Abstract.
Mosquito and virus surveillance systems are widely used in Western Australia (WA) to support public health efforts to reduce mosquito-borne disease. However, these programs are costly to maintain on a long-term basis. Therefore, we aimed to assess the validity of mosquito numbers and Ross River virus (RRV) isolates from surveillance trap sites as predictors of human RRV cases in south-west WA between 2003 and 2014. Using negative binomial regression modeling, mosquito surveillance was found to be a useful tool for predicting human RRV cases. In eight of the nine traps, when adjusted for season, there was an increased risk of RRV cases associated with elevated mosquito numbers detected 1 month before the onset of human cases for at least one quartile compared with the reference group. The most predictive urban trap sites were located near saltmarsh mosquito habitat, bushland that could sustain macropods and densely populated residential suburbs. This convergence of environments could allow enzootic transmission of RRV to spillover and infect the human population. Close proximity of urban trap sites to each other suggested these sites could be reduced. Ross River virus isolates were infrequent at some trap sites, so ceasing RRV isolation from mosquitoes at these sites or where isolates were not predictive of human cases could be considered. In future, trap sites could be reduced for routine surveillance, allowing other environments to be monitored to broaden the understanding of RRV ecology in the region. A more cost-effective and efficient surveillance program may result from these modifications.
INTRODUCTION
Ross River virus (RRV) disease is the most common mosquito-borne disease of humans in Australia, with an average of 4,800 cases reported each year.1 The disease is nonfatal and characterized by a fever, rash, and often long-term joint pain, which typically resolves over 3–6 months.2 Symptomatic infection is most common in people aged between 20 and 60 years old, whereas illness is uncommon in children.3
Ross River virus is predominantly maintained in nature by mosquito-macropod-mosquito cycles. The primary mosquito vectors in coastal regions are the saltmarsh mosquitoes Aedes camptorhynchus and Aedes vigilax, in inland regions the freshwater species Culex annulirostris, and in urban areas the container breeding Aedes notoscriptus.4 The natural reservoir hosts are nonmigratory native Australian macropods, such as kangaroos and wallabies.5,6 Spillover to humans and epidemic activity has been linked to reservoir host population dynamics and changing herd immunity.7
The risk of human infection is also a function of spatial and temporal abundance of vector mosquito species, the season, environmental conditions, and human activities.8 Weather conditions influence mosquito survival, reproduction, abundance, and distribution and also virus replication.9 Residential developments in close proximity to wetlands and saltmarsh habitats have been associated with increased RRV transmission to humans,10,11 while outdoor activities, especially camping have also been identified as major risks.12,13
Reducing the risk of human infection with RRV relies on avoidance of biting mosquitoes. Thus, public health interventions include educating the public on behavioral practices,14 such as avoiding high-risk areas and peak mosquito biting times, minimizing opportunities for breeding of domestic mosquito species, wearing light-colored protective clothing, and using personal insect repellent containing N,N-diethyl-3-methylbenzamide.5,13,15 Physical barriers, such as using bed nets and window and door screens, are also recommended.13,16 In Western Australia (WA), mosquito and virus surveillance are key strategies to detect increased mosquito and RRV activity to inform timely and specific public health warnings.17 For this reason, the WA Department of Health resources a mosquito and virus surveillance program in recognized areas of RRV activity in populated areas of the south-west of the state. There are costs associated with long-term maintenance of the program, so we aimed to assess the validity of using mosquito numbers and RRV isolates as predictors of human cases in south-west WA, and to compare the predictive capacity of different surveillance trap sites.
MATERIALS AND METHODS
Study design and setting.
This was a retrospective study of the association between adult mosquito species abundance data, RRV detections in mosquitoes and surrounding human RRV cases for nine mosquito surveillance trap sites in the Peel region, south-west WA over a 12-year period from January 2003 to December 2014 (144 months).
Study region.
The Peel region (defined here as the Local Government Areas of Rockingham, Mandurah, Murray, and Waroona) is situated on the south-west coast of WA, 70–130 km south of the state capital Perth. The region had a combined population of 225,000 in 2014.18 A substantial number of cases of RRV disease are reported annually from the Peel region, and large outbreaks are experienced every few years despite significant mosquito control efforts. Several hundred hectares of saltmarsh and seasonal tidal wetlands are situated along the margins of the Peel Inlet and Harvey Estuary and lower reaches of three rivers (Harvey, Murray, and Serpentine) that flow into these tidal waterways. Mosquito surveillance over more than 30 years in the region has shown that two recognized RRV vector species, Ae. camptorhynchus and Ae. vigilax are the predominant species between April and December, and between January and March, respectively.19 Furthermore, the region also has substantial areas of native bushland in close proximity to mosquito breeding habitat that supports populations of western grey kangaroos (Macropus fuliginosus), an important vertebrate host of RRV in southern WA.6
Human cases.
Ross River virus is a notifiable disease in WA. All WA RRV cases meeting the national case definition,20 including confirmation by laboratory testing are required to be notified to the WA Department of Health and are recorded in the Western Australian Notifiable Infectious Diseases Database (WANIDD). Western Australia also undertakes an “enhanced surveillance” program in which RRV cases are followed up via patient interviews to determine the most likely location and timing of exposure. Enhanced surveillance data were available for approximately 40% of cases. Where enhanced surveillance data were not available, residential address was assumed to be the location of exposure. The dataset created for spatial analyses consisted of all cases for which the most likely place of exposure could be precisely geocoded to a specific cadastral lot (a legally defined property boundary). In addition, if place of exposure data or residential data were not given as an exact location but could be pinpointed with reasonable confidence (e.g., a street corner within 250 m) then these cases were also geocoded. All other cases were excluded from the dataset for spatial analyses.
For this study, de-identified RRV notification data from 2003 to 2014 were downloaded from WANIDD. Ross River virus cases were allocated to trap sites if their location of exposure was within a 3 km, 10 km, or 20 km radius of the trap site. Ross River virus cases were extracted using QGIS 2.6.1 (QGIS Development Team).
Ethics approval was not required as this study evaluated data collected during the routine public health response to RRV as a notifiable disease.
Trap sites and mosquito isolates.
Adult mosquito data from all nine Peel region surveillance sites were collated. Trap sites included locations that were representative of different aspects of the RRV transmission cycle, including areas with high levels of mosquito breeding, human habitation, and native bushland that may harbor susceptible reservoir vertebrate hosts (M. Lindsay, unpublished doctoral thesis). Encephalitis virus surveillance light traps baited with carbon dioxide (EVS/CO2) traps were set midafternoon, run overnight and collected early the following morning. Further details on trap deployment and sampling collection have been described elsewhere.17,21 Trapping was carried out fortnightly between August and April (late winter to mid-autumn) and monthly from May to July each year. Data collected for each trap site included location ID, date, global positioning system coordinates; trap outcome (success, failure with sample, failure, stolen, or not set), mosquito species name, and count by species. Mosquitoes were identified to species using appropriate keys.22 Once identified, all mosquitoes were separated into pools of ≤ 20 for virus isolation according to collection site and date, species, and sex.17 If more than 500 mosquitoes were obtained in a single trap the first 350–500 specimens were processed and the remainder estimated by extrapolation by weight. Virus isolation was undertaken as previously described.23 For the modeling, total mosquito abundance and individual mosquito species were divided each month by the number of successful trap runs (trap successes and trap failures with sample) allowing for comparison of mosquito abundance between trap sites and over time.
Inclusion criteria.
Mosquito species known to feed on humans and confirmed as competent RRV vectors were included in the study and contributed to the mosquito counts for each trap site (primarily Ae. camptorhynchus and Ae. vigilax, but also nine other minor mosquito species Aedes alboannulatus, Aedes clelandi, Ae. notoscriptus, Aedes hesperonotius, Anopheles annulipes sensu lato, Cx. annulirostris, Culex australicus, Culex globocoxitus, and Culex quinquefasciatus).
Exclusion criteria.
Human RRV cases from June 1 to August 31 (winter months) were not included in the models because of the low positive predictive value of the RRV immunoglobulin M test that was used for most RRV notifications in the low-risk season in the south-west of WA, including the Peel region.24
Statistical analysis.
Negative binomial regression analysis was used as the human RRV case data were over-dispersed. The outcome variable for the analysis was the count of human RRV cases in each month. Independent variables included in the initial models were as follows: mosquito abundance (categorized into four equal groups, first quartile [reference group], second quartile, third quartile, and fourth quartile), presence of RRV isolate/s (Yes and No [reference group]), and season (spring, summer, and autumn [reference group]). Models were developed separately for each trap using a backward stepwise elimination. All independent variables were initially included, and then the least significant variable was removed one at a time, until remaining variables in the models were significantly associated with the outcome (P value < 0.05). Lags of 1 or 2 months were applied separately to both mosquito abundance and RRV isolate variables and retained where significant. If multiple lags were significant the smallest Bayesian information criterion number was used to select the best model. All data analysis was performed using SPSS v 23 (IBM, New York, NY).
RESULTS
Human RRV cases.
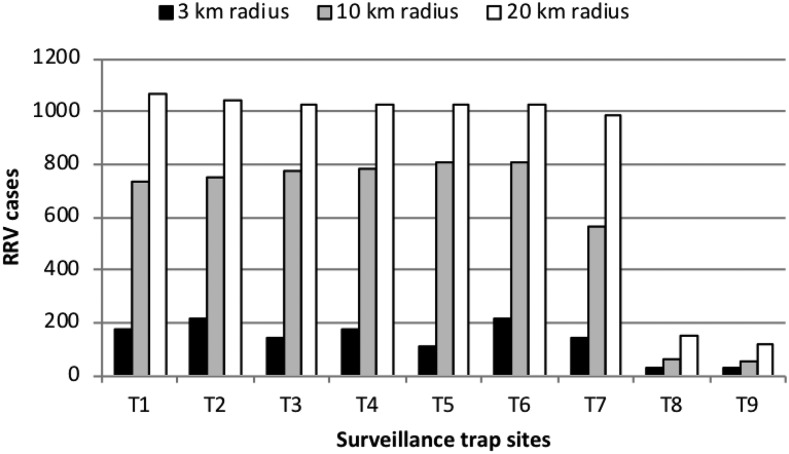
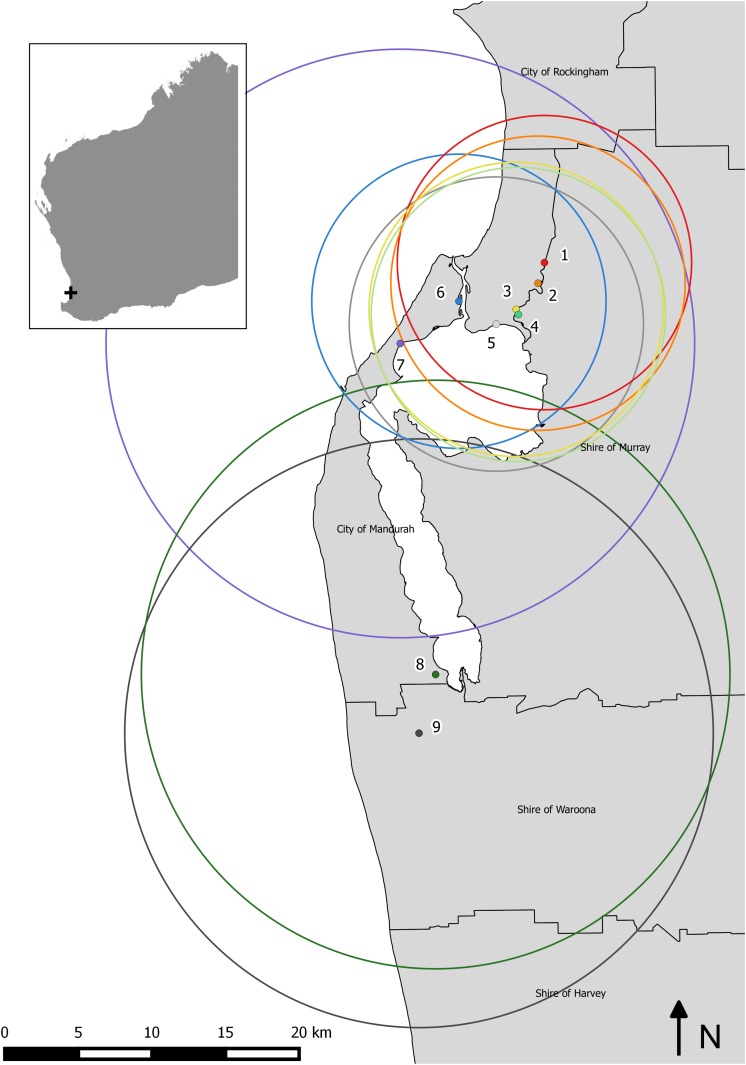
Assignment of RRV cases to traps was explored using three different radius sizes 3 km, 10 km, and 20 km. Increasing the radius out from each surveillance trap site increased the number of RRV cases available for inclusion in the regression models. Seven sites were located in a built-up urban environment (trap sites 1–7); however a total range of only 113–212 RRV cases was included across these sites over the 12-year time period using the smallest radii (3 km) (Figure 1). This small sample size was not considered large enough for modelling, as it would represent on average between 10 and 20 cases per year. Therefore, the results for the 3 km radii are not provided. Instead, 10 km radii were allocated to trap sites 1–6 as this captured a larger number of human RRV cases around each trap site location. Trap sites 8 and 9 were located within rural farmland, with only a small number of cases occurring in the vicinity of both sites. To reach a suitable sample size, human cases within 20 km radii of trap sites 7–9 were included for the analysis (Figure 2).
Figure 1.
Total number of Ross River virus (RRV) notifications assigned to each surveillance trap site using various radii (3 km, 10 km, and 20 km) from January 2003 to December 2014.
Figure 2.
Location of the nine surveillance trap sites in the Peel region, south-west Western Australia and the 10 km and 20 km radii used to capture sufficient Ross River virus cases for regression modeling. This figure appears in color at www.ajtmh.org.
The total number of RRV cases assigned to each trap over the 12-year study period ranged between 119 (trap site 9) and 984 (trap site 7) and the median monthly RRV cases ranged from zero (trap sites 8 and 9) to 5.5 (trap site 7) (Table 1). The maximum numbers of monthly human RRV cases assigned with an accurate exposure location ranged from 18 to 71.
Table 1.
Summary statistics of monthly human RRV cases assigned to trap sites in the Peel region, south-west Western Australia (excluding winter months)
| Trap site | T1 10 km | T2 10 km | T3 10 km | T4 10 km | T5 10 km | T6 10 km | T7 20 km | T8 20 km | T9 20 km |
|---|---|---|---|---|---|---|---|---|---|
| Min | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q1* | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 |
| Median | 4.5 | 4.5 | 4.5 | 4.5 | 5 | 4.5 | 5.5 | 0 | 0 |
| Q3† | 10 | 10 | 10 | 10.75 | 10.75 | 10.75 | 13 | 2 | 1 |
| Max | 43 | 47 | 50 | 50 | 51 | 53 | 71 | 19 | 18 |
| Total‡ | 738 | 752 | 774 | 780 | 810 | 807 | 984 | 147 | 119 |
RRV = Ross River virus.
First quartile.
Third quartile.
Total RRV cases over the study period (January 2003 to December 2014).
Mosquito identification and abundance.
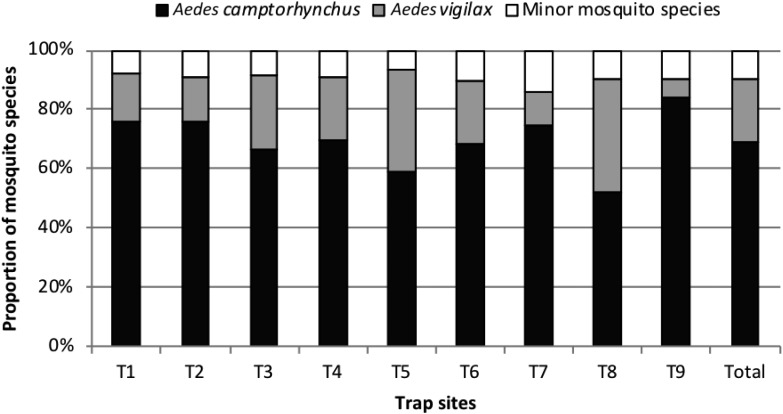
The most dominant mosquito species across the nine surveillance sites was the southern saltmarsh mosquito Ae. camptorhynchus, accounting for 69%, of all mosquitoes collected. This was followed by the saltmarsh mosquito Ae. vigilax (21%) (Figure 3). The remaining 10% of mosquitoes collected included nine other species from which RRV has been isolated and which are known to bite humans (Ae. alboannulatus, Ae. clelandi, Ae. notoscriptus, Ae. hesperonotius, An. annulipes sensu lato, Cx. annulirostris, Cx. australicus, Cx. globocoxitus, and Cx. quinquefasciatus). The median number of mosquitoes collected by month and trap site ranged from 142.5 (trap site 5) to 427 (trap site 4) mosquitoes over the 12-year time period (Table 2).
Figure 3.
Proportion of major and minor mosquito species known to transmit Ross River virus and bite humans collected in surveillance trap sites in the Peel region, south-west Western Australia, January 2003 to December 2014.
Table 2.
Summary statistics of known human biting mosquitoes collected from monthly EVS/CO2 surveillance trap sites from 2003 to 2014 from the Peel region, south-west Western Australia
| Trap site | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 |
|---|---|---|---|---|---|---|---|---|---|
| Median | 216.00 | 340.50 | 263.00 | 427.00 | 142.50 | 314.00 | 213.50 | 310.50 | 211.00 |
| Q1* | 66.25 | 105.75 | 107.50 | 134.75 | 36.75 | 92.00 | 84.50 | 132.50 | 62.50 |
| Q3† | 707.75 | 961.75 | 716.00 | 1,245.75 | 401.75 | 696.00 | 880.75 | 727.00 | 611.75 |
| IQR‡ | 641.50 | 856.00 | 608.50 | 1,111.00 | 365.00 | 604.00 | 796.25 | 594.50 | 549.25 |
First quartile.
Third quartile.
Interquartile range.
Ross River virus isolates.
Isolation of RRV from mosquitoes analyzed for this study was uncommon, but closely preceded periods of elevated reporting of human cases for RRV infection. Trap sites 8 and 9 recorded the greatest number of months with at least one RRV isolate (N = 7 months of a total of 144 months) (Table 3). Both of these trap sites were located in rural farmland. Ross River virus isolates from trap sites 7 and 8 frequently occurred in the same or successive months, whereas isolates detected from other traps did not consistently align with neighboring trap isolates. No RRV isolates were detected from any pools of mosquitoes collected from trap site 6, which was located on the western margin of the main channel connecting the Peel Inlet with the Indian Ocean.
Table 3.
Frequency of months in which RRV isolate/s were detected in female adult mosquitoes by trap site (over the 12-year period)
| Trap site | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 |
|---|---|---|---|---|---|---|---|---|---|
| Months with RRV detection (n) | 2 | 2 | 5 | 4 | 2 | 0 | 6 | 7 | 7 |
| Percent of months with RRV (%) | 1.4 | 1.4 | 3.5 | 2.8 | 1.4 | 0.0 | 4.2 | 4.9 | 4.9 |
RRV = Ross River virus.
Negative binomial regression analysis.
In all trap site locations, except trap site 7, there was an increased risk of human cases of RRV associated with elevated mosquito numbers detected 1 month before the onset of human cases for at least one quartile compared with the first quartile in a model that adjusted for season. However, there was not a consistent increase in RRV cases with increasing quartile (Tables 4 and 5). Isolation of RRV in mosquitoes trapped 1 month before the onset of human cases was associated with an increased risk of human RRV cases in trap sites 3, 7, and 8 but not in the other traps. In general, mosquito counts greater than the second quartile were predictive of an increased risk of human RRV cases. Mosquito counts greater than the first quartile were predictive of an increased risk of human RRV cases at trap sites 6 and 9 only.
Table 4.
Negative binomial regression models for human RRV cases for trap sites 1–6 using a 10-km radius with 1-month lag for mosquito abundance and RRV isolate detection
| 10 km radius | Trap 1 | Trap 2 | Trap 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | IRR | 95% CI | P | IRR | 95% CI | P | IRR | 95% CI | ||||
| Mosquito Q4 | 0.021 | 2.557 | 1.154 | 5.666 | 0.038 | 2.058 | 1.04 | 4.072 | 0.020 | 2.086 | 1.121 | 3.78 |
| Mosquito Q3 | 0.243 | 1.501 | 0.759 | 2.972 | 0.004 | 2.501 | 1.346 | 4.649 | 0.060 | 1.919 | 0.974 | 3.78 |
| Mosquito Q2 | 0.753 | 1.105 | 0.593 | 2.058 | 0.284 | 1.392 | 0.76 | 2.553 | 0.375 | 1.325 | 0.712 | 2.467 |
| Mosquito Q1 | – | 1 | – | – | – | 1 | – | – | – | 1 | – | – |
| Isolate = Yes | NS | – | – | – | NS | – | – | – | 0.022 | 4.249 | 1.236 | 14.611 |
| Isolate = No | – | – | – | – | – | – | – | – | – | 1 | – | – |
| Spring | 0.537 | 0.794 | 0.381 | 1.653 | 0.917 | 1.033 | 0.554 | 1.927 | 0.633 | 0.862 | 0.469 | 1.584 |
| Summer | 0.001 | 2.451 | 1.435 | 4.185 | 0.000 | 2.535 | 1.514 | 4.246 | 0.000 | 2.564 | 1.515 | 4.34 |
| Autumn (ref) | – | 1 | – | – | – | 1 | – | – | – | 1 | – | – |
| Trap 4 | Trap 5 | Trap 6 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 km radius | P | IRR | 95% CI | P | IRR | 95% CI | P | IRR | 95% CI | |||
| Mosquito Q4 | 0.022 | 2.165 | 1.116 | 4.2 | 0.013 | 2.185 | 1.182 | 4.039 | 0.079 | 1.818 | 0.933 | 3.544 |
| Mosquito Q3 | 0.005 | 2.519 | 1.325 | 4.788 | 0.180 | 1.533 | 0.821 | 2.863 | 0.043 | 1.84 | 1.019 | 3.323 |
| Mosquito Q2 | 0.883 | 1.048 | 0.563 | 1.952 | 0.748 | 1.115 | 0.573 | 2.170 | 0.010 | 2.206 | 1.207 | 4.032 |
| Mosquito Q1 | – | 1 | – | – | – | 1 | – | – | – | 1 | – | – |
| Isolate = Yes | NS | – | – | – | NS | – | – | – | No iso | – | – | – |
| Isolate = No | – | – | – | – | – | – | – | – | – | – | – | – |
| Spring | 0.937 | 0.977 | 0.546 | 1.747 | 0.131 | 1.519 | 0.883 | 2.614 | 0.370 | 1.28 | 0.746 | 2.197 |
| Summer | 0.000 | 2.538 | 1.513 | 4.249 | 0.001 | 2.586 | 1.496 | 4.472 | 0.000 | 2.911 | 1.747 | 4.851 |
| Autumn (ref) | – | 1 | – | – | – | 1 | – | – | – | 1 | – | – |
IRR = incidence rate ratio; No iso = no isolate was recorded from trap site 6 between January 2003 and December 2014; NS = not significant, therefore not included in final model; Q = quartile; RRV = Ross River virus; CI = confidence interval.
Table 5.
Negative binomial regression models for human RRV cases for trap sites 7–9 using a 20-km radius with 1-month lag for mosquito abundance and RRV isolate detection
| 20 km radius | Trap 7 | Trap 8 | Trap 9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | IRR | 95% CI | P | IRR | 95% CI | P | IRR | 95% CI | ||||
| Mosquito Q4 | NS | – | – | – | 0.010 | 3.548 | 1.359 | 9.266 | 0.000 | 7.014 | 2.389 | 20.594 |
| Mosquito Q3 | NS | – | – | – | 0.001 | 4.861 | 1.967 | 12.01 | 0.000 | 6.717 | 2.307 | 19.555 |
| Mosquito Q2 | NS | – | – | – | 0.433 | 1.499 | 0.545 | 4.121 | 0.007 | 3.709 | 1.419 | 9.693 |
| Mosquito Q1 | – | 1 | – | – | – | 1 | – | – | – | 1 | – | – |
| Isolate = Yes | 0.004 | 3.534 | 1.505 | 8.296 | 0.001 | 4.657 | 1.81 | 11.982 | NS | – | – | – |
| Isolate = No | – | 1 | – | – | – | 1 | – | – | – | – | – | – |
| Spring | 0.331 | 1.286 | 0.774 | 2.135 | 4.657 | 1.637 | 0.731 | 3.668 | 0.985 | 0.991 | 0.397 | 2.473 |
| Summer | 0.000 | 2.529 | 1.531 | 4.178 | 0.002 | 3.405 | 1.58 | 7.337 | 0.000 | 5.240 | 2.250 | 12.202 |
| Autumn (ref) | – | 1 | – | – | – | 1 | – | – | – | 1 | – | – |
IRR = incidence rate ratio; NS = not significant, therefore not included in final model; Q = quartile; RRV = Ross River virus; CI = confidence interval.
DISCUSSION
Adult mosquito surveillance systems are widely used to support public health efforts to reduce mosquito-borne disease. Evaluation of mosquito surveillance typically assesses the performance of different trapping methods.25–33 For example, CO2 baited versus unbaited32 or light traps with CO2 versus Biogents™-Sentinel traps with artificial attractants.28 More broadly, mosquito and virus surveillance systems combine vector data with climatic variables (temperature, rainfall, and tide height) and where appropriate sentinel animal seroconversions to create better calibrated predictive models of human disease risk.34–39 In this study, we used a quantitative approach to assess individual mosquito trap sites in an attempt to improve and streamline surveillance. We found that mosquito abundance and RRV detection in mosquitoes were effective predictors of human RRV risk across the Peel region in south-west WA. From this, it would appear that mosquito surveillance is a useful tool for predicting RRV incidence. The identified lag of 1 month was biologically plausible in view of the incubation period of RRV disease, the time taken for the patient to seek medical services, subsequent pathology tests, and notification of confirmed cases to the Department of Health. The incubation period of RRV ranges from 3 to 21 days, with an average of 7–9 days.40 Time lag effects for mosquito-borne disease surveillance are commonly supported in the literature. In Zhejiang, China, increased risk of human mosquito-borne disease followed a rise of mosquito abundance by 0–2 months.41 Similarly, increases in mosquito density in Manaus in the Brazilian Amazon and San Juan, Puerto Rico, preceded maximum malaria cases and dengue incidence, respectively.42,43 Although RRV isolates were rare, with the exception of trap site 9, they were predictive of human RRV cases if the total number of months with isolates over the 12-year period was five or more. As RRV isolation adds additional complexity and expense to mosquito surveillance, consideration could be given to ceasing attempts to isolate RRV from mosquitoes pooled from trap sites with small numbers of isolates or where RRV isolates were not predictive of human cases.
The mosquito trap sites located in urban areas (trap sites 1–7) were all located on or near to extensive saltmarsh mosquito–breeding areas. Given that these traps are also in close proximity to each other (within a radius of less than 10 km) it is likely mosquito breeding at all sites would be driven by similar environmental conditions. Therefore, consideration could be given to reducing the number of trap sites used for routine surveillance in this area. Similarly, consideration could be given to only undertaking mosquito trapping at trap site 8 rather than both trap sites 8 and 9.
Trap sites 2 and 4, on the western banks of the tidally influenced reaches of the Serpentine River were the most predictive of human RRV cases using the third quartile mosquito abundance (Q3 incidence rate ratio [IRR] 2.501; 95% confidence interval [CI]: 1.346, 4.649 and IRR 2.519; 95% CI: 1.325, 4.788, respectively). Trap site 1, located on the southwest corner of Lake Goegrup was the most predictive of human RRV cases using the fourth quartile mosquito abundance (Q4 IRR 2.557; 95% CI: 1.154, 5.666). The predictive capacity of these three trap sites may be because they are situated in close proximity to extensive natural saltmarsh mosquito–breeding habitat, bushland that can sustain macropod populations, and densely populated residential suburbs. This convergence of environments may be crucial in allowing the spillover of RRV into the human population when environmental conditions support enhanced enzootic activity of RRV in the region.
Surveillance systems should take into account the potential or risk of mosquito-borne disease in a community as well as available resources.44 This study has evaluated the individual predictive capacity of mosquito trap sites in the Peel region. A reduction in the number of trap sites comes with some fundamental issues, predominately the marked spatiotemporal variabilities in mosquito infection.45 Our study nevertheless broadly suggests mosquito trap sites located in urban environments adjacent to residential housing and large areas of bushland are better predictors of human RRV cases compared with trap sites located in small parks adjacent to residential housing, as larger areas of bushland can support both mosquito harborage and macropod populations. This outcome is important when considering future housing developments, which encroach on wetland and saltmarsh environments and will increase the risk of RRV transmission to humans. We therefore recommend a review of trap locations so that traps that are not as predictive of human cases can be moved to other locations where there is a focus of human RRV cases but where no traps are currently located.
Our study was limited by the small number of human RRV cases in the region and required a wide geographical area to gather enough cases for modeling. Mosquito dispersal experiments indicate 75% of all mosquito species are recaptured within 3 km from their release point.46,47 Therefore, our approach did not enable modeling for subtle spatial features of individual trap sites because of the lack of sufficient human cases within 3 km radii. Furthermore, even smaller numbers of cases were recorded near trap sites 7–9 requiring us to use 20 km radii, which further diluted the robustness of those models. This was evident from the wide confidence intervals for the risk ratio estimates for these two sites. The requirement to use a large radius around each trap site also resulted in substantial overlap of human cases across models, as was apparent from the lack of variation in case numbers across trap sites 1–6 (738 cases to 810 cases) which are no more than 6.5 km apart. Small population sizes meant we were unable to predict small differences in disease risk within the small geographical area. On the other hand, this is unlikely to change public health practice as the current trap sites are predictive of human cases in a wider geographical area. In addition, the severity of human RRV infection is known to vary from asymptomatic to debilitating, so the accurate definition of the true incidence of RRV disease is difficult as milder infections may go undetected or unreported.13 A larger pool of RRV cases would enable a finer assessment of the usefulness of mosquito surveillance for predicting human cases.
In conclusion, we found that mosquito surveillance was useful for the prediction of RRV cases in south-west WA. It may be possible to rationalize trap site locations for routine surveillance, with additional sites being used opportunistically to broaden the understanding of RRV ecology in the region. Evaluating mosquito surveillance for its effectiveness in predicting human arboviral infections is a useful exercise that should be undertaken periodically and may enable rationalization of resources. Adoption of alternative methods of virus detection, such as polymerase chain reaction may improve analysis through increased virus sensitivity or allow for testing of larger numbers of samples. In the future, this may further reduce surveillance program costs.
Acknowledgments:
Mosquito, virus isolate, and human case data for this study were generously provided by the Medical Entomology program within the Environmental Health Directorate of the Department of Health Western Australia. Special thanks to Mike Lindsay who made this work possible and Jay Nicholson for sharing the experience of mosquito trapping.
REFERENCES
- 1.Knope KE, et al. National Arbovirus and Malaria Advisory Committee , 2016. Arboviral diseases and malaria in Australia, 2013–14: annual report of the National Arbovirus and Malaria Advisory Committee. Commun Dis Intell Q Rep 40: E400–E436. [DOI] [PubMed] [Google Scholar]
- 2.Harley D, Bossingham D, Purdie DM, Pandeya N, Sleigh AC, 2002. Ross River virus disease in tropical Queensland: evolution of rheumatic manifestations in an inception cohort followed for six months. Med J Aust 177: 352–355. [DOI] [PubMed] [Google Scholar]
- 3.Russell RC, 2002. Ross River virus: ecology and distribution. Annu Rev Entomol 47: 1–31. [DOI] [PubMed] [Google Scholar]
- 4.Claflin SB, Webb CE, 2015. Ross River virus: many vectors and unusual hosts make for an unpredictable pathogen. PLoS Pathog 11: e1005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harley D, Sleigh A, Ritchie S, 2001. Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin Microbiol Rev 14: 909–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potter A, Johansen CA, Fenwick S, Reid SA, Lindsay MD, 2014. The seroprevalence and factors associated with Ross River virus infection in western grey kangaroos (Macropus fuliginosus) in Western Australia. Vector Borne Zoonotic Dis 14: 740–745. [DOI] [PubMed] [Google Scholar]
- 7.Carver S, Kilpatrick AM, Kuenzi A, Douglass R, Ostfeld RS, Weinstein P, 2010. Environmental monitoring to enhance comprehension and control of infectious diseases. J Environ Monit 12: 2048–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muhar A, Dale PE, Thalib L, Arito E, 2000. The spatial distribution of Ross River virus infections in Brisbane: significance of residential location and relationships with vegetation types. Environ Health Prev Med 4: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu W, Dale P, Turner L, Tong S, 2014. Projecting the impact of climate change on the transmission of Ross River virus: methodological challenges and research needs. Epidemiol Infect 142: 2013–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong S, 2004. Ross River virus disease in Australia: epidemiology, socioecology and public health response. Intern Med J 34: 58–60. [DOI] [PubMed] [Google Scholar]
- 11.Vally H, Peel M, Dowse GK, Cameron S, Codde JP, Hanigan I, Lindsay MD, 2012. Geographic Information Systems used to describe the link between the risk of Ross River virus infection and proximity to the Leschenault estuary, WA. Aust N Z J Public Health 36: 229–235. [DOI] [PubMed] [Google Scholar]
- 12.Tong S, Dale P, Nicholls N, Mackenzie JS, Wolff R, McMichael AJ, 2008. Climate variability, social and environmental factors, and Ross River virus transmission: research development and future research needs. Environ Health Perspect 116: 1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DW, Speers DJ, Mackenzie JS, 2011. The viruses of Australia and the risk to tourists. Travel Med Infect Dis 9: 113–125. [DOI] [PubMed] [Google Scholar]
- 14.Webb CE, 2015. Are we doing enough to promote the effective use of mosquito repellents? Med J Aust 202: 128–129. [DOI] [PubMed] [Google Scholar]
- 15.Harley D, Ritchie S, Bain C, Sleigh AC, 2005. Risks for Ross River virus disease in tropical Australia. Int J Epidemiol 34: 548–555. [DOI] [PubMed] [Google Scholar]
- 16.Murray-Smith S, Weinstein P, Skelly C, 1996. Field epidemiology of an outbreak of dengue fever in Charters Towers, Queensland: are insect screens protective? Aust N Z J Public Health 20: 545–547. [DOI] [PubMed] [Google Scholar]
- 17.Johansen CA, Nicholson J, Power S, Wong S, Burley MMW, Imrie A, Smith D, Shellam G, 2015. Arbovirus Surveillance and Research Laboratory Annual Report: 2013–2014. Nedlands, Western Australia: The University of Western Australia. [Google Scholar]
- 18.Australian Bureau of Statistics , 2014. Data by Local Government Areas Available at: http://stat.abs.gov.au/itt/r.jsp?databyregion#/. Accessed May 27, 2018.
- 19.Lindsay M, Oliveira N, Jasinska E, Johansen C, Harrington S, Wright AE, Smith D, 1996. An outbreak of Ross River virus disease in southwestern Australia. Emerg Infect Dis 2: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Department of Health , 2016. Ross River Virus Infection Case Definition Available at: http://www.health.gov.au/internet/main/publishing.nsf/content/cda-surveil-nndss-casedefs-cd_rrv.htm. Accessed May 27, 2018.
- 21.Lindsay M, Broom A, Oliveira N, Jasinska E, van Heuzen B, Caulfield S, McMinn P, Smith D, Shellam G, 1999. Arbovirus Surveillance and Research Laboratory Annual Report: 1997–1998. Nedlands, Western Australia: The University of Western Australia. [Google Scholar]
- 22.Liehne P, 1991. An Atlas of the Mosquitoes of Western Australia. Perth, Western Australia: Health Department of Western Australia. [Google Scholar]
- 23.Johansen CA, et al. 2017. Characterization of Fitzroy River virus and serologic evidence of human and animal infection. Emerg Infect Dis 23: 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvey LA, Donnelly JA, Lindsay M, Pottumarthyboddu S, D’Abrera VC, Smith DW, 2014. Ross River virus infection surveillance in the Greater Perth Metropolitan area—has there been an increase in cases in the winter months? Commun Dis Intell Q Rep 38: E114–E122. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie SA, Kline DL, 1995. Comparison of CDC and EVS light traps baited with carbon dioxide and octenol for trapping mosquitoes in Brisbane, Queensland (Diptera: Culicidae). Aust Entomol 34: 215–218. [Google Scholar]
- 26.Bisevac L, Franklin DC, Williamson GJ, Whelan PI, 2009. A comparison of two generic trap types for monitoring mosquitoes through an annual cycle in tropical Australia. J Am Mosq Control Assoc 25: 58–65. [DOI] [PubMed] [Google Scholar]
- 27.Hall-Mendelin S, Ritchie SA, Johansen CA, Zborowski P, Cortis G, Dandridge S, Hall RA, van den Hurk AF, 2010. Exploiting mosquito sugar feeding to detect mosquito-borne pathogens. Proc Natl Acad Sci USA 107: 11255–11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roiz D, Roussel M, Munoz J, Ruiz S, Soriguer R, Figuerola J, 2012. Efficacy of mosquito traps for collecting potential West Nile mosquito vectors in a natural Mediterranean wetland. Am J Trop Med Hyg 86: 642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.L’Ambert G, Ferre JB, Schaffner F, Fontenille D, 2012. Comparison of different trapping methods for surveillance of mosquito vectors of West Nile virus in Rhône Delta, France. J Vector Ecol 37: 269–275. [DOI] [PubMed] [Google Scholar]
- 30.Onyango SA, Kitron U, Mungai P, Muchiri EM, Kokwaro E, King CH, Mutuku FM, 2013. Monitoring malaria vector control interventions: effectiveness of five different adult mosquito sampling methods. J Med Entomol 50: 1140–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azil AH, Ritchie SA, Williams CR, 2015. Field worker evaluation of dengue vector surveillance methods: factors that determine perceived ease, difficulty, value, and time effectiveness in Australia and Malaysia. Asia Pac J Public Health 27: 705–714. [DOI] [PubMed] [Google Scholar]
- 32.Sriwichai P, Karl S, Samung Y, Sumruayphol S, Kiattibutr K, Payakkapol A, Mueller I, Yan G, Cui L, Sattabongkot J, 2015. Evaluation of CDC light traps for mosquito surveillance in a malaria endemic area on the Thai-Myanmar border. Parasit Vectors 8: 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pezzin A, Sy V, Puggioli A, Veronesi R, Carrieri M, Maccagnani B, Bellini R, 2016. Comparative study on the effectiveness of different mosquito traps in arbovirus surveillance with a focus on WNV detection. Acta Trop 153: 93–100. [DOI] [PubMed] [Google Scholar]
- 34.Tong S, Hu W, Nicholls N, Dale P, MacKenzie JS, Patz J, McMichael AJ, 2005. Climatic, high tide and vector variables and the transmission of Ross River virus. Intern Med J 35: 677–680. [DOI] [PubMed] [Google Scholar]
- 35.Woodruff RE, Guest CS, Garner MG, Becker N, Lindsay M, 2006. Early warning of Ross River virus epidemics: combining surveillance data on climate and mosquitoes. Epidemiology 17: 569–575. [DOI] [PubMed] [Google Scholar]
- 36.Hu W, Tong S, Mengersen K, Oldenburg B, 2006. Rainfall, mosquito density and the transmission of Ross River virus: a time-series forecasting model. Ecol Modell 196: 505–514. [Google Scholar]
- 37.Jacups SP, Whelan PI, Markey PG, Cleland SJ, Williamson GJ, Currie BJ, 2008. Predictive indicators for Ross River virus infection in the Darwin area of tropical northern Australia, using long-term mosquito trapping data. Trop Med Int Health 13: 943–952. [DOI] [PubMed] [Google Scholar]
- 38.Borah J, Dutta P, Khan SA, Mahanta J, 2013. Epidemiological concordance of Japanese encephalitis virus infection among mosquito vectors, amplifying hosts and humans in India. Epidemiol Infect 141: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvey LA, Johansen CA, Broom AK, Antao C, Lindsay MD, Mackenzie JS, Smith DW, 2014. Rainfall and sentinel chicken seroconversions predict human cases of Murray Valley encephalitis in the north of Western Australia. BMC Infect Dis 14: 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackenzie JS, Lindsay MD, Coelen RJ, Broom AK, Hall RA, Smith DW, 1994. Arboviruses causing human disease in the Australasian zoogeographic region. Arch Virol 136: 447–467. [DOI] [PubMed] [Google Scholar]
- 41.Guo S, Ling F, Hou J, Wang J, Fu G, Gong Z, 2014. Mosquito surveillance revealed lagged effects of mosquito abundance on mosquito-borne disease transmission: a retrospective study in Zhejiang, China. PLoS One 9: e112975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tadei WP, Thatcher BD, Santos JM, Scarpassa VM, Rodrigues IB, Rafael MS, 1998. Ecologic observations on Anopheline vectors of malaria in the Brazilian Amazon. Am J Trop Med Hyg 59: 325–335. [DOI] [PubMed] [Google Scholar]
- 43.Barrera R, Amador M, MacKay AJ, 2011. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl Trop Dis 5: e1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goddard J, 2012. Vector-Borne Disease Surveillance. Public Health Entomology. Boca Raton, FL: CRC Press, 57–82. [Google Scholar]
- 45.Gu W, Unnasch TR, Katholi CR, Lampman R, Novak RJ, 2008. Fundamental issues in mosquito surveillance for arboviral transmission. Trans R Soc Trop Med Hyg 102: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jardine A, Neville PJ, Dent C, Webster C, Lindsay MDA, 2014. Ross River virus risk associated with dispersal of Aedes (Ochlerotatus) camptorhynchus (Thomson) from breeding habitat into surrounding residential areas: Muddy Lakes, Western Australia. Am J Trop Med Hyg 91: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jardine A, Neville PJ, Lindsay MD, 2015. Proximity to mosquito breeding habitat and Ross River virus risk in the Peel region of Western Australia. Vector Borne Zoonotic Dis 15: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]