [PubMed] [Google Scholar]
- 27.Testani JM, Brisco MA, Turner JM et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. 2014;7:261–70. doi: 10.1161/CIRCHEARTFAILURE.113.000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felker GM, Lee KL, Bull DA et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knauf H, Mutschler E. Sequential nephron blockade breaks resistance to diuretics in edematous states. J Cardiovasc Pharmacol. 1997;3:367–72. doi: 10.1097/00005344-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Schrier RW. Role of Diminished Renal Function in Cardiovascular Mortality. Marker or Pathogenetic Factor? J Am Coll Cardiol. 2006;47:1–8. doi: 10.1016/j.jacc.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 31.Verbrugge FH, Grieten L, Mullens W. Management of the cardiorenal syndrome in decompensated heart failure. Cardiorenal Med. 2014;4:176–88. doi: 10.1159/000366168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moranville MP, Choi S, Hogg J et al. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther. 2015;33:42–9. doi: 10.1111/1755-5922.12109. [DOI] [PubMed] [Google Scholar]
- 33.Dhalla IA, Gomes T, Yao Z et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158:447–55. doi: 10.7326/0003-4819-158-6-201303190-00004. [DOI] [PubMed] [Google Scholar]
- 34.Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56:1527–34. doi: 10.1016/j.jacc.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 35.Hasselblad V, Stough WG, Shah MR et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE Trial. Eur J Heart Fail. 2007;9:1064–9. doi: 10.1016/j.ejheart.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrier RW. Body fluid volume regulation in health and disease: A unifying hypothesis. Ann Intern Med. 1990;113:155–9. doi: 10.7326/0003-4819-113-2-155. [DOI] [PubMed] [Google Scholar]
- 37.Bayliss J, Norell M, Canepaanson R et al. Untreated heartfailure – clinical and neuroendocrine effects of introducing diuretics. Br Heart J. 1987;57:17–22. doi: 10.1136/hrt.57.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Paterna S, Di Pasquale P, Parrinello G et al. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as a bolus, in refractory congestive heart failure. Eur J Heart Fail. 2000;2:305–13. doi: 10.1016/s1388-9842(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 40.Paterna S, Fasullo S, Parrinello G et al. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association Class III (Class C) (SMAC-HF study) Am J Med Sci. 2011;342:27–37. doi: 10.1097/MAJ.0b013e31820f10ad. [DOI] [PubMed] [Google Scholar]
- 41.Verbrugge FH, Steels P, Grieten L et al. Hyponatremia in Acute Decompensated Heart Failure. J Am Coll Cardiol. 2015;65:480–92. doi: 10.1016/j.jacc.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Jermyn R, Rajper N, Estrada C et al. Triple Diuretics and Aquaretic Strategy for Acute Decompensated Heart Failure due to Volume Overload. Case Rep Cardiol. 2013;2013:750794. doi: 10.1155/2013/750794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konstam M, Gheorghiade M, Burnett J et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–31. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 44.Costanzo MR, Saltzberg MT, Jessup M et al. Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) Investigators Ultrafiltration is associated with fewer rehospitalizations than continuous diuretic infusion in patients with decompensated heart failure: Results from UNLOAD. J Card Fail. 2010;16:277–84. doi: 10.1016/j.cardfail.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Bart BA, Goldsmith SR, Lee KL et al. Ultrafiltration in decompensated HF with CRS. N Engl J Med. 2012;367:2296–304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]


