Abstract
The mismatch repair (MMR) system, exemplified by the MutS/MutL proteins, is widespread in Bacteria and Eukarya. However, molecular mechanisms how numerous archaea and bacteria lacking the mutS/mutL genes maintain high replication fidelity and genome stability have remained elusive. EndoMS is a recently discovered hyperthermophilic mismatch-specific endonuclease encoded by nucS in Thermococcales. We deleted the nucS from the actinobacterium Corynebacterium glutamicum and demonstrated a drastic increase of spontaneous transition mutations in the nucS deletion strain. The observed spectra of these mutations were consistent with the enzymatic properties of EndoMS in vitro. The robust mismatch-specific endonuclease activity was detected with the purified C. glutamicum EndoMS protein but only in the presence of the β-clamp (DnaN). Our biochemical and genetic data suggest that the frequently occurring G/T mismatch is efficiently repaired by the bacterial EndoMS-β−clamp complex formed via a carboxy-terminal sequence motif of EndoMS proteins. Our study thus has great implications for understanding how the activity of the novel MMR system is coordinated with the replisome and provides new mechanistic insight into genetic diversity and mutational patterns in industrially and clinically (e.g. Mycobacteria) important archaeal and bacterial phyla previously thought to be devoid of the MMR system.
INTRODUCTION
In all three domains of life, the fidelity of DNA replication is crucial for faithful transfer of genetic information between generations, as uncorrected errors may lead to mutations potentially causing cell death, cancer and neurogenerative diseases (1). The fidelity of DNA replication is ensured by several distinct mechanisms including the capacity of DNA polymerases to select the correct nucleotide to be incorporated by the replisome (2). During the replication process, errors occasionally occur on the nascent (daughter) strand, and are detected and corrected by either the proofreading activity of DNA polymerases or the post-replicative mismatch repair (MMR) system (3). The functional role of the core MMR proteins MutL and MutS in the maintenance of the replication fidelity is exemplified by the fact that the genetic inactivation of the MMR system typically lowers the replication fidelity by two or three orders of magnitude and results in the accumulation of transition mutations. MMR also acts on mismatches and strand misalignments during recombination and controls recombination between similar, but non-identical sequences (4). Consequently, MMR inhibits interspecies recombination and gene exchange, and thus has wide implications for understanding the evolution of species (5).
In Escherichia coli and some other γ-proteobacteria, the ATP-dependent assembly of the MutS and MutL heteroduplex at the sites of mismatched bases leads to the activation of the MutH endonuclease activity, which selectively cleaves transiently non-methylated dam methylation sites (GATC), thus facilitating the selective unwinding and degradation of the mismatch by different exonucleases (6). The resulting daughter strand ‘nick’ created by MutH thus directs MMR to the neosynthesized strand (methyl-directed MMR). In E. coli, MutS and MutL strongly interact with the replication clamp (β-subunit) and the catalytic α-subunit of the DNA polymerase III (7), indicating that MMR and DNA replication are intimately coupled. In most other species, including humans, for which the mismatch repair systems have been characterized, hemimethylation does not play a role in strand discrimination (8). In these cases, the strand specificity signal have been proposed to correspond to DNA strand discontinuities created by Okazaki fragment processing and/or processing of frequently misincorporated ribonucleotides (8). These daughter strand ‘nicks’ may facilitate the asymmetric loading of the MutSα-MutLα complex mediated by the replication clamp PCNA (9), resulting in strand-specific MMR due to the selective incision made by MutL in the vicinity of the mismatch (nick-directed MMR). Therefore, at least two independent solutions coordinated by replication clamps have emerged during evolution to differentiate the newly synthetized DNA strand with the replication errors (mismatches) from the template strand with the correct genetic information.
Previous bioinformatics studies failed to identify the genes encoding the canonical MMR proteins in the majority of archaeal species and many actinobacteria (10), thus raising the question of how high fidelity DNA replication in these species is achieved. Recent biochemical (11) and structural (12) studies revealed a mismatch-specific endonuclease activity for the archaeal EndoMS/NucS family proteins, which use a dual base flipping mechanism for mismatch recognition (13). These proteins were first identified as PCNA-interacting endonuclease acting on branched DNA substrates (14–16). Quite recently, a bacterial homolog of these archaeal proteins from Mycobacterium smegmatis has been implicated in mutation avoidance through genetic studies, but in contrast to the archaeal proteins, the purified and correctly folded M. smegmatis EndoMS/NucS lacked nuclease activity with substrates bearing a single mismatch within a duplex DNA (17).
Here we report that the purified Corynebacterium glutamicum EndoMS/NucS (CglEndoMS) homodimers showed a mismatch-specific endonuclease activity that was markedly stimulated by the β-clamp encoded by dnaN in vitro. The C-terminal five residues of the CglEndoMS were found to be essential for the β-clamp interaction and were required for mutation avoidance activity. The replication clamp may thus direct the EndoMS to mismatches that are left unrepaired by the replisome. Our study provides new mechanistic insight into coordination of the novel mismatch repair activity and causally links the biochemical and cellular DNA repair activities of EndoMS proteins. These data provide the biochemical basis for understanding the rate and the spectrum of spontaneous mutations in archaea, actinobacteria and other bacterial phyla, which were previously thought to lack the MMR system.
MATERIALS AND METHODS
Bacterial strains and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli strains XL1-Blue and XL10-Gold were used as hosts for cloning experiments. E. coli strains JM109 and BL21 CodonPlus (DE3)-RIL were used for the overproduction of His-tagged EndoMS/NucS and β-clamp proteins, respectively. E. coli strains were grown at 37°C on solid or liquid Luria-Bertani media. C. glutamicum strains were grown at 30°C on solid or liquid brain heart infusion (BHI) media (Oxoïd). Antibiotics for the selection of recombinant E. coli strains were used at the following final concentrations: ampicillin (Amp), 100 μg/ml; kanamycin (Kan), 20 μg/ml; chloramphenicol (Cam), 30 μg/ml and tetracycline (Tet), 10 μg/ml. For the selection of C. glutamicum transformants, kanamycin and chloramphenicol were added at concentrations of 20 and 10 μg/ml, respectively.
Table 1. Bacterial strains and plasmids used in this study.
| Strains or plasmids | Genotype/characteristics | Reference or origin |
|---|---|---|
| E. coli strains | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Agilent |
| XL10-Gold | Tetr Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac Hte [F′ proAB lacIqZΔM15 Tn10 (Tetr) Amy Camr] | Agilent |
| JM109 | endA1, recA1, gyrA96, thi, hsdR17 (rk–, mk+), relA1, supE44, Δ(lac-proAB), [F′ traD36, proAB, laqIqZΔM15]. | Takara Bio |
| BL21 CodonPlus (DE3)-RIL | E. coli B F− ompT hsdS(rBmB) dcm+ Tetr gal λ(DE3) endA Hte [argU ileY leuW Camr] | Agilent |
| C. glutamicum strains | ||
| Cg ATCC 13032 | Wild-type parental strain | (20) |
| Cg (ΔnucS) | Cg 13032 (ΔnucS::aphA-3) (replacement of nucS by a non-polar kanamycin resistance cassette (aphA-3)) | This work |
| Cg (nucSΔCter) | Cg 13032 (nucSΔCter::aphA-3) (replacement of nucS by a copy of nucS with its 15 last nucleotides deleted, followed by a non-polar kanamycin resistance cassette) | This work |
| Cg (ΔnucS) + pXMJ19 | Cg 13032 (ΔnucS::aphA-3) transformed with the empty pXMJ19 vector | This work |
| Cg (ΔnucS) + pHM473 | Cg 13032 (ΔnucS::aphA-3) transformed with the pHM473 plasmid carrying a wild-type copy of the nucS gene | This work |
| Plasmids | ||
| pMCS5 | ori pMB1 (colE1), plac, lacZα, Ampr, MCS of pUC18 / Unable to replicate in C. glutamicum (suicide vector) | MoBiTec |
| pXMJ19 | ori pMB1 (colE1), ptac, lacIq, Camr, MCS of pUC18 / E. coli-C. glutamicum (shuttle vector) | (37) |
| pQE80L | ori colE1, pT5, lacIq, Ampr, 6xHis tag (N-ter) | Qiagen |
| pG-KJE8 | ori p15A, paraB, dnaK, dnaJ, grpE, Camr | Takara Bio |
| pUC18K | ori pMB1 (colE1), plac, lacZα, Ampr, Kanr / Promotor less non-polar kanamycin cassette (aphA-3) cloned into pUC18 | (38) |
| pHM449 | nucS of Cg ATCC 13032 cloned into pQE80L (BamHI / PstI) | This work |
| pHM455 | aphA-3 cassette flanked with the upstream and downstream regions of nucS and cloned into pMCS5 (Ampr,Kanr) | This work |
| pHM473 | nucS of Cg ATCC 13032 cloned into pXMJ19 (HindIII / EcoRI) | This work |
| pHM476 | nucS with its 15 last nucleotides deleted followed by the aphA-3 cassette and cloned into pMCS5 (Ampr,Kanr) | This work |
| pHM477 | A stop codon was introduced into nucS in pHM449 to delete five amino acids from the C-terminus of CglEndoMS | This work |
| pET-Cglβ-clamp | dnaN of Cg ATCC 13032 cloned into a modified version of pET28a (NdeI / NotI), ori pMB1 (colE1),pT7, Ampr, 6xHis tag (N-ter) | This work |
Construction of plasmids and C. glutamicum mutants
The nucS gene of the C. glutamicum ATCC 13032 strain was deleted by allelic exchange using the non-polar kanamycin resistance cassette aphA-3 (867 pb), subcloned from pUC18K (Table 1). First, this cassette was inserted between the EcoRI and BamHI sites of pMCS5, which is unable to replicate in C. glutamicum. Then, the 500 bp fragment located upstream from nucS and the 510 bp fragment located downstream from nucS were PCR-amplified with the primer pairs SK94/SK95 and SK96/SK97 (Supplementary Table S1), respectively. The two PCR products were subsequently digested by NdeI/EcoRI and BamHI/PstI and inserted into the flanking regions of the aphA-3 cassette previously cloned into pMCS5, yielding pHM455 (Table 1) (Figure 1A). This plasmid was used as a suicide vector and introduced into C. glutamicum ATCC 13032 competent cells by electroporation as described previously (18). Transformants were selected on BHI plates containing kanamycin after an incubation at 30°C for 3 days. The allelic exchange was then checked by PCR using the following primer pairs: SK83/SK84, SK153/H50 (absence of nucS) and H17/SK154 (integration of aphA-3 at the correct locus) (see Supplementary Table S1 and Supplementary Figure S1). A deletion of 15 nucleotides from the 3′ terminus of nucS was performed by PCR-amplification using the primer pair SK177/SK169 (Supplementary Table S1). The PCR product was digested by NdeI and EcoRI and subsequently used to replace the upstream region of nucS previously cloned into pHM455, yielding pHM476. This plasmid was then introduced into ATCC 13032 competent cells by electroporation and transformants were selected as previously described. The allelic exchange was monitored by PCR-amplification and sequencing using the following primer pairs: SK177/SK84, SK153/H50 and H17/SK154 (see Supplementary Table S1 and Supplementary Figure S1).
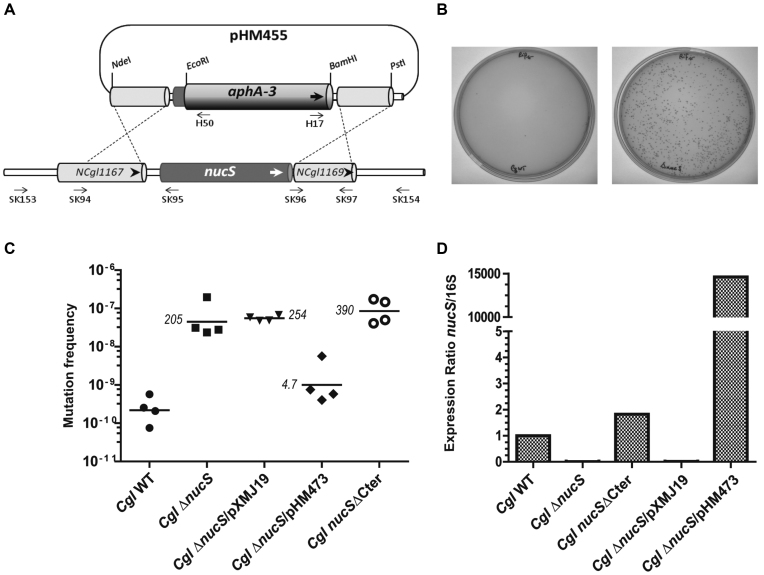
Figure 1.
The functional role of CglEndoMS/NucS in mutation avoidance. (A) The physical and genetic organization of the C. glutamicum nucS locus are shown. Approximate positions of the oligonucleotides used for construction and confirmation of the ΔnucS::aphA-3 (KanR) are also indicated. (B) The plates showing the formation of spontaneous RifR colonies for wildtype (left panel) and ΔnucS::aphA-3 (right panel) strains. The plates demonstrate the significant increase in the spontaneous mutation rate for the deletion strain. (C) Mutation frequencies observed for the C. glutamicum wild type, ΔnucS, ΔnucS/pXMJ19 (empty plasmid control), ΔnucS/pHM473 (carrying nucS in trans) and nucSΔCter strains. The last strain lacks the five last amino acid residues of CglEndoMS/NucS. The line indicates the geometric mean of the four different biological replicates. Numbers refer to relative fold differences compared to CglEndoMS-WT. (D) Relative nucS expression levels for the different strains are shown. The values shown are averages of two biological replicates determined using RT-PCR as described in the materials and methods section.
Complementation of the C. glutamicum ΔnucS mutant
To produce the trans complementation plasmid pHM473, the nucS gene was PCR-amplified with the primer pair SK163/SK164 and inserted between the HindIII/EcoRI sites of the non-integrative E. coli - C. glutamicum shuttle vector pXMJ19 carrying the cat gene (Table 1). The pHM473 plasmid was then transferred into the C. glutamicum ΔnucS::aphA-3 (KanR) strain by electroporation. Transformants were selected on BHI plates containing chloramphenicol (10 μg/ml) and kanamycin (20 μg/ml) after an incubation at 30°C for 2 days.
Estimation of mutation frequencies
C. glutamicum and its derivatives were grown overnight using 2 ml 96-deep-well plates at 30°C in BHI liquid medium, including antibiotics when required. Appropriate dilutions were plated on BHI medium with or without rifampicin (Rif) at 100 μg/ml. Rifampicin resistant (RifR) colonies originating from the independent cultures obtained were isolated and sequenced for the wild-type strain 13032 and the ΔnucS mutant, respectively. Spontaneous mutations conferring rifampicin resistance were then identified from these mutants, by PCR-amplification and sequencing a well characterized RifR-determining region in the rpoB gene (NP_599733; NCgl0471), using the primer pair SK157/SK158 [Supplementary Table S1 (19)]. Where indicated, streptomycin was used in analogous experiments at the final concentration of 100 μg/ml.
Cloning of the C. glutamicum genes encoding EndoMS/NucS and β-clamp (DnaN) for protein purification
The nucS (CAF19919; NCgl1168) and dnaN (NP_599254; NCgl0002) genes, encoding the CglEndoMS/NucS and the DNA polymerase III β-subunit (β-clamp) respectively, were amplified by PCR from C. glutamicum ATCC 13032 genomic DNA, using Pfu DNA polymerase (Agilent) and the following primer pairs: SK83/SK84 for nucS and Cglβ-clamp-F/Cglβ-clamp-R for dnaN [Supplementary Table S1 (20)]. The amplified nucS gene was inserted between the BamHI and PstI sites of pQE80L (Qiagen), to generate pHM449 (Table 1). To delete the last five amino acids of the EndoMS/NucS protein, a stop codon was introduced into the nucS gene on the pHM449 plasmid by PCR-mediated mutagenesis (QuikChange site-directed mutagenesis kit; Agilent), using the primer pair ΔCter-F/ΔCter-R (Supplementary Table S1). The DNA product corresponding to dnaN was digested by NdeI and NotI (New England Biolabs), and inserted into the respective sites of a modified pET28a vector (Novagen). In this modified vector, the recognition sequence for thrombin was changed to that for Tobacco Etch Virus (TEV) protease, and the Kan-resistance gene was changed to an Amp-resistance gene. The resulting plasmid was designated as pET-Cglβ-clamp (Table 1). Both inserted sequences were confirmed by sequencing.
Protein expression and purification
His-tagged CglEndoMS and CglEndoMS-ΔCter were prepared from E. coli JM109 cells transformed with pHM449 and pHM477, respectively. The chaperone plasmid pG-KJE8 was co-transformed with both plasmids. E. coli cells were cultured in LB medium, containing 50 μg/ml ampicillin, 34 μg/ml chloramphenicol, 10 ng/ml tetracycline and 0.5 mg/ml l-arabinose, at 37°C until the culture attained an OD600 of 0.5. IPTG was then added to a final concentration of 0.2 mM, and the cells were further grown for 18 h at 25°C. The cells were collected, and were resuspended in buffer A (25 mM HEPES, pH 7.5, 1 M NaCl, and 5 mM imidazole), and were disrupted by sonication. The soluble cell extracts were applied to TALON metal affinity resin (Clontech), and the resin was washed with buffer A. The bound fraction was eluted with 25 mM HEPES, pH 7.5, 0.5 M NaCl and 100 mM imidazole, and the eluate was applied to Superdex 200 5/150 column (GE Healthcare), which was eluted with 25 mM HEPES, pH 7.5, 0.3 M NaCl, 0.5 mM DTT, and 0.1 mM EDTA. The fraction containing CglEndoMS was stored at –80°C. His-tagged Cglβ-clamp was prepared from E. coli BL21 CodonPlus (DE3)-RIL (Agilent) cells transformed with pET-Cglβ-clamp. E. coli cells were cultured in LB medium, containing 50 μg/ml ampicillin and 34 μg/ml chloramphenicol, at 37°C until the culture attained an OD600 of 0.5. IPTG was then added to a final concentration of 1 mM, and the cells were further grown for 18 h at 25°C. The cells were collected, and were resuspended in buffer B (25 mM HEPES, pH 7.5, 0.5 M NaCl and 5 mM imidazole), and were disrupted by sonication. The soluble cell extracts were applied to TALON metal affinity resin, and the resin was washed with buffer B. The bound fraction was eluted with 25 mM HEPES, pH 7.5, 0.3 M NaCl and 100 mM imidazole, and the eluate was applied to a Superdex 200 5/150 column, which was eluted with 25 mM HEPES, pH 7.5, 0.3 M NaCl, 0.5 mM DTT and 0.1 mM EDTA. The protein concentrations were calculated by measuring the absorbance at 280 nm. The theoretical molar extinction coefficients of CglEndoMS, CglEndoMS-ΔCter, and Cglβ-clamp are 19 940, 19 940 and 21 430 M−1 cm−1, respectively. The purity of the proteins was confirmed by SDS-PAGE.
Gel filtration chromatography analyses
Purified recombinant CglEndoMS (1.2 nmol), CglEndoMS-ΔCter (1.2 nmol), Cglβ-clamp (2 nmol) were applied to a Superdex 200 3.2/30 column (GE Healthcare), and were eluted at a flow rate of 40 μl/min in buffer containing 25 mM HEPES, pH 7.5, 0.5 mM DTT, 0.1 mM EDTA and 0.15 M NaCl using SMART system (Amersham Pharmacia). The standard marker proteins, including thyroglobulin (MW, 670 000), γ-globulin (MW, 158 000), ovalbumin (MW, 44 000) and myoglobin (MW, 17 000), were subjected to gel filtration as a control.
Cleavage assay of mismatch-containing DNA
Cleavage reactions of the Cy5-labeled substrate dsDNAs, formed with the oligonucleotides listed in Supplementary Tables S2 and S3, by the proteins were generally performed at 30°C in an optimized reaction mixture containing 25 mM Bis–Tris, pH 6.4, 2.5 mM MnCl2, 5 mM DTT and 0.1% Triton X-100. The modified reaction conditions are described in each section. The reaction was quenched by the addition of 25 mM EDTA and 0.3% SDS, and the products were analyzed by either 10% native polyacrylamide gel electrophoresis (PAGE) or 8 M urea–15% PAGE in TBE buffer (89 mM Tris, 89 mM boric acid and 2.5 mM EDTA, pH 8.3). The products were visualized with a Typhoon Trio+ image analyzer (GE Healthcare).
Electrophoretic mobility shift assay
CglEndoMS (0.8 μM as a monomer) and Cglβ-clamp (8 μM as a monomer) were incubated with 5 nM 5′-Cy5-labeled ssDNA (45 nt) or dsDNA (45 bp) in a reaction solution (25 mM Bis–Tris, pH 6.4, 2.5 mM MnCl2, 5 mM DTT and 0.1% Triton X-100) at 30°C for 30 min. An aliquot was fractionated on an 8% PAGE in TBE buffer and was visualized and quantified with a Typhoon Trio+ image analyzer.
Homology modeling of the bacterial EndoMS/NucS
We constructed a model of the C. glutamicum EndoMS/NucS dimer with the modelling program Modeller (21) version 9.18 by using the EndoMS-dsDNA structure carrying a mismatch (PDB code 5GKE) as the template. Five initial models were constructed and the best model was chosen using molpdf and the DOPE score and by evaluating the stereochemical quality with PROCHECK (22). Molecular graphics and structures superposition were performed with the UCSF Chimera package (23). Chimera was developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS P41-GM103311).
RNA extraction, reverse transcription and real-time PCR quantification
Total RNA was extracted and purified from the C. glutamicum wild type strain and the mutants carrying a deletion of 15 nucleotides from the 3′ terminus of nucS using a Qiagen miRNeasy Mini kit (Qiagen, Courtaboeuf, France) with a final elution volume of 50 μl. The concentration of the RNA fraction was quantified using a Nanodrop 2000c system (Thermo Fisher Scientific). The RT-PCR was performed with a Maxima First Strand cDNA Synthesis Kit for RT-PCR (Thermo Scientific). For each strain, 200 ng of total RNA was used for the synthesis of the first-strand complementary DNA (cDNA). We used 50 pg of cDNA for the real-time PCR assay with SensiFast™ SYBR No-ROX reagents (Bioline Reagent) and the MiniOpticon System (BioRad). The real-time PCR was performed using a concentration range of nucS and 16S rRNA (house-hold) genes to calculate the quantitative expression ratio of nucS to 16S rRNA in each sample from two separate experiments. The following primer pairs were used: qPCR-NucS-F/qPCR-NucS-R (nucS) and qPCR-16S-F/qPCR-16S-R (16S rRNA; NCg1r01) (Supplementary Table S1). Where indicated, the percentage of the expression ratio of nucS to 16S for the wild type sample was set to 100%.
RESULTS
Identification and preparation of a C. glutamicum EndoMS
Previous structural studies have demonstrated that the EndoMS proteins possess a two-domain structure (12,16). The amino-terminal dimerization domain has been implicated in the mismatch (MM) binding, whereas the carboxyl-terminal region bears an endonuclease active site, which is structurally related to many families of restriction endonucleases (11,12). As we previously reported, the EndoMS homologs were identified not only in archaeal, but also in some of bacterial phyla, particularly actinobacteria (11,16). EndoMS also reportedly interacts with proliferating cell nuclear antigen (PCNA) via a PCNA-interacting peptide (PIP) motif at its C-terminal region, although the PIP motif is not conserved in all homologs (11,12). The β-clamp, identified as the β subunit of DNA polymerase III, in Bacteria has the same functions as the eukaryotic and archaeal PCNAs. The sequence, [EK]-[LY]-[TR]-L-F, which is similar to the consensus sequences of the β-clamp binding motif (24) was found in the C-terminal region of the putative sequences of the actinobacterial EndoMS homologs (Supplementary Figure S1A). Among the bacterial sequences, we selected the C. glutamicum EndoMS (encoded by nucS) and the β-clamp (encoded by dnaN) homologs for further studies. CglEndoMS shares only 29.2%, 26.5% and 20.7% amino acid identities, with the EndoMS proteins from Thermococcus kodakarensis, Pyrococcus abyssi and Methanopyrus kandleri, respectively, but the critical residues for the mismatch binding and nuclease activity are highly conserved among them.
The functional role of CglEndoMS in mutation avoidance
The nucS gene is surrounded by the genes NCgl1167 (upstream) and NCgl1169 (downstream) genes, which have been annotated as ‘universal stress protein UspA’ and ‘nucleoside-diphosphate-sugar epimerase’ in the C. glutamimum genome, respectively (Figure 1A). These proteins have predicted functions in multiple stress responses (including oxidative stress) and the metabolism of nucleotide sugars that act as activated donors in glycosylation reactions. Our strategy to inactivate the nucS gene, by replacing it with the aphA-3 gene conferring kanamycin resistance (KanR), is also illustrated in Figure 1A. The obtained plasmid-borne deletion-insertion construct (ΔnucS::aphA-3) was inserted into the suicide plasmid to yield the plasmid pHM455 (Table 1), which was transferred to C. glutamicum by electroporation. We screened twenty KanR colonies by PCR for the absence of nucS resulting from an expected double recombination event. This led to the identification of one C. glutamicum ΔnucS::aphA-3 candidate strain where nucS had been replaced with aphA-3. The correct chromosomal localization of aphA-3 was confirmed through additional PCR analyses (see Supplementary Figure S1B and C). The isolation of this deletion strain allowed the subsequent testing of whether nucS is required for mutation avoidance by estimating the mutation frequencies of RifR in the wild type and ΔnucS::aphA-3 C. glutamicum strains (Figure 1B). Using four biological replicates and by comparing the geometric means of the measured mutation rates, we observed that the lack of nucS increased the spontaneous mutation rate by a factor of 205 in comparison to the wild type strain (Figure 1C). This hypermutable phenotype was suppressed by expressing nucS in trans from the plasmid pHM473 (Figure 1C and D), thus establishing the role of nucS in mutation avoidance. To support this result, we measured mutation frequencies using a different marker gene. The mutation frequencies observed with streptomycin were 1.37 × 10−8 and 1.47 × 10−6 for the wild type and ΔnucS strains, respectively. To investigate the possible functional importance of the putative β-clamp interaction motif in CglEndoMS (see Figure 2A), we deleted 15 nucleotides corresponding to five carboxy-terminal residues from the chromosomal nucS gene and measured the level of RifR colony production (Table 1). Our results revealed that although this construct was still transcribed in cells, the five carboxyterminal residues, ELTLF, are absolutely required for the mutation avoidance function of CglEndoMS in our assays (Figure 1C and D).
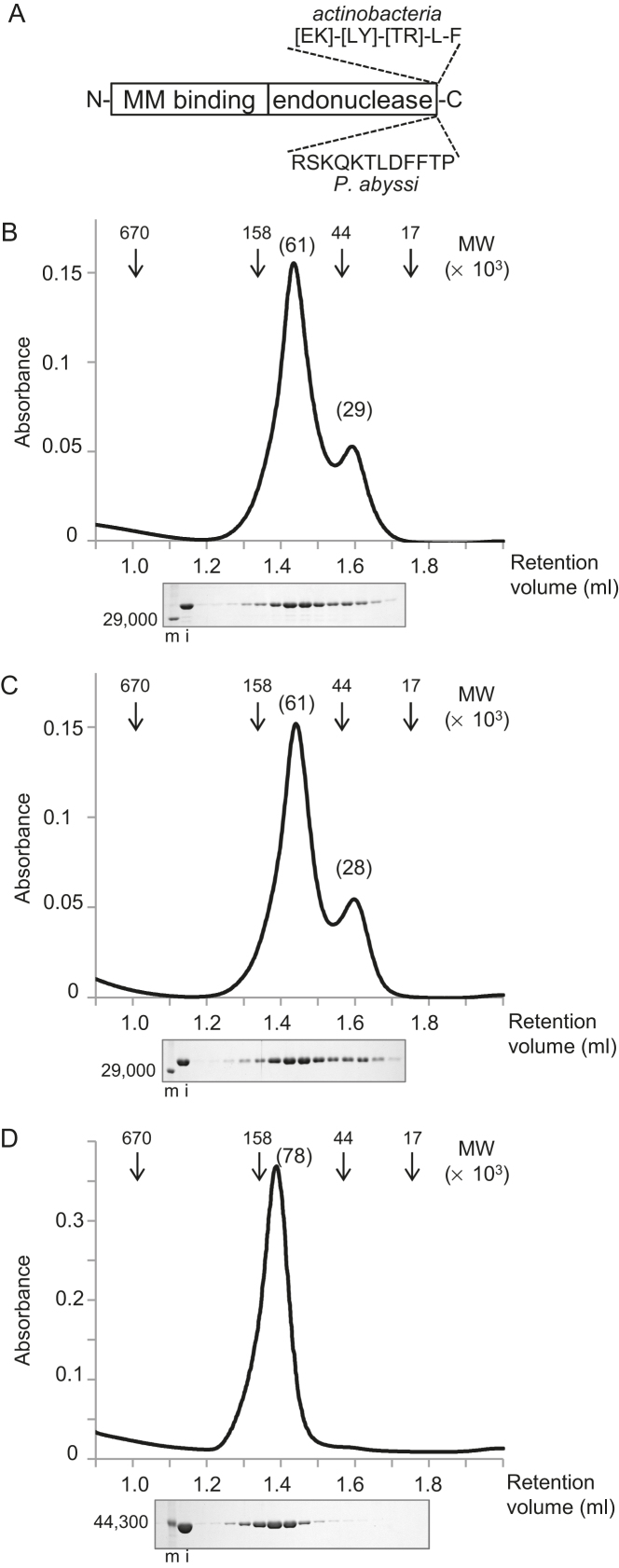
Figure 2.
Properties of CglEndoMS-WT and Cglβ-clamp. (A) The two-domain structure of the EndoMS/NucS proteins. ‘MM binding’ and ‘endonuclease’ refer to mismatch binding and endonuclease active site domains, respectively. The positions of the experimentally determined and putative replication clamp binding motif for the Thermococcales and actinobacterial EndoMS/NucS proteins are shown. (B–D) Gel filtration chromatography analyses of CglEndoMS-WT (B), CglEndoMS-ΔCter (C), and Cglβ-clamp (D). Elution profiles, monitored by the absorbance at 280 nm, are shown in the upper part. The arrowheads indicate the elution positions of the standard marker proteins, and the numbers in parentheses on the peak tops indicate the relative molecular masses estimated by the standard curve from the marker proteins. Aliquots of each fraction were subjected to an SDS-12% PAGE followed by Coomassie Brilliant Blue staining (lower part). Protein size markers were run in lanes m, and their sizes are indicated on the left sides of the gels. The loading samples (20%) were run in lanes i.
Purification of CglEndoMS and Cglβ-clamp
The recombinant His-tagged proteins, CglEndoMS, Cglβ-clamp, and CglEndoMS-ΔCter, among which the latter lacks five carboxyterminal residues of the wild type CglEndoMS, were successfully overproduced by cultivating the E. coli cells bearing the expression plasmids, pHM449, pET-Cglβ-clamp and pHM477 with IPTG induction (Table 1). As shown in Supplementary Figure S2, the three proteins were purified to near homogeneity. The migrations of the protein bands for His-tagged CglEndoMS and CglEndoMS-ΔCter were obviously slower than those estimated from the calculated molecular weights, 27 014.5 and 26 410.8, respectively, probably because of the open conformation of the EndoMS protein without a DNA substrate. The migration positions of the protein band for the His-tagged Cglβ-clamp protein corresponded well to the calculated molecular weight of 44 872.9. The gel filtration of CglEndoMS and CglEndoMS-ΔCter showed similar chromatograms. The estimated molecular weights of the peaks were 61 000 and 29 000 or 28 000, which appeared to be a dimer and a monomer, respectively (Figure 2B and C). This result suggests that, similar to its archaeal orthologues, CglEndoMS is mainly a dimer in solution. The main peak was isolated and applied to the same column again. However, this rechromatography resulted in the same profiles with two peaks, suggesting that the dimer form of CglEndoMS is unstable in both cases of WT and ΔCter. This might also reflect the presence of the monomer-dimer equilibrium for the two CglEndoMS variants. The elution profile of the Cglβ-clamp showed a clear single peak, with an estimated molecular weight of ∼77 900, suggesting that it exists as a stable homodimer in solution (Figure 2D). A similar result was previously described for the E. coli β-clamp (25).
The mismatch-specific cleavage activity of CglEndoMS is activated by the β-clamp
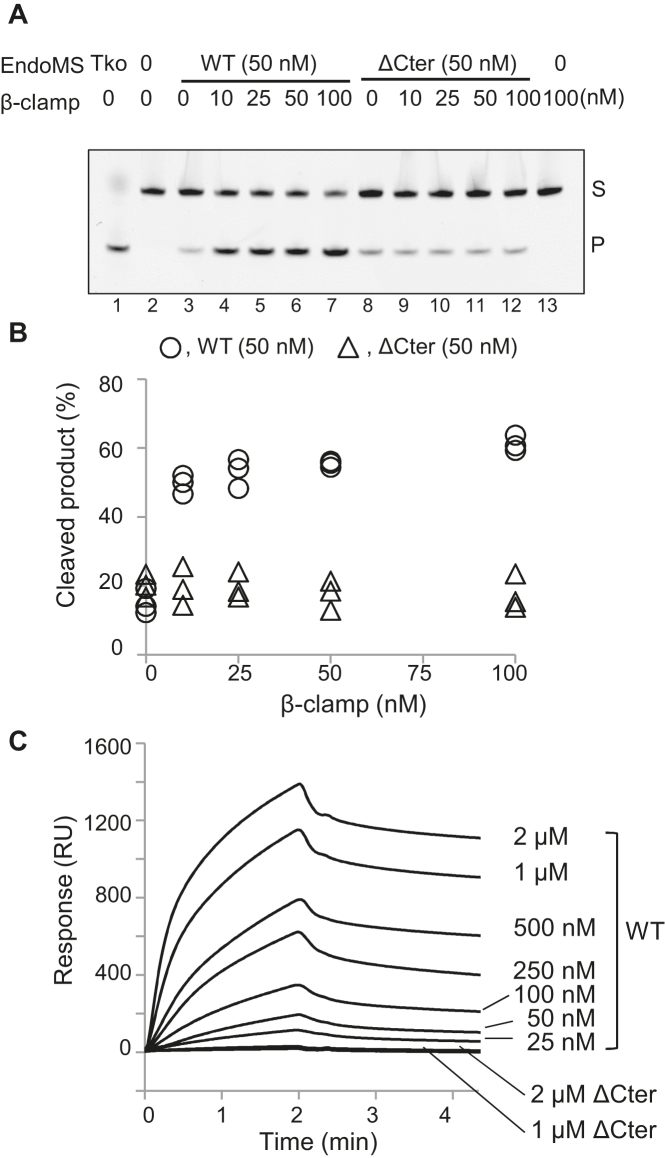
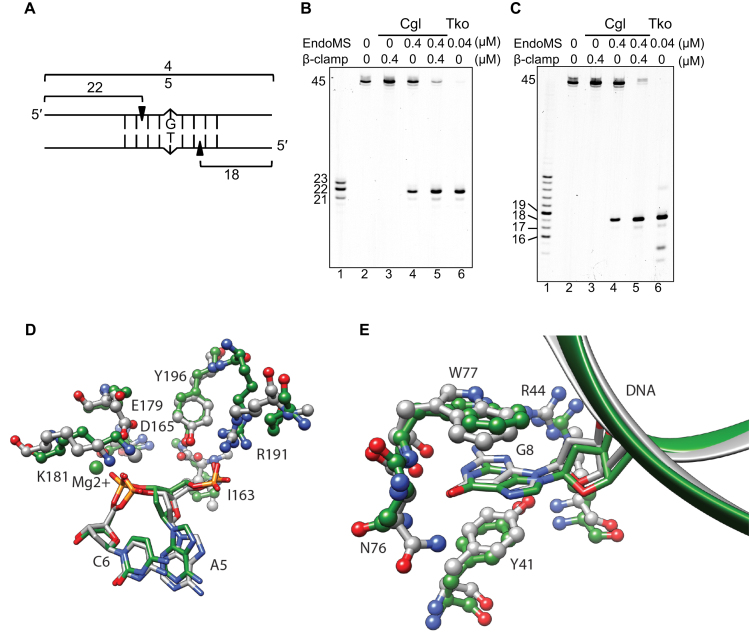
To investigate whether CglEndoMS has mismatch DNA-specific endonuclease activity, the purified protein was mixed with oligodeoxynucleotides containing a mismatched base-pair, and the cleavage reactions were monitored by native PAGE. We found that in comparison to T. kodakarensis EndoMS (20 nM), CglEndoMS had less activity with the mismatched DNA, even when more than twice the protein concentration (50 nM) was used (Figure 3A, lanes 1 and 3). However, the cleavage activity was markedly stimulated by the addition of the Cglβ-clamp, and maximal stimulation was reached at equimolar amounts of the Cglβ-clamp and CglEndoMS (Figure 3A, lanes 3–7, and 3B). Furthermore, no stimulation was observed when the CglEndoMS-ΔCter mutant was used (lanes 8–12). SPR analysis with immobilized Cglβ-clamp were performed to quantify the physical interactions of CglEndoMS with Cglβ-clamp (Figure 3C). A KD value of 88 nM was estimated through global fitting analysis, accounting for both association and dissociation phases using a 1:1 interaction model. The apparent binding affinity of EndoMS to β-clamp is comparable to that of P. abyssi EndoMS to PCNA (KD = 15 nM) (16). On the other hand, CglEndoMS-ΔCter did not show any response with Cglβ-clamp even at a high concentration up to 2 μM (Figure 3C). These results indicated that the Cglβ-clamp has a critical function in the activation of the mismatch-specific endonuclease activity of CglEndoMS, and that the last five residues of CglEndoMS are crucial for the interaction with the Cglβ-clamp. To determine the exact cleavage site of CglEndoMS in the mismatch-containing DNA, synthetic DNA substrates, labeled with Cy5 at the 5′-terminus of the upper or lower strand were used as substrates. The products generated by CglEndoMS with and without the Cglβ-clamp were analyzed by denaturing PAGE. These experiments revealed that CglEndoMS cleaved the third phosphodiester bond on the 5′ side of the mismatched base in both strands, to produce a cohesive end with a five-nucleotide long 5′-protrusion (Figure 4). The cleavage pattern by CglEndoMS was not different with or without Cglβ-clamp (Figure 4B and C), and was exactly the same as that observed in the presence of TkoEndoMS (11). This observation is also fully compatible with our structural models of the CglEndoMS dimer using the TkoEndoMS-dsDNA structure (PDB code 5GKE), indicating the excellent superimposition of the catalytic and substrate binding sites (Figure 4D and E).
Figure 3.
Cleavage of mismatch-containing DNA by CglEndoMS-WT and CglEndoMS-ΔCter with Cglβ-clamp. (A) The 5′-Cy5-labeled DNA substrates (5 nM) containing the GT base pair were incubated with the proteins. The products were analyzed by native 10% PAGE followed by laser scanning (cropped gel image). Lanes 1, positive control (20 nM TkoEndoMS, as a monomer); 2, negative control (no proteins); 3–7, 50 nM CglEndoMS-WT (as a monomer); 8–12, 50 nM CglEndoMS-ΔCter (as a monomer); 13, no EndoMS. The indicated concentrations (as a monomer) of Cglβ-clamp were added to the reactions. Representative results are shown. The band assignments are indicated on the side of the panels: s, substrates: p, cleaved products. (B) Quantification of the cleaved products. Independent data points from three measurements are plotted. (C) The physical interactions of CglEndoMS with Cglβ-clamp were characterized by SPR using a BIACORE J system (Biacore Inc.). Purified Cglβ-Clamp was immobilized on the sensor Chip CM5, and various concentrations (indicated on the right sides of the sensorgrams) of CglEndoMS-WT and CglEndoMS-ΔCter were loaded onto the chip for 2 min at a flow rate of 30 μl/min in running buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, and 0.05% surfactant P20). Regeneration was achieved using a 1-min injection of 0.025% SDS. Each background response was subtracted. Data were analyzed using BIAevaluation v.3 software (Biacore Inc.)
Figure 4.
Cleavage pattern of mismatch-containing DNA by CglEndoMS. (A) The dsDNA substrate (45 bp) containing the mismatched base pair and the cleaved products are shown schematically. The 5′-Cy5-labeled upper strand (B) and the 5′-Cy5-labeled lower strand (C) were used to make the dsDNA. The numbers indicate the length of the each strand cleaved by CglEndoMS. (B and C) The substrates (5 nM) were incubated with various proteins. The products were separated by 8 M urea–12% PAGE in TBE buffer followed by laser scanning. The size markers were loaded in lanes 1 in panels b and c, and the sizes are indicated on the left side of each band. Lanes 2, no proteins; 3, 0.4 μM Cglβ-clamp (as monomer); 4, 0.4 μM CglEndoMS (as monomer); 5, 0.4 μM CglEndoMS (as monomer) and 0.4 μM Cglβ-clamp (as monomer); 6, 0.04 μM TkoEndoMS (as monomer). (D) Catalytic site structure superposition of EndoMS/NucS model (green) and template 5GKE (gray). DNA and protein are depicted in stick and ball-and-stick representations respectively. Residue numbering is relative to the template sequence. R191 is not a conserved amino acid, but in the case of CglEndoMS/NucS R173 is predicted to present a similar interaction with DNA phosphate. (E) Mismatch base recognition site structure superposition of EndoMS/NucS model (green) and template 5GKE (gray). The DNA and protein are depicted in stick-and-ribbon and ball-and-stick representations, respectively. Residue numbering is relative to the template sequence.
DNA binding ability of CglEndoMS
A recent report revealed that the EndoMS/NucS protein from M. smegmatis has some ssDNA binding ability, although no cleavage activity for mismatched DNA was shown in vitro (17). For comparison with the M. smegmatis EndoMS/NucS, the DNA binding ability of CglEndoMS was examined by an electrophoresis mobility shift assay (EMSA). The EMSA revealed that CglEndoMS cleaved the G/T-containing DNA and no shifted band was observed. In the case of normal dsDNA and ssDNA, some shifted bands were observed as reported for M. smegmatis EndoMS/NucS (Supplementary Figure S3). These shifts may reflect nonspecific binding.
Optimized reaction conditions for actinobacterial EndoMS with mismatch-containing DNA
To further characterize CglEndoMS and Cglβ-clamp, the cleavage reactions were performed under various conditions. The effects of divalent cations, temperature, and pH on the activity are shown in Supplementary Figure S4. Whereas the mismatch endonuclease activity of TkoEndoMS can use both Mg2+ and Mn2+ for catalysis and functions in a broad pH range ranging from 6.0 to 11.0, the activity of the bacterial protein is strictly Mn2+-dependent and has a very narrow pH optimum around 6.4. In agreement with the mesophilic lifestyle of C. glutamicum, the activity of CglEndoMS rapidly diminishes above 30°C. In the future, comparisons of bacterial and archaeal EndoMS orthologues will be interesting to further investigate the structural basis of their peculiar catalytic differences, although they do not seem to play major roles in the determination of the substrate specificity as described below.
In vitro mismatch specificity of the CglEndoMS protein
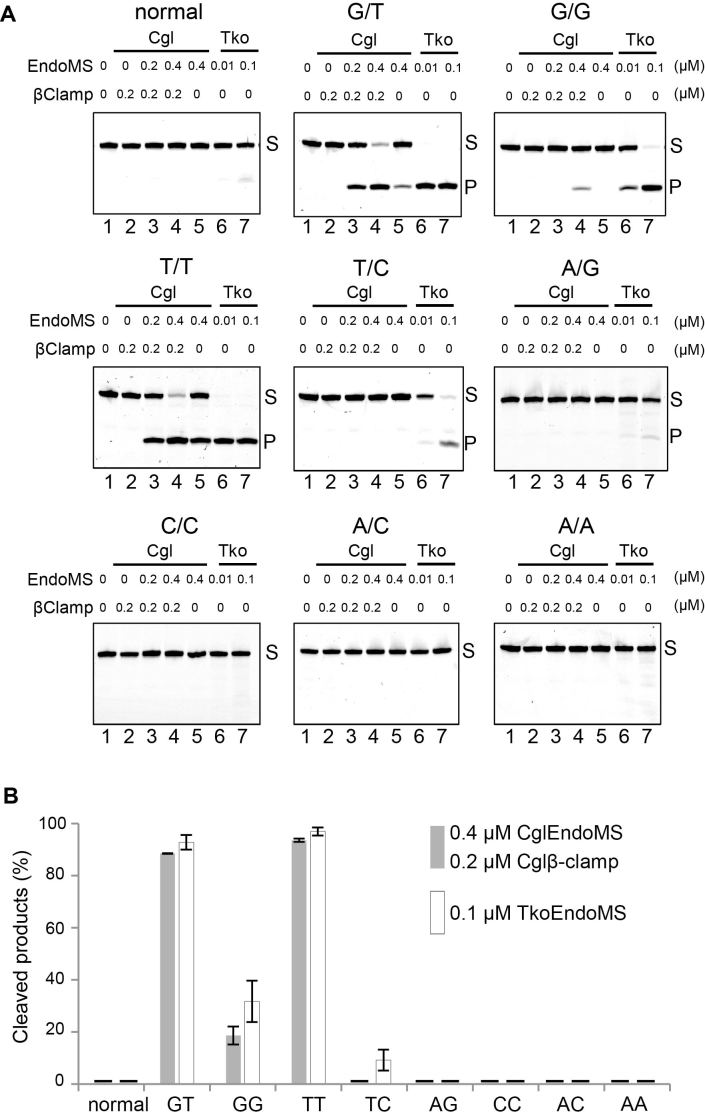
To investigate the mismatch specificity of the CglEndoMS nuclease, we used substrates containing all possible combinations of mismatched bases in cleavage reactions in the presence of the Cglβ-clamp (Figure 5). We observed very clear endonuclease activities not only with the G/T mismatch-containing dsDNA substrate used in the above experiments and preferred by the TkoEndoMS, but also with DNA duplexes containing T/T and, to a lesser extent, G/G mismatches. However, T/C, A/G, C/C and A/C mismatches were either cut very poorly or not all. We conclude that the preferred in vitro substrates of CglEndoMS are G/T (mismatch resulting in ‘transition’ mutations if left unrepaired) and T/T (‘transversion’ mismatch), which were cleaved with high efficiencies under these experimental conditions. Notably, the other possible ‘transition mismatch’, A/C, was not cleaved at all under these optimized conditions. We also stress that a previous study indicated that the EndoMS proteins lack ‘nicking’ activity that would cleave only one of the two strands of the DNAs with the mismatch-containing DNA substrates (11). Nevertheless, there is a possibility that CglEndoMS, in particular complexed with the DNA-bound Cglβ-clamp, might introduce a nick into a dsDNA. To address this point, a normal DNA or DNAs containing C/C or A/C mismatches were reacted with CglEndoMS with or without β-clamp. The reaction mixtures were fractionated by electrophoresis on a denaturing gel in parallel with a native gel. A cleaved band was detected from the G/T mismatch, but not from either the C/C or A/C mismatch, indicating that CglEndoMS did not clearly cleave even one of the two strands of the DNAs in the vicinity of these mismatches (Supplementary Figure S5).
Figure 5.
Preference for base-pair mismatches. (A) Five nanomolar Cy5-labeled dsDNA (45 bp), containing single base-pair mismatches (G/T, G/G, T/T, T/C, A/G, C/C, A/C, A/A), were incubated with various proteins as indicated on the panels. The products were separated by 10% PAGE. The representative results are shown. The base pairs are indicated on the top of each panel. The band assignments are indicated on the side of the panels, s, substrates; p, cleaved products. (B) Quantification of the cleaved products by 0.4 μM CglEndoMS and 0.2 μM Cglβ-clamp, and by 0.1 μM TkoEndoMS, as shown in lanes 4 and 7 in (A). The bars show the averages and the error bars are standard error of the mean (s.e.m.) from three independent experiments.
Accumulation of transition mutations in the absence of the CglEndoMS
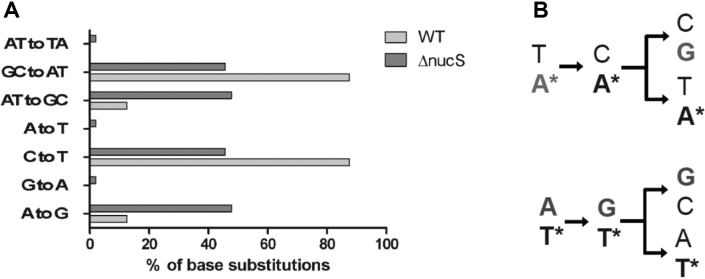
To investigate the mutational patterns of the C. glutamicum wild type and ΔnucS strains, we isolated and sequenced independent RifR mutants for the two strains (Figure 6). Many different types of mutations within the rpoB gene can cause resistance to rifampicin (19,26). We found that up to 98% of base substitutions in the isolated RifR mutants were transition mutations (Supplementary Table S4, Figure 6A). The fact that transitions are strongly favored over transversions among the sequenced base substitutions is in agreement with earlier studies on spectra of spontaneous mutations in Bacteria [see for instance (27)]. In particular, our results suggested that the relative level of the appearance of A:T to G:C transitions were favored approximately three to four-fold in the absence of EndoMS (Figure 6A). A priori, these transitions could result from either non-repaired C:A or G:T mismatches, as indicated schematically in Figure 6B.
Figure 6.
Mutational patterns of C. glutamicum wild type (WT) and ΔnucS strains. (A) Patterns of transition mutations (RifR) observed for wild type and ΔnucS C. glutamicum strains. (B) A simplified schema demonstrating how A to G transitions accumulating in the absence of nucS can result from either non-repaired G/T or C/A mismatches. Asterisks refer to the template strand bases.
DISCUSSION
Our previous study, searching for proteins involved in mismatch repair (MMR) in archaea that lack the homologs for MutS/MutL proteins, revealed an endonuclease family dubbed EndoMS that is encoded by nucS. This protein family cleaves both strands of double-stranded DNA carrying mismatches in vitro, thus generating double-strand breaks (11). In addition, a structural analysis of this archaeal homodimeric EndoMS, complexed with a double-stranded DNA carrying G/T mismatches, revealed that these mismatched bases were flipped out into binding sites located in the N-terminal domain of EndoMS. The active site for cleavage, which resembles that of the restriction endonuclease, is located in the C-terminal domain (12), and the archaeal EndoMS structures in the absence of DNA, together with earlier SAXS analyses (14), indicated the movement of the C-terminal catalytic domain brought about by the DNA binding that is required for activity. The nucS genes are widely distributed in the domain Archaea, but not in most Bacteria and not at all in Eukarya. It is remarkable that a nucS homolog was also found in Actinobacteria, which lack mutS/mutL homologs. A recent report showed that the nucS gene in M. smegmatis cells is required for mutation avoidance and antirecombination in the cells (17). This genetic study strongly supported the MMR function of EndoMS/NucS in this mycobacterial species. However, the purified EndoMS from M. smegmatis did not show the mismatch-specific endonuclease activity in vitro. Therefore, in the absence of combined biochemical and genetic studies in a single archaeal or actinobacterial species lacking canonical MutS/MutL-directed MMR proteins, the existence and specificity of an alternative MMR system was not fully understood or demonstrated.
Here, we have linked biochemical and genetic evidence for the mutation avoidance activities of the EndoMS family of proteins in C. glutamicum. We provide convincing evidence indicating that the physical interaction between the C. glutamicum replication β-clamp (DnaN) and the EndoMS homodimer is required for the recognition and cleavage of the mismatches in vitro and in vivo to protect corynebacterial cells from accumulation of mutations. Our biochemical data revealed that the physical interaction of the β-clamp substantially activates the mismatch-specific cleavage activity of the EndoMS/NucS proteins, and the carboxyl-terminal β-clamp-binding motif of CglEndoMS is necessary for this activation and mutation avoidance in the cells. A priori, the replication clamp could act directly on the catalytic step of EndoMS proteins or modulate its DNA binding properties. In the first scenario, the clamp could increase the catalytic rate constant up to four orders of magnitude, as has been demonstrated with other DNA repair nucleases (28). This is not fully compatible with our observations indicating ‘only’ four to five fold activation. In the future experiments, we will thus address whether the replication clamp helps EndoMS to scan DNA molecules that carry mismatches in order to recognize and process them.
Our observations already suggest a functional coupling of EndoMS/NucS-directed mismatch repair with the replisome. The role of the replication clamp, the β-clamp in Bacteria and PCNA in Archaea, is important for coordinating this non-canonical MMR, and thus diminishing the formation of unwanted double-strand chromosomal breaks by free EndoMS/NucS. After a double-strand break occurs at the mismatch site by EndoMS/NucS, this break would be processed by end resection to produce 3′-protruding ends, followed by homologous recombination. A recent articles showing that Corynebacteria are polyploidic organisms, resembling archaea (29), strongly supports the involvement of the homologous recombination in the repair of double strand breaks created around the repaired mismatches without strand discrimination.
CglEndoMS shows the order of preferences for G/T, T/T and G/G mismatches in vitro and these substrate preferences are quite similar to those of TkoEndoMS (11), signifying that the actinobacterial and archaeal EndoMS/NucS orthologues may share a common DNA repair function. The large majority of the RifR base substitutions occurring in the absence of EndoMS are transitions (Figure 6A) indicating that they must result from unrepaired purine mispairing with a wrong pyrimidine (G/T or C/A transition mismatches). Strikingly, whereas G/T mismatches act as preferred substrates for EndoMS/NucS proteins in vitro (Figure 5), C/A mismatches are processed very poorly (or not all). This is different from E. coli MutS/MutL system that corrects both transition mismatches [see for instance the early reference (30)]. The fact that both MMR systems work efficiently on the G/T mismatch is likely to have a biological meaning, as G/T mismatches are very common replication errors (8). This may reflect the relatively high capacity of at least some DNA polymerases to efficiently create G/T mismatches (31) that are stabilized by rare tautomeric forms of guanosine and thymidine (32). Transitions may thus occur more frequently in the cell than tranversions because molecular mechanisms generating and stabilizing them are different. It thus appears feasible that G/T mismatches frequently escaping proofreading activity of the replisome are efficiently repaired by the EndoMS:β-clamp complex. We stress that the EndoMS/NucS system is quite different from the very short patch repair system (Vsr) found for instance in E. coli (30). Although EndoMS and VSR endonucleases both efficiently cleave G/T mismatches, the Vsr system corrects G/T mismatches resulting from 5-methylcytosine deamination in a very specific sequence context. Moreover, the recognition of the G/T mismatch is achieved differently in the two systems. Cytosine methylation does not appear to occur in Corynebacteria and Mycobacteria, as experimentally suggested by PacBio sequencing (33,34). Our combined biochemical and genetic study thus suggests that EndoMS/NucS proteins have been fine-tuned to correct the frequently occurring transition mismatches in cells. We do not exclude the possibility that other types of mismatches (for instance T/T or G/G mismatches) resulting in transversions will also be processed by this novel DNA repair pathway, when they occur in cells.
In conclusion, we demonstrated the existence of a non-canonical MMR system in C. glutamicum, one of the most important industrial organisms used for the production of L-glutamate and L-lysine and other various products (35,36). The measured frequencies and the spectra of transition mutations for the wild type and ΔnucS C. glutamicum strains are consistent with the enzymatic properties of EndoMS/NucS-β–clamp complex in vitro. Our study may also be applicable for the industrial engineering of the useful strains and could provide a rational format for understanding the extent of genetic variation and the development of antibiotic resistant phenotypes in important human pathogens including Mycobacterium tuberculosis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Takeshi Yamagami and Namiko Imai for technical assistance. We also thank Christine Houssin and Célia De Sousa-d’Auria (I2BC, Univ. Paris-Sud, France) for their expertise in C. glutamicum manipulation. We also thank Julia Gross for help with DNA sequencing experiments and Ursula Liebl for helpful comments on the manuscript.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Ministry of Education, Culture, Sports, Science and Technology of Japan [JP26242075 to Y.I.]; Japan Society for the Promotion of Science (JSPS) (to Y.I.); CNRS, INSERM and E. polytechnique (to H.M and S.S.). Funding for open access charge: INSERM.
Conflict of interest statement. None declared.
REFERENCES
- 1. Jackson S.P., Bartek J.. The DNA-damage response in human biology and disease. Nature. 2009; 461:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ganai R.A., Johansson E.. DNA replication—a matter of fidelity. Mol. Cell. 2016; 62:745–755. [DOI] [PubMed] [Google Scholar]
- 3. Jiricny J. Postreplicative mismatch repair. Cold Spring Harb. Perspect. Biol. 2013; 5:a012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tham K.C., Kanaar R., Lebbink J.H.. Mismatch repair and homeologous recombination. DNA Repair (Amst.). 2016; 38:75–83. [DOI] [PubMed] [Google Scholar]
- 5. Matic I., Rayssiguier C., Radman M.. Interspecies gene exchange in bacteria: the role of SOS and mismatch repair systems in evolution of species. Cell. 1995; 80:507–515. [DOI] [PubMed] [Google Scholar]
- 6. Putnam C.D. Evolution of the methyl directed mismatch repair system in Escherichia coli. DNA Repair (Amst.). 2016; 38:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pluciennik A., Burdett V., Lukianova O., O’Donnell M., Modrich P.. Involvement of the beta clamp in methyl-directed mismatch repair in vitro. J. Biol. Chem. 2009; 284:32782–32791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kunkel T.A., Erie D.A.. Eukaryotic mismatch repair in relation to DNA replication. Annu. Rev. Genet. 2015; 49:291–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pluciennik A., Dzantiev L., Iyer R.R., Constantin N., Kadyrov F.A., Modrich P.. PCNA function in the activation and strand direction of MutLalpha endonuclease in mismatch repair. Proc. Natl. Acad. Sci. U.S.A. 2010; 107:16066–16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin Z., Nei M., Ma H.. The origins and early evolution of DNA mismatch repair genes–multiple horizontal gene transfers and co-evolution. Nucleic Acids Res. 2007; 35:7591–7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishino S., Nishi Y., Oda S., Uemori T., Sagara T., Takatsu N., Yamagami T., Shirai T., Ishino Y.. Identification of a mismatch-specific endonuclease in hyperthermophilic Archaea. Nucleic Acids Res. 2016; 44:2977–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakae S., Hijikata A., Tsuji T., Yonezawa K., Kouyama K.I., Mayanagi K., Ishino S., Ishino Y., Shirai T.. Structure of the EndoMS-DNA Complex as mismatch restriction endonuclease. Structure. 2016; 24:1960–1971. [DOI] [PubMed] [Google Scholar]
- 13. Ariyoshi M., Morikawa K.. A dual base flipping mechanism for archaeal mismatch repair. Structure. 2016; 24:1859–1861. [DOI] [PubMed] [Google Scholar]
- 14. Creze C., Ligabue A., Laurent S., Lestini R., Laptenok S.P., Khun J., Vos M.H., Czjzek M., Myllykallio H., Flament D.. Modulation of the Pyrococcus abyssi NucS endonuclease activity by replication clamp at functional and structural levels. J. Biol. Chem. 2012; 287:15648–15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meslet-Cladiere L., Norais C., Kuhn J., Briffotaux J., Sloostra J.W., Ferrari E., Hubscher U., Flament D., Myllykallio H.. A novel proteomic approach identifies new interaction partners for proliferating cell nuclear antigen. J. Mol. Biol. 2007; 372:1137–1148. [DOI] [PubMed] [Google Scholar]
- 16. Ren B., Kuhn J., Meslet-Cladiere L., Briffotaux J., Norais C., Lavigne R., Flament D., Ladenstein R., Myllykallio H.. Structure and function of a novel endonuclease acting on branched DNA substrates. EMBO J. 2009; 28:2479–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castaneda-Garcia A., Prieto A.I., Rodriguez-Beltran J., Alonso N., Cantillon D., Costas C., Perez-Lago L., Zegeye E.D., Herranz M., Plocinski P. et al. A non-canonical mismatch repair pathway in prokaryotes. Nat. Commun. 2017; 8:14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Portevin D., De Sousa-D’Auria C., Houssin C., Grimaldi C., Chami M., Daffe M., Guilhot C.. A polyketide synthase catalyzes the last condensation step of mycolic acid biosynthesis in mycobacteria and related organisms. Proc. Natl. Acad. Sci. U.S.A. 2004; 101:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell E.A., Korzheva N., Mustaev A., Murakami K., Nair S., Goldfarb A., Darst S.A.. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell. 2001; 104:901–912. [DOI] [PubMed] [Google Scholar]
- 20. Ikeda M., Nakagawa S.. The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl. Microbiol. Biotechnol. 2003; 62:99–109. [DOI] [PubMed] [Google Scholar]
- 21. Sali A., Blundell T.L.. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993; 234:779–815. [DOI] [PubMed] [Google Scholar]
- 22. Laskowski R.A., Rullmannn J.A., MacArthur M.W., Kaptein R., Thornton J.M.. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR. 1996; 8:477–486. [DOI] [PubMed] [Google Scholar]
- 23. Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E.. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004; 25:1605–1612. [DOI] [PubMed] [Google Scholar]
- 24. Dalrymple B.P., Kongsuwan K., Wijffels G., Dixon N.E., Jennings P.A.. A universal protein-protein interaction motif in the eubacterial DNA replication and repair systems. Proc. Natl. Acad. Sci. U.S.A. 2001; 98:11627–11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johanson K.O., McHenry C.S.. Purification and characterization of the beta subunit of the DNA polymerase III holoenzyme of Escherichia coli. J. Biol. Chem. 1980; 255:10984–10990. [PubMed] [Google Scholar]
- 26. Garibyan L., Huang T., Kim M., Wolff E., Nguyen A., Nguyen T., Diep A., Hu K., Iverson A., Yang H. et al. Use of the rpoB gene to determine the specificity of base substitution mutations on the Escherichia coli chromosome. DNA Repair (Amst.). 2003; 2:593–608. [DOI] [PubMed] [Google Scholar]
- 27. Schaaper R.M., Dunn R.L.. Spectra of spontaneous mutations in Escherichia coli strains defective in mismatch correction: the nature of in vivo DNA replication errors. Proc. Natl. Acad. Sci. U.S.A. 1987; 84:6220–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hutton R.D., Roberts J.A., Penedo J.C., White M.F.. PCNA stimulates catalysis by structure-specific nucleases using two distinct mechanisms: substrate targeting and catalytic step. Nucleic Acids Res. 2008; 36:6720–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bohm K., Meyer F., Rhomberg A., Kalinowski J., Donovan C., Bramkamp M.. Novel chromosome organization pattern in Actinomycetales-Overlapping replication cycles combined with diploidy. MBio. 2017; 8:e00511-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kramer B., Kramer W., Fritz H.J.. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984; 38:879–887. [DOI] [PubMed] [Google Scholar]
- 31. Batra V.K., Beard W.A., Pedersen L.C., Wilson S.H.. Structures of DNA polymerase mispaired DNA termini transitioning to Pre-catalytic complexes support an Induced-Fit fidelity mechanism. Structure. 2016; 24:1863–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kimsey I.J., Szymanski E.S., Zahurancik W.J., Shakya A., Xue Y., Chu C.C., Sathyamoorthy B., Suo Z., Al-Hashimi H.M.. Dynamic basis for dG*dT misincorporation via tautomerization and ionization. Nature. 2018; 554:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blow M.J., Clark T.A., Daum C.G., Deutschbauer A.M., Fomenkov A., Fries R., Froula J., Kang D.D., Malmstrom R.R., Morgan R.D. et al. The epigenomic landscape of prokaryotes. PLoS Genet. 2016; 12:e1005854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu L.X., Zhong J., Jia X.M., Liu G., Kang Y., Dong M.X., Zhang X.L., Li Q., Yue L.Y., Li C.D. et al. Precision methylome characterization of Mycobacterium tuberculosis complex (MTBC) using PacBio single-molecule real-time (SMRT) technology. Nucleic Acids Res. 2016; 44:730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heider S.A., Wendisch V.F.. Engineering microbial cell factories: Metabolic engineering of Corynebacterium glutamicum with a focus on non-natural products. Biotechnol. J. 2015; 10:1170–1184. [DOI] [PubMed] [Google Scholar]
- 36. Wendisch V.F., Jorge J.M.P., Perez-Garcia F., Sgobba E.. Updates on industrial production of amino acids using Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2016; 32:105. [DOI] [PubMed] [Google Scholar]
- 37. Jakoby M., Ngouoto-Nkili C.E., Burkovski A.. Construction and application of new Corynebacterium glutamicum vectors. Biotechnol. Tech. 1999; 13:437–441. [Google Scholar]
- 38. Menard R., Sansonetti P.J., Parsot C.. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 1993; 175:5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.