Abstract.
Urinary schistosomiasis causes damage to the urological system. Ultrasound is a method that detects the burden of secondary disease, individually and in epidemiological studies. In this study, the Schistosoma haematobium–associated urinary tract pathology is analyzed before and after treatment in a short period of time. Seventy children who had previously participated in an epidemiological study on schistosomiasis in the city of Cubal, Angola, and had also performed urinary ultrasound between August 2013 and February 2014 were cited 6–8 months later to assess the possible reinfection and repeat new urinary ultrasound, analyzing changes at the level of urinary pathology. The presence of hematuria and proteinuria was also analyzed. Of the 70 children analyzed, 29 (41.4%) were girls, with an average age of 10.4 years (standard deviation 2.3). Fifty-three (75.7%) had an improvement in their bladder and/or kidney scores, whereas 12 (17.1%) had no change and five (7.1%) had progression of the disease. None of the parameters analyzed completely disappeared. After one single course of treatment with praziquantel, all the analyzed parameters showed regression. Improvement was greater in the urinary bladder than in the upper urinary tract, though these lesions also reversed; the reversion of all parameters was greater among children older than 10 years old than the younger ones. Proteinuria was the parameter with a smaller reduction. Ultrasound should be a usual tool for diagnosis and follow-up in urinary schistosomiasis, particularly in children; more accurate recommendations about follow-up in the case of children whose lesions do not reverse should be established.
INTRODUCTION
Human schistosomiasis is one of the most prevalent parasitic infections in the world, and after malaria, produces the greatest consequences for public health and the economy in endemic countries.1,2 It is estimated that 240 million people are infected,3,4 mainly in sub-Saharan Africa. It accounts for at least 3.3 million disability-adjusted life years.5
At present, the primary means of schistosomiasis control is mass drug administration (MDA) with praziquantel.6 The current World Health Organization (WHO) guidelines on how to conduct MDA for the control of severe morbidity are applied depending on the initial prevalence and intensity of infection.7,8 The main objective of this strategy is the control of infection-associated morbidities to limit the cumulative morbidity and internal damage from infection and any subsequent reinfection.8
In urinary schistosomiasis, the adult worms live mainly in the venous plexus of the urinary bladder. Morbidity is caused by egg deposition around the urinary tract, causing inflammation and lesions; thus, the related pathology is found mainly in the urinary bladder, ureters, and kidney.1,2 The most common lesions are irregularities of the bladder wall, a distorted bladder shape, and wall thickening; bladder masses may be also present. Lesions in the upper urinary tract, such as ureter dilatation and hydronephrosis, are less frequent but usually more severe, indicating a higher level of pathology.9,10 Despite lacking specificity, microhematuria, and proteinuria are the best markers of morbidity due to urinary schistosomiasis.17 Although there is an association between the prevalence and intensity of infection and morbidity, this is not a perfect correlation.11,12 Some recent studies suggest that to establish the periodicity of chemotherapy administration, it may be useful to know the dynamics of some of the morbidity outcomes, including the evolution of urologic lesions after taking praziquantel considering initial morbidity, frequency of reinfection, age, and other factors.13–15
After roughly 40 years of war, Angola has been recovering control and surveillance activities in recent years. A recent parasitological survey in the Cubal district in Central Angola showed a prevalence of urinary schistosomiasis of at least 61% in school-age children.16 Thus, this area is considered a highly endemic area where no MDA or other preventive activities have yet been undertaken.
Our aim was to analyze the Schistosoma haematobium–associated urinary tract pathology among school-age children in this area, both pre- and shortly posttreatment, assess the effects of age and reinfection, and analyze the association of pathologic lesions with indirect measures of urinary schistosomiasis infections such as hematuria and proteinuria.
METHODS
Study population and data collection.
Between the months of June and November of 2013 a cross-sectional study was carried out in the city of Cubal. Urine samples were collected in the fourth and fifth grade classes, corresponding to 9 and 10 years old. We analyzed 1,283 urine samples, of which 785 (61%) were positive for the presence of S. haematobium eggs.16
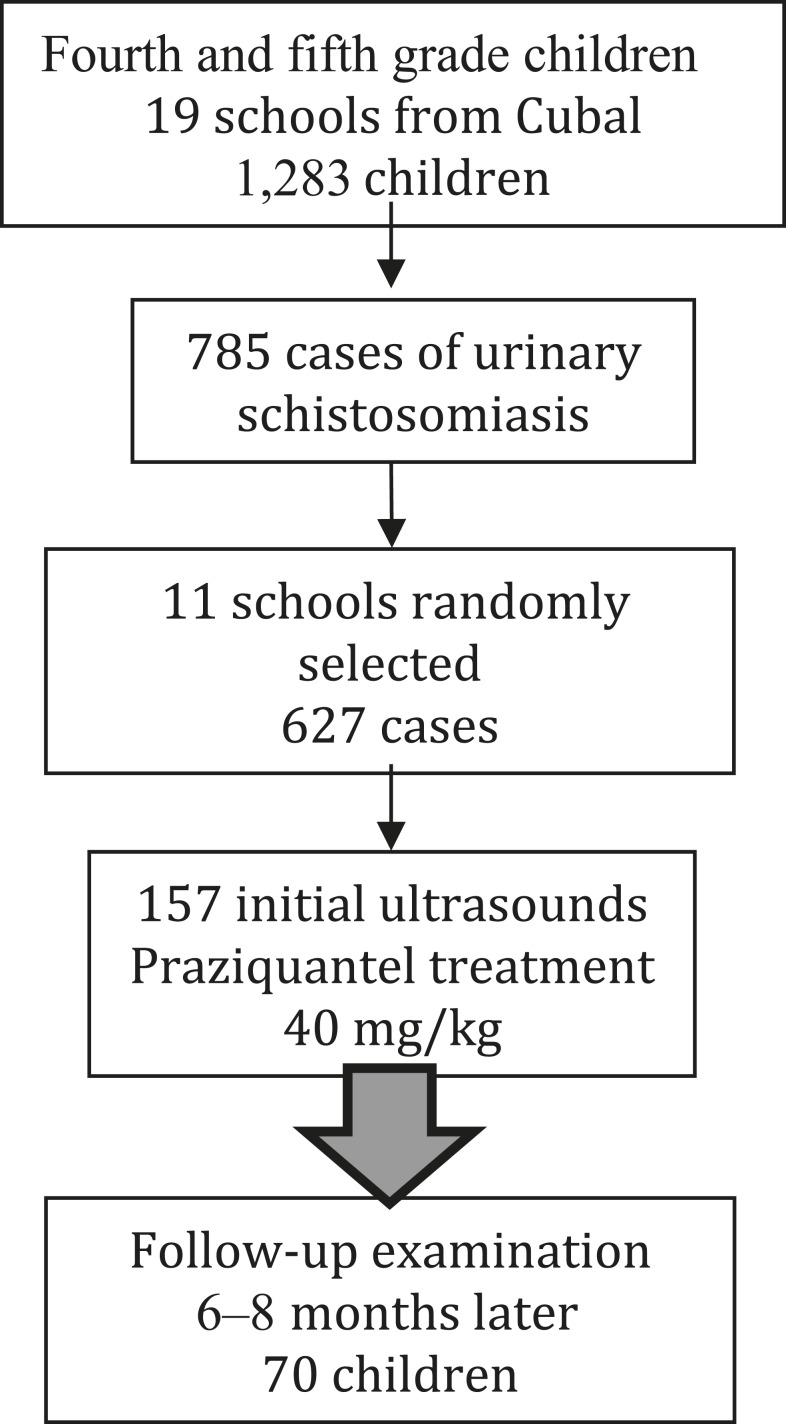
Subsequently, 11 of these schools were randomly selected; all patients diagnosed with schistosomiasis in these 11 schools (627 children) were cited for urinary ultrasound; of them, 127 (25%) attended. Treatment with praziquantel was administered at a dose of 40 mg/kg on the same day. The children and their parents also received information about the pathophysiology and epidemiology of the disease, along with recommendations to avoid bathing in river water.17 A flow chart explaining inclusion in the study is shown in Figure 1.
Figure 1.
Flow chart of study enrollment.
Six to eight months later, all of the children who previously underwent ultrasound and praziquantel treatment were given a second ultrasound examination. A new urine sample was collected; a urine dipstick test was simultaneous performed, and a microscopic analysis followed later the same day. If hematuria, proteinuria, or Schistosoma eggs were detected from the urine strip and microscopic examinations, and/or any alteration consistent with schistosomiasis was found in the ultrasound, a new treatment regimen of praziquantel 40 mg/kg was administered. All patients were also asked whether they had bathed in the river during that time.
Participation in the study was voluntary and had prior parental consent. We excluded children whose parents or legal guardians objected to their participation.
Ultrasound examination.
Ultrasonographic assessments were performed at the Hospital Nossa Senhora da Paz with a portable ultrasonography device (myLab 25; ESAOTE, Genova, Italy). All examinations were performed by the same clinician who performed the first round of ultrasounds. A second clinician supervised and compared the results.
Pathological changes caused by S. haematobium were assessed following the same criteria as in the first round of ultrasounds. The shape of the urinary bladder was recorded, lesions detected on the bladder wall were defined, and the degree of dilatation of the ureters and renal pelvis was measured, following current WHO guidelines.18 The exact categorization of the pathologic changes was calculated using the global score as an index of the severity of the morbidity and lesions. Pregnancy was recorded if present. Children were asked to drink water abundantly before the ultrasound examination, which took place only when the bladder was full. In cases where any dilatation of the renal pelvis was detected, the ultrasound was repeated after urination to rule out the possibility of dilatation due to bladder and ureteral repletion.
Urinary tract abnormalities were assessed using the WHO score. For successive analysis, children with at least one point on the WHO score were classified as having “urinary tract abnormalities” and those with at least one point for the upper urinary tract as having “upper urinary tract abnormalities.”
Statistical analysis.
Continuous variables were expressed by the mean and standard deviation, and quantitative variables by the median and p25–p75 quartiles. Normal distribution was tested using the Shapiro–Wilk test. Differences in normally distributed variables were evaluated using the t test for independent variables and analysis of variance. The MacNemar test was used to measure the percentage differences in the paired groups. The data collection and calculations were performed using SPSS v 18 (IBM Corp., Armonk, NY).
Ethical aspects.
The project was approved by the Vall d’Hebron Research Institute Ethics Committee and the regional health and education institutions. Before commencement, we conducted informative talks with the school principals, teachers, parents, and community leaders, and informed consent was obtained from all parents or legal guardians.
RESULTS
Seventy of the 157 children who performed the initial ultrasound (44.8%) came to the control between 6 and 8 months later.” Among them, 29 (41.4%) were girls, the mean age was 10.4 (SD 2.3) years, and 22 (31.4%) children and their parents or legal guardians stated that they were still bathing in the river.
Table 1 shows the presence of macrohematuria, microhematuria, proteinuria, and S. haematobium eggs at the initial ultrasounds. Those children who did not undergo any ultrasound were significantly younger than those who underwent all of the follow-up examinations; microscopic hematuria was significantly lower in the group who completed all the follow-ups than in the other two groups. We found no other differences among groups, as shown in Table 1.
Table 1.
Basal demographic and clinical data of children who completed or not all the study follow-up
| Cases | Attended follow-up examination (N = 70) n (%) | Performed first ultrasound did not attend follow-up examination (N = 87) n (%) | Statistical significance test | Did not perform ultrasound (N = 638) n (%) | Statistical significance test |
|---|---|---|---|---|---|
| Mean age | 10.4 | 10.6 | P = 0.834 | 9 | P < 0.01 |
| Female | 29 (41.4%) | 42 (48.3%) | – | 323 | – |
| Macroscopic hematuria | 10 (14.3%) | 19 (21.9%) | P = 0.603 | 184 (28.8%) | – |
| Microscopic hematuria | 62 (88.5%) | 83 (95.4%) | P = 0.011 | 608 (95.3%) | P = 0.430 |
| Proteinuria | 43 (61.4%) | 54 (62.1%) | P = 0.848 | 490 (76.8%) | P = 0.015 |
| Mean ultrasound WHO classification | 5.5 | 5.3 | P = 0.603T | – | P = 0.601 |
| Lower urinary tract | 3.3 | 2.9 | P = 0.815 | – | – |
| Upper urinary tract | 2.2 | 2.4 | P = 0.614 | – | – |
Bold values indicate significative statistical test (< 0.05).
Table 2 shows the differences in clinical parameters before and after praziquantel treatment, including macroscopic and microscopic hematuria, proteinuria, the presence of eggs of S. haematobium in urine, and the median ultrasound score. The difference in improvement between children 10 years old and younger and those older than 10 was not statistically significant for any of the evaluated parameters: reduction in macroscopic hematuria, P = 0.11; in microscopic hematuria, P = 0.15; in proteinuria, P = 0.08; in S. haematobium eggs, P = 0.27; and the median ultrasound score, P = 0.14.
Table 2.
Clinical parameters before and after treatment with praziquantel (dose 40 mg/kg)
| Before praziquantel n (%) | After praziquantel n (%) | Odds ratio (CI 95%) | P value | |
|---|---|---|---|---|
| Macroscopic hematuria | 10 (14.2%) | 3 (4.3%) | 3.7 (0.9–21.8) | 0.04 |
| ≤ 10y (N = 39) | 5/39 (12.8%) | 3/39 (7.7%) | 1.76 (0.3–12.1) | 0.46 |
| > 10y (N = 31) | 5/31 (16.1%) | 0 | – | – |
| Microscopic hematuria | 62 (88.5%) | 22 (31.4%) | 16.9 (6.5–46.9) | < 0.01 |
| ≤ 10y (N = 39) | 35/39 (89.7%) | 15/39 (38.4%) | 14 (3.7–62.7) | < 0.01 |
| > 10y (N = 31) | 27/31 (87.1%) | 7/31 (22.6%) | 23.1 (5.2–115.5) | < 0.01 |
| + | 15 (21.4%) | 4 (5.7%) | 4.5 (1.3–19.5) | < 0.01 |
| ++ | 10 (14.3%) | 5 (7.1%) | 2.2 (0.6–8.5) | 0.17 |
| +++ | 37 (52.8%) | 13 (18.6%) | 4.9 (2.2–11.5) | < 0.01 |
| Proteinuria | 43 (61.4%) | 21 (30%) | 3.7 (1.7–7.9) | < 0.01 |
| ≤ 10y (N = 39) | 21/39 (53.8%) | 15/39 (38.4%) | 1.9 (0.7–5.1) | 0.17 |
| > 10y (N = 31) | 22/31 (70.9%) | 6/31 (19.3%) | 10.2 (2.8–39.9) | < 0.01 |
| + | 22 (31.4%) | 11 (15.7%) | 2.5 (1.0–6.2) | 0.03 |
| ++ | 9 (12.8%) | 7 (10%) | 1.3 (0.4–4.5) | 0.59 |
| +++ | 12 (17.1%) | 3 (4.3%) | 4.6 (1.2–26.5) | 0.01 |
| Schistosoma haematobium eggs | 70 (100%) | 18 (25.7%) | 199.3 (25.8–541.6) | < 0.01 |
| ≤ 10y (N = 39) | 39/39 (100%) | 12/39 (30.7%) | 85.5 (10.5–697.4) | < 0.01 |
| > 10y (N = 31) | 31/31 (100%) | 6/31 (19.3%) | 125.0 (14.1–108.6) | < 0.01 |
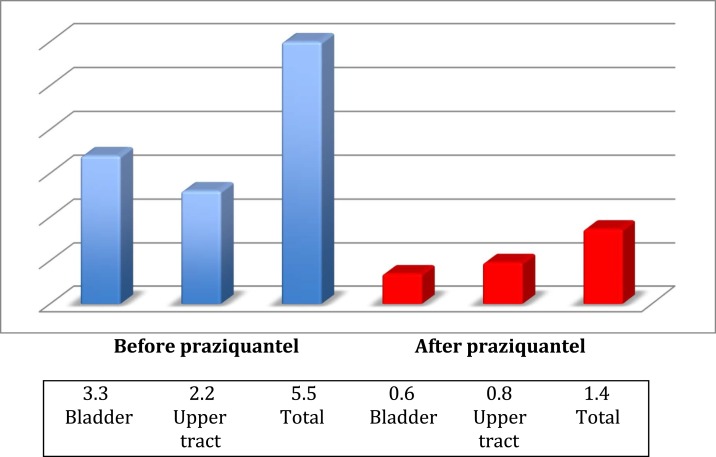
| Median ultrasound score | 5,50 | 1.40 | – | < 0.01 |
| ≤ 10y (N = 39) | 5,33 (IQR 2–8) | 1,72 (IQR 0–2) | < 0.01 | |
| > 10y (N = 31) | 5,71 (IQR 2–9) | 1,00 (IQR 0–1) | < 0.01 | |
| Bladder | 3,34 (IQR 2–5) | 0,56 (IQR 0–1) | < 0.01 | |
| Upper urinary tract | 2,16 (IQR 0–4) | 0,79 (IQR 0–0) | < 0.01 |
CI = confidence interval; IQR = interquartilic range.
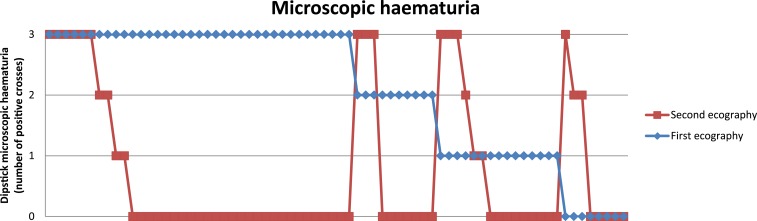
The differences between children older than 10 years or 10 years old and younger are also shown. Individual changes in relation to microhaematuria are shown in Figure 2 and the median ultrasound score before and after praziquantel is also shown graphically in Figure 3.
Figure 2.
Microscopic hematuria evolution before and after praziquantel. This figure appears in color at www.ajtmh.org.
Figure 3.
Median ultrasound before and after praziquantel according to WHO score. This figure appears in color at www.ajtmh.org.
Children who stated to keep bathing in the river had worse overall ultrasound mean scores after treatment than children who stated compliance with counseling of non-bathing; the same trend was also observed in clinical parameters, although they had not statistical significance. These results are shown in Table 3.
Table 3.
Relationship between clinical and ultrasound evolution and self-referred advice compliance (not bathing in the river)
| Pretreatment | Posttreatment | P value | |
|---|---|---|---|
| Macroscopic hematuria | 0.7 | ||
| Noncompliant children (N = 22) | 4 (18.2%) | 2 (9.2%) | |
| Compliant children (N = 48) | 7 (14.6%) | 1 (2.1%) | |
| Microscopic hematuria | 0.4 | ||
| Noncompliant children (N = 22) | 19 (86.4%) | 3 (13.6%) | |
| Compliant children (N = 48) | 43 (88.6%) | 5 (10.4%) | |
| Proteinuria | 0.08 | ||
| Noncompliant children (N = 22) | 12 (54.6%) | 14 (63.3%) | |
| Compliant children (N = 48) | 31 (64.6%) | 8 (16.7%) | |
| Median ultrasound score | 0.15 | ||
| Non-compliant children (N = 22) | 7 (IQR 2–8) | 2.64 (IQR 1–3) | |
| Compliant children (N = 48) | 4.8 (IQR 2–7) | 0.83 (IQR 0–2) |
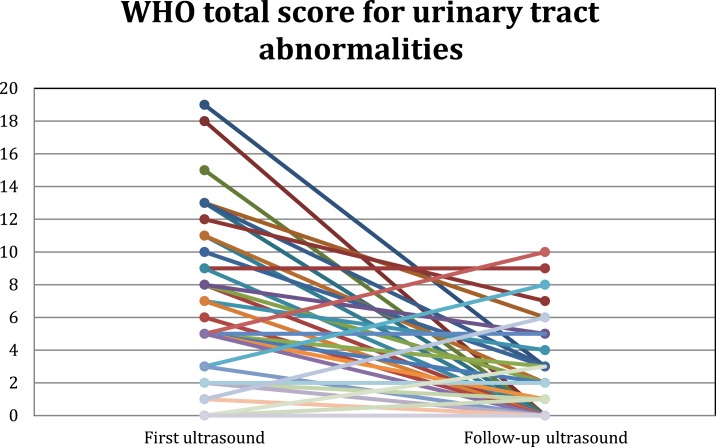
Of the 70 participating children, 53 (75.7%) showed improved bladder and/or kidney scores, whereas 12 (17.1%) had no change, and 5 (7.1%) showed disease progression. These data are displayed in Figure 4. Of the 25 children with moderate to severe upper tract lesions, 14 (56%) showed complete resolution of their abnormalities, as did 37/61 (60.6%) of those with bladder abnormalities.
Figure 4.
Follow-up ultrasound evolution in the 70 participating children. This figure appears in color at www.ajtmh.org.
Among those older than 10 years, 22/31 (70.9%) experienced an improvement in their lesions, compared with 30/39 (76.9%) in the younger group, whereas seven in the older group and two in the younger group showed no lesions in either of the two ultrasounds. Given that only children with lesions could experience improvement, this amounted to 22/24 (91.7%) in the older group and 30/37 (81.1%) in the younger group.
DISCUSSION
The present study shows data for an area in Angola, where the only schistosomiasis species present is S. haematobium, endemicity is high, and no public health measures such as MDA have yet been undertaken.
Ultrasound examination allows assessment of the actual pathology of the urinary tract in S. haematobium infection, providing a more accurate evaluation of internal damage than by parasitological determination of eggs or urine analysis. Furthermore, it is an effective method for evaluating the evolution of the damage, as it is safe to allow repeat examination of the same individual. Although it is widely accepted that this should be a standard tool in the management of schistosomiasis, there is still limited use of this in wide areas of sub-Saharan Africa where the disease is endemic. A recent review of morbidity reduction associated with urinary schistosomiasis found just 15 studies that used ultrasound to detect urinary tract improvement after treatment; among them, nine analyzed both abnormalities in the bladder and in the upper urinary tract, six analyzed only the urinary bladder13; just one was performed in central Africa,15 and none were carried out in southern Africa. Moreover, the last of these studies was performed in 2008, and none included long-term assessment.
After a single standard 40 mg/kg dose of praziquantel, those with renal and bladder ultrasound findings showed a regression of these abnormalities within a 6–8 month period. Although improvement was greater in the urinary bladder than in the upper urinary tract, even some of the most severe lesions were reversed, such as ureter dilatation and hydronephrosis. Recovery was even found in children who still had hematuria, proteinuria, or S. haematobium eggs in their urine. This is an important finding showing the benefits of treatment in areas with very high endemicity.
The reversal of all parameters was greater in the older group than in those aged 10 or less. This finding is surprising because age is usually considered an important proxy for cumulative parasite exposure and subsequent tissue damage, and lesions are more difficult to reverse as infection progresses to a more chronic and fibrotic stage.13,19 One explanation may be that the older children were more receptive to health education advice, and therefore became reinfected less frequently; another reason may be that younger children in this area, compared with older ones, are usually more affected by other comorbidities such as malnutrition and anemia.20 As praziquantel requires a competent immune system to take effect,21 it may be less effective in these children. In addition, as each praziquantel tablet provides 600 mg, it can be difficult to adjust the dose for children weighing less than 15 kg. Further studies are needed to verify these hypotheses, as big gaps in knowledge remain.
All laboratory data improved after treatment, including macroscopic and microscopic hematuria, proteinuria, and the presence of S. haematobium eggs in urine, although proteinuria showed the smallest reduction, and none disappeared completely. The presence of eggs in urine some months after treatment is common in children in endemic areas.22–24 One reason may be reinfection25 but other possibilities are the presence of immature forms at the time of treatment,26 and the ineffectiveness of 40 mg/kg single-dose praziquantel to eliminate the full parasite burden in children with other comorbidities or high parasite levels before treatment.27
Hematuria and proteinuria parameters are surrogates for pathology, which respond to the inflammatory process unleashed by the presence of S. haematobium eggs. As proteinuria has been noted as a marker of upper tract pathology,28,29 it seems logical that this parameter showed the smaller reduction. We suggest that children with high proteinuria levels should be more closely monitored during follow-up.
After the initial work of the research team in the area, emphasis was placed on educational activities such as informational activities in schools. All children who participated in the study along with their teachers, parents, and guardians received information about the transmission of the disease and how to avoid it. Nevertheless, 31.4% of these children or their companions acknowledged having bathed in the river after the first ultrasound. Despite that it is likely that many others denied bathing to gain positive recognition, we found differences in pathology between children who recognized to continue bathing in the river compared with those who stated that they no longer bathed, although these differences were nonsignificant statistically. This indicates that educational measures must be continuous and accompanied by sanitation and hygiene measures to be effective.
The children who dropped out during the study were slightly younger and had more microscopic hematuria than those who completed the entire follow-up; no other significant differences were found between the two groups. Therefore, we consider that although the number of children who did not complete the study was high, this did not bias the results and would in any case represent a reduction in the significance of the results. The reasons why so many children failed to complete the follow-up may be related to the low awareness of disease in relation to schistosomiasis in the area, as no educational campaigns had been yet conducted in relation to its importance; the high frequency of hematuria in children makes it to be generally considered a pathology of minor importance. Another reason may be that the children had to go to the hospital accompanied by a responsible adult to perform the ultrasound. In some cases, the schools were distant and this displacement could be difficult for some families.
The follow-up time was short compared with other studies,14 yet even in such a short time the upper urinary tract lesions could be reversed, possibly because at this early age most lesions are due to congestive uropathy and very few to the more chronic, precancerous lesions that are more difficult to reverse. Although we do not know if the pathology detected would end up degenerating in precancerous or cancerous lesions, as there are enormous gaps in the knowledge on this pathway,30 they represent an inflammatory response, with consequences by itself. Nevertheless, 25% of the children experienced no change (10% having no lesions in either the first or second ultrasound); and five children got worse. This finding may be due to longer-standing disease that continued to evolve despite treatment, bacterial superinfections that hastened progression, or other immunity-related factors in these patients. It may be also because of the fact that a single dose of praziquantel may not be enough to cure completely the infection in very high intensity infections.31 In our study, egg burden quantification could not be performed in the initial epidemiological report because of technical and economical constraints. Another reason may be reinfection, although it is difficult to prove that new infections would lead to these lesions in such a short period. Further studies are needed to assess these possibilities.
CONCLUSIONS
The severity of morbidity was very high in this group of children, and our findings highlight the importance of treatment at a young age, even in the presence of high reinfection levels, as treatment can dramatically change morbidity in school-age children. In this area, mass treatment should be initiated as soon as possible.
Ultrasound should be a standard tool for the diagnosis and follow-up of urinary schistosomiasis, particularly in children, and more accurate recommendations for follow-up should be established in cases where children’s lesions do not reverse.
REFERENCES
- 1.Chitsulo L, Engels D, Montresor A, Savioli L, 2000. The global status of schistosomiasis and its control. Acta Trop 77: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King CH, Dickman K, Tisch D, 2005. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability related outcomes in endemic schistosomiasis. Lancet 365: 1561–1569. [DOI] [PubMed] [Google Scholar]
- 3.Gryseels B, Polman K, Clerinx J, Kestens L, 2006. Human schistosomiasis. Lancet 368: 1106–1118. [DOI] [PubMed] [Google Scholar]
- 4.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J, 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6: 411–425. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, et al. 2013. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization , 1985. The control of schistosomiasis: report of a WHO Expert Committee. World Health Organ Tech Rep Ser 728: 1–113. [PubMed] [Google Scholar]
- 7.WHO , 2002. Prevention and Control of schistosomiasis and Soil-Transmitted helminthiasis: Report of a WHO Expert Committee. Report No.: 912. Technical Report Series. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 8.WHO , 2006. Preventive Chemotherapy in Human helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 9.Hatz CF, 2001. The use of ultrasound in schistosomiasis. Adv Parasitol 48: 225–284. [DOI] [PubMed] [Google Scholar]
- 10.Akpata R, Neumayr A, Holtfreter MC, Krantz I, Singh DD, Mota R, Walter S, Hatz C, Richter J, 2015. The WHO ultrasonography protocol for assessing morbidity due to Schistosoma haematobium. Acceptance and evolution over 14 years. Systematic review. Parasitol Res 114: 1279–1289. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer KC, Ndhlovu PD, Wagatsuma Y, Munatsi A, Shiff CJ, 2003. Epidemiological assessment of Schistosoma haematobium-induced kidney and bladder pathology in rural Zimbabwe. Acta Trop 85: 339–347. [DOI] [PubMed] [Google Scholar]
- 12.Kahama AI, Vennervald BJ, Kombe Y, Kihara RW, Ndzovu M, Mungai P, Ouma JH, 1999. Parameters associated with Schistosoma haematobium infection before and after chemotherapy in school children from two villages in the coast province of Kenya. Trop Med Int Health 4: 335–340. [DOI] [PubMed] [Google Scholar]
- 13.Andrade G, Bertsch DJ, Gazzinelli A, King CH, 2017. Decline in infection-related morbidities following drug-mediated reductions in the intensity of Schistosoma infection: a systematic review and meta-analysis. PLoS Negl Trop Dis 11: e0005372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y, et al. 2017. Protocol and baseline data for a multi-year cohort study of the effects of different mass drug treatment approaches on functional morbidities from schistosomiasis in four African countries. BMC Infect Dis 17: 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doehring E, Ehrich JH, Bremer HJ, 1986. Reversibility of urinary tract abnormalities due to Schistosoma haematobium infection. Kidney Int 30: 582–585. [DOI] [PubMed] [Google Scholar]
- 16.Bocanegra C, et al. 2015. Epidemiology of schistosomiasis and usefulness of indirect diagnostic tests in school-age children in Cubal, central Angola. PLoS Negl Trop Dis 9: e0004055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bocanegra García C, et al. 2018. Ultrasound findings and associated factors to morbidity in Schistosoma haematobium infection in a highly endemic setting. Trop Med Int Health 23: 221–228. [DOI] [PubMed] [Google Scholar]
- 18.Richter J, Hatz C, Campagne G, Bergquist N, Jenkins J, 2000. Ultrasound in schistosomiasis: A Practical Guide to the Standardized Use of Ultrasonography for the Assessment of schistosomiasis-Related Morbidity. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 19.Magak P, Chang-Cojulun A, Kadzo H, Ireri E, Muchiri E, Kitron U, King CH, 2015. Case-control study of posttreatment regression of urinary tract morbidity among adults in Schistosoma haematobium-endemic communities in Kwale County, Kenya. Am J Trop Med Hyg 93: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeAlegria MLAR, et al. 2017. Prevalence of Strongyloides stercoralis and other intestinal parasite infections in school children in a rural area of Angola: a cross-sectional study. Am J Trop Med Hyg 97: 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vale N, Gouveia MJ, Rinaldi G, Brindley PJ, Gärtner F, Correia da Costa JM, 2017. Praziquantel for schistosomiasis: single-drug metabolism revisited, mode of action, and resistance. Antimicrob Agents Chemother 61: pii:e02582–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senghor B, Diaw OT, Doucoure S, Seye M, Diallo A, Talla I, Bâ CT, Sokhna C, 2016. Impact of annual praziquantel treatment on urogenital schistosomiasis in a seasonal transmission focus in central Senegal. PLoS Negl Trop Dis 10: e0004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwang J, Olliaro PL, 2014. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis-a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl Trop Dis 8: e3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nalugwa A, Nuwaha F, Tukahebwa EM, Olsen A, 2015. Single versus double dose praziquantel comparison on efficacy and Schistosoma mansoni re-infection in preschool-age children in Uganda: a randomized controlled trial. PLoS Negl Trop Dis 9: e0003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garba A, et al. 2013. Efficacy and safety of two closely spaced doses of praziquantel against Schistosoma haematobium and S. mansoni and re-infection patterns in school-aged children in Niger. Acta Trop 128: 334–344. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed AM, Abbas H, Mansour FA, Gasim GI, Adam I, 2012. Schistosoma haematobium infections among schoolchildren in central Sudan one year after treatment with praziquantel. Parasit Vectors 5: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olliaro PL, et al. 2011. A multicentre randomized controlled trial of the efficacy and safety of single-dose praziquantel at 40 mg/kg vs. 60 mg/kg for treating intestinal schistosomiasis in the Philippines, Mauritania, Tanzania and Brazil. PLoS Negl Trop Dis 5: e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell Stothard J, Sousa-Figueiredo JC, Simba Khamis I, Garba A, Rollinson D, 2009. Urinary schistosomiasis-associated morbidity in school children detected with urine albumin to creatinin ratio (UACR) reagent strips. J Pediatr Urol 5: 287–291. [DOI] [PubMed] [Google Scholar]
- 29.Murare HM, Taylor P, 1987. Haematuria and proteinuria during Schistosoma haematobium infection: relationship to intensity of infection and the value of chemical reagent strips for pre and post-treatment diagnosis. Trans R Soc Trop Med Hyg 81: 426–430. [DOI] [PubMed] [Google Scholar]
- 30.Honeycutt J, Hammam O, Fu CL, Hsieh MH, 2014. Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends Parasitol 30: 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danso-Appiah A, Utzinger J, Liu J, Olliaro P, 2008. Drugs for treating urinary schistosomiasis. Cochrane Database Syst Rev 200: CD000053. [DOI] [PubMed] [Google Scholar]