Abstract
Familial hypercholesterolaemia is the most common monogenic disorder associated with premature coronary artery disease. Mutations are most frequently found in the LDL receptor gene. Clinical criteria can be used to make the diagnosis; however, genetic testing will confirm the disorder and is very useful for cascade screening. Early identification and adequate treatment can improve prognosis, reducing negative clinical cardiovascular outcomes. Patients with familial hypercholesterolaemia are considered at high cardiovascular risk and the treatment target is LDL cholesterol <2.6 mmol/l or at least a 50 % reduction in LDL cholesterol. Patients require intensive treatment with statins and ezetimibe and/or colesevelam. Recently, proprotein convertase subtilisin/kexin type 9 inhibitors have been approved for the management of familial hypercholesterolaemia on top of statins.
Keywords: Familial hypercholesterolaemia, genetic testing, cascade screening, statins
Familial hypercholesterolaemia (FH) is the genetic disorder most commonly associated with elevated LDL cholesterol (LDL-C) levels from birth and with premature atherosclerotic cardiovascular disease (ASCVD).[1] It is caused by mutations in genes related to the clearance of LDLs such as LDL receptor (LDLR), apolipoprotein B-100 (APOB) and proprotein convertase subtilisin/kexin type 9 (PCSK9).[2] The prognosis for patients with FH has improved in the past 30 years, with statins improving clinical outcomes and reducing total mortality.[3,4]
Different strategies have been proposed to improve early detection of the disorder and ensure adequate treatment to prevent the development of ASCVD. The detection of index cases (IC; the first individual diagnosed in the family) through different case-finding strategies and cascade screening in relatives using LDL-C levels and/or genetic testing is feasible and cost-effective, especially for identifying cases in young people.[5–7]
This comprehensive review focuses on epidemiology, diagnosis and screening programmes, the goals of treatment and current lipid-lowering therapy in FH.
Prevalence
Traditionally, the prevalence of heterozygous FH has been estimated to be one case in 500 in the general population (0.2%).[2] However, a recent systematic review and meta-analysis has shown a prevalence of one in 250 individuals – higher than initially thought.[8]
Due to a founder effect, the prevalence of the disorder can be higher– up to one in 50–67 of the general population – in specific populations.[9] In patients with genetically confirmed acute coronary syndromes, the prevalence of FH is up to 8.7%.[10,11]
The prevalence of homozygous FH (HoFH) has been estimated to be one in 1 million, based on the frequency of heterozygous FH among relatives’ survivors of MI.[12] However, a recent analysis established the prevalence of molecularly defined HoFH as being one in 300,000 individuals.[13]
Molecular Defects
FH is autosomal-dominantly inherited and >80% of cases are caused by functional mutations in the LDLR gene. As of October 2017, >1,700 different functional mutations in the LDLR gene had been described worldwide.[14,15] Five to 10% of FH cases are caused by mutations in the APOB gene[16,17] and <1% are caused by some specific ‘gain-of-function’ mutations in the PCSK9 gene.[14,18] These later mutations enhance the binding of PCSK9 to LDLR, increasing degradation of the receptor in the endosome, resulting in high cholesterol levels.[19] There is emerging evidence that some patients with FH phenotype in whom a mutation in these three genes has not been detected may have a polygenic hypercholesterolaemia. These individuals have significantly higher number of LDL-C-increasing variations and LDL-C levels than controls.[20]
A very rare recessive form of FH that is clinically similar to HoFH is caused by mutations in low-density lipoprotein receptor adaptor protein 1 (LDLRAP1). The remaining FH cases are driven by monogenic mutations in other genes related to high cholesterol levels, such as APOE, SREBP2, STAP1 and LIPA.[21]
Cardiovascular Disease in Familial Hypercholesterolaemia
ASCVD in FH results from prolonged exposure to very high levels of LDL-C from birth.[2] Vascular imaging studies in children have confirmed that carotid intima-media thickness is greater than in unaffected siblings by 8 years of age.[22] Other studies using coronary angiography or electron beam tomography have shown that coronary atherosclerosis is evident from age 17 in males and 25 in females, and that 25 % of adolescents with FH have detectable coronary calcium.[23,24]
The first manifestation of the disorder is usually MI, which occurs as early as the third decade of life, and on average 20 years earlier than in individuals without FH.[25,26] In the pre-statin era, a cumulative risk of fatal and non-fatal coronary events was observed in around 50 % of men and 30 % of women by the age of 60.[27] The first UK Simon Broome Register analysis in 1991 showed that younger FH patients had a 100-fold increase in coronary mortality and nearly 10-fold increase in total mortality compared to the general normolipidaemic population.[28] A recent analysis of the Copenhagen General Population Study showed a multifactorial adjusted odds ratio for coronary artery disease of 3.3 in carriers of a FH mutation.[29] Data from the Spanish Familial Hypercholesterolemia Longitudinal Cohort Study (SAFEHEART) showed a prevalence of ASCVD in molecularly-defined FH subjects of 13 %, which is threefold higher than their unaffected relatives.[30]
Cardiovascular manifestations are highly variable depending on the molecular basis of the FH, LDL-C levels and the presence of other risk factors including lipoprotein(a).[30–35] Patients with severe mutations are at higher risk than those patients with milder mutations.[32,33] Lipoprotein(a) >1.8 μmol/l has been established as a cardiovascular risk factor in FH and its interaction with the type of mutation has been observed in molecularly-defined FH patients.[34,35]
HoFH is characterised by accelerated atherosclerosis that mainly affects the aortic root and coronary ostium. Absolute LDL-C levels are related to the severity of cardiovascular disease. The first cardiovascular event often occurs during early adolescence, especially in those cases with severe mutations (LDLR-negative). LDLR-defective patients usually develop clinical cardiovascular disease by the age of 30.[36,37]
Subclinical Atherosclerosis
The atherosclerotic burden of FH can be demonstrated using non-invasive imaging techniques.[38–40] In recent years, coronary CT angiography has emerged as a safe and non-invasive method of assessing coronary atherosclerosis.[41] Cross-sectional studies have shown that FH patients have higher coronary artery calcium scores than non-FH individuals (Figure 1).[42,43] However, it is still necessary to determine whether imaging studies improve risk stratification, intensity of treatment and clinical outcomes if they are incorporated as part of FH management.
Figure 1: Coronary Artery Calcifications (Red Arrows) in a 37-year-old Man with Familial Hypercholesterolaemia.
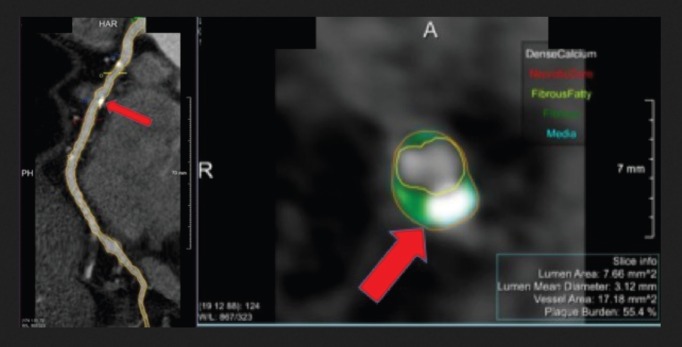
Cardiac CT allows us to establish the presence of coronary atherosclerotic plaques in asymptomatic subjects with familiar hypercholesterolaemia.
Clinical Diagnosis of Heterozygous FH
A clinical diagnosis of FH is made based on high plasma levels of LDL-C, family history of hypercholesterolaemia, a history of premature ASCVD and the presence of tendon xanthomas.[1,2] In general, LDL-C levels in adult patients are >4.9 mmol/l; however, lower cholesterol levels may be observed in some FH patients and their relatives, especially younger individuals, and an overlap in the distribution of LDL-C levels can be observed.[44,45] Triglyceride levels are usually normal; however, high levels do not exclude the diagnosis if other criteria strongly suggest FH. Tendon xanthomas are pathognomonic for the disorder and are associated with higher cardiovascular risk.[46,47] Xanthomas are present in <20 % of FH patients with a functional mutation;[30,48] therefore, the absence of xanthomas does not exclude the diagnosis of FH.
In the past 30 years, three different clinical criteria have been developed for the diagnosis of FH. The MEDical PEDigrees with FH to Make Early Diagnosis and Prevent Early Deaths (MedPed) programme focuses on lipid levels, and does not incorporate clinical characteristics or genetic testing.[49] The Simon Broome Register criteria for IC include lipid levels, tendon xanthomas, family history of hypercholesterolaemia, premature ASCVD and the presence of a functional mutation on genetic testing.[6] Relatives should be diagnosed using gender- and age-specific LDL-C levels.[50]
The most accepted and commonly used FH diagnosis criteria are the Dutch Lipid Clinic Network criteria (Table 1). These criteria calculate a score based on LDL-C levels, the presence of arcus cornealis and tendon xanthomas, hypercholesterolaemia and premature CVD in relatives, and positive genetic testing. A total score ≥8 makes the diagnosis definite.[51] These criteria should only be used for the identification of ICs.
Table 1: Clinical Criteria for the Diagnosis of Heterozygous Familial Hypercholesterolaemia from the Dutch Lipid Clinic Network.
| Family history | Score | |
| 1. First-degree relative with premature coronary heart disease or 2. First-degree relative with LDL cholesterol >95th percentile by age and gender for country 3. First-degree relative with xanthoma and/or arcus cornealis or 4. Children <18 years with LDL cholesterol >95th percentile by age and gender for country |
1 1 2 2 |
|
| Clinical history | ||
| 1. Premature coronary heart disease 2. Premature cerebral or peripheral vascular disease |
2 1 |
|
| Physical examination | ||
| 1. Tendon xanthoma 2. Arcus cornealis <45 years |
6 4 |
|
| LDL cholesterol | ||
| 1. >8.5 mmol/l 2. 6.5–8.4 mmol/l 3. 5.0–6.4 mmol/l 4. 4.0–4.9 mmol/l |
8 5 3 1 |
|
| DNA analysis | ||
| 1. Causative mutation in LDLR, APOB or PCSK9 | 8 | |
| Clinical diagnosis | ||
| Definite Probable Possible Unlikely |
>8 6–8 3–5 <3 |
|
Source: Nordestgaard et al., 2018.[7] Published with permission from Oxford University Press.
Diagnosis in Children and Adolescents
FH should be suspected in children and adolescents where LDL-C >4.9 mmol/l after the exclusion of secondary causes of hypercholesterolaemia, or where LDL-C >3.9 mmol/l and one parent has confirmed FH.[5–7,49] In the Netherlands, LDL-C >3.5 mmol/l can predict the presence of a LDLR mutation with a post-test probability of 0.98.[52] Lipid levels vary with age, especially during puberty, and some overlap in LDL-C levels might be observed. Total cholesterol and LDL-C discriminate better among children with and without FH at 1–9 years of age.[53] The affected parent should undergo genetic testing and, once the diagnosis has been confirmed, the implications of genetic testing in children should be discussed with them.[5,54,55]
Diagnosis of Homozygous Familial Hypercholesterolaemia
The diagnosis of HoFH is typically based on very high levels of LDL-C (untreated LDL-C >13 mmol/l or treated LDL-C >7.8 mmol/l on maximum lipid-lowering treatment) and the presence of cutaneous and tendon xanthomas in the first decade of life. Both parents must be heterozygous FH and should have elevated LDL-C levels.[36] Phenotypic expression of this condition is highly variable depending on the type of mutation.[37]
Genetic Testing
Genetic testing is the gold standard for the diagnosis of the disorder and facilitates cascade screening. A pathogenic mutation in one of the LDLR, APOB and PCSK9 genes is identified in 70–80 % of definite FH cases and in 20–40 % in those with a milder phenotype.[14,56] The Dutch Lipid Clinic Network criteria have better sensitivity and specificity than genetic testing.[57] The absence of a known mutation does not exclude a diagnosis of FH, especially in those cases with a strong phenotype. Different studies have shown that using only cholesterol levels in ICs or relatives leads to the misdiagnosis of approximately 18 % of carriers and non-carriers of a mutation.[58,59]
In addition to the importance of confirming the diagnosis, a positive result from genetic testing is associated with prognosis. For any LDL-C level, individuals carrying a mutation are at higher risk for ASCVD than those who do not have a mutation.[60] The type of mutation is also related to LDL-C levels and ASCVD risk, as shown in several studies.[32,61]
Screening Strategies
Early identification of FH is important for the prevention of coronary artery disease. At diagnosis, most patients are unaware of their condition and are receiving inadequate lipid-lowering therapy.[62–65] ICs should be identified among individuals with total cholesterol >8 mmol/l, with cardiovascular disease <60 years of age and/or with tendon xanthomas or premature arcus cornealis (Table 2).[6,7] When an IC is identified, the individual should be referred to a specialist for genetic testing, if available. Cascade screening using LDL-C measurement should be conducted in their relatives. If the mutation is known in the IC, consenting family members should also be offered a genetic test.[6,7,49] Molecular testing avoids misclassification and is included as part of the screening algorithm in countries like Spain, the Netherlands and Norway.[66,67] Different analyses have demonstrated that the identification of an adult IC through different case-finding strategies and cascade screening in relatives using lipid and/or genetic testing is the most efficient and cost-effective method of identifying new FH cases.[64,68,69] Analysis of the SAFEHEART Registry predicted that identifying 9,000 cases of FH in 10 years could prevent 847 coronary events and 203 coronary deaths and add 767 quality-adjusted life years.[69] Universal screening has the potential to detect more affected people in the community; for this, LDL-C levels should be measured in adults by the age of 20.[49]
Table 2: Criteria for Suspected Familial Hypercholesterolaemia According to Guidelines and Consensus Panels.
| Spanish Familial Hypercholesterolaemia Foundation[5] | European Atherosclerosis Society[7] | National Institute for Health and Care Excellence[6] | National Lipid Association[49] | |
|---|---|---|---|---|
| Adults | >18 years LDL-C >5.7 mmol/l plus one of the following:
|
>18 years Total cholesterol >8.0 mmol/l Premature coronary heart disease in case or family member Xanthoma in case or family member Sudden cardiac death in family member |
>16 years Total cholesterol >7.8 mmol/l LDL-C >4.9 mmol/l |
>20 years LDL-C >4.9 mmol/l Non-HDL-C >5.7 mmol/l |
| Children | <18 years LDL-C >4.9 mmol/l LDL >3.9 mmol/l plus high cholesterol and/or premature cardiovascular disease in one parent or DNA-positive in one parent |
<18 years LDL-C >4.9 mmol/l on two occasions after 3-month diet LDL-C >4.1 mmol/l plus premature coronary heart disease and/or high cholesterol in one parent |
<16 years LDL-C >4.1 mmol/l |
<20 years LDL-C >4.1 mmol/l Non-HDL-C >4.9 mmol/l |
| Exclusion of secondary causes | Exclusion of secondary causes | Exclusion of secondary causes | Exclusion of secondary causes |
HDL-C = HDL cholesterol; LDL-C = LDL cholesterol.
Screening in children is still controversial, especially in relation to the age at which testing should be carried out and lipid-lowering therapy started.[70,71] The US National Lipid Association recommends universal lipid screening of all children aged 9–11, and as early as 2 years of age if there is a family history of hypercholesterolaemia or premature ASCVD.[49] In some countries, children are screened between 2 and 5 years of age as part of cascade screening in families with a known diagnosis of the disorder.[5,53,55] In the UK, the National Institute for Health and Care Excellence guidelines recommend a genetic test is performed by the age of 10 in children who are at risk because they have one parent with FH.[6]
LDL-C Targets and Treatment of FH
Observational data have shown that statins significantly reduce the risk of coronary and total mortality in FH.[3,4,72] Intensive LDL-C reduction with statins or LDL apheresis also has beneficial effects on surrogate endpoints for ASCVD.[73–75] Despite aggressive lipid-lowering therapy, FH patients still experience cardiovascular events, partly due to uncontrolled risk factors.[76] Many studies have found that patients with FH are undertreated.[77,78] A recent analysis of the SAFEHEART Registry showed that although most patients were on the maximum lipid-lowering therapy after 5 years of follow-up, only 11 % had reached the target of LDL-C <2.6 mmol/l.[78] Despite the elevated lifetime risk for cardiovascular disease associated with FH, individuals’ risk profiles are heterogeneous. A cardiovascular risk equation including some classic risk factors and lipoprotein(a) and able to accurately predict incident ASCVD in molecularly-defined FH has recently been developed (Figure 2). Although recalibration with other populations is needed, this tool will improve risk stratification and treatment in FH patients.[79]
Figure 2: Five- Versus 10-year Risk of Developing Incident Atherosclerotic Cardiovascular Disease for 66-year-old Men with Familial Hypercholesterolaemia.
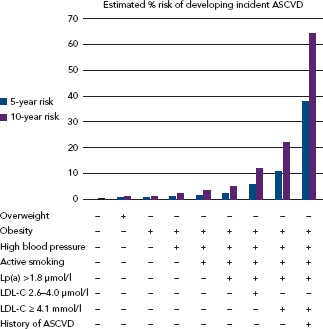
Changes in risk profile can be estimated according the modification of cardiovascular risk factors. ASCVD = atherosclerotic cardiovascular disease; LDL-C = LDL cholesterol; Lp(a) = lipoprotein(a). Source: Pérez de Isla et al., 2017,[30] with permission from Wolters Kluwer.
International guidelines consider LDL-C <2.6 mmol/l as the optimal target in adults with FH; <1.8 mmol/l in adults with FH and cardiovascular disease or type 2 diabetes; and at least a 50 % reduction in LDL-C levels if these goals cannot be achieved with maximally tolerated lipid-lowering therapy.[5–7,49] Adults with FH should be treated from the moment of diagnosis.
There is no evidence to support a target LDL-C level in children. Expert consensus-recommended targets are <4.1 mmol/l,[5,55] <3.5 mmol/l,[5,70] <2.6 mmol/l[80] and a reduction of 50 % from pre-treatment levels in children aged 8–10 years.[70] The recommended age at which to start lipid-lowering therapy varies between 8[81] and 10 years,[5,6,49,70,80] with[5] or without[70] differences between boys and girls. Analysis of 217 children <18 years old with FH in the SAFEHEART Registry found that the percentage achieving LDL-C <3.4 mmol/l increased from 20 % to 42 % after 4.7 years of follow-up. This result was principally explained by the use of statins.[82]
When pharmacological treatment is offered, the adult or child/adolescent or their parents or carer should be informed that treatment is lifelong.
Statins
All statins have been used in FH; however, most adult patients will require high-intensity statin therapy, such as atorvastatin 40–80 mg or rosuvastatin 20–40 mg.
In children with FH, treatment should be started at the lowest recommended dose and titrated up according to response and tolerability.[5,70,82] In HoFH, statins can be started at age 2 and can produce a modest and variable effect on LDL-C, depending on the type of mutation.[36]
Women with FH should receive pre-pregnancy counselling. They should be given instructions to stop any lipid-lowering treatment at least 4 weeks before discontinuing contraception and should not use statins during pregnancy and lactation.
Ezetimibe
In patients with primary hypercholesterolaemia, ezetimibe monotherapy reduced LDL-C levels by about 18 %. It can be safely co-administered with statins, resulting in up to a 23 % incremental decrease in LDC-C in FH patients.[83]
Bile Acid Sequestrants
Bile acid sequestrants are rarely used due to their adverse gastrointestinal side-effects and poor patient compliance. Colesevelam has a greater potency to bind bile acids, providing a much better tolerability profile than the other sequestrants. Colesevelam reduces LDL-C by 13–19 % when administered as monotherapy and by an additional 18 % when prescribed in combination with statins.[84]
PCSK9 Inhibitors
Evidence that loss-of-function mutations in the PCSK9 gene produce low levels of LDL-C and lower the incidence of cardiovascular disease[85] has further substantiated the role of PCSK9 as a potential target for the new generation of cholesterol-lowering drugs.
In 2015, the US Food and Drug Administration and European Medicines Agency approved evolocumab and alirocumab for the treatment of FH in patients who do not achieve LDL-C targets with maximum tolerated doses of conventional lipid-lowering therapy. The efficacy and safety of both these PCSK9 inhibitors have been demonstrated in FH patients with LCL-C inadequately controlled by statins and/or other lipid-lowering therapy.
A significant 50–60 % reduction in LDL-C over the reduction achieved by statins was obtained compared to placebo and they were well tolerated. Furthermore, >60 % of patients achieved LDL-C <1.8 mmol/l with PCSK9 inhibitors.[86,87]
The efficacy and tolerance of evolocumab have also been demonstrated in HoFH. A significant reduction in LDL-C levels of 20 % maintained after 48 weeks of treatment was observed in patients with and without apheresis.[88] This reduction is modest compared with its effect in heterozygous FH. The reduced efficacy is because PCSK9 inhibition requires some LDLR activity, which is almost absent in HoFH.
Recent results from long-term outcome trials of PCSK9 inhibitors in patients with ASCVD have shown a significant 15 % relative risk reduction for major cardiovascular events, supporting their cardiovascular benefit.[89,90] Although these trials were not carried out specifically in a FH cohort, they may also support the benefit of this class of drugs in this high-risk population.
Microsomal Triglyceride Transfer Protein Inhibitor
Lomitapide inhibits microsomal triglyceride transfer protein at the hepatocytes and enterocytes, preventing the assembly of triglycerides into very-low-density lipoprotein and chylomicrons. Lomitapide has been approved for the treatment of HoFH in people >18 years of age. LDL-C reductions of 50 % and 40 % at week 26 and 78, respectively, have been described.[91] Due to its mechanism of action, gastrointestinal side-effects (mild transaminase elevations and diarrhoea) are the most common adverse events. These effects are managed by gradual titration of the dose and adherence to the recommended low-fat diet. Hepatic fat was found to be increased by up to 8 % at week 26 in patients taking lomitapide, but no further increase was reported for the 18-month duration of the study.[91]
LDL Apheresis
LDL apheresis is safe and is the only long-term treatment with the potential to slow early atherosclerosis and prolong survival in HoFH patients.[92] LDL apheresis is also an option for patients with severe heterozygous FH, especially if pharmacological treatment insufficiently controls their LDL-C and their cardiovascular risk is still high.
Practical considerations have to be taken into account with this treatment. The cost, problems with insurance, venous access and the biweekly apheresis sessions are important issues that must be considered for each patient before proceeding.
Conclusion
FH is a common and treatable disorder. Early diagnosis and treatment will improve clinical cardiovascular outcomes. Identification of ICs and cascade screening using lipids and genetic testing in their relatives is cost-effective. Screening programmes are necessary to increase the number of cases identified and treated. Patients require high-intensity statin therapy and ezetimibe. For those not achieving target LDL-C, the new iPCSK9 are a good option for reducing LDL-C and cardiovascular risk. For severe HoFH, lomitapide and LDL apheresis are indicated.
Acknowledgments
The authors thank the Spanish Familial Hypercholesterolemia Foundation, especially Maria Teresa Pariente for the development of cascade screening and the SAFEHEART Registry.
References
- 1.Gidding SS, Champagne MA, de Ferranti SD et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167–92. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein JL, Hobbs HH, Brown MS. Volume 2. New York: McGraw-Hill: 2001. Familial hypercholesterolemia. In: Scriver CR Beaudet AL Sly WS Valle D The Metabolic and Molecular Basis of Inherited Disease. pp. 2863–913. [Google Scholar]
- 3.Scientific Steering Committee on behalf of the Simon Broome Register Group. Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Atherosclerosis. 1999;142:105–12. doi: 10.1016/S0021-9150(98)00200-7. [DOI] [PubMed] [Google Scholar]
- 4.Versmissen J, Oosterveer DM, Yazdanpanah M et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mata P, Alonso R, Ruiz A et al. Diagnosis and treatment of familial hypercholesterolemia in Spain: Consensus document. Aten Primaria. 2015;47:56–65. doi: 10.1016/j.aprim.2013.12.015. [in Spanish]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wierzbicki AS, Humphries SE, Minhas R. Familial hypercholesterolaemia: summary of NICE guidance. BMJ. 2008;337:509–11. doi: 10.1136/bmj.a1095. [DOI] [PubMed] [Google Scholar]
- 7.Nordestgaard BG, Chapman MJ, Humphries SE et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–90a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akioyamen LE, Genest J, Shan SD et al. Estimating the prevalence of heterozygous familial hypercholesterolemia: a systematic review and meta-analysis. BMJ Open. 2017;7:e016461. doi: 10.1136/bmjopen-2017-016461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leitersdorf E, Tobin EJ, Davignon J et al. Common low-density lipoprotein receptor mutations in the French Canadian population. J Clin Invest. 1990;85:1014–23. doi: 10.1172/JCI114531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanchen D, Gencer B, Auer R et al. Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur Heart J. 2015;36:2438–45. doi: 10.1093/eurheartj/ehv289. [DOI] [PubMed] [Google Scholar]
- 11.Amor-Salamanca A, Castillo S, Gonzalez-Vioque E et al. Genetically confirmed familial hypercholesterolemia in patients with acute coronary syndrome. J Am Coll Cardiol. 2017;70:1732–40. doi: 10.1016/j.jacc.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein J, Schrott H, Hazzard W et al. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest. 1973;52:1544–68. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjouke B, Kusters DM, Kindt I et al. Homozygous autosomal dominant hypercholesterolemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J. 2015;36:560–5. doi: 10.1093/eurheartj/ehu058. [DOI] [PubMed] [Google Scholar]
- 14.Palacios L, Grandoso L, Cuevas N et al. Molecular characterization of familial hypercholesterolemia in Spain. Atherosclerosis. 2012;221:137–42. doi: 10.1016/j.atherosclerosis.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Leigh SE, Foster AH, Whittall RA et al. Update and analysis of the University College London low density lipoprotein receptor familial hypercholesterolemia database. Ann Hum Genet. 2008;72:485–98. doi: 10.1111/j.1469-1809.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 16.Andersern LH, Miserez AR, Ahmad Z et al. Familial defective apolipoproteina B-100: A review. J Clin Lipidol. 2016;10:1297–302. doi: 10.1016/j.jacl.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Meriño-Ibarra E, Castillo S, Mozas S et al. Screening of APOB gene mutations in subjects with clinical diagnosis of familial hypercholesterolemia. Human Biol. 2005;77:663–73. doi: 10.1353/hub.2006.0005. [DOI] [PubMed] [Google Scholar]
- 18.Humphries SE, Whittall RA, Hubbart CS et al. Genetic causes of familial hypercholesterolemia in patients in the UK: relation to plasma lipid levels and coronary heart disease risk. J Med Genet. 2006;43:943–9. doi: 10.1136/jmg.2006.038356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abifadel M, Varret M, Rabes JP et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 20.Talmud PJ, Shah S, Whittall R et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolemia: a case-control study. Lancet. 2013;381:1293–301. doi: 10.1016/S0140-6736(12)62127-8. [DOI] [PubMed] [Google Scholar]
- 21.Henderson R, O’Kane M, McGilligan V et al. The genetics and screening of familial hypercholesterolemia. J Biomed Sci. 2016;23:39. doi: 10.1186/s12929-016-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusters DM, Wiegman A, Kastelein JJ et al. Carotid intima-media thickness in children with familial hypercholesterolemia. Circ Res. 2014;114:307–10. doi: 10.1161/CIRCRESAHA.114.301430. [DOI] [PubMed] [Google Scholar]
- 23.Mabuchi H, Koizumi J, Shimizu M et al. Development of coronary heart disease in familial hypercholesterolemia. Circulation. 1989;79:225–332. doi: 10.1161/01.CIR.79.2.225. [DOI] [PubMed] [Google Scholar]
- 24.Gidding SS, Bookstein LC, Chomka EV. Usefulness of electron beam tomography in adolescents and young adults with heterozygous familial hypercholesterolemia. Circulation. 1998;98:2580–3. doi: 10.1161/01.CIR.98.23.2580. [DOI] [PubMed] [Google Scholar]
- 25.Alonso R, Castillo S, Civeira F et al. Hipercolesterolemia familiar en España. Estudio descriptive de 819 casos no relacionados. Med Clin (Barc) 2002;118:487–92. doi: 10.1016/S0025-7753(02)72428-7. [in Spanish]. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins P, Stephenson S, Wu L et al. Evaluation of coronary risk factors in patients with heterozygous familial hypercholesterolemia. Am J Cardiol. 2001;87:547–53. doi: 10.1016/S0002-9149(00)01429-6. [DOI] [PubMed] [Google Scholar]
- 27.Slack J. Risks of ischaemic heart disease in familial hyperlipidemic states. Lancet. 1969;2:1380–2. doi: 10.1016/S0140-6736(69)90930-1. [DOI] [PubMed] [Google Scholar]
- 28.Scientific Steering Committee on behalf of the Simon Broome Register Group. The risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ. 1991;303:893–6. doi: 10.1136/bmj.303.6807.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benn M, Watts GF, Tybjaerg-Hansen A et al. Mutations causative of familial hypercholesterolemia: screening of 98098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J. 2016;37:1384–94. doi: 10.1093/eurheartj/ehw028. [DOI] [PubMed] [Google Scholar]
- 30.Pérez de Isla L, Alonso R, Mata N et al. Coronary heart disease, peripheral arterial disease, and stroke in familial hypercholesterolaemia: Insights from the SAFEHEART Registry (Spanish Familial Hypercholesterolaemia Cohort Study). Arterioscler Thromb Vasc Biol. 2016;36:2004–16. doi: 10.1161/ATVBAHA.116.307514. [DOI] [PubMed] [Google Scholar]
- 31.Jansen AC, van Aalst-Cohen ES, Tanck MW et al. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: data in 2400 patients. J Intern Med. 2004;256:482–90. doi: 10.1111/j.1365-2796.2004.01405.x. [DOI] [PubMed] [Google Scholar]
- 32.Alonso R, Mata N, Castillo S et al. Cardiovascular disease in familial hypercholesterolaemia. Influence of low-density lipoprotein receptor mutation type and classic risk factors. Atherosclerosis. 2008;200:315–22. doi: 10.1016/j.atherosclerosis.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Umans-Eckenhausen MA, Sijbrands EJ, Kastelein JJ et al. Low-density lipoprotein gene mutations and cardiovascular risk in a large genetic cascade screening population. Circulation. 2002;106:3031–6. doi: 10.1161/01.CIR.0000041253.61683.08. [DOI] [PubMed] [Google Scholar]
- 34.Alonso R, Andres E, Mata N et al. Lipoprotein(a) levels in familial hypercholesterolaemia: an important predictor for cardiovascular disease independent of the type of LDL-receptor mutation. J Am Coll Cardiol. 2014;63:1982–9. doi: 10.1016/j.jacc.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 35.Nordestgaard BG, Chapman MJ, Ray K et al. European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factors: current status. Eur Heart J. 2010;31:2844–53. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuchel M, Bruckert E, Ginsbrg HN et al. Homozygous familial hypercholesterolemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial hypercholesterolemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146–57. doi: 10.1093/eurheartj/ehu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso R, Díaz-Díaz JL, Arrieta F et al. Clinical and molecular characteristics of homozygous familial hypercholesterolemia patients: Insights from SAFEHEART Registry. J Clin Lipidol. 2016;10:953–61. doi: 10.1016/j.jacl.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 38.De Groot E, Hovingh GK, Wiegman A et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109:33–8. doi: 10.1161/01.CIR.0000131516.65699.ba. [DOI] [PubMed] [Google Scholar]
- 39.Junyet M, Gilabert R, Zambón D et al. Femoral atherosclerosis in heterozygous familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2008;28:580–6. doi: 10.1161/ATVBAHA.107.153841. [DOI] [PubMed] [Google Scholar]
- 40.Caballero P, Alonso R, Rosado P et al. Detection of subclinical atherosclerosis in familial hypercholesterolemia using non-invasive imaging modalities. Atherosclerosis. 2012;222:468–72. doi: 10.1016/j.atherosclerosis.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 41.Min J, Shaw L, Berman D. The present state of coronary computed tomography angiography. J Am Coll Cardiol. 2010;55:957–65. doi: 10.1016/j.jacc.2009.08.087. [DOI] [PubMed] [Google Scholar]
- 42.Miname MH, Ribeiro MS, Parga Filho J et al. Evaluation of subclinical atherosclerosis by computed tomography coronary angiography and its association with risk factors in familial hypercholesterolemia. Atherosclerosis. 2010;213:486–91. doi: 10.1016/j.atherosclerosis.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Neefjes LA, Ten Kate GJ, Alexia R et al. Accelerated subclinical coronary atherosclerosis in patients with familial hypercholesterolemia. Atherosclerosis. 2011;219:721–7. doi: 10.1016/j.atherosclerosis.2011.09.052. [DOI] [PubMed] [Google Scholar]
- 44.Huigen R, Hutten BA, Kindt I et al. Discriminative ability of LDL-C to identify patients with familial hypercholesterolemia: a cross-sectional study in 26,406 individuals tested for genetic FH. Circ Cardiovasc Genet. 2012;5:354–9. doi: 10.1161/CIRCGENETICS.111.962456. [DOI] [PubMed] [Google Scholar]
- 45.Campagna F, Martino F, Bifolco M et al. Detection of familial hypercholesterolemia in a cohort of children with hypercholesterolemia: results of a family and DNA-based screening. Atherosclerosis. 2008;196:356–64. doi: 10.1016/j.atherosclerosis.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Civeira F, Castillo S, Alonso R et al. Tendon xanthomas in familial hypercholesterolemia are associated with cardiovascular risk independently of the low-density lipoprotein receptor gene mutation. Arterioscler Thromb Vasc Biol. 2005;25:1960–5. doi: 10.1161/01.ATV.0000177811.14176.2b. [DOI] [PubMed] [Google Scholar]
- 47.Oosterveer D, Versmissen J, Yazdanpanah M et al. Differences in characteristics and risk of cardiovascular disease in familial hypercholesterolemia patients with and without tendon xanthomas: A systematic review and meta-analysis. Atherosclerosis. 2009;207:311–7. doi: 10.1016/j.atherosclerosis.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Bourbon M, Alves AC, Alonso R et al. Mutational analysis and genotype-phenotype relation in familial hypercholesterolemia: The SAFEHEART registry. Atherosclerosis. 2017;262:8–13. doi: 10.1016/j.atherosclerosis.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg A, Hopkins P, Toth P et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adults patients clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1–8. doi: 10.1016/j.jacl.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Starr B, Hadfield SE, Hutten BA et al. Development of sensitive and specific age- and gender-specific low-density lipoprotein cholesterol cutoffs for diagnosis of first-degree relatives with familial hypercholesterolemia in cascade testing. Clin Chem Lab Med. 2008;46:791–803. doi: 10.1515/CCLM.2008.135. [DOI] [PubMed] [Google Scholar]
- 51.Familial Hypercholesterolemia – Report of a Second WHO Consultation. Geneva: World Health Organization;: 1999. World Health Organization. [Google Scholar]
- 52.Wiegamn A, Rodenburg J, de Jongh S et al. Family history and cardiovascular risk in familial hypercholesterolemia. Data in more than 1000 children. Circulation. 2003;107:1473–8. doi: 10.1161/01.CIR.0000058166.99182.54. [DOI] [PubMed] [Google Scholar]
- 53.Kusters DM, de Beaufort C, Widhalm K et al. Paediatric screening for hypercholesterolemia in Europe. Arch Dis Child. 2012;97:272–6. doi: 10.1136/archdischild-2011-300081. [DOI] [PubMed] [Google Scholar]
- 54.Watts G, Sulivan D, Poplawski N et al. Familial hypercholesterolaemia: a model of care from Australasia. Atherosclerosis. 2011;12:s221–63. doi: 10.1016/j.atherosclerosissup.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Descamps O, Tenoutasse S, Stephenne X et al. Management of familial hypercholesterolemia in children and young adults: Consensus paper developed by a panel of lipidologists, cardiologists, paediatricians, nutritionists, gastroenterologists, general practitioners and a patient organization. Atherosclerosis. 2011;218:272–80. doi: 10.1016/j.atherosclerosis.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 56.Humphries SE, Norbury G, Leigh S et al. What is the utility of DNA testing in patients with familial hypercholesterolemia. Curr Opinion Lipidol. 2008;19:362–8. doi: 10.1097/MOL.0b013e32830636e5. [DOI] [PubMed] [Google Scholar]
- 57.Civeira F, Ros E, Jarauta E et al. Comparison of genetic versus clinical diagnosis in familial hypercholesterolemia. Am J Cardiol. 2008;102:1187–93. doi: 10.1016/j.amjcard.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 58.Umans-Eckenhausen MA, Defesche JC, Sijbrands EJ et al. Review of first 5 years of screening for familial hypercholesterolemia. Lancet. 2001;357:165–8. doi: 10.1016/S0140-6736(00)03587-X. [DOI] [PubMed] [Google Scholar]
- 59.Damgaard D, Larsen ML, Nissen PH et al. The relationship of molecular genetic to clinical diagnosis of familial hypercholesterolemia in a Danish population. Atherosclerosis. 2005;180:155–60. doi: 10.1016/j.atherosclerosis.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Khera A, Won HH, Peloso G et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–89. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huijgen R, Kindt I, Defesche JC et al. Cardiovascular risk in relation to functionality of sequence variants in the gene coding for the low-density lipoprotein receptor: a study among 29365 individuals tested for 64 specific low-density lipoprotein-receptor sequence variants. Eur Heart J. 2012;33:2325–30. doi: 10.1093/eurheartj/ehs038. [DOI] [PubMed] [Google Scholar]
- 62.Mata N, Alonso R, Badimon L et al. Clinical characteristics and evaluation of LDL-cholesterol treatment of the Spanish Familial Hypercholesterolemia Longitudinal Cohort Study (SAFEHEART). Lipids Health Dis. 2011;10:94. doi: 10.1186/1476-511X-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neil HA, Hammond T, Huxley R et al. Extent of underdiagnosed of familial hypercholesterolemia in routine practice: prospective registry study: BMJ. 2000;321:148. doi: 10.1136/bmj.321.7254.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leren T, Finbourd T, Manshaus T et al. Diagnosis of familial hypercholesterolemia in general practice using clinical diagnostic criteria or genetic testing as part of cascade genetic screening. Community Genet. 2008;11:26–35. doi: 10.1159/000111637. [DOI] [PubMed] [Google Scholar]
- 65.Leren T, Berge KE. Subjects with molecularly defined familial hypercholesterolemia or familial defective apo B-100 are not being adequately treated. PLoS One. 2011;6:e16721. doi: 10.1371/journal.pone.0016721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Defesche J. Defining the challenges of FH Screening for familial hypercholesterolemia. J Clin Lipidol. 2010;4:338–41. doi: 10.1016/j.jacl.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 67.Mata P, Alonso R, Pérez-Jiménez F. Screening for familial hypercholesterolemia: A model for preventive medicine. Rev Esp Cardiol. 2014;67:685–8. doi: 10.1016/j.recesp.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 68.Marks D, Wonderling D, Thorogood M et al. Screening for hypercholesterolemia versus case finding for familial hypercholesterolemia: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2000;4:1–123. [PubMed] [Google Scholar]
- 69.Lázaro P, Pérez de Isla L, Watts GF et al. Cost-effectiveness of a cascade screening program for the early detection of familial hypercholesterolemia. J Clin Lipidol. 2017;11:260–71. doi: 10.1016/j.jacl.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Wiegman A, Gidding S, Watts G et al. Familial hypercholesterolemia in children and adolescent: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36:2425–37. doi: 10.1093/eurheartj/ehv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klancar G, Groselj U, Kovac J et al. Universal screening for familial hypercholesterolemia in children. J Am Coll Cardiol. 2015;66:1250–57. doi: 10.1016/j.jacc.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 72.Neil A, Cooper J, Betteridge J et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J. 2008;29:2625–33. doi: 10.1093/eurheartj/ehn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kroon AA, Aengevaeren WR, van der WT et al. LDL-Apheresis Atherosclerosis Regression Study (LAARS). Effect of aggressive versus conventional lipid lowering treatment on coronary atherosclerosis. Circulation. 1996;93:1826–35. doi: 10.1161/01.CIR.93.10.1826. [DOI] [PubMed] [Google Scholar]
- 74.Smilde TJ, van Wissen S, Awollersheim H et al. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001;357:577–81. doi: 10.1016/S0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 75.Alonso R, Mata P, De Andres R et al. Sustained long-term improvement of arterial endothelial function in heterozygous familial hypercholesterolemia patients treated with simvastatin. Atherosclerosis. 2001;157:423–9. doi: 10.1016/S0021-9150(00)00733-4. [DOI] [PubMed] [Google Scholar]
- 76.Galema-Boers AM, Lenzen MJ, Engelkes SR et al. Cardiovascular risk in patients with familial hypercholesterolemia using optimal lipid-lowering therapy. J Clin Lipidol. 2018;12:409–16. doi: 10.1016/j.jacl.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 77.Pijlman AH, Huijgen R, Verhagen SN et al. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: A large cross-sectional study in the Netherlands. Atherosclerosis. 2010;209:189–94. doi: 10.1016/j.atherosclerosis.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 78.Perez de Isla L, Alonso R, Watts G et al. Attainment of LDL-cholesterol treatments goals in patients with familial hypercholesterolemia. 5-year SAFEHEART registry follow-up. J Am Coll Cardiol. 2016;67:1278–85. doi: 10.1016/j.jacc.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 79.Pérez de Isla L, Alonso R, Mata N et al. SAFEHEART investigators. Predicting cardiovascular events in familial hypercholesterolemia: the SAFEHEART Registry. Circulation. 2017;135:2133–44. doi: 10.1161/CIRCULATIONAHA.116.024541. [DOI] [PubMed] [Google Scholar]
- 80.Jellinger PS, Handelsman Y, Rosenblit PD et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease – executive summary. Endocr Pract. 2017;23:479–97. doi: 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- 81.Watts GF, Gidding S, Wierzbicki AS et al. Integrated guidance on the care of familial hypercholesterolemia from the International FH Foundation. Int J Cardiol. 2014;171:309–25. doi: 10.1016/j.ijcard.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 82.Saltijeral A, Pérez de Isla L, Alonso R et al. Attainment of LDL cholesterol treatment coals in children and adolescents with familial hypercholesterolemia. The SAFEHEART Follow-up Registry. Rev Esp Cardiol. 2017;70:444–50. doi: 10.1016/j.recesp.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 83.van der Graaf A, Cuffie-Jackson C, Vissers MN et al. Efficacy and safety of coadministration of ezetimibe and simvastatin in adolescents with heterozygous familial hypercholesterolemia. J Am Coll Cardiol. 2008;52:1421–9. doi: 10.1016/j.jacc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Huijgen R, Abbink EJ, Bruckert E et al. Colesevelam added to combination therapy with a statin and ezetimibe in patients with familial hypercholesterolemia: a 12-week, multicenter, randomized, double-blind, controlled trial. Clin Ther. 2010;32:615–25. doi: 10.1016/j.clinthera.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 85.Cohen JC, Boerwinkle E, Mosley TH et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 86.Raal F, Stein E, Dufour R et al. PCSK9 inhibition with evolocumab (AMG145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–40. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 87.Kastelein JJ, Ginsberg H, Langslet G et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996–3003. doi: 10.1093/eurheartj/ehv370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raal F, Hovingh GK, Blom D et al. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol. 2017;5:280–90. doi: 10.1016/S2213-8587(17)30044-X. [DOI] [PubMed] [Google Scholar]
- 89.Sabatine MS, Giugliano RP, Keech AC et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 90.Steg G, Schwartz GG, Szarek M The ODYSSEY OUTCOMES Trial: Topline results Alirocumab in patients after acute coronary syndrome. Mar 10, 2018. Presented at 67th Scientific Sessions, American College of Cardiology, Orlando, Florida, US,
- 91.Cuchel M, Mehageer EA, du Toit Theron H et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–6. doi: 10.1016/S0140-6736(12)61731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thompson GR, Catapano A, Saheb S et al. Severe hypercholesterolaemia: therapeutic goals and eligibility criteria for LDL apheresis in Europe. Curr Opin Lipidol. 2010;21:492–8. doi: 10.1097/MOL.0b013e3283402f53. [DOI] [PubMed] [Google Scholar]


