Abstract.
Hepatitis A and hepatitis E viruses (HAV and HEV) are the most common etiologies of viral hepatitis in India. To better understand the epidemiology of these infections, laboratory surveillance data generated during 2014–2017, by a network of 51 virology laboratories, were analyzed. Among 24,000 patients tested for both HAV and HEV, 3,017 (12.6%) tested positive for HAV, 3,865 (16.1%) for HEV, and 320 (1.3%) for both HAV and HEV. Most (74.6%) HAV patients were aged ≤ 19 years, whereas 76.9% of HEV patients were aged ≥ 20 years. These laboratories diagnosed 12 HAV and 31 HEV clusters, highlighting the need for provision of safe drinking water and improvements in sanitation. Further expansion of the laboratory network and continued surveillance will provide data necessary for informed decision-making regarding introduction of hepatitis-A vaccine into the immunization program.
Viral hepatitis is an important public problem in developing countries. In India, sporadic hepatitis A virus (HAV) and hepatitis E virus (HEV) infections as well as waterborne outbreaks are frequent.1,2 Hepatitis A virus exposure typically occurs early in life, when the infection is often subclinical. Serological studies in India in recent decades indicate a consistent pattern of high HAV exposure during childhood, with more than 80% of children demonstrating antibodies against the virus by age 10 years.2,3 Because of early exposure, clinical cases are relatively rare among children. Hence, hepatitis A vaccine has not been included in the routine childhood immunization program in India.4 However, information about disease burden, as measured through serological surveys which estimate past infections, and disease surveillance programs which measure morbidity and mortality, is vital to document the changing epidemiology of the disease and for making informed decisions about introducing HAV vaccine.3
India does not have a case-based surveillance system for viral hepatitis. The Integrated Disease Surveillance Program (IDSP) in India conducts surveillance in all states for outbreak-prone diseases, including viral hepatitis. Aggregate numbers of patients with viral hepatitis seeking care from public health facilities and selected private health facilities are reported to district surveillance units every week. Suspected hepatitis outbreaks, based on a predetermined threshold of weekly number of suspected viral hepatitis cases, are investigated by the district surveillance units, and based on the results of laboratory investigations, outbreaks are laboratory confirmed.5 Aggregated data reported to the district surveillance units and the outbreak investigation reports do not include information about the age distribution of patients.
The laboratory capacity to diagnose outbreaks through the IDSP is variable in different Indian states. To strengthen the laboratory capacity for providing timely diagnosis of disease outbreaks, the Indian Council of Medical Research and the Department of Health Research have established a Virus Research and Diagnostic Laboratory (VRDL) network during 2013–2014 (http://www.dhr.gov.in/sites/default/files/about%20scheme.pdf). The number of laboratories in the network increased from 20 in 2014 to 34 in 2015, 37 in 2016, and 51 by October 2017, in 24 of India’s 29 states (Figure 1). Virus Research and Diagnostic Laboratories are established in medical colleges (N = 47) or research institutes of the Indian Council of Medical Research (N = 4). Virus Research and Diagnostic Laboratories receive samples from two sources: the district public health authorities for laboratory confirmation of disease clusters (suspected outbreaks) and individual patients seeking health care at the medical colleges or tertiary care hospitals.6 Demographic, clinical, and laboratory data from all patients are entered in a web-based data entry system. Virus Research and Diagnostic Laboratories follow a uniform protocol for laboratory testing.
Figure 1.
Map of India showing location of Virus Research and Diagnostic Laboratories.
As a part of the surveillance protocol, all patients presenting with a syndromic diagnosis of febrile jaundice attending medical colleges or hospitals, or samples from such patients referred by the district public health authorities in case of suspected outbreaks are enrolled. Sera samples from these patients are investigated for different hepatitis viruses, including HAV and HEV, using commercial enzyme-linked immunosorbent assays (ELISAs) to detect immunoglobulin M (IgM) antibodies. The ELISA kits recommended for use in the network for IgM antibodies against HAV included Hepavase MA-96 (General Biologicals, Hsinchu, Taiwan), ImmunoVision (ImmunoVision Springdale, AR), and DRG (DRG International Inc., Springfield Township, NJ). For IgM antibodies against HEV, the recommended kits are those manufactured by MP Biomedicals (MP Biomedicals Suisse, Geneva, Switzerland) and MBS-SRL (MBS-SRL, Milano, Italy). To better understand the epidemiology of HAV and HEV infections in the country, we analyzed the laboratory surveillance data generated by a network of 51 virology laboratories during January 2014–October 2017. The findings of etiologies other than HAV and HEV are not presented in this article.
During January 2014–October 2017, VRDLs tested specimens from 24,000 patients with suspected viral hepatitis. These included 713 (3.0%) specimens from patients in suspected clusters (outbreaks) and 23,287 (97.0%) specimens from patients seeking care from medical colleges. Overall, 7,202 (30.0%) of samples tested positive, including 3,017 (12.6%) for HAV, 3,865 (16.1%) for HEV, and 320 (1.3%) for both HAV and HEV (Table 1). Among children aged ≤ 9 years and those aged 10–19 years, the HAV:HEV positivity ratio was 5.9:1 and 1.2:1, respectively. On the other hand, in older age groups, HEV positivity was higher than that of HAV (Table 1). Of the 3,050 HAV-positive patients whose age was known, 2,276 (74.6%) were aged ≤ 19 years, whereas most of the 3,639 HEV-positive patients were aged ≥ 20 years (N = 2,800, 76.9%). The median ages of HAV- and HEV-positive patients were eight (interquartile range = 5–18 years) and 28 (interquartile range = 21–40 years) years, respectively. Males predominated among both HAV and HEV cases (P = 0.000). They made up 61.1% of HAV patients and 65.5% of HEV patients. The number of patients investigated and the HAV/HEV positivity varied by region. In south, north, and northeastern regions, HAV:HEV positivity ratio was 2.57, 1.19, and 2.63, respectively, whereas in the remaining regions, HEV positivity was higher than that of HAV (Table 1).
Table 1.
Hepatitis A and E virus positivity by age group, gender, and region, Virus Research and Diagnostic Laboratory network, India, 2014–2017
| Number tested | No. HAV positive (%) | No. HEV positive (%) | No. positive for both HAV and HEV (%) | HAV:HEV positivity ratio | |
|---|---|---|---|---|---|
| Age group (years) | |||||
| ≤ 9 | 5,060 | 1,493 (29.5) | 182 (3.6) | 86 (1.7) | 5.89 |
| 10–19 | 3,552 | 636 (17.9) | 510 (14.4) | 61 (1.7) | 1.22 |
| 20–29 | 3,892 | 234 (6.0) | 1,147 (29.5) | 51 (1.3) | 0.24 |
| 30–39 | 3,245 | 182 (5.6) | 674 (20.8) | 24 (0.7) | 0.30 |
| 40–49 | 2,621 | 130 (5.0) | 428 (16.3) | 20 (0.8) | 0.33 |
| 50–59 | 1,558 | 54 (3.5) | 248 (15.9) | 26 (1.7) | 0.29 |
| ≥ 60 | 1,286 | 36 (2.8) | 165 (12.8) | 17 (1.3) | 0.29 |
| Not available | 2,786 | 252 (9.0) | 511 (18.3) | 35 (1.3) | 0.53 |
| Gender | |||||
| Male | 15,094 | 1,826 (12.1) | 2,546 (16.9) | 192 (1.3) | 0.74 |
| Female | 8,834 | 1,162 (13.2) | 1,317 (14.9) | 125 (1.4) | 0.89 |
| Not available | 72 | 29 (40.3) | 2 (2.8) | 3 (4.2) | 6.40 |
| Region | |||||
| South | 1,517 | 340 (22.4) | 120 (7.9) | 20 (1.3) | 2.57 |
| North | 9,255 | 1,597 (17.3) | 1,325 (14.3) | 142 (1.5) | 1.19 |
| East | 2,908 | 311 (10.7) | 444 (15.3) | 40 (1.4) | 0.73 |
| Northeast | 770 | 123 (16.0) | 40 (5.2) | 11 (1.4) | 2.63 |
| West/central | 9,550 | 646 (13.3) | 1,936 (35.6) | 107 (1.9) | 0.37 |
| Total | 24,000 | 3,017 (12.6) | 3,865 (16.1) | 320 (1.3) | 0.80 |
HAV = Hepatitis A virus; HEV = Hepatitis E virus.
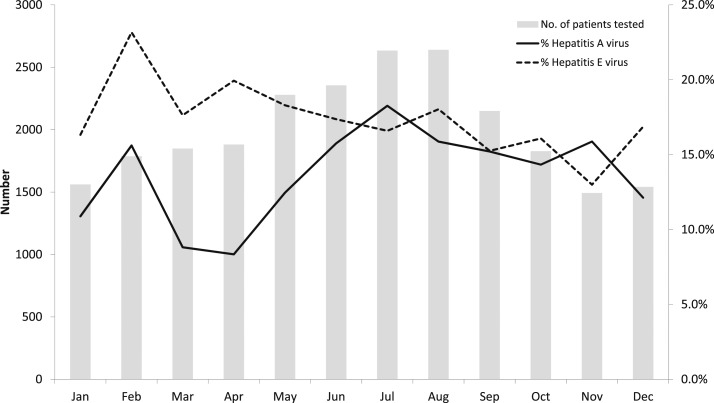
Figure 2 shows the percentage positivity for hepatitis A and E by months. The mean monthly rate of HAV positivity ranged from about 8.3% to 18.3%, with higher positivity during June to November, compared with the period from December to May. The HEV positivity which ranged from 13% to 23.2% exhibited less seasonal variation throughout the year (Figure 1).
Figure 2.
Average number of patients tested for hepatitis A virus and hepatitis E virus per month and percentage of positive tests—India, 2014–2017.
During 2014–2017, VRDLs diagnosed 43 clusters of viral hepatitis; of these, 12 (28%) were caused by HAV and 31 (72%) were caused by HEV (Supplemental Figure 1).
Laboratory surveillance data from the national VRDL network indicated that HAV and HEV were frequent causes of viral hepatitis during 2014–2017. About a third (30.0%) of viral hepatitis cases investigated by VRDLs were due to HAV and/or HEV. The laboratory surveillance data indicated that about three-fourths of the laboratory-confirmed HAV cases were aged ≤ 19 years, whereas 76.9% of the HEV cases were aged > 19 years.
In countries with high HAV endemicity, clinical disease is infrequent among children, whereas susceptible adults are at higher risk for infection and clinical illness. The overall disease rates are generally low, and outbreaks are rare because of the high level of population immunity.1–3 The seroprevalence data in India also indicate higher prevalence of HAV among children, although a few pockets of low seroprevalence have been reported in urban areas with higher levels of socioeconomic development.2 The data from VRDLs showed that HAV accounted for 13.9% of the viral hepatitis cases tested by these laboratories and nearly three-fourths of these patients were aged < 19 years. On the other hand, 76.9% of HEV-positive cases diagnosed by the VRDLs were aged ≥ 20 years, which is consistent with data from epidemiological studies from many countries, which have found low HEV seroprevalence during early childhood.7 Higher HAV:HEV positivity ratio among children and lower HAV:HEV positivity ratio among adults observed in our analysis are consistent with the published literature from India.8–11 The lower median age of patients with HAV infection compared with that of patients with HEV suggests that young children in India are susceptible to HAV infection. Although most of the large hepatitis outbreaks in India in the past were caused by HEV, and HAV outbreaks have been rare because of high population immunity, in recent years, HAV outbreaks have been reported from Kerala, Tamil Nadu, Himachal Pradesh, and Maharashtra, affecting children and adults.12–15 The predominance of males among both HAV and HEV cases has been reported previously.2,7 This might be related to a higher level of exposure of males to contaminated water or differences in the health-seeking behavior of males and females. Higher HAV positivity during rainy and post-rainy seasons compared with the dryer months of the year observed in our data is consistent with the data from IDSP.5
The findings of this analysis are subject to certain limitations. First, the data presented pertain to patients seeking care from the selected sentinel sites, who were predominantly from urban areas, and hence might not be representative of the entire country. Second, medical colleges, being tertiary care hospitals, serve populations which might reside outside the city where they are located, including neighboring districts and states. Precise estimates of the populations served by these medical colleges were not available. In light of this, it was not possible to calculate the incidence of these infections. Third, as most VRDLs do not investigate outbreaks, information about age and gender was only available for patients whose blood samples were referred for laboratory diagnosis and does not necessarily reflect characteristics of all persons affected during the outbreaks. Virus Research and Diagnostic Laboratories also did not investigate to find out if the viral hepatitis patients attending medical colleges are outbreak related.
The virology laboratory network is established to strengthen the capacity for timely diagnosis of disease outbreaks in India. During 2014–2017, the network identified 43 HAV and HEV clusters, and thus complemented the IDSP in laboratory confirmation. This network also generated case-based data on HAV and HEV infection to understand the epidemiology of these infections. Expansion of the laboratory network to more sites in the country will provide additional epidemiologic data, which will be useful to inform policymakers about the need for introduction of hepatitis A vaccine in routine immunization programs.
Supplementary Material
Acknowledgments:
Authors gratefully acknowledge principal investigators and staff of the following Virus Research and Diagnostic Laboratories: Manipal Centre for Virus Research, Manipal University, Karnataka; Regional Medical Research Centre, Port Blair, Andaman and Nicobar; King George’s Medical University, Lucknow, Uttar Pradesh; Regional Medical Research Centre, Bhubaneswar, Odisha; Late Sri Baliram Kashyap Memorial Govt. Medical College, Jagdalpur, Chattisgarh; SMS Medical College, Jaipur, Rajasthan; Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala; National Institute of Virology (field unit), Alappuzha, Kerala; King Institute of Preventive Medicine and Research, Chennai, Tamil Nadu; Rajendra Institute of Medical Sciences, Ranchi, Jharkhand; Andhra Medical College, Vishakhapatnam, Andhra Pradesh; National Institute of Research in Tribal Health, Jabalpur, Madhya Pradesh; Rajendra Memorial Research Institute of Medical Sciences, Patna, Bihar; Regional Medical Research Centre for NE region, Dibrugarh, Assam; Government Medical College, Agartala, Tripura; Osmania Medical College, Hyderabad, Andhra Pradesh; Indira Gandhi Medical College, Shimla, Himachal Pradesh; Government Medical College, Jammu, Jammu and Kashmir; Sher-i-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir; BJ Medical College, Ahmadabad, Gujarat; Government Medical College, Patna, Bihar; Government Medical College, Amristar, Punjab; Pt. B. D. Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana; Post Graduate Institute of Medical Education and Research, Chandigarh; North Eastern Indira Gandhi Regional Institute of Health and Medical Research, Shillong, Meghalaya; Government Medical College, Jamnagar, Gujarat; Indira Gandhi Medical College, Nagpur, Maharashtra; Madurai Medical College, Madurai, Tamil Nadu; Sri Venkateswara Institute of Medical Science, Tirupati, Andhra Pradesh; Govt. Theni Medical College, Theni, Tamil Nadu; Guwahati Medical College, Guwahati, Assam; Govt. Medical College, Thiruvananthapuram, Kerala; All India Institute of Medial Sciences, Bhopal, Madhya Pradesh; Govt. Siddhartha Medical College, Vijayawada, Andhra Pradesh; Jawaharlal Nehru Institute of Medical Sciences, Imphal, Manipur; NICED, Kolkatta, West Bengal; Dr. Rajendra Prasad Govt. Medical College, Kangra, Himachal Pradesh; JNIMSH Imphal, Manipur; JN Medical College, AMU, Aligarh, Uttar Pradesh; Govt. Medical College, Patiala, Punjab; UP RIMSR, Etawah, Uttar Pradesh; JMCH, Borbheta, Jorhat, Assam; Bangalore Medical College, Karnataka; Govt. Medical College, Kozikode, Kerala.
Note: Supplemental figure appears at www.ajtmh.org.
REFERENCES
- 1.Satsangi S, Chawla YK, 2016. Viral hepatitis: Indian scenario. Med J Armed Forces India 72: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO , 2010. The Global Prevalence of Hepatitis A Virus Infection and Susceptibility: A Systematic Review Available at: http://apps.who.int/iris/bitstream/10665/70180/1/WHO_IVB_10.01_eng.pdf. Accessed May 29, 2018.
- 3.Aggarwal R, Goel A, 2015. Hepatitis A: epidemiology in resource-poor countries. Curr Opin Infect Dis 28: 488–496. [DOI] [PubMed] [Google Scholar]
- 4.Mathur P, Arora NK, 2008. Epidemiological transition of hepatitis A in India: issues for vaccination in developing countries. Indian J Med Res 128: 699–704. [PubMed] [Google Scholar]
- 5.Kumar T, Shrivastava A, Kumar A, Laserson KF, Narain JP, Venkatesh S, Chauhan LS, Averhoff F, 2015. Viral hepatitis surveillance–India, 2011–2013. MMWR Morb Mortal Wkly Rep 64: 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshua V, Murhekar MV, Ashok M, Kanagasabai K, Ravi M, Sabarinathan R, Kirubakaran BK, Ramachandran V, Gupta N, Mehendale S, 2016. Mapping dengue cases through a national network of laboratories, 2014–15. Indian J Med Res 144: 938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO , 2010. The Global Prevalence of Hepatitis E Virus Infection and Susceptibility: A Systematic Review Available at: http://apps.who.int/iris/bitstream/10665/70513/1/WHO_IVB_10.14_eng.pdf.
- 8.Jain P, Prakash S, Gupta S, Singh KP, Shrivastava S, Singh DD, Singh J, 2013. Prevalence of hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis D virus and hepatitis E virus as causes of acute viral hepatitis in north India: a hospital based study. Indian J Med Microbiol 31: 261–265. [DOI] [PubMed] [Google Scholar]
- 9.Chadha MS, Walimbe AM, Chobe LP, Arankalle VA, 2003. Comparison of etiology of sporadic acute and fulminant viral hepatitis in hospitalized patients in Pune, India during 1978–81 and 1994–97. Indian J Gastroenterol 22: 11–15. [PubMed] [Google Scholar]
- 10.Talukdar AJ, Islam S, Kashyap P, Dutta S, 2016. Sporadic adult acute viral hepatitis in northeast India is predominantly hepatitis A virus related. Indian J Gastroenterol 35: 401–402. [DOI] [PubMed] [Google Scholar]
- 11.Nandi B, Hadimani P, Arunachalam R, Ganjoo RK, 2009. Spectrum of acute viral hepatitis in southern India. Med J Armed Forces India 65: 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raveendran S, Rakesh PS, Dev S, Vijayakumar N, Prasannakumar P, 2016. Investigation of an outbreak of hepatitis a in a coastal area, Kerala, southern India. J Prim Care Community Health 7: 288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chobe LP, Arankalle VA, 2009. Investigation of a hepatitis A outbreak from Shimla Himachal Pradesh. Indian J Med Res 130: 179–184. [PubMed] [Google Scholar]
- 14.Chadha MS, Lole KS, Bora MH, Arankalle VA, 2009. Outbreaks of hepatitis A among children in western India. Trans R Soc Trop Med Hyg 103: 911–916. [DOI] [PubMed] [Google Scholar]
- 15.Sowmyanarayanan TV, Mukhopadhya A, Gladstone BP, Sarkar R, Kang G, 2008. Investigation of a hepatitis A outbreak in children in an urban slum in Vellore, Tamil Nadu, using geographic information systems. Indian J Med Res 128: 32–37. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.